Abstract
Low intensity (< 2 Vpp/cm (peak to peak voltage/cm)), high frequency (10–30 MHz), and 10 min alternating electric fields (sine wave with no DC component) induce non-contact and enzyme-free cell detachment of anchorage-dependent cells directly from commercially available cell culture flasks and stack plates. 0.25 Vpp/cm, 20 MHz alternating electric field for 10 min at room temperature (RT) induced maximum detachment and separated 99.5 ± 0.1% (mean ± SEM, n = 6) of CHO-K1 and 99.8 ± 0.2% of BALB/3T3 cells from the culture flasks. Both vertical and lateral alternating electric field applications for 10 min at RT detach the CHO-K1 cells from 25 cm2 culture flasks. The alternating electric field application induced cell detachment is almost noncytotoxic, and over 90% of the detached cells remained alive. The alternating electric field applied CHO-K1 cells for 90 min showed little or no lag phase and immediately enter exponential phase in cell growth. Combination of the 20 MHz alternating electric field and enzymatic treatment for 4 min at 37 °C showed synergetic effect and quickly detached human induced pluripotent stem cells from a laminin-coated culture flask compared with the only enzymatic treatment. These results indicate that the rapid cell detachment with both the electric field application and the enzymatic treatment could be applied to subcultures of cells that are susceptible to prolonged enzymatic digestion damage for mass culture of sustainable clinical use.
Keywords: Cell detachment, Alternating electric field, Induced pluripotent stem cells, Stack plates, CHO-K1 cells, BALB/3T3 cells
Introduction
Anchorage-dependent cells are of immense interest for various biotechnological applications. With the advent of stem cells and their use in clinics for cell therapy and regenerative medicine purposes, progress of novel culture devices and technologies for adherent cells has accelerated greatly with a view to a large-scale expansion of these cells (Guedes et al. 2012; Chen et al. 2013; Heathman et al. 2015; Merten 2015). The adherent cells represent a great production means of viruses for vaccination purposes at large scales using microcarriers, and a large number of novel viral vaccines have been developed using this production technology (Merten 2015). Although much research investigating the cell culture has focused on the expansion of cells in vitro, much less involves cell detachment for passaging or as a step towards cryopreservation and storage.
Cell detachment from cell culture platforms is commonly used by enzymatic digestion such as trypsin. However, this method of cell detachment leads to damage to proteins on cell surface or in tissues and affects differentiation efficiency of human mesenchymal stem cells (Hirai et al. 2002; Potapova et al. 2008; Goh et al. 2013). Hirai and colleagues (2002) showed that prolonged trypsinization delayed the first cell division due to the requirement for the recovery from the cell surface damage. Potapova et al. (2008) reported that human mesenchymal stem cell markers such as CD29, CD44, CD54, CD55, CD90, CD105, and HLA-I decreased expressions on the cells with time of exposure to trypsin over a range of 5, 30, and 90 min. Goh et al. (2013) showed that human fetal mesenchymal stem cells on Cytodex 3 detached with protease treatments significantly reduced osteogenic differentiation efficiency. These results clearly indicate that the exposure to the trypsin or the other proteolytic agent should be as short as possible (Nienow et al. 2014; Merten 2015). Therefore, it is necessary to enhance the detachment efficiency of the cells by simultaneous treatment with both the proteolytic agent and a novel non-enzymatic cell detachment technique from commercially available cell culture platforms. The required non-enzymatic cell detachment technique should be based on simple and non-contact physical stimulation without impairing the cells.
Non-enzymatic cell detachment techniques have been reported by several groups and fall into five major classifications. The first is temperature-responsive polymer coated platforms such as culture dishes and microcarriers (Nakajima et al. 2001; Hendrick et al. 2001; Yang et al. 2010). Seeded cells were expanded on the platforms, after which the culture temperature decreased from 37 °C to below 30 °C where it was suggested that the cells were able to detach from the platforms. The second is based on a gold surface modified with a thiol-functionalized RGD peptide (Kakegawa et al. 2013; Yoon and Mofrad 2011). Cells on a zwitterionic oligopeptide monolayer coated gold surface were detached along with desorption of the monolayer by applying a negative electrical potential. The third is ultraviolet light responsive SiO2/TiO2 nanocomposite films (Hong et al. 2013; Cheng et al. 2017). The surface of the SiO2/TiO2 nanocomposite film can transform into super-hydrophilic condition by 365 nm ultraviolet radiation. Cells cultured on a SiO2/TiO2 nanocomposite films were detached by the 365 nm ultraviolet radiation. The fourth is resonance vibration with low temperature mediated cell detachment (Kurashina et al. 2017). With vibration amplitude of 2 μm at 15.9 kHz and cooling at 10 °C, cells cultured on piezoelectric ceramic plate chamber were detached after 10 min of the application. The fifth is high-frequency wave potential induced cell detachment (Koyama 2011). Cultured cells on an indium tin oxide/glass electrode were detached by ± 10 mV versus Ag/AgCl, 9 MHz triangular wave potential application. These 5 methodologies are also able to be classified from view point of cell–surface or cell–cell detachment. The cell–surface detachment is capable of detaching cell sheets from the platforms. The first three methods of the temperature-responsive polymer coated platforms, the gold surface modified with a thiol-functionalized RGD peptide, and the ultraviolet light responsive SiO2/TiO2 nanocomposite films are categorized in the cell–surface detachment. The cell–cell detachment is capable of detaching single cells from the platforms such as the enzymatic digestion. The other 2 methods of the resonance vibration with low temperature and the high-frequency wave potential application are categorized in the cell–cell detachment. These five non-enzymatic cell detachment techniques use original devices and/or fabricated materials and cannot detach cells directly from commercially available cell culture platforms, however.
To develop the simple and non-contact physical cell detachment technique without impairing the cells, we have focused attention on high frequency alternating electric field application. Between 1 and 300 MHz of the alternating electric fields involve only minute dielectric losses (heating) (Hippel 1954). Such electric fields of low intensities (< 2 Vpp/cm) are commonly considered to have no biological effect (Elson 1995; Kirson et al. 2004). Peak-to-peak voltage (Vpp) is full vertical length of a voltage waveform from the very top to the very bottom. The field intensities throughout the manuscript are expressed in peak to peak voltage per centimeter (Vpp/cm).
In the present study we show for the first time, to our knowledge, that the low-intensity (< 2 Vpp/cm) and high frequency (10–30 MHz) alternating electric fields induce non-contact and enzyme-free cell detachment of anchorage-dependent cells directly from commercially available cell culture flasks and stack plates. This novel non-contact and enzyme-free cell detachment method maintains more than 90% of cell survival rate.
