Abstract
We found that strawberry (Fragaria x ananassa) extract has an IgE production suppressive activity and its oral administration improved skin manifestation in atopic dermatitis model mice. In present study, we identified an active substance using the IgE-producing human myeloma cell line U266. Gel filtration experiment indicated that the IgE suppressor was more than 6 kDa in molecular size. In addition, its pectinase treatment inhibited the activity, suggesting that the active substance in strawberry extract is pectin. Among solutions of water-(WP), hexametaphosphate-(HXP), acid-(HP) and alkali soluble pectin (OHP) extracted from strawberry, only OHP suppressed IgE production, and their suppressive activity was cancelled by pectinase treatment. In addition, OHP extracted from apple also inhibited IgE production. Furthermore, OHP also suppressed IgE production and did not affect IgG and IgM production in human peripheral blood mononuclear cells in an in vitro immunization condition. From these results, we concluded that OHP was an IgE suppressor in strawberry extract.
Keywords: IgE, Allergy, Strawberry, Pectin, U266, PBMC
Introduction
Strawberry (Fragaria x ananassa) is one of the most widely consumed fruits in either fresh or processed forms such as juice, puree and jam in the world. It possesses remarkably nutritional compositions such as vitamin C and folates, and non-nutrient element such as anthocyanins, flavonols, ellagic tannin and cinnamic acid (Aaby et al. 2007a, b; Giampieri et al. 2012). It has also some beneficial effects such as anti-allergic activity, antioxidative activity, anticancer activity, inhibitory effect of cardiovascular disease risk and cognitive decline (Choi and Yan 2009; Devore et al. 2012; Giampieri et al. 2014; Park et al. 2007; Ninomiya et al. 2010; Zhang et al. 2008). Among them, anti-allergic effect is attracted much attention because allergies such as allergic asthma, rhinitis and atopic dermatitis are increasing all over the world (Johansson et al. 2004).
It is well known that IgE plays a critical role in acute hypersensitivity reactions. IgE binds to the high-affinity IgE receptor (FcεRI) on mast cells and basophils. Cross-linking of IgE bound to FcεRI with allergen induces the release of inflammatory mediators such as histamine and leukotriene (Corry and Kheradmand 1999; Gould and Sutton 2008; Galli and Tsai 2012). Thus, IgE triggers allergic symptoms. A number of studies show that IgE production in B cells is induced by some cytokines such as IL-4 and IL-13 which is secreted by type 2 T (Th2) cells, and type 1 T (Th1) cells do not provide help for IgE synthesis (Romagnani 1992). Th1 and Th2 cells are differentiated from CD4+ T cells by expression of the master regulator, T-bet and GATA3, respectively (Kanhere et al. 2012; Skapenko et al. 2004). These findings strongly suggest that Th2 lead to allergic reactions through the induction of IgE production in B cells. Therefore, the suppression of IgE and Th2 differentiation is key to the palliation of allergic symptoms.
There are some reports on anti-allergic activity of strawberry components. Choi and Yan (2009) reported that ellagic acid, which is rich in strawberry, attenuated IgE mediated degranulation in vitro and in vivo, and Ninomiya et al. (2010) showed that linocinnamarin and cinnamic acid inhibited antigen-stimulated degranulation thorough the inactivation of spleen tyrosine kinase (Syk)/phopholipase Cγ (PLCγ) pathways in rat basophilic leukemia RBL-2H3 cells. We also found that some species of strawberry extracts suppressed IgE production and decreased the expression level of GATA3 in human peripheral blood mononuclear cells (PBMCs) in an in vitro immunization (IVI), and its oral administration inhibited IgE level in sera and improved the skin manifestation in atopic dermatitis model mice NC/Nga (Iwamoto et al. 2013). In addition, we have showed that one of the IgE suppressors in strawberry is glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in vitro (Iwamoto et al. 2012). However, anti-allergic activity of strawberry was not fully understood yet. In recent study, we found that strawberry extract suppressed IgE production in human myeloma cell line U266, whereas the suppressive effect was not due to GAPDH. These findings suggested that strawberry has some IgE suppressors other than GAPDH.
In present study, based on the results of IgE suppressive effect in U266, we purified an IgE suppressor derived from strawberry extract, and identified alkali-soluble pectin as a novel suppressor.
Materials and methods
Sample preparation
‘Toyonoka’ strawberry (Fragaria x ananassa) was purchased in Fukuoka prefecture, Japan and homogenized after the addition of equal weight of phosphate buffered saline (PBS) to extract components. The homogenate was centrifuged at 9400g at 4 °C for 60 min, the supernatant was filtrated through a 0.22 µm membrane (Millipore, Billerica, CA, USA) and used as the strawberry extract.
