Abstract
Background
To evaluate the efficacy and safety of Xuebijing injection (XBJ) treatment for acute organophosphorus pesticide poisoning.
Methods
Using the PubMed, Cochrane, Embase, Sinomed, Wanfang, CNKI, and Weipu (VIP) databases from the beginning of the datasets until December 2017, all of the relevant randomized controlled trials were identified. Relative risks (RR), weighted mean difference (WMD), along with 95% confidence interval (95% CI) were used to analyze the main outcomes. Statistical analysis was performed using the RevMan software version 5.3. The qualities of the involved studies were assessed by the risk of bias according to the Cochrane handbook.
Results
Twenty-six randomized controlled trials with 1,880 participants were collected in total. Compared with just conventional therapy alone, XBJ combined with conventional therapy significantly reduced the 7-day mortality rate (RR: 0.33; 95% CI: 0.22–0.49), CRP in the 50 mL group (WMD: −11.60; 95% CI: −14.38 to −8.83), CRP in the 100 mL group (WMD: −1.73; 95% CI: −2.91 to −0.55), AChE in the 50 mL group (WMD: −4.58;95% CI: −5.87 to −3.28), AChE in the 100 mL group (WMD: −1.73; 95% CI: −2.07 to −1.39), hospital stays in the 50 mL group (WMD: −4.26; 95% CI: −4.89 to −3.64), TNF-α in the 50 mL group (WMD: −2.66; 95% CI: −4.99 to −0.32), NF-κB in the 50 mL group (WMD: −13.07; 95% CI: −14.67 to −11.47), and CK-MB in the 50 mL group (WMD: −32.28; 95% CI: −40.62 to −23.93). However, there was no statistical difference of TNF-α in the 100 mL group (WMD: −2.17; 95% CI: −4.66 to 0.32).
Conclusions
XBJ has a significant clinical efficacy for the treatment of patients with acute organophosphorus pesticide poisoning. The most effective dose is 50 mL, while the most effective frequency is twice a day. However, more studies are needed to confirm the extract efficacy of XBJ.
Keywords: Xuebijing injection (XBJ), acute organophosphorus pesticide poisoning (AOPP), systematic review, meta-analysis
Introduction
Acute organophosphorus pesticide poisoning (AOPP) is among the most common medical acute conditions with complex symptoms and a high mortality rate (1). Data from the World Health Organization indicate that approximately three million people each year are at risk of organophosphorus pesticide poisoning (2). Research has shown that the major mechanism that causes organophosphorus pesticide poisoning is the inhibition of organic phosphorus acting on cholinesterase. The inhibition leads to a large accumulation of acetylcholine in the tissue space, which further results in a series of dysfunctions in the nervous system, myocardial cells, and respiration (3,4). Severe AOPP can cause acute cholinergic crisis, including the stimulation of the nicotinic receptors and muscarinic receptors, which leads to the main cause of patient death, namely respiration failure (5). Current therapeutic strategies for patients with AOPP mainly consist of resuscitation, gastric lavage, intravenous atropine and pralidoxime, and supportive cares. However, the mortality rate is still as high as 43.94% in Southeast Asia and India (6). Therefore, more therapies for the better management for AOPP are urgently needed.
Xuebijing injection (XBJ), a traditional Chinese medicine, is widely used in the treatment of sepsis in China. The possible mechanisms are believed to be associated with antagonizing the pro-inflammatory factors (7-9). XBJ is extracted from 5 Chinese herbs including Radix Salviae Miltiorrhiae, Rhizoma Chuanxiong, Flos Carthami, Angelica Sinensis and Radix Paeoniae Rubra. At least 21 compounds have been found in XBJ, including amino acids, phenolic acids, flavonoid glycoside, terpene glycoside, and phthalide (10). Radix Salviae Miltiorrhiae (11-15), Rhizoma Chuanxiong (16,17), Flos Carthami (18,19), and Angelica Sinensis (20-22) are known to exert an anti-inflammatory effect. The quality of XBJ has been strictly controlled according to the standard set by the China Ministry of Public Health.
Methods
Database and search strategies
The Cochrane, EMbase, PubMed, Sinomed, CNKI, WanFang, and Weipu databases were searched from their inceptions until December 2017. The following search terms were used individually or combined: “randomized controlled trials”, “Acute organophosphorus pesticide poisoning” and “Xuebijing injection”, et al. The references of the included records were searched to find any additional articles. All literature reviews were conducted by two investigators. When a disagreement occurred, a third investigator was involved until an agreement was reached.
Eligibility criteria for original studies
This systematic review and meta-analysis were composed of 27 items according to the PRISMA statement checklist (23).