Materials and methods
Cultivation of CHO-K1, BALB/3T3, and human iPS cells
Chinese hamster ovary cell line CHO-K1 (JCRB9018, JCRB cell bank, Osaka, Japan), mouse fibroblast cell line BALB/3T3 clone A31 (JCRB9005, JCRB cell bank), and human induced pluripotent stem cells (human iPS cell 771-2 strain, passage number 6, ReproCELL Inc., Kanagawa, Japan; Hirano et al. 2017) were used to evaluate the proposed method as adherent cells. The CHO-K1 cells were cultured in Ham’s F-12 medium (Wako, Osaka, Japan) supplemented with 10 v/v % fetal bovine serum (10 v/v % FBS; Biological Industries Ltd., Cromwell, CT, USA), 100 units/mL penicillin, and 100 μg/mL streptomycin (1 v/v % PS; Wako). The BALB/3T3 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Wako) supplemented with the 10 v/v % FBS and 1 v/v % PS. The human iPS cells were cultured in StemFit AK02N medium (Ajinomoto, Tokyo, Japan) using 0.5 μg/cm2 laminin-511 E8 (iMatrix-511, Nippi, Tokyo, Japan) coated culture flasks. The CHO-K1, BALB/3T3, and human iPS cells were cultured in 25 cm2 Falcon culture flasks (Cat. 353108, Corning incorporated, New York, USA) under a 5% CO2 humidified atmospheric condition at 37 °C.
Cell detachment from culture flask using alternating electric field application
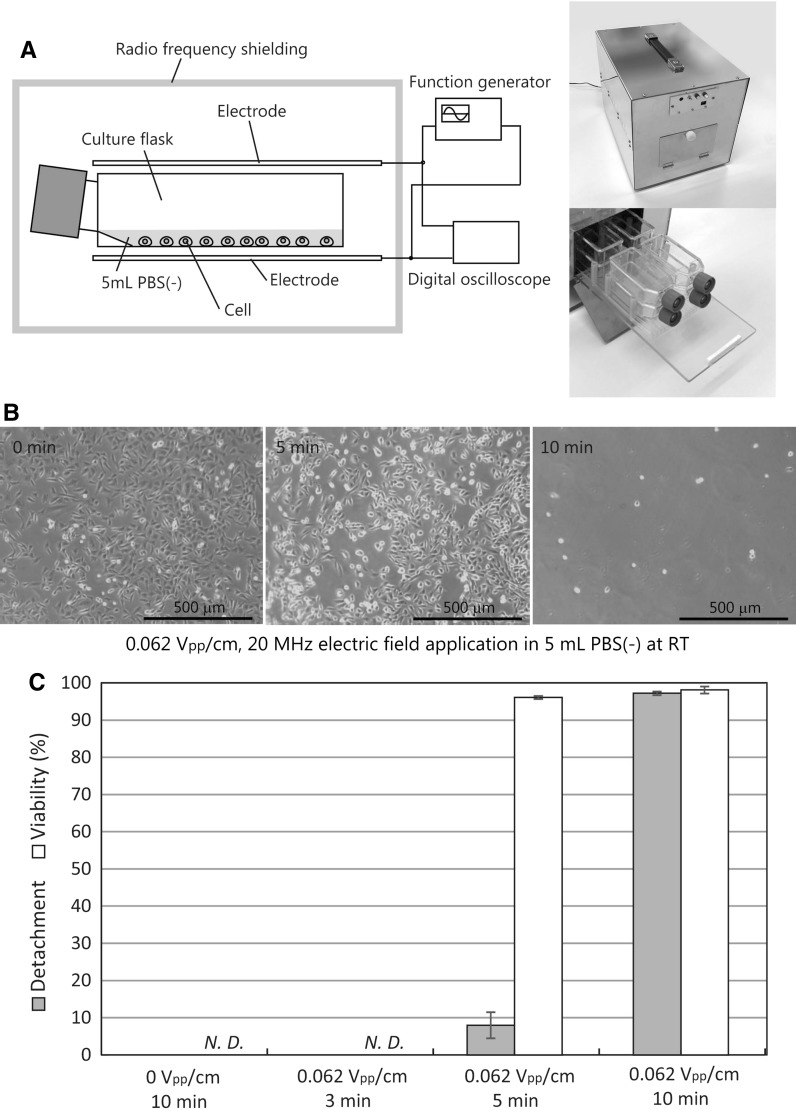
Over 70% sub-confluence of either CHO-K1 or BALB/3T3 cells in the 25 cm2 culture flask were washed with Dulbecco’s calcium and magnesium free phosphate-buffered saline (PBS(−), Wako), and then 5 mL fresh PBS(−) was added into the flasks. The cells in each culture flask were subjected to an alternating electric field (sine wave with no DC component) at room temperature (RT). 10 × 10 cm2 of two stainless-steel plate electrodes were placed on a top and a bottom or both lateral sides of the flask(s) (Figs. 1a, 2a, b). The parallel plate electrodes were connected to a function generator and a digital storage oscilloscope that generated the required sine-wave signals (Fig. 1a). To avoid emitting radiation at radio wavelengths, the cell detachment system was covered with radio frequency shielding (Cell detachment system CD-01, ABLE Corporation, Tokyo, Japan) (Fig. 1a). After the application, the cells were collected from the culture flask by pipetting 7–10 times.
Fig. 1.
Alternating electric field induced CHO-K1 cell detachment from culture flasks. a A schematic illustration and photographs of cell detachment system CD-01. The radio frequency shielding was composed of stainless steel. b 0.062 Vpp/cm, 20 MHz alternating electric field application at RT detached CHO-K1 cells. The detachment of CHO-K1 cells from the culture flask began after 5 min of the electric field application. c Detachment and viability rates of the 20 MHz alternating electric field applied CHO-K1 cells. Data are mean ± SEM (n = 6 in detachment; n = 4 in viability). N.D. not determined
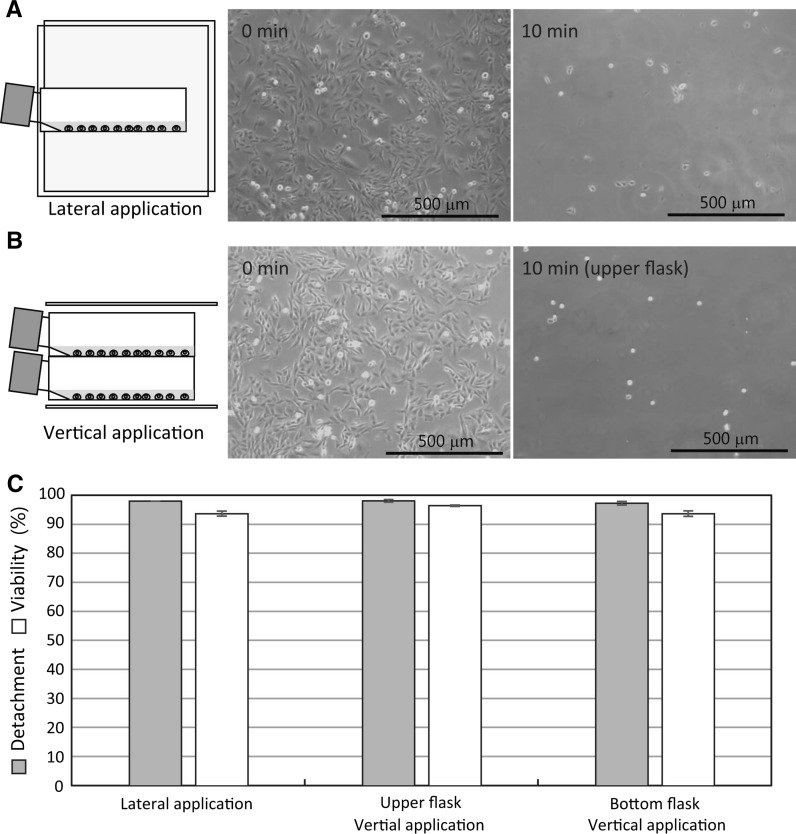
Fig. 2.