To investigate the molecular size of active substance, strawberry extract was separated by gel filtration using Econo-Pac 10 DG column (Bio-Rad, Hercules, CA, USA). Column was equilibrated with 20 mL of PBS and 3 mL of strawberry extract was added to it. The eluents were collected in fraction of 4 mL by the addition of PBS.
To evaluate the effect of heat-treatment, strawberry extract was heated at 100 °C for 10 min. To examine the trypsin and pectinase sensitivity, 100 µL of strawberry extract and fraction 1 were treated with 410 U of trypsin from porcine pancreas (Sigma, St. Luis, MO, USA) at 37 °C for 3 h or 57 U of pectinase from Aspergillus niger (Sigma) at 25 °C for 3 h. The IgE production suppressive activity of these samples was examined by using U266 cells.
Extraction of pectin solutions from strawberry
The extraction of strawberry pectin solutions was performed according to the method reported by Rombouts and Thibault (1986) and Rnard et al. (1990) with some modifications. Briefly, the sliced strawberry was boiled with four times volume of 99.5% of ethanol for 15 min and slurry was collected by filtration. The slurry was fractionated in 70% ethanol and washed with 70% ethanol until the filtrate gave negative reaction in the phenol–sulfuric acid method. The residue was washed in the order of 70%, 80%, 90%, 99% ethanol and then with diethyl ether, and dried at room temperature. This residue was used as alcohol-insoluble residue (AIS). To extract water-soluble pectin (WP), AIS was stirred with water at 4 °C for 12 h and the supernatant containing WP was collected by filtration. The slurry was washed with water until the filtrate gave negative reaction in phenol–sulfuric acid test, and stirred with 0.4% sodium hexametaphosphate at room temperature for 2 h. The supernatant containing hexametaphosphate-soluble pectin (HXP) was collected by centrifugation at 9400g for 30 min. To extract acid-soluble pectin (HP), residue was washed with water until the sugar was not detected by phenol–sulfuric acid test, and boiled in 0.05 N hydrochloric acid for 1 h, then cooled, the supernatant was collected by centrifugation at 9400g for 30 min. The slurry was washed until the sugar was not detected, stirred with 0.05 N sodium hydroxide for 30 min at 4 °C, and supernatant containing alkali-soluble pectin (OHP) was collected by centrifugation at 9400g for 1 h. The extracted pectin was concentrated by ultrafiltration using 3 kDa cut membrane (Millipore). The total sugar content was determined by the phenol–sulfuric method.
Protein assay
The protein concentration was measured by using BIO-RAD protein assay (Bio-rad) with BSA as a standard.
Cell and cell culture
2.0 × 105 cells/mL of human myeloma cell line U266 were cultured in the 10% fetal bovine serum (FBS, Thermo, Waltham, MA, USA) containing RPMI1640 medium (Thermo) with or without prepared samples at 37 °C under humidified 5% CO2–95%. After 3 days, IgE concentration in the supernatant was measured by an enzyme-linked immunosorbent assay (ELISA).
Human peripheral blood mononuclear cells (PBMCs) were separated from heparinized human blood of healthy donors by using Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden). The separated cells were stored at − 85 °C in deep freezer. Informed consent from all healthy volunteers was obtained in accordance with the Declaration of Helsinki. To investigate the effect of sample on immunoglobulin production, in vitro immunization (IVI) was performed as described by Kawahara et al. (2000). Human PBMCs were cultured at 1.0 × 106 cells/mL in the 5% FBS and 10% human plasma containing E-RDF medium (Kyokuto Pharmaceuticals, Tokyo, Japan) supplemented with 10 ng/mL of recombinant human IL-4, IL-6 (R&D systems, Minneapolis, MN, USA), 10 µg/mL of muramyl dipeptide (Sigma) and 100 ng/mL of the cedar pollen allergen Cry j 1 (Hayashibara, Okayama, Japan) with or without prepared samples. After 10 days, total Ig concentration in the supernatant was measured by ELISA.
Measurement of IgE antibodies
Total IgE concentration in culture medium was measured by sandwich ELISA. Ninety-six well microplates were coated with anti-human IgE (Biosource, Camarillo, CA, USA) diluted with PBS. The antibody-coated wells were blocked with 1.0% BSA/PBS, and then, each sample was added to them. After washing with 0.05% Tween 20 containing PBS (TPBS) three times, biotin-conjugated anti-human IgE antibody (Biosource, Camarillo, CA, USA) and horseradish peroxidase (HRP)-conjugated streptavidin (Vector laboratories, Burlingame, CA, USA) were added to them. After washing with TPBS three times, a substrate solution (0.1 M citrate buffer (pH4.0) containing 0.003% H2O2 and 0.3 mg/mL p-2, 2′-azino-di(3-ethylbenzothiazoline-6-sulphonic acid) diammonium salt) was added to them. Total IgG and IgM was detected by using anti-human IgG, IgM (Biosource) and anti-human IgG, IgM HRP conjugated (Biosource). After 15 min, the absorbance at 415 nm was measured by a microplate reader (Bio-Rad).
