Abstract
Bone marrow-derived early outgrowth cells play an important role in endothelial repair. In vitro isolation techniques have identified two distinct morphological early outgrowth cell populations, but it is still unknown whether they present some functional phenotypic differences. Accordingly, the aim of the present study was to determine whether there are phenotypic differences in cellular function between two putative early outgrowth cells in culture. Peripheral blood samples were collected from 18 healthy adults. Thereafter, mononuclear cells were isolated by Ficoll density-gradient centrifugation and plated on 6-well plates coated with human fibronectin. After 2 and 7 days, respectively, non-adherent cells (NAC) and adherent cells (AC) underwent functional assays in order to measure the migratory capacity (Boyden chamber), angiogenic growth factor release (ELISA) and apoptosis (TUNEL). Migration to both VEGF (517 ± 74 vs. 273 ± 74 AU) and SDF-1 (517 ± 68 vs. 232 ± 68 AU) were approximately twofold higher (P < 0.05) in the NAC when compared to AC. Release of angiogenic factors, granulocyte colony-stimulating and hepatocyte growth factor, were not different between cell types. Apoptotic response to staurosporine was significantly lower in NAC (20 ± 32 vs. 125 ± 32%). In summary, NAC and AC demonstrated functional phenotypic differences in migratory capacity and apoptotic susceptibility, which makes it difficult to compare these two early outgrowth cell populations in literature.
Keywords: Early outgrowth cells, Endothelium, Angiogenic growth factors, Cell migration, Cell apoptosis
Introduction
Asahara et al. (Asahara et al. 1997) first described a population of human circulating CD34-positive cells that could differentiate ex vivo into cells with endothelial cell-like characteristics, called endothelial progenitor cells. These bone marrow-derived progenitor cells are considered to have a pivotal role in maintaining the integrity of the endothelium and postnatal vasculogenesis (Asahara et al. 1997; Urbich and Dimmeler 2004).
They are able to migrate to local sites of endothelial damage, secrete potent growth factors and participate in vascular repair (Kawamoto et al. 2001). Declines in these angiogenic cells bioavailability and function, including reduced colony formation and migration capacity, have been linked with accelerated atherosclerotic disease progression, poor cardiovascular disease prognosis and adverse outcomes (Hill et al. 2003; MacEneaney et al. 2010). However, subsequent studies have indicated these cells may play minimal or no role in neovascularization of tumors, vessel repair, or normal vessel growth and development (Gothert et al. 2004; Stadtfeld and Graf 2005). Controversies surrounding these fundamental questions may in part originate from the heterogeneous phenotypic definitions of endothelial progenitor cells and a lack of functional clonogenic assays to isolate and accurately describe these subpopulations (Yoder et al. 2007).
Two early endothelial outgrowth populations can be isolated in culture using the non-adherent (NAC) or adherent cells (AC) from peripheral blood mononuclear cells (PBMC) plated on fibronectin-coated plates (Hirschi et al. 2008; Sonnenschein et al. 2011). However, it is still uncertain whether the two early endothelial outgrowth cells are functionally distinct populations. Accordingly, we tested the hypothesis that there are phenotypic differences in cellular function between angiogenic adherent and non-adherent cells in culture.
Materials and methods
Subjects
Peripheral blood samples were obtained from 18 healthy adults (56 ± 2 years). All subjects were sedentary (< 3 days of moderate exercise/week for at least 1 year before the study), nonsmokers, normotensive (arterial blood pressure ≤ 140/90 mmHg), non-medicated, and free of overt cardiovascular and metabolic disease, as assessed by medical history, physical examination, and fasting blood chemistries. Subjects over the age of 40 years were further evaluated for clinical evidence of coronary artery disease with electrocardiogram and blood pressure measurements at rest and during incremental exercise performed to exhaustion. The study was reviewed and approved by the University of Colorado at Boulder Institutional Review Board. Before participation, all of the subjects provided written informed consent according to the guidelines of the University of Colorado at Boulder.
Body composition and metabolic measurements
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy X-ray absorptiometry (Lunar, Madison, WI). Body mass index was calculated as weight (kg) divided by height (m) squared. Minimal waist circumference was measured according to published guidelines (Lohmann et al. 1988). Fasting plasma lipid and lipoprotein, glucose, and insulin concentrations were determined using standard techniques by the clinical laboratory affiliated with the General Clinical Research Center.