Vertical and lateral alternating electric field application induced detachment of CHO-K1 cells from culture flasks. a 0.062 Vpp/cm, 20 MHz lateral alternating electric field application induced detachment of CHO-K1 cells. b 0.062 Vpp/cm, 20 MHz vertical alternating electric field application induced detachment of CHO-K1 cells. c Detachment (one way ANOVA, p = 0.66) and viability rates of the 0.062 Vpp/cm, 20 MHz lateral and vertical alternating electric field applied CHO-K1 cells. Data are mean ± SEM (n = 6 in detachment; n = 4 in viability)
In the cell detachment with a small amount of PBS(−), CHO-K1 cells on the culture flasks were washed by PBS(−), and then 0 or 1 mL fresh PBS(−) added in each of the flasks. The cells in each culture flask were subjected to the vertical alternating electric field for 10 min at RT. After the application, the cells in each culture flask were replaced with 5 mL fresh PBS(−) and were collected by the pipetting 7–10 times. In the cell detachment with a calcium containing phosphate-buffered saline, CHO-K1 cells on the culture flask were washed with 0.9 mM CaCl2 containing Dulbecco’s magnesium free phosphate-buffered saline (PBS(+)), and then 5 mL fresh PBS(+) added into the culture flask. The cells were subjected to the vertical alternating electric field for 10 min at RT. After the application, the cells were collected from the culture flask by pipetting 7–10 times with the 5 mL of PBS(+). In the cell detachment experiment with the medium, CHO-K1 cells on the culture flask were replaced with 5 mL fresh corresponding medium and were subjected to the vertical alternating electric field for 10 min at RT. After the application, the cells were replaced with 5 mL fresh PBS(−) and were collected by the pipetting 7–10 times.
The human iPS cells cultured in the 25 cm2 culture flasks for a week were washed with PBS(−), and then 5 mL fresh 0.5 × TrypLE [mixture of 2.5 mL TrypLE select (Thermo Fisher scientific, Waltham, MA, USA) and 2.5 mL of 0.5 mM EDTA in PBS(−)] was added into the flask. The human iPS cells on the culture flask were vertically subjected to 0.25 Vpp/cm, 20 MHz alternating electric field for 4 min at 37 °C in a humidified 5% CO2 atmosphere. After the application, the human iPS cells were collected from the culture flask by tapping 20 times. In control experiments, the human iPS cells were incubated with 5 mL fresh 0.5 × TrypLE for 4 min at 37 °C in a humidified 5% CO2 atmosphere. After the incubation, the human iPS cells on the culture flask were collected by pipetting 10 times. Images of the human iPS cell colonies on the culture flask were recorded before and after the application using the microscope (CK2, Olympus, Tokyo, Japan) connected with the USB digital camera (Wraycam-G500, Wraymer Inc., Osaka, Japan).
Cell detachment from stack plates using lateral alternating electric field application
Over 70% sub-confluence of CHO-K1 cells in 1264 cm2 two-tiered stack plates (Cell factory, Thermo fisher scientific, MA, USA) were washed twice with PBS(−), and then 350 mL fresh PBS(−) was added into the stack plates. The cells in the stack plates were subjected to 0.25 Vpp/cm, 20 MHz lateral alternating electric field application with intermittent horizontal turn vortex (vortex at 1000 rpm for 2 s and static for 3 s) (radius gyration = 1.6 mm, Cell detachment system, ABLE Corporation) at RT for 10 min. 35 × 15 cm2 of two stainless-steel plate electrodes (21 cm in distance between the electrodes) were placed on both lateral sides of the stack plates. After the 10 min application, the stack plates were further agitated for 10 s at 2300 rpm and static for 10 s 3 times. After the vortex, the cell suspension in the 350 mL PBS(−) was collected and then 150 mL fresh PBS(−) was added into the stack plates. The stack plates were further agitated for 10 s at 2300 rpm and static for 10 s 3 times. After the remaining PBS(−) was collected, cell detachment rates, cell viability, and detached total cell number were analyzed, respectively.
Analyses of cell detachment rates, cell viability, and detached cell number
Number of the CHO-K1 or the BALB/3T3 cells attached on the culture flasks and the stack plates before and after the alternating electric field application were counted in six random areas of 0.25 mm2 culture substrate using a microscope (CK2, Olympus) connected with an USB digital camera (Wraycam-G500, Wraymer Inc.). The cell detachment rate was calculated as a ratio of the decrease in the cell density of the treated cells compared with the cell density of the non-treated cells [(before cell density − after cell density)/before cell density × 100].
Detached cell number and cell viability of the cells were determined using the trypan blue exclusion method with a disposable hemocytometer (Cell counter plate, As one corp., Osaka, Japan). More than 100 cells were counted in in each of the cell viability tests.
Cell growth measurement
The cultured CHO-K1 cells in the 25 cm2 culture flasks were detached by either 0.05 w/v % trypsin–EDTA (Wako) at 37 °C or 20 MHz vertical alternating electric field application at RT (Cell detachment system, ABLE Corporation). The detached CHO-K1 cells were seeded at a cell density of 1 × 105 cells in each Falcon ϕ35 mm culture dishes (Becton, Dickinson and company, NJ, USA) in Ham’s F-12 medium containing 10 v/v % FBS and 1 v/v % PS. When the CHO-K1 cells attached on the culture dishes after 3 h of incubation at 37 °C in a humidified 5% CO2 atmosphere, we measured the cell growth of either the trypsinized or the alternating electric field applied cells. The cells in each of the dishes were treated with 0.5 mL 10 × trypsin–EDTA (0.5 w/v % trypsin—5.3 mM EDTA solution, Wako) for 3 min at 37 °C and were detached by cell scraping. The trypsinization was stopped by adding 0.5 mL culture medium. After the cell collection, total cell number in the culture dish was measured by cell viability analyzer Vi-cell XR (Beckman coulter, Inc., CA, USA).