Statistics
Data obtained are expressed as the means ± SEM. One-way ANOVA was used to assess the statistical significance of the difference against the control. Each value of *p < 0.05, **p < 0.01 and ***p < 0.001 was considered to be statistically significant.
Results and discussion
Effect of strawberry extract on IgE production of U266 cells
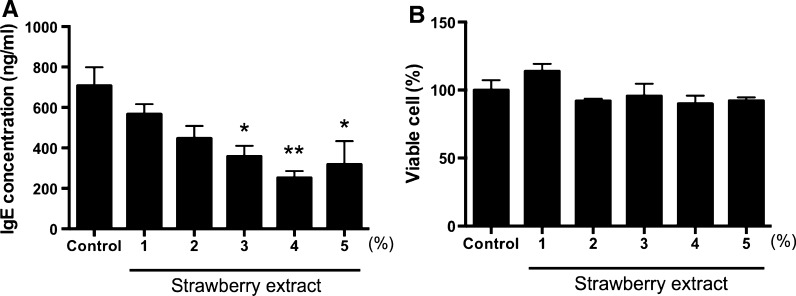
At first, the effect of strawberry extract on IgE production in U266 cells was examined. U266 cells were cultured in 10% FBS containing RPMI1640 medium with various concentrations of ‘Toyonoka’ strawberry extract. As shown in Fig. 1a, strawberry extract suppressed IgE production in a dose-dependent manner. Significant differences were observed at 3% or higher concentration of extract. The IgE suppressive effect of strawberry extract was not caused by cytotoxicity (Fig. 1b). We reported that GAPDH in strawberry is one of the IgE suppressors in human PBMCs in IVI condition (Iwamoto et al. 2012), whereas GAPDH did not decrease IgE production in U266 cells (data not shown). These results suggest that strawberry extract includes the IgE suppressors other than GAPDH.
Fig. 1.
Effect of strawberry extracts on IgE production in U266 cells. U266 cells were cultured in 10% FBS containing RPMI1640 for 2 days. a The amounts of IgE in U266 cells were measured by ELISA. b Cell viability was measured using Cell counting kit-8. As a control, PBS only was added to the medium. The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01
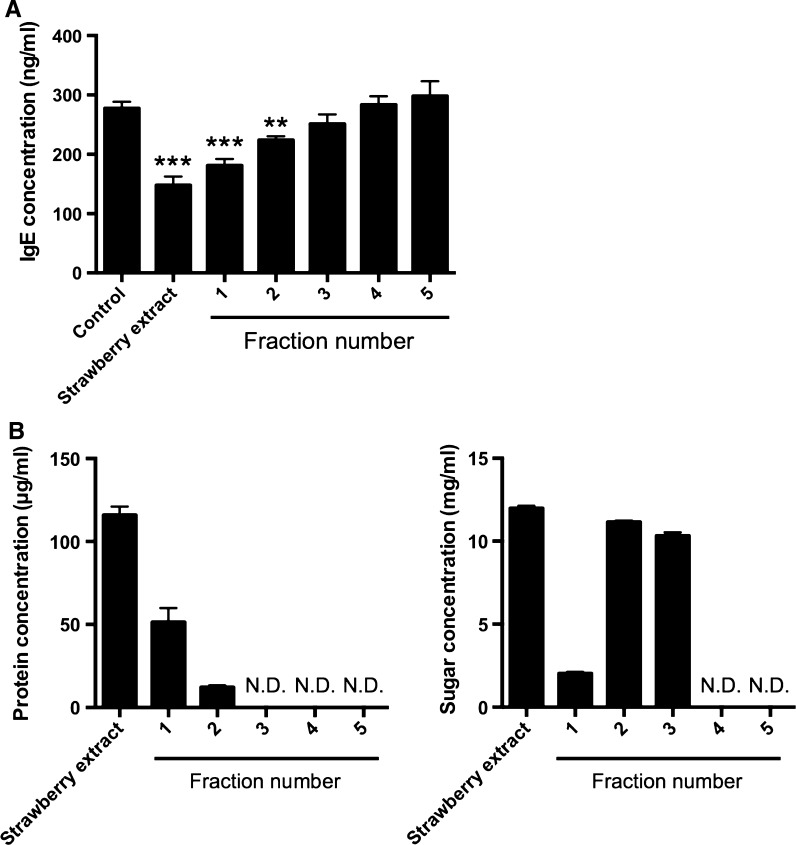
Next, to investigate the molecular size of an IgE suppressor, the effect of gel filtration fractions of strawberry on IgE production was evaluated. As the result, fraction 1 and 2 significantly suppressed IgE production in U266 cells and in particular, fraction 1 decreased IgE production more strongly than fraction 2 (Fig. 2a). The molecular size of fraction 1 was more than 6 kDa, and included proteins and saccharides (Fig. 2b). Therefore, it was suggested that an IgE suppressor in strawberry extract is high molecular size component such as proteins and polysaccharides.