PBMC isolation
PBMC were isolated from peripheral blood by Ficoll density-gradient centrifugation (Histopaque 1077; Sigma Aldrich, St Louis, MO, USA), washed and resuspended in phosphate buffered saline (Gibco, Grand Island, NY, USA). PBMC’s were plated on 6-well fibronectin-coated plates (BD Biosciences, San Jose, CA, USA) at 37 °C and 5% CO2. Two different protocols of culture were developed in order to isolate the early EPC derived from non-adherent and adherent cells in culture.
NAC isolation
PBMC’s were cultured in fibronectin-coated plates for 48 h using 199 medium (ThermoFisher, Massachusetts, USA) supplemented with 20% fetal bovine serum (ThermoFisher, Massachusetts, USA), penicillin (100 U/mL; ThermoFisher, Massachusetts, USA), and streptomycin (100 mg/mL; ThermoFisher, Massachusetts, USA) (MacEneaney et al. 2010). After 2 days in culture, non-adherent cells underwent migration, angiogenic factors release and apoptosis assays.
AC isolation
PBMC’s were cultured in fibronectin-coated plates with endothelial growth medium type 2 (EGM-2; Lonza, Visp, Switzerland) containing 2% fetal bovine serum, hydrocortisone, ascorbic acid, gentamicin-amphotericin, heparin and growth factors (hFGF-B, hEGF, VEGF, R3-IGF-1). After 4 days of incubation, non-adherent cells were removed after thorough washing with EGM-2, and the adherent cells underwent the functional assays at day 7 (Rocha et al. 2015).
Migration assay
Migratory capacity was assessed using a modified Boyden chamber technique as previously described by our laboratory (Hoetzer et al. 2005). Briefly, NAC and AC (4 × 105), resulting from the isolation techniques noted above, were resuspended in culture medium, consisting of medium (199 or endothelial basal medium, respectively), penicillin (100 U/mL), and streptomycin (100 μg/mL), and then placed in the upper chamber of a 24-well modified Boyden chamber coated with fibronectin (FluoroBlok, BD Biosciences, New Jersey, USA). The upper chamber was placed in the lower chamber containing culture medium and vascular endothelial growth factor (VEGF, 2 ng/mL) or stromal cell-derived factor-1alpha (SDF-1alpha; 10 ng/mL), both factors considered crucial in the differentiation and mobilization of angiogenic cells, for 22 h at 37 °C. Cells were then labeled with calcein AM (Molecular Probes, Oregon, USA), and the fluorescence of the migrated cells was determined in duplicate and the mean relative fluorescent units presented.
Angiogenic growth factor release
A concentration of 5 × 105 NAC and AC from each subject were seeded onto six wells of 24-well fibronectin-coated plates (BD Biosciences, New Jersey, USA) with 199 medium and EGM-2, respectively. Phytohemagglutinin (10 μg/mL) was added to three wells to stimulate growth factor release. Conditioned growth medium was collected after 24 h and the concentrations of granulocyte colony-stimulating factor (G-CSF) and hepatocyte growth factor (HGF) were determined by enzyme immunoassay (R&D Systems, Minesota, USA).
Apoptosis
NAC and AC were fixed in 3.7% buffered formaldehyde solution and apoptosis was determined by enzyme-linked immunoassay (Trevigen, Maryland, USA) for terminal deoxynucleotidyl (transferase-mediated dUTP nick end-labeling TUNEL), according to the manufacturer’s instructions.
Statistical analysis
Group and condition differences were determined by analysis of variance (ANOVA). When indicated by a significant F value, Bonferroni’s post hoc tests were performed to compare specific group means. Importantly, there were no main effects or interactions of gender observed, so, the data were combined and presented together. Student’s t test was used for the simple comparisons between groups. All data are expressed as means ± S.E.M. Statistical significance was set a priori at P < 0.05.
Results
The anthropometric, biochemical and hemodynamic variables of selected subjects are presented in Table 1. All subjects presented normal levels of fasting glucose, total cholesterol and subfractions, triglycerides and blood pressure.
Table 1.