Statistical analysis
Statistical analysis was performed using Student’s t test (2-tailed). In Fig. 2, statistical analysis of detachment rates was performed using a one way ANOVA test. The calculations were performed using Microsoft Excel.
Results
Detachment of CHO-K1 cells from culture flask
Figure 1 shows vertical alternating electric field application induced detachment of CHO-K1 cells from the 25 cm2 culture flask. The CHO-K1 cells in 5 mL PBS(−) were vertically exposed to 0.062 Vpp/cm, 20 MHz alternating electric field for 3, 5, and 10 min at RT, respectively (Fig. 1). The detachment of CHO-K1 cells from the culture flask began after 5 min, and separation was completed 10 min after the electric field application (Fig. 1b). Figure 1c shows cell detachment rates and cell viabilities in the CHO-K1 cells. The 0.062 Vpp/cm, 20 MHz alternating electric field detached 8.0 ± 3.5% (mean ± SEM, n = 6) of the CHO-K1 cells after 5 min application and almost all (97.1 ± 0.4%) of the cells were detached after 10 min application (Fig. 1c). The alternating electric field application induced cell detachment is almost noncytotoxic, and 96.0 ± 0.4% and 98.0 ± 0.9% (mean ± SEM, n = 4) of the detached CHO-K1 cells remained alive after 5 and 10 min application, respectively (Fig. 1c). Only PBS(−) treatment for 10 min and the 3 min vertical electric field application did not detach the CHO-K1 cells from the culture flasks (Fig. 1c).
Figure 2a, c show lateral alternating electric field application induced detachment of CHO-K1 cells from the 25 cm2 culture flask. The CHO-K1 cells in 5 mL PBS(−) were laterally exposed to 0.062 Vpp/cm, 20 MHz alternating electric field for 10 min at RT (Fig. 2a, c). 97.8 ± 0.5% of the CHO-K1 cells were detached from the culture flask and 93.5 ± 0.9% of the cells kept alive after 10 min application (Fig. 2c). Next, we investigated whether the CHO-K1 cells in stacked two culture flasks were detached by the vertical alternating electric field application. The cells in both the upper and the bottom culture flasks were detached after 10 min application (Fig. 2b, c). Cell detachment rates of the upper and the bottom culture flasks were 97.9 ± 0.7% and 97.1 ± 0.8%, respectively (Fig. 2b, c). The results in Fig. 2c indicated that no significant difference between vertical and lateral alternating electric field applications induced detachment of CHO-K1 cells from the culture flask substrates (one way ANOVA, P = 0.66). The detached CHO-K1 cells in the upper and the bottom culture flasks remained alive after 10 min application. The cell viabilities of the detached CHO-K1 cells were 96.3 ± 0.3% and 93.5 ± 1.0% in the upper and the bottom flasks, respectively (Fig. 2c).
We investigated whether the CHO-K1 cells in calcium ion containing solutions were detached from the culture flask by the alternating electric field application (Fig. 3). The CHO-K1 cells in either 5 mL Ham’s F-12 medium or 5 mL 0.9 mM CaCl2 containing Dulbecco’s magnesium free phosphate-buffered saline (PBS(+)) were vertically exposed to 0.062 Vpp/cm, 20 MHz alternating electric field for 10 min at RT (Fig. 3b, c, g). The alternating electric field applications did not detach the CHO-K1 cells from the culture flasks in the medium and in the PBS(+), respectively (Fig. 3b, c, g). Next, we investigated whether the cell detachment is dependent on the fluid volume of PBS(−). The cells were replaced with 0, 1, or 5 mL of PBS(−) in each of the culture flasks and were exposed to the vertical alternating electric field for 10 min (Fig. 3d–g). In the 1 mL PBS(−), 42.7 ± 10.0% of the CHO-K1 cells were detached from the culture flask and 97.0 ± 1.6% of the cells remained alive (Fig. 3e, g). In the 5 mL PBS(−), the alternating electric field application detached 97.1 ± 0.4% of the CHO-K1 cells and showed the survival rate of 98.0 ± 0.9% (Figs. 1, 3d, g). Although cell rounding of the CHO-K1 cells was observed in both the 0 mL PBS(−) with the alternating electric field application and only 5 mL PBS(−) treatments for 10 min, both the cells did not detach from the culture flasks (Fig. 3a, f, g). The results in Fig. 3 indicated that the CHO-K1 cell detachment depends on fluid volume of the PBS(−).
Fig. 3.