Inclusion criteria: (I) patients who had been pathologically diagnosed as AOPP, with no limitation on the patient gender and ethnicity, and the diagnostic criteria was both explicit and normative; (II) the intervention performed used both XBJ and conventional therapies; (III) the comparison intervention used conventional therapies (anticholinergic agents, cholinesterase reactivators, and other supporting therapy); (IV) primary and secondary outcomes were evaluated. Primary outcomes were 7-day mortality. Secondary outcomes included the level of CRP, the time to ChE recovery, the length of hospital stay, the level of TNF-ɑ, the level of NF-κB, and the level of CK-MB; (V) the study design was based on a randomized controlled trial.
A study was excluded for the following reasons: (I) it investigated the treatment group or the control group for Tanreqing injection combined with similar efficacy of Chinese patent medicines; (II) the data was statistically flawed.
Study selection
Two reviewers independently identified the trials which met the inclusion criteria by screening the title and abstract of each record and retrieved their full-text if necessary. Any disagreement between the two reviewers was solved by discussion with the third reviewer. Otherwise, the agreement was reached by consensus.
Data extraction
We designed a pre-defined data extraction form, and two reviewers independently extracted the following information from the selected trials: the first author, published year, study location, sample size, mean age, intervention, control, course, and outcomes. Any disagreement between the two reviewers was discussed with the third reviewer until a consensus was reached.
Assessment for risk of bias of included studies
Two reviewers assessed the risk of bias for each study using the Cochrane risk of bias tool. The random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and conflict of interest were taken into account for the assessment. Disagreements about the risk of assessment were resolved through a discussion between an additional two reviewers.
Data synthesis
The RevMan software (version 5.3) provided by the Cochrane Collaboration was used for data analysis. Dichotomous variable was presented as relative risk (RR). Continuous outcomes were presented as the mean difference and with a 95% confidential interval (CI) rate. Statistical heterogeneity was assessed according to the Cochrane Handbook of Systematic Review of Interventions (version 5.1). Chi-square and I2 tests were used to test the heterogeneity. If there was heterogeneity between the studies (I2>50%, P<0.05), the cause of heterogeneity such as gender, age, course and severity of disease, dosage and duration of treatment, and subgroup analysis was searched for. If the cause was not found, the random effects model for metanalysis was used. Subgroup analysis or sensitivity analysis was performed to eliminate heterogeneity based on possible heterogeneous factors; if there was no heterogeneity between the studies, the fixed effect model was applied. Publication bias was assessed by using a funnel plot where there were more than 10 trials in the meta-analysis. In addition, we used the GRADE pro (Version 3.6) software to rate the quality of evidence.
Subgroup analysis
Subgroup analysis was performed based on the dosage of XBJ (50 mL group, 100 mL group and 150 mL group) in this systematic review.
Results
Description of included studies
A total of 354 records were identified based on search strategy, and 287 potentially eligible records were obtained after removing duplicate publications. After screening the titles and abstracts, a total of 255 trials were excluded. This left 26 trials which were included in the meta-analysis, and 6 more trials were later excluded for various reasons (Figure 1). A total of 1,880 participants were allocated between the XBJ group (n=962) and the control group (n=918). The characteristics of the included studies are presented in Table 1. The methodological quality of most studies was “poor” (Figure S1).
Figure 1.
Study flow diagram.
Table 1. The characteristics of included studies.