Selected subject characteristics
| Variable | |
|---|---|
| N | 18 |
| Age (years) | 56 ± 2 |
| Body mass (kg) | 83.5 ± 5.9 |
| BMI (kg/m2) | 27.8 ± 1.2 |
| Waist circumference (cm) | 90.5 ± 4.2 |
| Systolic BP (mmHg) | 119 ± 3 |
| Diastolic BP (mmHg) | 73 ± 2 |
| Total cholesterol (mg/dL) | 202.8 ± 9.7 |
| LDL-cholesterol (mg/dL) | 122.7 ± 7.2 |
| HDL-cholesterol (mg/dL) | 56.9 ± 4.8 |
| Triglycerides (mg/dL) | 118 ± 15 |
| Glucose (mg/dL) | 92 ± 3 |
| Insulin (μU/mL) | 11 ± 1.5 |
Values expressed as mean ± SEM. BMI, body mass index; BP, blood pressure; LDL, low-density lipoprotein; HDL, high-density lipoprotein
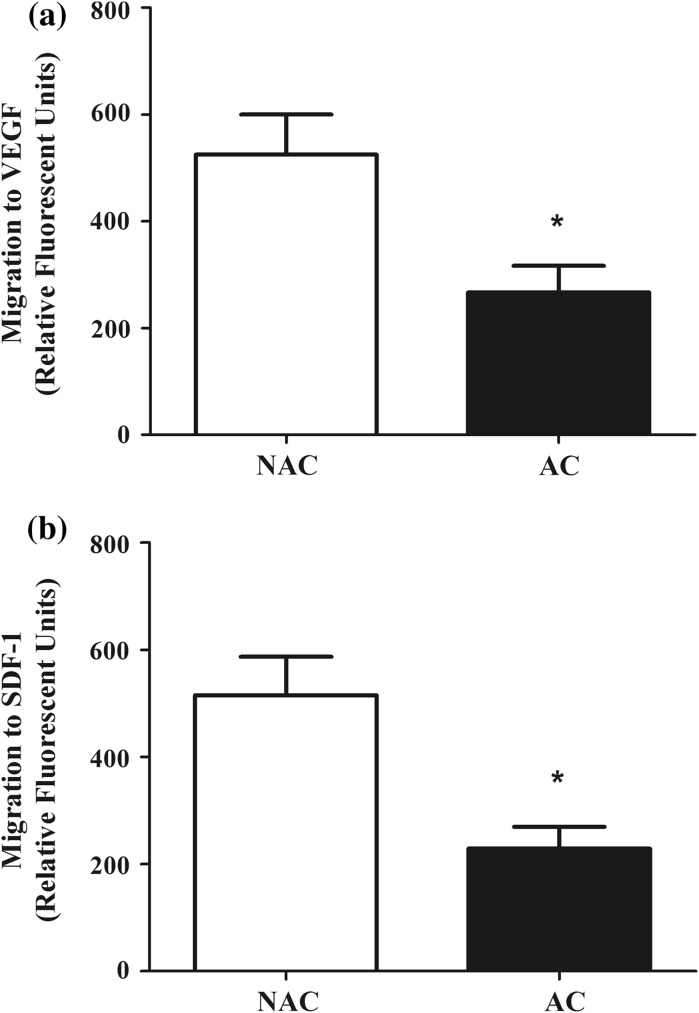
Migration of NAC and AC to chemotactic factors was demonstrated in Fig. 1. Overall, migratory capacity to VEGF (NAC: 524.7 ± 75.2 vs. AC: 266.9 ± 49.6; P < 0.05) and SDF-1 (NAC: 515.1 ± 72.2 vs. AC: 229.1 ± 40.6; P < 0.05) were approximately 50% lower in AC when compared to NAC.
Fig. 1.
Migration to vascular endothelial growth factor (VEGF, a) and stromal-derived factor-1 (SDF-1, b) in non-adherent cells (NAC) and adherent cells (AC) in culture. *P < 0.05 versus NAC
Release of angiogenic growth factors in culture is also an important function of angiogenic cells. Both NAC and AC increased G-CSF release after phytohemagglutinin stimulation (P < 0.05, Table 2). No differences were observed between groups, regarding the release of angiogenic factors G-CSF and HGF (Table 2).
Table 2.