Fig. 2.
Effect of fractionated strawberry extracts on IgE production in U266 cells. U266 cells were cultured in 10% FBS containing RPMI1640 for 2 days. Strawberry extract was fractionated by gel filtration. a The amounts of IgE in U266 cells were measured by ELISA. As a control, PBS only was added to the medium. The fractionated strawberry extracts were added to the medium at a final concentration of 5% (v/v). b Protein and sugar concentration in strawberry extract. ND Not detected. The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01, ***p < 0.001
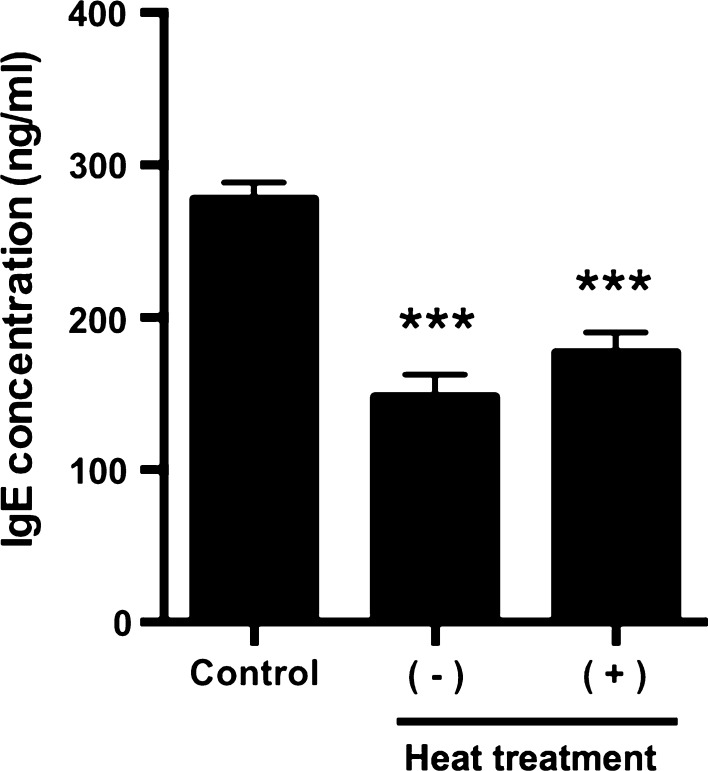
Furthermore, to examined the properties of an active substance, the effect of strawberry extract heated at 100 °C for 10 min on IgE production was investigated. As shown in Fig. 3, heat-treated strawberry extract also decreased IgE production and there was not significantly different between the non- and heat-treated extract, indicating that the active substance is stable to heat.
Fig. 3.