| Study ID | Sample size (N) | Mean age (x±s, years) | Intervention | Control | Course (qd) | Outcome measure |
|---|---|---|---|---|---|---|
| Zhang 2016 (15) | N=30 (T: 15; C: 15) | 39.14±4.91 | CT + XBJ | CT | 7 d | b |
| Jiao 2009 (16) | N=50 (T: 25; C: 25) | T: 32.1±11.8; C: 31.0±12.7 | CT + XBJ | CT | 7 d | a, b |
| Hu 2012 (17) | N=58 (T: 30; C: 28) | 41.35±8.56 | CT + XBJ | CT | 7 d | d |
| Guo 2010 (18) | N=108 (T: 55; C: 53) | T: 34.69±17.47; C: 35.14±18.35 | CT + XBJ | CT | 7 d | a |
| Li 2014 (19) | N=51 (T: 31; C: 20) | NA | CT + BP + XBJ | CT+BP | 5 d | a |
| Pan 2015 (20) | N=89 (T: 51; C: 38) | T: 39.9±7.1; C: 39.4±6.5 | CT + XBJ | CT | 7 d | a, b, d |
| Wang 2017 (21) | N=150 (T: 75; C: 75) | T: 51.13±8.38; C: 51.46±8.15 | CT + XBJ | CT | 7 d | f |
| Ma 2010 (22) | N=40 (T: 20; C: 20) | T: 35.6±16.6; C: 39.4±18.7 | CT + XBJ | CT | 7 d | a, e, g |
| Wang 2008 (23) | N=70 (T: 35; C: 35) | T: 32.12±10.51; C: 32.43±9.81 | CT + XBJ | CT | 7 d | a, d, f |
| Zou 2008 (24) | N=15 (T: 7; C: 8) | T: 32.4±9.8; C: 32.1±10.5 | CT + XBJ | CT | 7 d | a, f |
| Tian 2017 (25) | N=72 (T: 36; C: 36) | 51.7±12.3 | CT + XBJ | CT | 7 d | b, e, g |
| Li 2010 (26) | N=42 (T: 23; C: 19) | T: 35.0±6.1; C: 32.46±4.58 | CT + XBJ | CT | 7 d | g |
| Zhao 2013 (27) | N=107 (T: 53; C: 54) | 34.3±10.1 | CT + BP + XBJ | CT + BP | 5 d | a, g |
| Jiang 2017 (28) | N=93 (T: 47; C: 46) | T: 45±14; C: 46±15 | CT + XBJ | CT | 7 d | c |
| Zhang 2017 (29) | N=82 (T: 46; C: 36) | 46.0±12.9 | CT + XBJ | CT | 7 d | a, c, e |
| Li 2013 (30) | N=65 (T: 35; C: 30) | NA | CT + XBJ | CT | 7 d | c, e |
| Zhang 2011 (31) | N=30 (T: 14; C: 16) | 40.21±6.22 | CT + XBJ | CT | 7 d | b |
| Yu 2015 (32) | N=60 (T: 30; C: 30) | T: 40.8±12.9; C: 40.9±12.8 | CT + XBJ | CT | 7 d | a |
| Yuan 2011 (33) | N=60 (T: 30; C: 30) | 31.6±9.2 | CT + XBJ | CT | 7 d | a, d |
| Chen 2014 (34) | N=192 (T: 97; C: 95) | T: 34.6±8.2; C: 35.7±7.3 | CT + XBJ | CT | 6 d | a, d, e |
| Wang 2013 (35) | N=60 (T: 30; C: 30) | T: 38.6±6.01; C: 37.10±7.54 | CT + XBJ | CT | 7 d | a, b |
| Liao 2010 (36) | N=82 (T: 40; C: 42) | 40.82±5.43 | CT + XBJ | CT | 4 d | a, e |
| Lin 2012 (37) | N=72 (T: 36; C: 36) | T: 36.3±7.4; C: 36.8±7.6 | CT + XBJ | CT | 7 d | a, d |
| Liu 2009 (38) | N=56 (T: 28; C: 28) | 41.6±27.0 | CT + XBJ | CT | 7 d | d, g |
| Chen 2016 (39) | N=68 (T: 34; C: 34) | T: 49.5±5.8; C: 49.4±5.9 | CT + XBJ | CT | 7 d | b, c, e, g |
| Yang 2014 (40) | N=78 (T: 39; C: 39) | T: 52.0±1.5; C: 52.5±1.0 | CT + XBJ | CT | 7 d | b |
T, treatment group; C, control group; NA, not applicable; CT, conventional therapy; XBJ, xuebijing injection; BP, blood perfusion; a, all-cause mortality in 7 days; b, the level of C-reactive protein; c, the time of ChE came back to normal level; d, the level of CK-MB; e, the duration of hospital stays; f, the level of NF-κB; g, the level of TNF-α.
Figure S1.
Risk of bias summary.
7-day mortality
Fifteen studies (24-37,41) provided data on the 7-day mortality rate based on the Chi-square test P=0.79>0.05 and I2 =0%<50%. We selected the fixed-effects model to analyze the 7-day mortality. In Figure 2A, the results show the 7-day mortality (RR: 0.33; 95% CI: 0.22–0.49) of the XBJ group was lower than in the control group. Funnel plot is shown as visually asymmetrical (Figure 2B).
Figure 2.
7-day mortality: (A) forest plot, (B) funnel plot.
The level of C-reactive protein (CRP)
Eight studies (24,27,35,38,39,42-44) analyzed the level of CRP. Subgroup analysis was performed in the 50 mL group and the 100 mL group. In the 50 mL group, we selected the random-effects model based on the Chi-square test P=0.0009<0.05 and I2 =79%>50%. In Figure 3, the results show the level of CRP (WMD: −11.60; 95% CI: −14.38 to −8.83) was lower than in the control group. In the 100 mL group, the random-effects model was selected for a Chi-square test P=0.02<0.05 and I2 =73%>50%. Compared with the control group, the XBJ group could reduce the level of CRP (WMD: −1.73; 95% CI: −2.91 to −0.55). The results indicated that the 50 mL dose of XBJ dose was more useful than the 100 mL one for reducing the level of CRP.
Figure 3.
Forest plot: the level of C-reactive protein (CRP).