Release of angiogenic growth factors in culture
| Growth factors | NAC | AC | ||
|---|---|---|---|---|
| Unstimulated | Stimulated | Unstimulated | Stimulated | |
| G-CSF (pg/mL) | 56.6 ± 5.2 | 627.5 ± 68.0† | 55.8 ± 4.3 | 587.8 ± 52.1† |
| HGF (pg/mL) | 127.8 ± 10.4 | 126.8 ± 13.3 | 126.9 ± 15.2 | 138.2 ± 18.7 |
Values expressed as mean ± SEM. G-CSF, granulocyte colony-stimulating factor; HGF, hepatocyte growth factor; NAC, non-adherent cells; AC, adherent cells
†P < 0.05 versus Unstimulated cells
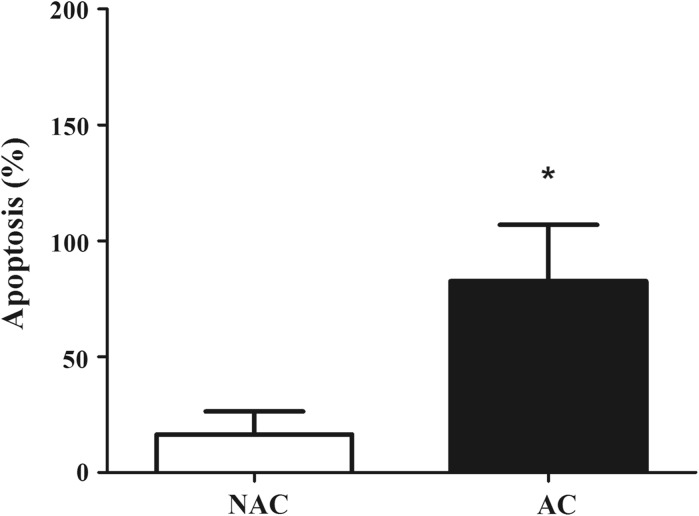
Apoptotic response was determined after the stimulation with a pro-apoptotic substance, called staurosporine (Fig. 2). AC presented approximately five-fold higher apoptotic response to staurosporine when compared to NAC (NAC: 16.5 ± 9.9 vs. AC: 82.6 ± 24.5; P < 0.05).
Fig. 2.
Apoptotic response to staurosporine in non-adherent cells (NAC) and adherent cells (AC) in culture. *P = 0.03 versus NAC
Discussion
The novel findings of the present study were threefold: (1) NAC presented higher migration capacity to VEGF and SDF-1 than AC; (2) both early outgrowth cell types presented similar angiogenic growth factor release; and (3) NAC showed lower apoptotic level than AC. Although both types of cells are related to reendothelialization and vascular repair, they clearly represent distinct angiogenic subpopulations.
Previous studies have demonstrated that NAC, also called endothelial cell colony-forming units (CFU-EC) (Hill et al. 2003; Yoder et al. 2007), present similar functional characteristics to hematopoietic-derived monocytes/macrophages (Ingram et al. 2005; Yoder et al. 2007). They showed that NAC express the macrophage-specific antigen (i.e., CD115) and contain sodium fluoride-inhibitable nonspecific esterase activity, which is a unique characteristic of monocytes and macrophages (Yoder et al. 2007). This idea is supported by our study, because high migration potential and resistance to apoptosis found in NAC are usually present in monocytes/macrophages (Galimi et al. 2001; Zhang et al. 2006).
In addition, AC presented an increased apoptosis susceptibility. It was demonstrated that elevated expression of stem cell-associated transcription factors is associated with a reduction in cell apoptosis (Ramasamy et al. 2012). AC is suggested to represent a subpopulation in higher stage of differentiation process from early outgrowth cells towards endothelial cells than NAC, and so they present a greater number of apoptotic cells (Guan et al. 2013).
Previous studies have grouped NAC and AC as early endothelial outgrowth cells, even using different media during isolation in culture, because they present similarities in expression of “endothelial-like” proteins. However, according to the present data, there are two functionally distinct early outgrowth cells (i.e., AC and NAC) in those conditions. Evidences have shown that progenitor cells could adjust their morphological phenotypes to different microenvironments (Yang et al. 2011; Guan et al. 2013). Then, caution should be taken in considering different methods or culture media to isolate the same cell population.
There is a technical issue regarding this study that should be mentioned. No hematopoietic and endothelial markers in NAC and AC were measured by flow cytometry in the present study. However, previous studies showed that both NAC and AC present similar endothelial progenitor markers, such as CD34, VEGFR2 and eNOS (Mobius-Winkler et al. 2009).