Alternating electric field applied CHO-K1 cell detachment depends on fluid volume of the PBS(−). a Only 5 mL PBS(−) treatment for 10 min at RT. The 0.062 Vpp/cm, 20 MHz vertical alternating electric field applied the CHO-K1 cells for 10 min at RT in b 5 mL Ham’s F-12 medium, c 5 mL PBS(+), d 5 mL PBS(−), e 1 mL PBS(−), and f 0 mL PBS(−). g Detachment and viability rates of the CHO-K1 cells. Only 5 mL PBS(−) treatment for 10 min at RT indicates 0 V/cm, 5 mL PBS(−). 0.062 Vpp/cm, 20 MHz vertical alternating electric field was applied to the CHO-K1 cells in 5 mL Ham’s F-12 medium, 5 mL PBS(+), 0 mL PBS(−), 1 mL PBS(−), or 5 mL PBS(−) for 10 min at RT, respectively. Data are mean ± SEM (n = 6 in detachment; n = 4 in viability). N.D. not determined
Frequency and electric field intensity dependencies of cell detachment
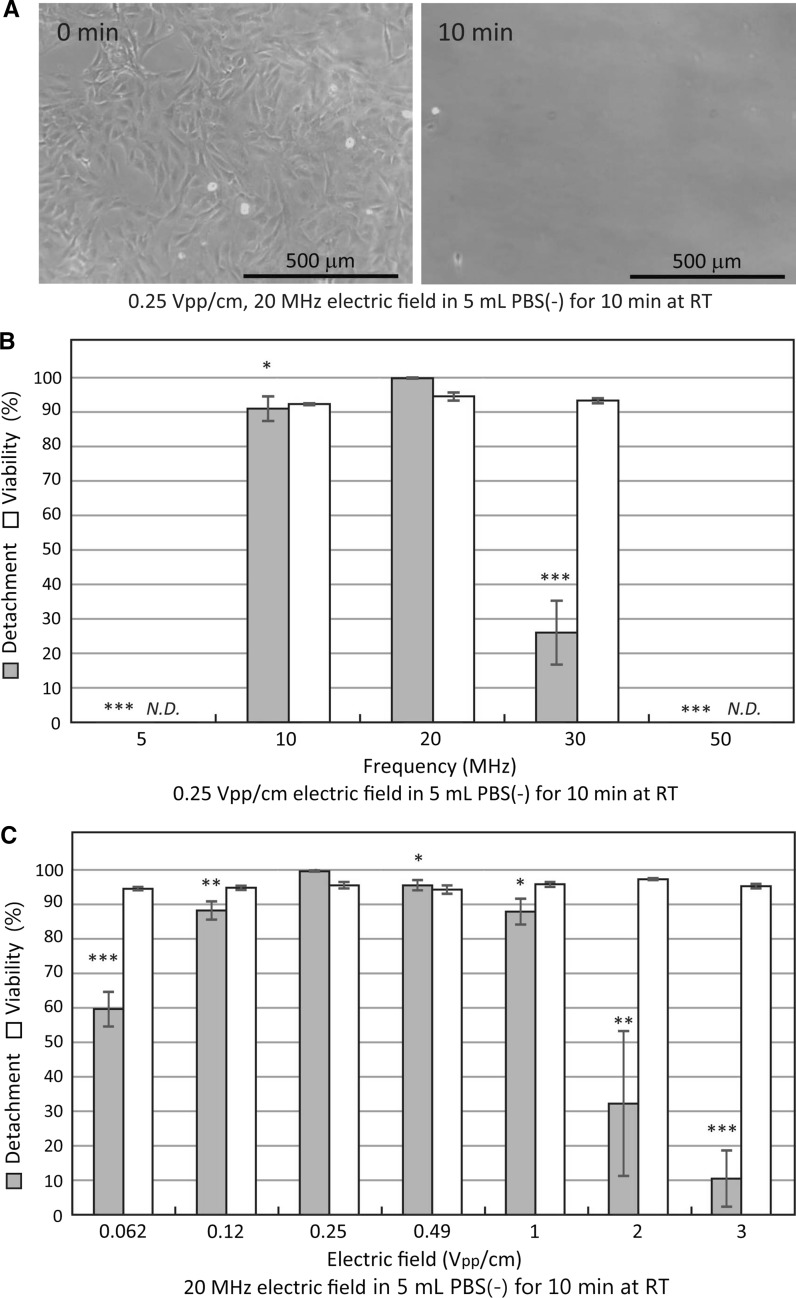
We investigated frequency dependency of cell detachment rates and cell viabilities in CHO-K1 cells (Fig. 4). The CHO-K1 cells on the culture flasks were vertically exposed to 0.062 Vpp/cm, between 0.5 and 150 MHz of alternating electric fields for 10 min at RT (Fig. 4a). We found that between 2 and 30 MHz of the alternating electric field applications detached over 80% of the CHO-K1 cells (Fig. 4a). The alternating electric field induced cell detachment peaked at 20 MHz (Fig. 4a). Using the 20 MHz electric field, we examined the electric field intensity dependency of cell detachment rates and cell viabilities (Fig. 4b). The CHO-K1 cells in the culture flasks were vertically exposed to 0.0077–3 Vpp/cm, 20 MHz alternating electric field for 10 min at RT (Fig. 4b). Over 90% of the CHO-K1 cells on the culture flask substrates were detached by 0.015–0.49 Vpp/cm, 20 MHz alternating electric fields for 10 min at RT (Fig. 4b). The 0.25 Vpp/cm, 20 MHz alternating electric field application reached maximum detachment and separated 99.5 ± 0.1% of the CHO-K1 cells from the culture flask after 10 min application (Fig. 4b). Higher and lower alternating electric field intensities tend to decrease the cell detachment rates (Fig. 4b). Over 90% of the detached cells were remained alive (Fig. 4) and grew normally on the culture flask substrates.
Fig. 4.
Frequency and electric field intensity dependencies of detachment and viability rates in CHO-K1 cells. a Detachment and viability rates of 0.062 Vpp/cm alternating electric field applied CHO-K1 cells for 10 min at RT. **P < 0.01, ***P < 0.001 compared with 20 MHz of the detachment. b Detachment and viability rates of 20 MHz alternating electric field applied CHO-K1 cells for 10 min at RT. Data are mean ± SEM (n = 6 in detachment; n = 4 in viability). **P < 0.01, ***P < 0.001 compared with 0.25 Vpp/cm of the detachment
Next, we examined frequency dependency of cell detachment rates and cell viabilities in BALB/3T3 cells (Fig. 5). The BALB/3T3 cells in the culture flasks were vertically exposed to 0.25 Vpp/cm, between 5 and 50 MHz of alternating electric fields for 10 min at RT (Fig. 5b). Detachment of the BALB/3T3 cells was observed in narrow frequency band of between 10 and 30 MHz alternating electric fields for 10 min at RT (Fig. 5b). The alternating electric field induced cell detachments peaked at 20 MHz (Fig. 5b). Little or no cells were detached by either 5 or 50 MHz alternating electric field applications for 10 min at RT (Fig. 5b). Using the 20 MHz peak cell detachment, we examined the alternating electric field intensity dependency of cell detachment rates and cell viabilities in BALB/3T3 cells (Fig. 5c). The BALB/3T3 cells were vertically exposed to 0.062–3 Vpp/cm, 20 MHz alternating electric fields for 10 min at RT (Fig. 5c). Over 80% of the cells on the culture flasks were detached by 0.12–1 Vpp/cm, 20 MHz alternating electric field applications for 10 min at RT (Fig. 5c). The 0.25 Vpp/cm and 20 MHz alternating electric field application induced cell detachment reached maximum and separated 99.8 ± 0.2% of the BALB/3T3 cells from the culture flask after 10 min application (Fig. 5). Over 90% of the detached cells were remained alive after the 10 min applications (Fig. 5) and grew normally on the culture flask substrates. The results in Figs. 4 and 5 clearly showed that 0.25 Vpp/cm, 20 MHz alternating electric field application for 10 min at RT reached maximum detachments and separated 99.5 ± 0.1% and 99.8 ± 0.2% of CHO-K1 and BALB/3T3 cells from the culture flasks, respectively. The cell viabilities of CHO-K1 and BALB/3T3 cells at the 0.25 Vpp/cm, 20 MHz alternating electric field applications were both 95.8 ± 0.9% (Figs. 4 and 5).
Fig. 5.