The time to AChE recovery
Four studies (31,40,43,45) analyzed the time to AChE recovery. In the 50 mL group, we selected the fixed-effects model based on the Chi-square test P=0.5>0.05 and I2=0%<50%. In Figure 4, the results show the time for AChE recovery (WMD: −4.58; 95% CI: −5.87 to −3.28) was lower than in the control group. In the 100 mL group, the fixed-effects model was selected for the Chi-square test P=0.47>0.05 and I2 =0%<50%. When compared with the control group, the XBJ group had a reduced time for AChE recovery (WMD: −1.73; 95% CI: −2.07 to −1.39). The results indicated that the XBJ dose of 50 mL was more useful than the 100 mL one for reducing the time for AChE recovery.
Figure 4.
Forest plot: the time to AChE recovery.
The length of hospital stays
Seven studies (28,31,34,36,42,43,45) provided data about the length of hospital stays. In the 50 mL group, we selected the fixed-effects model based on the Chi-square test P=0.61>0.05 and I2 =0%<50%. In Figure 5, the results show the length of hospital stay (WMD: −4.26; 95% CI: −4.89 to −3.64) was shorter than in the control group. In the 100 mL group, the fixed-effects model was selected for the Chi-square test P=0.27>0.05 and I2 =23%<50%. When compared with the control group, the XBJ group’s length of hospital stay was shorter (WMD: −2.48; 95% CI: −3.13 to −1.82). The results indicated that the XBJ dose of 50 mL was more useful than the 100 mL one for reducing the length of hospital stay.
Figure 5.
Forest plot: the length of hospital-stays.
The level of TNF-ɑ
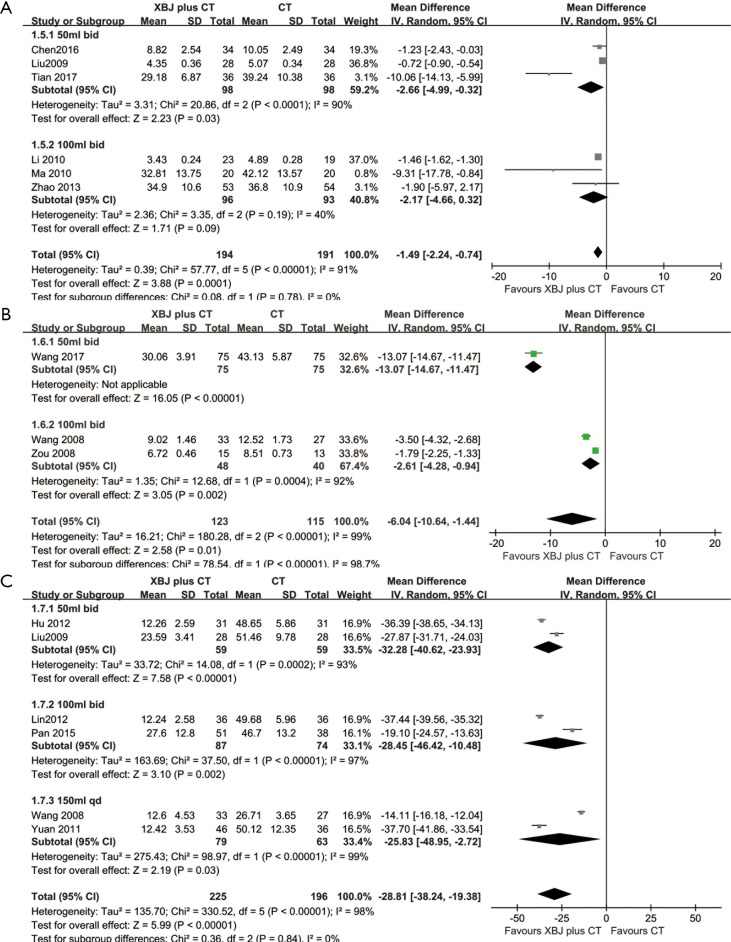
Six studies (28,30,42,43,46,47) provided data for the level of TNF-ɑ. In the 50 mL group, we selected the random-effects model based on the Chi-square test P=0.0001<0.05 and I2 =90%>50%. In Figure S2A, the results show that the level of TNF-ɑ (WMD: −2.66; 95% CI: −4.99 to −0.32) was lower than in the control group. In the 100 mL group, there was no statistical difference between the two groups (WMD: −2.17; 95% CI: −4.66 to 0.32). The results indicated that the XBJ dose of 50 mL could reduce the level of TNF-ɑ.
The level of NF-κB
Three studies (29,41,48) analyzed the level of NF-κB. In the 50 mL group, there was only 1 study which had the results showing the level of NF-κB (WMD: −13.07; 95% CI: −14.67 to −11.47). In the 100 mL group, the random-effects model was selected for the Chi-square test P=0.0004<0.05 and I2 =92%>50%. Compared with the control group, the XBJ group could reduce the level of NF-κB (WMD: −2.61; 95% CI: −4.28 to −0.94) (Figure S2B). The results indicated that the XBJ dose of 50 mL is more useful than 100 mL for reducing the level of NF-κB.