Effect of heat-treated strawberry extract on IgE production in U266 cells. U266 cells were cultured in 10% FBS containing RPMI1640 for 2 days. The amounts of IgE in U266 cells were measured by ELISA. As a control, PBS only was added to the medium. Strawberry extract was added to the medium at a final concentration of 5% (v/v). The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01, **p < 0.001
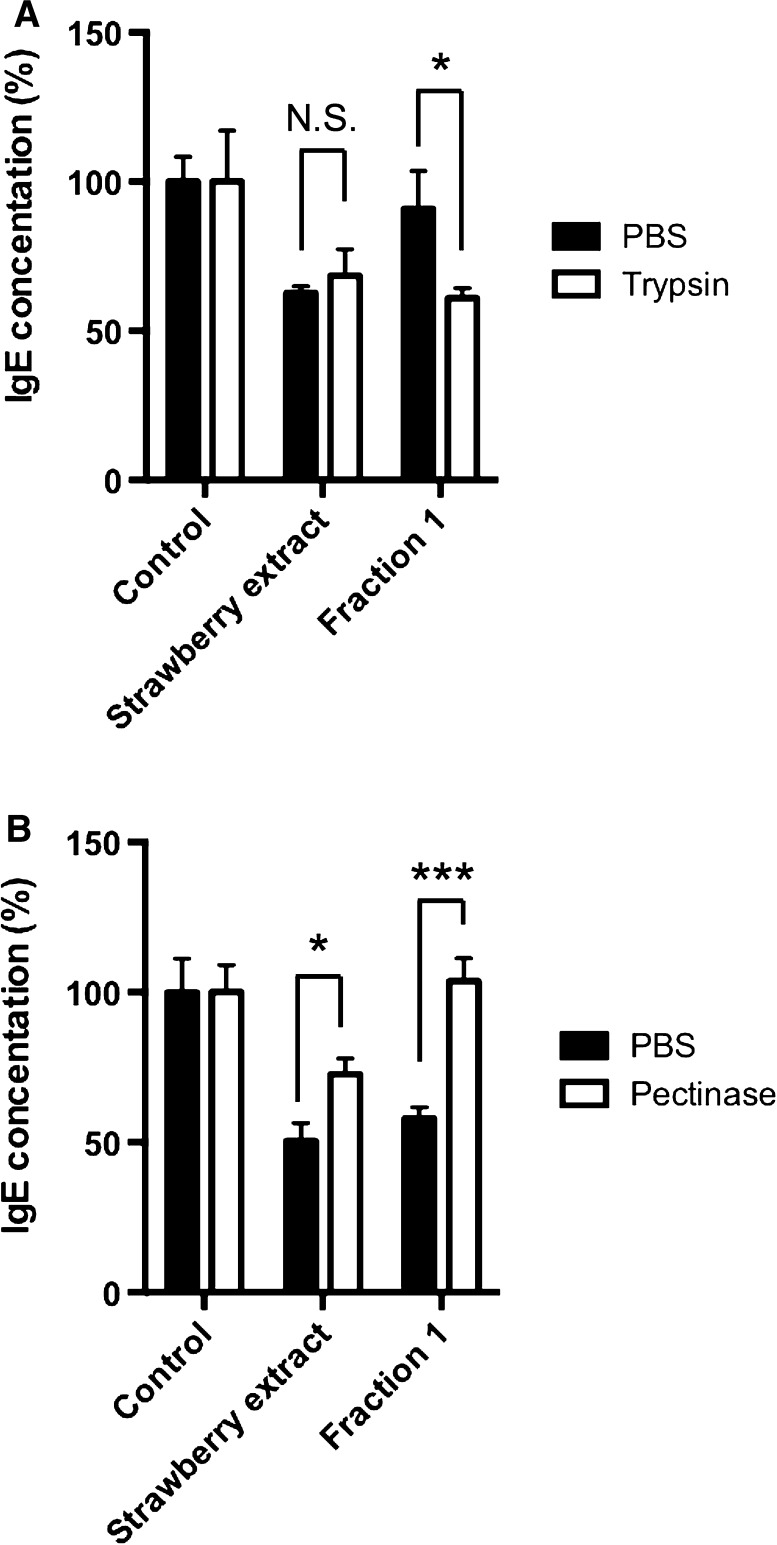
Then, the effect of trypsin and pectinase treated strawberry extract and fraction 1 on IgE production was examined. As shown in Fig. 4a, trypsin treatment did not affect IgE suppression in strawberry extract, but rather promoted it in fraction 1. This result suggests that trypsinized proteins in fraction 1 may have a possibility to suppress IgE production in U266 cells. In Fig. 4b, pectinase treatment significantly cancelled IgE suppression in strawberry extract and fraction 1. But, its rate of strawberry extract was small when compared with fraction 1, suggesting that low molecular fraction in strawberry extract may contain certain water-soluble pectinase inhibitor. From these results, it was considered that pectin was an IgE suppressor that was abundantly contained in strawberry.
Fig. 4.