Frequency and electric field intensity dependencies of detachment and viability rates in BALB/3T3 cells. a Photographs of 0.25 Vpp/cm, 20 MHz vertical alternating electric field application detached BALB/3T3 cells. b Detachment and viability rates of 0.25 Vpp/cm alternating electric field applied BALB/3T3 cells for 10 min at RT. *P < 0.05, ***P < 0.001 compared with 20 MHz of the detachment. c Detachment and viability rates of 20 MHz alternating electric field applied BALB/3T3 cells for 10 min at RT. Data are mean ± SEM (n = 6 in detachment; n = 4 in viability). N.D. not determined. *P < 0.05, **P < 0.01, ***P < 0.001 compared with 0.25 Vpp/cm of the detachment
Initial cell growth of alternating electric field detached CHO-K1 cells
Next, we compared initial cell growth of the alternating electric field detached CHO-K1 cells with that of the trypsinized cells (Fig. 6). The CHO-K1 cells on the culture flask were detached by either 20 MHz vertical alternating electric field applications for 10 min and 90 min at RT or trypsin–EDTA treatments for 10 min and 90 min at 37 °C (Fig. 6). The detached CHO-K1 cells were seeded at a cell density of approximately 1 × 105 cells/35 mm culture dish (Fig. 6). After 3 h of the cell culture, total cell number in the culture dishes were counted as 0 h of cell growth (Fig. 6). Generally, doubling time (Dt) of CHO-K1 cells is from 14 h to 20 h under optimum conditions and initial proliferation rate of the cells is affected by seeding cell density. We found that all of the 0.062 Vpp/cm, 0.25 Vpp/cm, and 1 Vpp/cm, 20 MHz alternating electric field application for 10 and 90 min at RT showed little or no lag phase of the CHO-K1 cells and immediately enter exponential phase, respectively (Fig. 6a–c). Meanwhile, length of lag phase in the trypsinized CHO-K1 cells depends on exposure time of the trypsin–EDTA solution (Fig. 6d).
Fig. 6.
Initial cell growth of CHO-K1 cells after the vertical alternating electric field application or trypsinization. The each CHO-K1 cells were subcultured by a 0.062 Vpp/cm, 20 MHz alternating electric field at RT, b 0.25 Vpp/cm, 20 MHz alternating electric field at RT, c 1 Vpp/cm, 20 MHz alternating electric field at RT, and d trypsin EDTA treatment at 37 °C. Data are mean ± SEM of two independent experiments in duplicate (n = 4)
Detachment of human iPS cells from culture flask
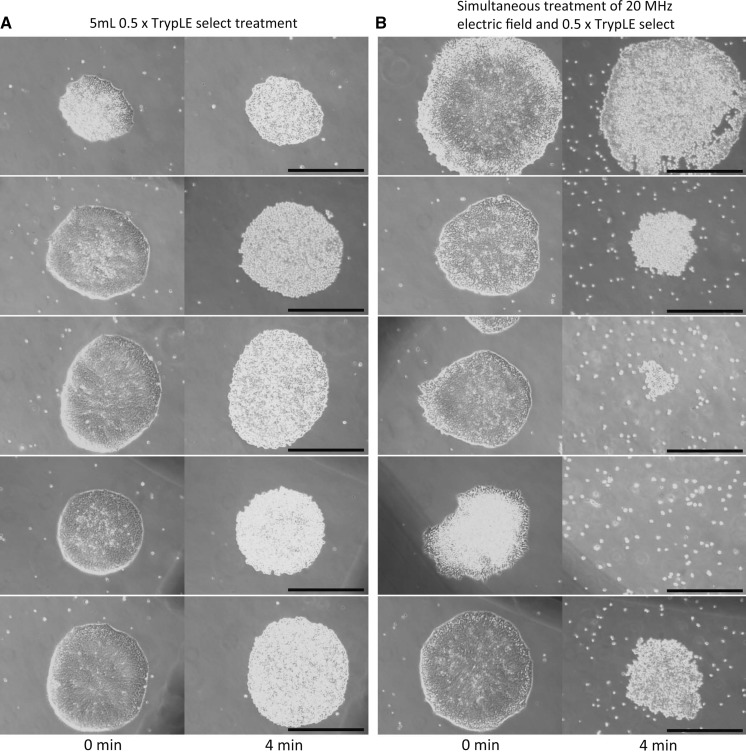
Next, we examined whether human iPS cells were quickly detached from the culture flask by simultaneous treatment of enzymatic digestion and alternating electric field application. The cultured human iPS cells attached tightly to the 0.5 μg/cm2 laminin-511 E8 coated culture flask. Therefore, 0.5 × TrypLE select enzymatic treatment for 4 min at 37 °C with pipetting 10 times did not detach the human iPS cells from the culture flask (Fig. 7a). In addition, only 0.25 Vpp/cm, 20 MHz alternating electric field application in 5 mL PBS(−) for 4 min at 37 °C with pipetting 10 times did not detach the human iPS cells. We found that simultaneous stimulation of 0.25 Vpp/cm, 20 MHz alternating electric field application and 0.5 × TrypLE select treatment for 4 min at 37 °C detached the human iPS cells from the culture flask (Fig. 7b). Because in monolayer culture, the cell culture flask is usually manually tapped to aid detachment after removal from the incubator, we decide to tap the culture flask 20 times instead of the pipetting of the human iPS cells. After the simultaneous stimulation and 20 times of tapping at a side of the culture flask, a part or whole of the human iPS cells in the colony were detached from the culture flask (Fig. 7b). Cell viability of the detached human iPS cells was 97% (= 138/142). The results in Fig. 7 demonstrated that combination of the alternating electric field application and the enzymatic treatment exhibits synergetic effect of the rapid cell detachment.
Fig. 7.