The level of CK-MB
Six studies (27,29,33,37,47,49) analyzed the level of CK-MB. In the 50 mL group, we selected the random-effects model based on the Chi-square test P=0.0002<0.05 and I2=93%>50%. In Figure S2C, the results show that the level of CK-MB (WMD: −32.28; 95% CI: −40.62 to −23.93) was lower than in the control group. In the 100 mL group, a random-effects model was selected for the Chi-square test P=0.00001<0.05 and I2=97%>50%. Compared with the control group, the XBJ group had a reduced level of CK-MB (WMD: −28.45; 95% CI: −46.42 to −10.48). In the 150 mL group, the random-effects model was selected for the Chi-square test P=0.00001<0.05 and I2=99%>50%. Compared with the control group, the XBJ group had a reduced level of CK-MB (WMD: −25.83; 95% CI: −48.95 to −2.72). The results indicated that the XBJ dose of 50 mL was the most useful dose, and the most effective frequency to reduce the level of CK-MB was twice a day.
Grade quality of evidence
Based on the five factors (risk of bias, inconsistency, indirectness, imprecision, publication bias), the quality of evidence was assessed as “low” by GRADE profiler (Figure 6).
Figure 6.
The grade quality of evidence.
Discussion
Summary of findings
This meta-analysis reveals that XBJ combined with conventional therapy could reduce the 7-day mortality rate. In addition, it showed benefit in reducing the level of CRP, TNF-ɑ, NF-kB, CK-MB, the length of hospital-stay, and the time for AChE recovery. Meanwhile, the results also show that the most effective dosage of XBJ is 50 mL, and the most effective frequency is twice a day.
We speculate that this significant benefit could be due to two reasons. Firstly, it is well-known that the major mechanism for organophosphorus pesticide poisoning is the inhibition of organic phosphorus acting on cholinesterase. According to the theory of traditional Chinese medicine, XBJ has the function of blood-activation, toxin-scattering, and possible acetylcholine acceleration and excretion of (50). Secondly, an increasing amount of evidence has documented that XBJ inhibits inflammatory responses and thereby protects against secondary multiple organ injury in many clinical scenarios such as severe sepsis (51) and heat stroke (52). Therefore, this inhibition of XBJ to inflammatory responses may play a pivotal role in alleviating organophosphorus-induced multiple vital organ injury.
Of note, the results show that the benefit will decay with an increasing dosage of XBJ. We speculate that a small dose of XBJ could inhibit inflammatory responses, by decreasing the level of TNF-ɑ, and NF-κB. Furthermore, according to the included studies, we speculate that the researchers may use a small dose of XBJ for moderate AOPP, and a large dose of XBJ for severe AOPP. Severe AOPP is more difficult to treat in spite of the use of XBJ. Therefore, it may be more useful for moderate AOPP to use XBJ with a dose of 50 mL.
Sensitivity analysis
In this systematic review, to account for the heterogeneity among the studies, we adopted the random effects model and analyzed the subgroups according to different dosages of Xuebijing in the experimental group. A total of 15 studies described the 7-day mortality, with the results of the meta-analysis showing low heterogeneity. This indicates a high level of credibility to this research. In the meta-analysis of other outcomes (including the level of CRP, TNF-ɑ, NF-κB, CK-MB), the severity of AOPP could have been the main reason for the substantial heterogeneity, considering the possible influence factor. According to the expert consensus for AOPP [2016], the severity of AOPP is different with different kinds and dosages of the toxicants (53). Meanwhile, the therapeutic method is different according to the severity of AOPP (e.g., blood perfusion could be used for severe patients with AOPP). Therefore, there is still some heterogeneity between the subgroups. The different age and condition of the patients would have different reactions to the toxicants. Therefore, the level of CRP and other inflammatory factors would be different. With these combined factors, the larger heterogeneity in partial outcomes were produced.
Limitations of this systematic review
Some limitations should be taken into consideration in this systematic review. Firstly, even though we comprehensively searched the databases, the gray literature was not collected. All of the included studies were conducted in the mainland China, which may have also resulted in a sampling bias. Second, the significance between-study heterogeneity detected in some outcomes might have influenced the validity of the meta-analysis.
Implication for further research
According to the expert consensus of AOPP, the severity of patients with AOPP is dependent on the kind and the dosage of organophosphorus. However, it is rarely classified in the current literature. Therefore, in order to improve the management of AOPP, more studies are needed to evaluate the efficacy of XBJ for different kinds of organophosphorus poisoning. A recent study showed that the level of CRP and APPACHE II scoring may be associated with the prediction of an AOPP prognosis (54). Hence, more studies should pay attention to the level of CRP and APPACHE II score.