Effect of strawberry extracts treated by enzyme on IgE production in U266 cells. U266 cells were cultured in 10% FBS containing RPMI1640 for 2 days. The amounts of IgE in U266 cells were measured by ELISA. a Effect of trypsinized strawberry extract on IgE production. b Effect of pectinase treated strawberry extract on IgE production. As a control, enzyme only was added to the medium. Samples were added to medium at a concentration of 5% (v/v). The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01, ***p < 0.001. N.S. Not significant
The effect of pectin on IgE production in U266 cells
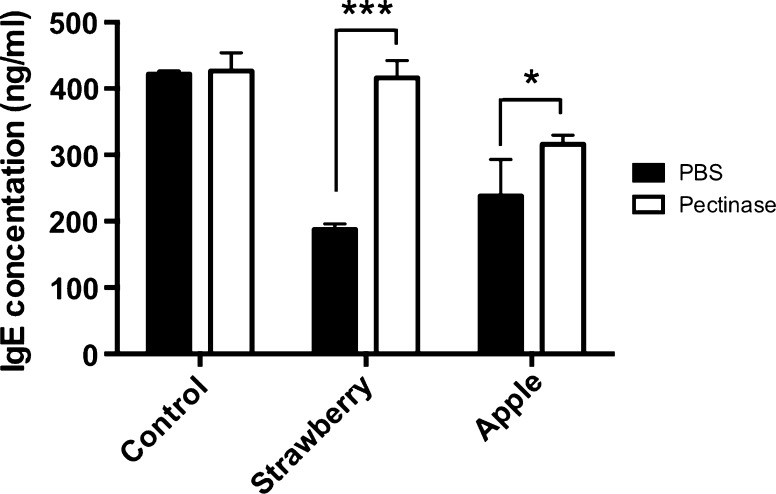
Pectin was further fractionated as shown in Fig. 5a, and then the effect of water-(WP), hexametaphosphate-(HXP), acid-(HP) and alkali-soluble pectin (OHP) solution on IgE production in U266 cells was examined. OHP suppressed IgE production, whereas WP, HXP and HP did not suppress IgE production without the decrease of cell viability (Fig. 5b,c). In addition, OHP extracted from ‘Sanfuji’ apple also decreased IgE production. Furthermore, the IgE suppressive activities of OHP fraction from strawberry and apple were cancelled by the pectinase treatment (Fig. 6). We considered that the difference of cancellation rate between strawberry and apple OHPs was due to their pectinase sensitivity based on pectin structure. These results suggest that alkali-soluble pectin is one of the IgE suppressors in strawberry.
Fig. 5.
Effect of pectin extracted from strawberry and apple on IgE production. U266 cells were cultured in 10% FBS containing RPMI1640 for 2 days. The amounts of IgE in U266 cells were measured by ELISA. a Flow chart of pectin extraction from strawberry. b IgE concentration. c Viable cell. As a control, each solvent only was added to the medium. WP water-soluble pectin, HXP hexametaphosphate-soluble pectin, HP acid-soluble pectin, OHP alkali-soluble pectin. The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 6.
Effect of pectinase treated OHP on IgE production. U266 cells were cultured in 10% FBS containing RPMI1640 for 2 days. The amounts of IgE in U266 cells were measured by ELISA. As a control, PBS only was added to the medium. OHP was added to medium at the concentration of 15 µg/mL. The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01, ***p < 0.001
The effect of alkali-soluble pectin solution on immunoglobulin production in human PBMCs
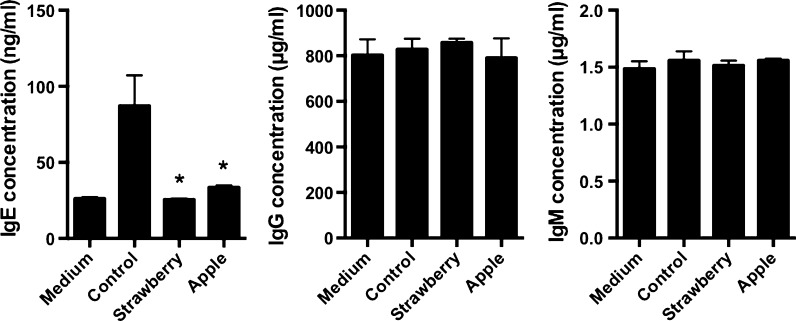
The effect of OHP on immunoglobulin productions in human PBMCs was examined. As shown in Fig. 7, strawberry and apple OHP markedly suppressed IgE production and did not affect IgG and IgM production in human PBMCs under IVI condition. Therefore, it is suggested that the suppressive effect of OHP acts only the IgE production.
Fig. 7.
Effect of OHP on Ig productions in human PBMCs. Human PBMCs were cultured in 10% human plasma and 5% FBS containing E-RDF medium supplemented with IL-2, -4, -6, MDP and Cry j 1 for 10 days. As a background of IgE, medium without cells was set up. As a control, PBS only was added to the medium. Strawberry and apple OHP were added to the medium at a final concentration of 5 µg/mL. The result showed the average value of three independent measurements. Data are means ± SEM (n = 3). Statistically significant differences from control were represented as *p < 0.05, **p < 0.01, ***p < 0.001
Sakurai et al. (1996) reported that the orally administrated pectin was detected in the liver and peyer’s patch in mice, suggesting the dietary pectin was transferred to blood. Many studies have shown that the water-soluble pectin is an important material for the activation and normalization of intestinal immunity. It is reported that the serum IgE level in the water-soluble pectin fed rats was lower than that of the cellulose and chitosan fed rats whereas its IgA, IgG levels were higher (Lim et al. 1997). In addition, the dietary pectin enhanced production of IgA, IgG and IgM in mesenteric lymph node (MLN) lymphocytes, while reduced IgE production (Lim et al. 2002). Their productions of IFN-γ and TNF-α in the water-soluble pectin fed rats were increased and the rate of IL-2 receptor positive cell population was significantly higher than that of the cellulose fed rats, suggesting the immunoglobulin modulating effect of pectin was mediated by the Th1 cell activation. However, there is no report about the immunological function of pectic polysaccharide except water-soluble pectin. Then, we examined WP, HXP, HP and OHP solutions extracted from strawberry and apple. As the results, OHP suppressed IgE production in U266 cells, whereas WP, HXP and HP did not affect. Inari and Takeuchi (1997) showed that total sugar of their pectin fractions extracted from ‘Houkouwase’ strawberry was WP 25.9%, HXP 15.2%, HP 40.5% and OHP 18.4%, and the molecular size of OHP was approximately 10 kDa, while WP, HXP and HP was 1000 kDa, 1000 kDa, 300 kDa, respectively. In addition, the rate of galacturonic acid in OHP was lower than other fractions, whereas total neutral sugar rate was higher than them. The pectin molecule consists mainly of galacturonic acid and neutral sugar such as rhamnose, arabinose, xyrose, mannose, galactose and glucose (Darvill et al. 1978). Among them, the rate of arabinose and galactose in alkali-soluble pectin was higher than others in ‘Senga sengana’ strawberry (Legentil et al. 1995). From these finding, the IgE production suppressive activity of OHP may be caused by its structural difference such as molecular size and sugar constituent.