In conclusion, NAC and AC, both considered as early outgrowth cells, are functional distinct populations. NAC present higher migration potential and lower apoptotic susceptibility than AC. Early outgrowth cells are a heterogenic population involved in angiogenesis and vascular repair. However, this diversity makes it difficult to compare these two early outgrowth cell subpopulations. Further studies are necessary to better characterize this giant group of cells and effectively separate them according functional and morphological properties.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Galimi F, Cottone E, Vigna E, Arena N, Boccaccio C, Giordano S, Naldini L, Comoglio PM. Hepatocyte growth factor is a regulator of monocyte-macrophage function. J Immunol. 2001;166(2):1241–1247. doi: 10.4049/jimmunol.166.2.1241. [DOI] [PubMed] [Google Scholar]
- Gothert JR, Gustin SE, van Eekelen JA, Schmidt U, Hall MA, Jane SM, Green AR, Gottgens B, Izon DJ, Begley CG. Genetically tagging endothelial cells in vivo: bone marrow-derived cells do not contribute to tumor endothelium. Blood. 2004;104(6):1769–1777. doi: 10.1182/blood-2003-11-3952. [DOI] [PubMed] [Google Scholar]
- Guan XM, Cheng M, Li H, Cui XD, Li X, Wang YL, Sun JL, Zhang XY. Biological properties of bone marrow-derived early and late endothelial progenitor cells in different culture media. Mol Med Rep. 2013;8(6):1722–1728. doi: 10.3892/mmr.2013.1718. [DOI] [PubMed] [Google Scholar]
- Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348(7):593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28(9):1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoetzer GL, Irmiger HM, Keith RS, Westbrook KM, DeSouza CA. Endothelial nitric oxide synthase inhibition does not alter endothelial progenitor cell colony forming capacity or migratory activity. J Cardiovasc Pharmacol. 2005;46(3):387–389. doi: 10.1097/01.fjc.0000175456.53869.ab. [DOI] [PubMed] [Google Scholar]
- Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106(5):1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- Kawamoto A, Gwon HC, Iwaguro H, Yamaguchi JI, Uchida S, Masuda H, Silver M, Ma H, Kearney M, Isner JM, Asahara T. Therapeutic potential of ex vivo expanded endothelial progenitor cells for myocardial ischemia. Circulation. 2001;103(5):634–637. doi: 10.1161/01.CIR.103.5.634. [DOI] [PubMed] [Google Scholar]
- Lohmann TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign: Human Kinetics Books; 1988. [Google Scholar]
- MacEneaney OJ, Kushner EJ, Westby CM, Cech JN, Greiner JJ, Stauffer BL, DeSouza CA. Endothelial progenitor cell function, apoptosis, and telomere length in overweight/obese humans. Obesity (Silver Spring) 2010;18(9):1677–1682. doi: 10.1038/oby.2009.494. [DOI] [PubMed] [Google Scholar]
- Mobius-Winkler S, Hollriegel R, Schuler G, Adams V. Endothelial progenitor cells: implications for cardiovascular disease. Cytometry A. 2009;75(1):25–37. doi: 10.1002/cyto.a.20669. [DOI] [PubMed] [Google Scholar]
- Ramasamy R, Tong CK, Yip WK, Vellasamy S, Tan BC, Seow HF. Basic fibroblast growth factor modulates cell cycle of human umbilical cord-derived mesenchymal stem cells. Cell Prolif. 2012;45(2):132–139. doi: 10.1111/j.1365-2184.2012.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha NG, Sales AR, Miranda RL, Silva MS, Silva JF, Silva BM, Santos AA, Nobrega AC. Aerobic exercise modulation of mental stress-induced responses in cultured endothelial progenitor cells from healthy and metabolic syndrome subjects. Life Sci. 2015;123:93–99. doi: 10.1016/j.lfs.2014.12.026. [DOI] [PubMed] [Google Scholar]
- Sonnenschein K, Horvath T, Mueller M, Markowski A, Siegmund T, Jacob C, Drexler H, Landmesser U. Exercise training improves in vivo endothelial repair capacity of early endothelial progenitor cells in subjects with metabolic syndrome. Eur J Cardiovasc Prev Rehabil. 2011;18(3):406–414. doi: 10.1177/1741826710389373. [DOI] [PubMed] [Google Scholar]
- Stadtfeld M, Graf T. Assessing the role of hematopoietic plasticity for endothelial and hepatocyte development by non-invasive lineage tracing. Development. 2005;132(1):203–213. doi: 10.1242/dev.01558. [DOI] [PubMed] [Google Scholar]
- Urbich C, Dimmeler S. Endothelial progenitor cells: characterization and role in vascular biology. Circ Res. 2004;95(4):343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- Yang N, Li D, Jiao P, Chen B, Yao S, Sang H, Yang M, Han J, Zhang Y, Qin S. The characteristics of endothelial progenitor cells derived from mononuclear cells of rat bone marrow in different culture conditions. Cytotechnology. 2011;63(3):217–226. doi: 10.1007/s10616-010-9329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109(5):1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SJ, Zhang H, Wei YJ, Su WJ, Liao ZK, Hou M, Zhou JY, Hu SS. Adult endothelial progenitor cells from human peripheral blood maintain monocyte/macrophage function throughout in vitro culture. Cell Res. 2006;16(6):577–584. doi: 10.1038/sj.cr.7310075. [DOI] [PubMed] [Google Scholar]