Because of the low methodological quality, it is necessary to improve the quality of traditional Chinese medicine RCT and ensure that real random methods are adopted, which should be strictly controlled in terms of experimental design, research implementation, data analysis and reporting. Attention should be paid to multi-center participation, the generation of random sequences and the implementation of blind methods.
Conclusions
XBJ has a significant clinical efficacy in the treatment of patients with acute organophosphorus pesticide poisoning. The most effective dosage is 50 mL and the most effective frequency is twice a day. More randomized, larger sample-sized, high quality and multi-center studies are needed to confirm the extract efficacy of XBJ.
Figure S2.
Forest plot: the level of TNF-α (A), the level of NF-κB (B), the level of CK-MB (C).
Acknowledgements
Funding: This study was supported by the National Natural Science Foundation of China (No:81774146).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Wang Z, Lv Y, Cui T. Analysis of cause of death by acute organophosphorus pesticide poisoning. Zhong Guo She Qu Yi Shi 2012;14:106-7. [Google Scholar]
- 2.Cavaliere MJ, Puga FR, Calore EE, et al. Protective effect of pralidoxime on muscle fiber necrosis induced by organophosphate compounds. J Toxicol Clin Toxicol 1998;36:295-300. 10.3109/15563659809028024 [DOI] [PubMed] [Google Scholar]
- 3.Ryniak S, Harbut P, Gozdzik W, et al. Whole blood transfusion in the treatment of an acute organophosphorus poisoning-a case report. Med Sci Monit 2011;17:CS109-11. 10.12659/MSM.881922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamel F, Hoppin JA. Association of pesticide exposure with neurologic dysfunction and disease. Environ Health Perspect 2004;112:950-8. 10.1289/ehp.7135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu HX, Liu CF, Yang WH. Clinical study of continuous micropump infusion of atropine and pralidoxime chloride for treatment of severe acute organophosphorus insecticide poisoning. J Chin Med Assoc 2015;78:709-13. 10.1016/j.jcma.2015.08.006 [DOI] [PubMed] [Google Scholar]
- 6.Dash SK, Mohanty MK, Mohanty S, et al. Organophosphorus poisoning: victim specific analysis of analysis of mortality and morbidity. Med Sci Law 2008;48:241-5. 10.1258/rsmmsl.48.3.241 [DOI] [PubMed] [Google Scholar]
- 7.Jiang M, Zhou M, Han Y, et al. Identification of NF-κB Inhibitors in Xuebijing injection for sepsis treatment based on bioactivity-integrated UPLC-Q/TOF. J Ethnopharmacol 2013;147:426-33. 10.1016/j.jep.2013.03.032 [DOI] [PubMed] [Google Scholar]
- 8.Huang H, Ji L, Song S, et al. Identification of the major constituents in Xuebijing injection by HPLC-ESI-MS. Phytochem Anal 2011;22:330-8. 10.1002/pca.1284 [DOI] [PubMed] [Google Scholar]
- 9.Sun J, Xue Q, Guo L, et al. Xuebijing protects against lipopolysaccharide-induced lung injury in rabbits. Exp Lung Res 2010;36:211-8. 10.3109/01902140903312123 [DOI] [PubMed] [Google Scholar]
- 10.Jia P, Wang S, Meng X, et al. Effects of ionic liquid and nanogold particles on high-performance liquid chromatography-electrochemical detection and their application in highly efficient separation and sensitive analysis of five phenolic acids in Xuebijing injection. Talanta 2013;107:103-10. 10.1016/j.talanta.2012.12.031 [DOI] [PubMed] [Google Scholar]
- 11.Jiang WY, Jeon BH, Kim YC, et al. PF2401-SF, standardized fraction of Salvia miltiorrhiza shows anti-inflammatory activity in macrophages and acute arthritis in vivo. Int Immunopharmacol 2013;16:160-4. 10.1016/j.intimp.2013.03.028 [DOI] [PubMed] [Google Scholar]
- 12.Yin X, Yin Y, Cao FL, et al. Tanshinone IIA attenuates the inflammatory response and apoptosis after traumatic injury of the spinal cord in adult rats. PLoS One 2012;7:e38381. 10.1371/journal.pone.0038381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li M, Zhang L, Cai RL, et al. Lipid-soluble extracts from Salvia miltiorrhiza inhibit production of LPS-induced inflammatory mediators via NF-κB modulation in RAW 264.7 cells and perform antiinflammatory effects in vivo. Phytother Res 2012;26:1195-204. 10.1002/ptr.3680 [DOI] [PubMed] [Google Scholar]
- 14.Tang S, Shen XY, Huang HQ, et al. Cryptotanshinone suppressed inflammatory cytokines secretion in RAW264.7 macrophages through inhibition of the NF-κB and MAPK signaling pathways. Inflammation 2011;34:111-8. 10.1007/s10753-010-9214-3 [DOI] [PubMed] [Google Scholar]
- 15.Ren ZH, Tong YH, Xu W, et al. Tanshinone II A attenuates inflammatory responses of rats with myocardial infarction by reducing MCP-1 expression. Phytomedicine 2010;17:212-8. 10.1016/j.phymed.2009.08.010 [DOI] [PubMed] [Google Scholar]
- 16.Ran X, Ma L, Peng C, et al. Ligusticum chuanxiong Hort: a review of chemistry and pharmacology. Pharm Biol 2011;49:1180-9. 10.3109/13880209.2011.576346 [DOI] [PubMed] [Google Scholar]