Detachment of human iPS cells by combination of enzymatic digestion and vertical alternating electric field application. Each bar in the photographs indicate 500 μm. a Human iPS cells on the 0.5 mg/cm2 laminin-511 coated culture flask were treated with 0.5 × TrypLE select at 37 °C for 4 min. After incubation, the cells were detached by 10 times pipetting with the 0.5 × TrypLE select. b Human iPS cells on the 0.5 mg/cm2 laminin-511 coated culture flask were treated with both 0.5 × TrypLE select and 0.25 Vpp/cm, 20 MHz vertical electric field application at 37 °C for 4 min. After incubation, the cells were detached by 20 times tapping at a side of the culture flask. Cell viability of the detached cells was 97% (= 138/142)
Alternating electric field application detached CHO-K1 cells from stack plates
We examined whether CHO-K1 cells were detached from the 1264 cm2 two-tiered stack plates (Cell factory) by lateral alternating electric field application with vortex (Fig. 8). When the stack plates were exposed to continuous vortex at 1300 rpm for 3 min after the alternating electric field application for 10 min at RT, cell suspension in PBS(−) made bubbles and injured most of the detached CHO-K1 cells. Therefore, the cells in the stack plates were subjected to 0.25 Vpp/cm, 20 MHz lateral alternating electric field application with intermittent vortex (weak vortex at 1000 rpm for 2 s and static for 3 s) at RT for 10 min. After the 10 min application, the stack plates were agitated for 10 s at 2300 rpm and static for 10 s 3 times. After the vortex, the cell suspension in the 350 mL PBS(−) was collected and then 150 mL fresh PBS(–) was added into the stack plates. The stack plates were further agitated for 10 s at 2300 rpm and static for 10 s 3 times. We found that treatment of 0.25 Vpp/cm, 20 MHz lateral alternating electric field application with the intermittent vortex for 10 min in PBS(−) at RT detached 97.0 ± 1.2% (mean ± SEM, n = 6) of the CHO-K1 cells from the bottom plate (Fig. 8). We used the two-tiered 1264 cm2 stack plates and did not observe the cells on the upper plate. Therefore, we counted the detached total cell numbers by hemocytometer. The detached total cell number and the cell viability of the CHO-K1 cells were 12.7 ± 1.2 × 107 cells/1264 cm2 (n = 12) and 91.5 ± 0.6% (n = 4) after treatment of the lateral alternating electric field with the intermittent vortex, respectively (Fig. 8). The results in Fig. 8 indicated that the treatment of 0.25 Vpp/cm, 20 MHz lateral alternating electric field application with the intermittent vortex separated almost all of the CHO-K1 cells from the stack plates. The detached cells were remained alive and grew normally on the culture flask substrates.
Fig. 8.
Detachment of CHO-K1 cells from 1264 cm2 two-tiered stack plates by simultaneous treatment of lateral alternating electric field application and intermittent vortex. a Photographs of 0.25 Vpp/cm, 20 MHz lateral alternating electric field application with intermittent vortex detached CHO-K1 cells from the bottom plate. b Detachment rates, viability rates, and detached total cell number of 0.25 Vpp/cm, 20 MHz lateral alternating electric field application for 10 min at RT with intermittent vortex detached CHO-K1 cells. Data are mean ± SEM (n = 6 in detachment, n = 4 in viability, n = 12 in detached total cell number). Details of cell detachment method were described in the Materials and Methods
Discussion
The results presented in this paper demonstrated that (1) the low intensity (< 2 Vpp/cm) and high frequency of 10–30 MHz alternating electric field applications can detach anchorage-dependent animal cells directly from commercially available cell culture platforms and (2) simultaneous treatment of alternating electric field application and enzymatic digestion exhibits synergetic effect of the rapid cell detachment. This novel non-contact and non-enzymatic cell detachment method maintains more than 90% of cell survival rate.
We have demonstrated that both the vertical and lateral alternating electric field applications for 10 min at RT detach the CHO-K1 cells from 25 cm2 culture flasks (Figs. 1 and 2). The 0.25 Vpp/cm, 20 MHz alternating electric field for 10 min at RT induced maximum cell detachments and separated 99.5 ± 0.1% and 99.8 ± 0.2% of CHO-K1 and BALB/3T3 cells from the culture flasks, respectively (Figs. 4 and 5). The CHO-K1 cells were detached from the stack plates by the 0.25 Vpp/cm, 20 MHz lateral alternating electric field application with the intermittent vortex for 10 min at RT (Fig. 8). Therefore, the 0.25 Vpp/cm, 20 MHz alternating electric field applications would detach a wide range of anchorage-dependent animal cells for subculture. Detachment of the BALB/3T3 cells has the narrow frequency band and the small range of the electric field intensity compared to that of the CHO-K1 cells (Figs. 4 and 5). Because adhesion area of the BALB/3T3 cell to the culture flask is larger than that of the CHO-K1 cell (Figs. 1 and 5), strong adhesion of the BALB/3T3 cells on the culture flask might reflect the narrow frequency band and the small electric field intensity range of the cell detachment. Our finding results indicate that uniform alternating electric field application from parallel plate electrodes can detach anchorage-dependent animal cells from a wide range of sizes and shapes of cell culture platforms such as culture flasks (Figs. 2, 4, and 5) and stack plates (Fig. 8).
The alternating electric field applications did not detach the CHO-K1 cells from the culture flasks in the medium and in the PBS(+), respectively (Fig. 3b, c, g). The CHO-K1 cell detachment depends on fluid volume of the PBS(−) (Fig. 3). Moreover, the CHO-K1 cells did not detach from the 0 mL PBS(−) culture flasks (Fig. 3). Therefore, the mechanisms of alternating electric field application induced cell detachment would involve release of calcium ions from cell adhesion molecules such as cadherins and integrins into the PBS(−) (Fig. 3).
Over 1 Vpp/cm alternating electric field intensity decreases cell detachment rates of both CHO-K1 and BALB/3T3 cells (Figs. 4 and 5). Moreover, we found that both the vertical and lateral alternating electric field applications detach the CHO-K1 cells from 25 cm2 culture flasks (Figs. 1 and 2). Alternating electric field applications between 1 and 300 MHz involve only minute dielectric losses and little heating (Hippel 1954). Such fields of low intensities are generally considered to have no biological effect (Elson 1995; Kirson et al. 2004). Less than 1 MHz of low-frequency alternating electric field increases dielectric loss of liquid consists primarily of water (Hippel 1954). The low-frequency alternating electric field moves the ions such as H+ and OH−, hits surrounding water molecules, causes dissipation of energy, and increases the dielectric loss of the liquid. Therefore, non-thermal effects of minor biological consequence would affect the alternating electric field application induced cell detachment even at the low electric field intensity of both the vertical and lateral alternating electric field applications.
We found that length of lag phase in the trypsinized CHO-K1 cells depends on exposure time of the trypsin–EDTA solution (Fig. 6d). Meanwhile, all of the alternating electric field applied cells showed little or no lag phase and immediately enter exponential phase, respectively (Fig. 6a–c). Cell growth of animal cells shows four distinct phases: lag phase, the delay before the start of exponential growth; exponential phase, where cell division proceeds at a constant rate; stationary phase, when conditions become unfavorable for growth and cells stop replicating; death phase, when cells lose viability. Because Hirai et al. (2002) reported that prolonged trypsinization delayed the first cell division due to the recovery from cell surface damage, the alternating electric field applications induced cell detachments would be less damage to the cells (Fig. 6).