In conclusion, we reported here that strawberry extract suppressed IgE production in U266 cells and its IgE suppressor was alkali-soluble pectin. However, further study is needed to reveal the underlying mechanism in its activity. At present, some allergic symptoms were treated by anti-allergic drugs that inhibit the release and activity of chemical mediators, but they occasionally occur harmful side effect. This study may be useful for the development of functional foods that are able to improve the allergic manifestation or keep the physical condition.
Abbreviations
- AIS
Alcohol-insoluble residue
- BSA
Bovine serum albumin
- ELISA
Enzyme-linked immunosorbent assay
- FBS
Fetal bovine serum
- FcεRI
High-affinity IgE receptor
- GATA3
GATA binding protein 3
- HP
Acid-soluble pectin
- HRP
Horseradish peroxidase
- HXP
Hexametaphosphate-soluble pectin
- IFN
Interferon
- Ig
Immunoglobulin
- IL
Interleukin
- IVI
In vitro immunization
- MDP
Muramyl dipeptide
- OHP
Alkali-soluble pectin
- PBMCs
Peripheral blood mononuclear cells
- PBS
Phosphate buffered saline
- Th
Helper T
- WP
Water-soluble pectin
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aaby K, Ekeberg D, Skrede G. Characterization of phenolic compounds in strawberry (Fragaria × ananassa) fruits by different HPLC detectors and contribution of individual compounds to total antioxidant capacity. J Agric Food Chem. 2007;55:4395–4406. doi: 10.1021/jf0702592. [DOI] [PubMed] [Google Scholar]
- Aaby K, Wrolstad RE, Ekeberg D, Skrede G. Polyphenol composition and antioxidant activity in strawberry purees; impact of achene level and storage. J Agric Food Chem. 2007;55:5156–5166. doi: 10.1021/jf070467u. [DOI] [PubMed] [Google Scholar]
- Choi YH, Yan GH. Ellagic acid attenuates immunoglobulin E-mediated allergic response in mast cells. Biol Pharm Bull. 2009;32:1118–1121. doi: 10.1248/bpb.32.1118. [DOI] [PubMed] [Google Scholar]
- Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature. 1999;402:18–23. doi: 10.1038/35037014. [DOI] [PubMed] [Google Scholar]
- Darvill AG, McNeil M, Albersheim P. Structure of plant cell walls VIII. A new pectic polysaccharide. Plant Physiol. 1978;62:418–422. doi: 10.1104/pp.62.3.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Kang JH, Breteler M, Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M. IgE and mast cells in allergic disease. Nat Med. 2012;18:693–704. doi: 10.1038/nm.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giampieri F, Tulipani S, Alvarez-Suarez JM, Quiles JL, Mezzetti B, Battino M. The strawberry: composition, nutritional quality, and impact on human health. Nutrition. 2012;28:9–19. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Giampieri F, Alvarez-Suarez JM, Battino M. Strawberry and human health: effects beyond antioxidant activity. J Agric Food Chem. 2014;62:3867–3876. doi: 10.1021/jf405455n. [DOI] [PubMed] [Google Scholar]
- Gould HJ, Sutton BJ. IgE in allergy and asthma today. Nat Rev Immunol. 2008;8:205–217. doi: 10.1038/nri2273. [DOI] [PubMed] [Google Scholar]
- Inari T, Takeuchi T. Changes in pectic substances during the ripening of strawberry fruit. J Jpn Soc Food Sci Technol. 1997;44:319–324. doi: 10.3136/nskkk.44.319. [DOI] [Google Scholar]
- Iwamoto A, Mitsuda K, Inoue A, Kato T, Inoue Y, Kawahara H. Purification and identification of an IgE suppressor from strawberry in an in vitro immunization system. Cytotechnology. 2012;64:309–314. doi: 10.1007/s10616-012-9432-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto A, Inoue A, Inoue Y, Yamada K, Tachibana H, Kawahara H. Anti-allergic effect of strawberry extract. J Funct Food. 2013;5:1947–1955. doi: 10.1016/j.jff.2013.09.016. [DOI] [Google Scholar]