- 17.Liu L, Ning ZQ, Shan S, et al. Phthalide Lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-mediated NF-kappaB Activation. Planta Med 2005;71:808-13. 10.1055/s-2005-871231 [DOI] [PubMed] [Google Scholar]
- 18.Wang CC, Choy CS, Liu YH, et al. Protective effect of dried safflower petal aqueous extract and its main constituent, carthamus yellow, against lipopolysaccharide-induced inflammation in RAW264.7 macrophages. J Sci Food Agric 2011;91:218-25. 10.1002/jsfa.4172 [DOI] [PubMed] [Google Scholar]
- 19.Song L, Zhu Y, Jin M, et al. Hydroxysafflor yellow a inhibits lipopolysaccharide-induced inflammatory signal transduction in human alveolar epithelial A549 cells. Fitoterapia 2013;84:107-14. 10.1016/j.fitote.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 20.Saw CL, Wu Q, Su ZY, et al. Effects of natural phytochemicals in Angelica sinensis (Danggui) on Nrf2-mediated gene expression of phase II drug metabolizing enzymes and anti-inflammation. Biopharm Drug Dispos 2013;34:303-11. 10.1002/bdd.1846 [DOI] [PubMed] [Google Scholar]
- 21.Su YW, Chiou WF, Chao SH, et al. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol 2011;11:1166-72. 10.1016/j.intimp.2011.03.014 [DOI] [PubMed] [Google Scholar]
- 22.Chao WW, Hong YH, Chen ML, et al. Inhibitory effects of Angelica sinensis ethyl acetate extract and major compounds on NF-kappaB trans-activation activity and LPS-induced inflammation. J Ethnopharmacol 2010;129:244-9. 10.1016/j.jep.2010.03.022 [DOI] [PubMed] [Google Scholar]
- 23.Moher D, Liberati A, Tetzlaff J, et al. Preferred Reporting Items for Systematic Reviews and Meta-Analysis: The PRISMA Statement. PLoS Med 2009;6:e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiao P, Han XT. The influence of blood circulation removing extravasted blood degassing on blood serum C response protein (CRP) level and clinical signifi cance in acute ganophosphorus pesticide poisoning (AOPP) patients. Contemp Med 2009;15:2-3. [Google Scholar]
- 25.Guo JR, Mi H, Zhang XH. Effect of Xuebijing on Interleukin 18 in patients with Acute Severe Organophosphate Posioning. Med Recapitul 2010;16:3821-2. [Google Scholar]
- 26.Li Y. Clinical study on the treatment of severe organophosphorus pesticide poisoning by Xuebijing combined with blood perfusion. Public Med Forum Mag 2010;18:2949-50. [Google Scholar]
- 27.Pan YC, He SF, Tang XX. Clinical research of Xuebijing combined with penehyclidine hydrochloride in treatment of acute organophosphorus pesticide poisoning. Clin Med Engineer 2015;22:427-8. [Google Scholar]
- 28.Ma YY, Niu YQ, Yao MX. Study on the intervention of serum tumor necrosis factor and interleukin 10 in patients with acute organophosphorus pesticide poisoning. Clin Focus 2010;25:1998-9. [Google Scholar]
- 29.Wang Y, Xu RT, Zou YG, et al. Intervention of Xuebijing injection in acute organophosphate poisoning complicated with cardiovascular injury. J Shandong Univer Health Sci 2008;46:1170-1. [Google Scholar]
- 30.Zhao XS, Gao Y, Song Q, et al. The effect of Xuebijing injection on the treatment of severe organophosphorus poisoning. Chin J Clin 2013;7:4094-6. [Google Scholar]
- 31.Zhang QF. Clinical study on Xuebijing Injection combined with pralidoxime chloride injection in the treatment of organophosphorus pesticide poisoning. Drugs Clin 2017;32:1057-60. [Google Scholar]
- 32.Yu DC, Wen JQ, Tan JX, et al. Clinical observation on the treatment of acute organophosphorus pesticide poisoning by Xuebijing injection. Mod Diagn Treat 2015;26:546-7. [Google Scholar]
- 33.Yuan GH. Clinical observation of Xuebijing injection on the treatment of acute organophosphorus poisoning and 30 cases of cardiovascular injury. J Xianning Univ (Med Sci) 2011;25:37-8. [Google Scholar]
- 34.Chen KP. Penehyclidine Hydrochloride combined with Xuebijing injection in treating acute organophosphorus pesticide poisoning complicating myocardial injury in 97 cases. Clinical pharmaceuticals. Clin Med 2014;23:75-6. [Google Scholar]
- 35.Wang JH. Observation on the curative effect of Xuebijing injection combined with Penehyclidine Hydrochloride on severe organophosphorus pesticide poisoning complicating respiratory failure. Zhejiang JITCWM 2013;23:25-6. 10.1631/jzus.A1200139 [DOI] [Google Scholar]
- 36.Liao XL. Application of Xuebijing in acute organophosphorus pesticide poisoning. J Tai Zhou Polytechnic Coll 2010;10:34-5. [Google Scholar]
- 37.Lin SM, Gao YM, Ding HJ. The curative effect of Xuebijing injection on acute organophosphorus pesticide poisoning complicating respiratory failure. Chin J Prim Med Pharm 2012;19:2947-8. [Google Scholar]
- 38.Zhang L. The curative effect of Xuebijing injection on acute organophosphorus pesticide poisoning. World latest Med Inf 2016;16:110-2.