Subculture of human iPS cells commonly used sequential treatments of brief enzymatic digestion and mechanical detachment with cell scraper (Nakagawa et al. 2014). However, the cell scraper method might reduce cell viability of the human iPS cells significantly. Moreover, for large-scale cell expansion the cell scraper method cannot be used. We demonstrated that the human iPS cells were quickly detached by combination of enzymatic digestion and alternating electric field application for 4 min at 37 °C (Fig. 7). Cell viability of the detached human iPS cells was 97%. These results indicated that the combination of both the enzymatic and the non-enzymatic treatments could be applied to subcultures of cells that are susceptible to prolonged enzymatic digestion damage for mass culture of sustainable clinical use.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Chen AK-L, Reuveny S, Oh SKW. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotech Adv. 2013;31:1032–1046. doi: 10.1016/j.biotechadv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Cheng Z, Cheng K, Weng W. SiO2/TiO2 nanocomposite films on polystyrene for light-induced cell detachment application. ACS Appl Mater Interfaces. 2017;9:2130–2137. doi: 10.1021/acsami.6b14182. [DOI] [PubMed] [Google Scholar]
- Elson E. Biologic effects of radiofrequency and microwave fields: in vivo and in vitro experimental results. In: Bronzino JD, editor. The biomedical engineering handbook. Boca Raton: CRC Press Inc; 1995. pp. 1417–1423. [Google Scholar]
- Goh TK-P, Zhang Z-Y, Chen AK-L, Reuveny S, Choolan M, Chan JKY, Oh SK-W. Microcarrier culture for efficient expansion and osteogenic differentiation of human fetal mesenchymal stem cells. Biores Open Access. 2013;2:84–97. doi: 10.1089/biores.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guedes JC, Park J-H, Lakhkar NJ, Kim H-W, Knowles JC, Wall IB. TiO2-doped phosphate glass microcarriers: a stable bioactive substrate for expansion of adherent mammalian cells. J Biomater Appl. 2012;28:2–11. doi: 10.1177/0885328212459093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathman TRJ, Glyn VAM, Picken A, Rafiq QA, Coopman K, Nienow AW, Kara B, Hewitt CJ. Expansion, harvest and cryopreservation of human mesenchymal stem cells in a serum-free microcarrier process. Biotech Bioeng. 2015;112:1696–1706. doi: 10.1002/bit.25582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrick V, Muniz E, Geuskens G, Wérenne J. Adhesion, growth and detachment of cells on modified polystyrene surface. Cytotechnology. 2001;36:49–53. doi: 10.1023/A:1014041003617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippel RV. Dielectric materials and applications. Cambridge: MIT Press; 1954. p. 438. [Google Scholar]
- Hirai H, Umegaki R, Kino-Oka M, Taya M. Characterization of cellular motions through direct observation of individual cells at early stage in anchorage-dependent culture. J Biosci Bioeng. 2002;94:351–356. doi: 10.1016/S1389-1723(02)80176-1. [DOI] [PubMed] [Google Scholar]
- Hirano K, Konagaya S, Turner A, Noda Y, Kitamura S, Kotera H, Iwata H. Closed-channel culture system for efficient and reproducible differentiation of human pluripotent stem cells into islet cells. Biochem Biophys Res Commun. 2017;487:344–350. doi: 10.1016/j.bbrc.2017.04.062. [DOI] [PubMed] [Google Scholar]
- Hong Y, Yu M, Weng W, Cheng K, Wang H, Lin J. Light-induced cell detachment for cell sheet technology. Biomaterials. 2013;34:11–18. doi: 10.1016/j.biomaterials.2012.09.043. [DOI] [PubMed] [Google Scholar]
- Kakegawa T, Mochizuki N, Sadr N, Suzuki H, Fukuda J. Cell-adhesive and cell-repulsive zwitterionic oligopeptides for micropatterning and rapid electrochemical detachment of cells. Tisssue Eng A. 2013;19:290–298. doi: 10.1089/ten.tea.2011.0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirson ED, Gurvich Z, Schneiderman R, Dekel E, Itzhaki A, Wasserman Y, Schatzberger R, Palti Y. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64:3288–3295. doi: 10.1158/0008-5472.CAN-04-0083. [DOI] [PubMed] [Google Scholar]
- Koyama S (2011) Electrically modulated attachment and detachment of animal cells cultured on an optically transparent patterning electrode. J Biosci Bioeng 111:578–583. (Corrigendum in: Koyama S (2012) J Biosci Bioeng 114:240) [DOI] [PubMed]
- Kurashina Y, Hirano M, Imashiro C, Totani K, Komotori J, Takemura K. Enzyme-free cell detachment mediated by resonance vibration with temperature modulation. Biotech Bioeng. 2017;114:2279–2288. doi: 10.1002/bit.26361. [DOI] [PubMed] [Google Scholar]
- Merten O-W. Advances in cell culture: anchorage dependence. Philos Trans R Soc B. 2015;370:20140040. doi: 10.1098/rstb.2014.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa M, Taniguchi Y, Senda S, Takizawa N, Ichisaka T, Asano K, Morizane A, Doi D, Takahashi J, Nishizawa M, Yoshida Y, Toyoda T, Osafune K, Sekiguchi K, Yamanaka S. A novel efficient feeder-free culture system for the derivation of human induced pluripotent stem cells. Sci Rep. 2014;4:3594. doi: 10.1038/srep03594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima K, Honda S, Nakamura Y, López-Redondo F, Kohsaka S, Yamato M, Kikuchi A, Okano T. Intact microglia are cultured and non-invasively harvested without pathological activation using a novel cultured cell recovery method. Biomaterials. 2001;22:1213–1223. doi: 10.1016/S0142-9612(00)00270-2. [DOI] [PubMed] [Google Scholar]
- Nienow AW, Rafiq QA, Coopman K, Hewitt CJ. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem Eng J. 2014;85:79–88. doi: 10.1016/j.bej.2014.02.005. [DOI] [Google Scholar]
- Potapova IA, Brink PR, Cohen IS, Doronin SV. Culturing of human mesenchymal stem cells as three-dimensional aggregates induces functional expression of CXCR4 that regulates adhesion to endothelial cells. J Biol Chem. 2008;283:13100–13107. doi: 10.1074/jbc.M800184200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HS, Jeon O, Bhang SH, Lee S-H, Kim B-S. Suspension culture of mammalian cells using thermosensitive microcarrier that allows cell detachment without proteolytic enzyme treatment. Cell Transpl. 2010;19:1123–1132. doi: 10.3727/096368910X516664. [DOI] [PubMed] [Google Scholar]
- Yoon SH, Mofrad MR. Cell adhesion and detachment on gold surfaces modified with a thiol-functionalized RGD peptide. Biomaterials. 2011;32:7286–7296. doi: 10.1016/j.biomaterials.2011.05.077. [DOI] [PubMed] [Google Scholar]