- Johansson SGO, Bieber T, Dahl R, Friedmann PS, Lanier BQ, Lockey RF, Motala C, Ortega-Martell JA, Platts-Mills TA, Ring J, Thien F, Van Cauwenberge P, Williams HC. Revised nomenclature for allergy for global use: report of the nomenclature review committee of the World Allergy Organization, October 2003. J Allerg Clin Immunol. 2004;113:832–836. doi: 10.1016/j.jaci.2003.12.591. [DOI] [PubMed] [Google Scholar]
- Kanhere A, Hertweck A, Bhatia U, Gökmen MR, Perucha E, Jackson I, Lord GM, Jenner RG. T-bet and GATA3 orchestrate Th1 and Th2 differentiation through lineage-specific targeting of distal regulatory elements. Nat Commun. 2012;3:1268. doi: 10.1038/ncomms2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H, Maeda-Yamamoto M, Hakamata K. Effective induction and acquisition of human monoclonal IgE antibodies reactive with house-dust mite extracts. J Immunoll Methods. 2000;233:33–40. doi: 10.1016/S0022-1759(99)00183-0. [DOI] [PubMed] [Google Scholar]
- Legentil A, Guichard I, Piffaut B, Haluk JP. Characterization of strawberry pectin extracted by chemical means. LWT-Food Sci Technol. 1995;28:569–576. doi: 10.1016/0023-6438(95)90003-9. [DOI] [Google Scholar]
- Lim BO, Yamada K, Nonaka M, Kuramoto Y, Hung P, Sugano M. Dietary fibers modulate indices of intestinal immune function in rats. J Nutr. 1997;127:663–667. doi: 10.1093/jn/127.5.663. [DOI] [PubMed] [Google Scholar]
- Lim BO, Choe RW, Park DK, Kim HC, Kim SY, Yamada K, Sugano M. Effect of dietary level of pectin on immunoglobulin and cytokine production by mesenteric lymph node lymphocytes and interleukin-2 receptor in rats. Food Sci Technol Res. 2002;8:14–16. doi: 10.3136/fstr.8.14. [DOI] [Google Scholar]
- Ninomiya M, Itoh T, Ishikawa S, Saiki M, Narumiya K, Yasuda M, Koshikawa K, Nozawa Y, Koketsu M. Phenolic constituents isolated from Fragaria ananassa Duch. inhibit antigen-stimulated degranulation through direct inhibition of spleen tyrosine kinase activation. Bioorg Med Chem. 2010;18:5932–5937. doi: 10.1016/j.bmc.2010.06.083. [DOI] [PubMed] [Google Scholar]
- Park SJ, Shin WH, Seo JW, Kim EJ. Anthocyanins inhibit airway inflammation and hyperresponsiveness in a murine asthma model. Food Chem Toxicol. 2007;45:1459–1467. doi: 10.1016/j.fct.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Rnard CMGC, Voragen AGJ, Thibault JF, Pilnik W. Studies on apple protopectin:I. Extraction of insoluble pectin by chemical means. Carbohy Res. 1990;12:9–25. doi: 10.1016/0144-8617(90)90101-W. [DOI] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Aller Immunol. 1992;98:279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Rombouts FM, Thibault JF. Feruloylated pectic substances from sugar-beet pulp. Carbohy Res. 1986;154:177–187. doi: 10.1016/S0008-6215(00)90031-4. [DOI] [Google Scholar]
- Sakurai MH, Matsumoto T, Kiyohara H, Yamada H. Detection and tissue distribution of anti-ulser pectic polysaccharides from Bupleurum falctum by polyclonal antibody. Planta Med. 1996;62:341–346. doi: 10.1055/s-2006-957898. [DOI] [PubMed] [Google Scholar]
- Skapenko A, Leipe J, Niesner U, Devriendt K, Beetz R, Radbruch A, Kalden JR, Lipsky PH, Schulze-Koops H. GATA-3 in human T cell helper type 2 development. J Exp Med. 2004;199:423–428. doi: 10.1084/jem.20031323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Seeram NP, Lee R, Feng L, Heber D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J Agric Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]