- 39.Zhang XB. Observation on the curative effect of Xuebijing injection on acute organophosphorus pesticide poisoning. Med Inf 2011:1863-4. [Google Scholar]
- 40.Jiang Y. The effect of Xuebijing injection combined with Pralidoxime Iodide on the life quality and rehabilitation process of the patients with organophosphorus pesticide poisoning. Clin Pharm 2017;10:73-5. [Google Scholar]
- 41.Zou YG. Effects of Xuebijing injection on NF-kB in acute poisoning patients complicated with multiple organ dysfunction syndrome. D. Shandong University, 2008. [Google Scholar]
- 42.Tian YH, Chen QX, Deng XY, et al. Effects of Xuebing injection on serum CRP, TNF-ɑ and IL-6 levels in patients with severe acute organophosphorus pesticide poisoning. Chin Traditional Patent Med 2017;39:1365-8. [Google Scholar]
- 43.Chen YG, Ji YR, Song NY. Clinical observation on the treatment of acute organophosphorus pesticide poisoning by Xuebijing injection. Health Forefront 2016;7:200. [Google Scholar]
- 44.Yang WL. Clinical observation on the treatment of acute organophosphorus pesticide poisoning by Xuebijing injection. Med Inf 2014;27:318. [Google Scholar]
- 45.Li X, Liu JZ. Clinical observation of Xuebijing injection as adjuvant therapy on the treatment of severe organophosphorus pesticide poisoning. Rational Drug Use 2013;6:71. [Google Scholar]
- 46.Li XM. The effect of Xuebijing injection on inflammatory mediators in patients with severe acute organophosphorus pesticide poisoning. Tianjing Med J 2010;38:593-5. [Google Scholar]
- 47.Liu YH, Jiao H, Chen ZX, et al. The protective effect of Xuebijing injection on multiple organ damage in patients with acute organophosphorus pesticide poisoning and its mechanism. Chin J Crit Care Med 2009;29:884-7. [Google Scholar]
- 48.Wang YJ, Zhang CR. The efficacy and mechanism of Xuebijing injection on the treatment of myocardial damage induced by acute organophosphorus pesticide poisoning. Chin Commun Doc 2017;33:90-1. [Google Scholar]
- 49.Hu RS, Wang HY. Effect of Xuebijing injection on the plasma CK-MB and CTnT in patients with acute organophosphorus poisoning. Chin Foreign Med Res 2012;10:30-1. [Google Scholar]
- 50.Shi H, Hong Y, Qian J, et al. Xuebijing in the treatment of patients with sepsis. Am J Emerg Med 2017;35:285-91. 10.1016/j.ajem.2016.11.007 [DOI] [PubMed] [Google Scholar]
- 51.Qi F, Liang ZX, She DY, et al. A clinical study on the effects and mechanism of xuebijing injection in severe pneumonia patients. J Tradit Chin Med 2011;31:46-9. 10.1016/S0254-6272(11)60011-3 [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Tong H, Zhang X, et al. Xuebijing injection alleviates liver injury by inhibiting secretory function of Kupffer cells in heat stroke rats. J Tradit Chin Med 2013;33:243-9. 10.1016/S0254-6272(13)60133-8 [DOI] [PubMed] [Google Scholar]
- 53.Department of Emergency Medical attached to Chinese Medical Association. Clinical expert consensus on diagnosis and treatment of acute organophosphorus pesticide poisoning. Chin J Crit Care Med 2016,36:1057-65. [Google Scholar]
- 54.Guo CF, Wang Y, Liu JH, et al. Analysis on influencing factors of prognosis of patients with acute organophosphorus pesticide poisoning. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi 2016;34:372-4. [DOI] [PubMed] [Google Scholar]