Abstract
Background
Gastric cancer has been the second cause of cancer death worldwide. Chemical comprehensive treatment programs primarily were the main therapy method with modest efficacy to gastric cancer. Traditional Chinese medicine (TCM) has been reported to alleviate adverse events induced by chemotherapy, but has not yet developed clinical trials to test and needs scientific evidence for making policy. Single-patient (N-of-1) trials might be an eligible study design for TCM since it well represented the individualized treatment philosophy of TCM. The aim of this study is to obtain information necessary to design a more series trial.
Methods
Individuals who underwent gastrectomy were included. Each patient suffered 3-week standard chemotherapy and 3-day treatment periods (decoction with Astragalus mongholicus and Semen Cuscutae or placebo: decoction without Astragalus mongholicus and Semen Cuscutae). Each trial lasted up to a maximum of 30 weeks or a minimum of 20 weeks. Staffs and participants were blinded to the randomization. This study was approved by Ethics Committee of First Hospital, Lanzhou University in November, 2014.
Results
From August, 2014 to March, 2015, 6 participants were included. There were 16 cycles compared between intervention and control decoction (2.28, 95% CI: 1.24–5.47), P<0.0001. The quality of life (QoL) score after the trial was reported is a little higher than before, t=3.87, P=0.01. Two participants reported symptoms had improved after taken trial decoction.
Conclusions
This is the first N-of-1 trials of testing the effectiveness of TCM decoction on alleviative treatment to gastric cancer. The feasibility study will help to develop a practical design for the more series trial.
Keywords: N-of-1 trials, traditional Chinese medicine (TCM), chemotherapy-induced leukopenia, gastric cancer
Introduction
Gastric cancer is one of the major malignant carcinoma. With an incidence of 989,600, gastric cancer was the fourth most frequent malignant caner and the second most common cause of cancer death with 738,000 worldwide in 2008 (1). Most patients with gastric cancer are offered surgical therapies with chemotherapy because of a small but significant survival advantage (2). At present, a regimen based on platinum is regarded standard treatment with good performance status. Unfortunately, chemotherapeutic drugs that have various side effects, of which leukopenia is the most common and positively associated with survival in gastric cancer.
Overall, although the efficacy of chemotherapy is modest at best, cytotoxic drugs will probably continue to be part of standard treatment, and alleviative treatment is the trend in the next few decades. Traditional Chinese medicine (TCM) has been reported to alleviate adverse events induced by conventional cancer therapy, improve patient’s quality of life (QoL) (3), enhance cellular immunity of cancer patients receiving chemotherapy/radiotherapy (4), reduce cancer pain (5), relieve cancer-related fatigue (6) and improve anorexia and cachexia (7). Astragalus mongholicus and Semen Cuscutae has been reported to improve advanced treatment quality of cancer patients (8,9).
It is well known that treatment based on syndrome differentiation is one of the characteristics and essences of TCM which emphasizes individualized treatment (10). Population-based randomized controlled trials (RCTs) might not be the optimal study design for TCM because individualized TCM intervention often makes it difficult for population-based RCTs to carry out a standard form. The use of decoctions with field herbs or patent Chinese medicine hardly represents the superiority of individualized treatment effects (11). Single case RCTs (N-of-1 trials) have been paid more and more attention since clinicians started realizing the limitation of population-based RCTs when medical interventions work ubiquitously or under most circumstances for the majority of common chronic conditions (12). We designed an N-of-1 feasibility trials to: (I) determine the best treatment of alleviative treatment in advance gastric cancer; (II) develop and test N-of-1 trials used in TCM field; (III) assess the feasibility of recruiting and randomizing participants and the compliance; (IV) identify and evaluate designing, conducting, monitoring and managing the study.
Methods
Trial design
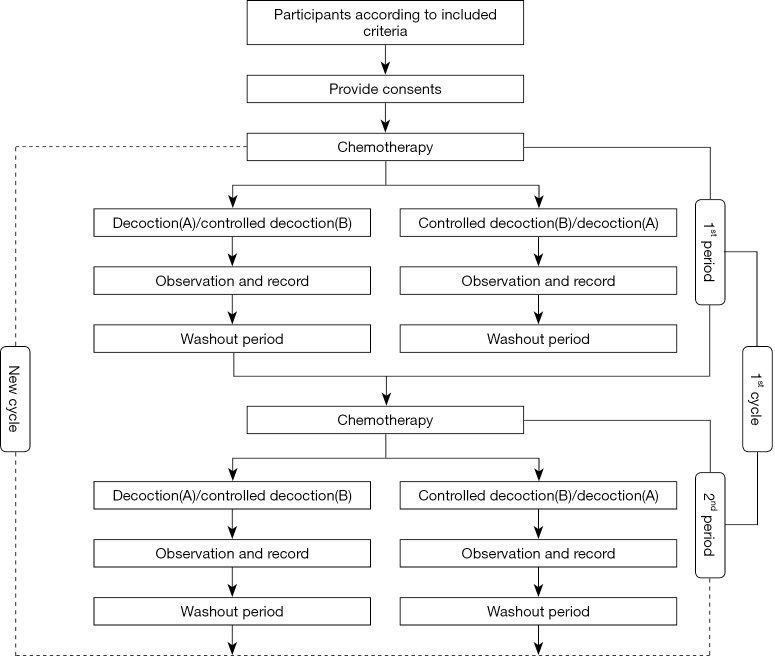
We designed the N-of-1 trials consistently with previously published guidelines by Guyatt et al. (13). This feasibility study was a series of N-of-1 trials with randomization, double-blind, and controlled treatment. In this study, the single participant who met the inclusion criteria of TCM clinical response underwent 3 pairs of treatment periods with individualized herbal decoction with standard chemotherapy. In our series, each N-of-1 trial consisted of two or three cycles with treatment and control assigned randomly. Every treatment or control periods were separated by 1-week washout intervals to make carryover effects minimized and observe the disease development (Figure 1).
Figure 1.
The flow chart of the whole process of the feasibility study.
Each trial lasted up to a minimum of 20 weeks or a maximum of 30 weeks. Each patient suffered 3-week standard chemotherapy and then used 3-day treatment periods (decoction A with Astragalus mongholicus and Semen Cuscutae or decoction B without Astragalus mongholicus and Semen Cuscutae) to allow for adequate time for relieving the discomfort caused by chemotherapy. For each patient, the daily herbal dose that had previously been associated with the self-rated symptom (RS) and development were administrated in the respective N-of-1 trial.
Because decoction we used is a mixture of herbs and water, it is a bit more difficult to make sure the pharmacological half-life period. We resumed the half-life periods of main herbs such as Astragalus mongholicus and Semen Cuscutae to determine the probable washout interval time.
If the symptoms changed and developed, patients were offered the option of stopping a treatment period early and waiting for the other treatment period after a washout period or withdrawing the trial.
Setting and participants
We recruited inpatients aged 18 years or older at the Oncological Surgery Center of the First Hospital of Lanzhou University between June 2014 and March 2015.
Patients were included if they had a history of radical gastrectomy requiring chemotherapy therapy according to the National Comprehensive Cancer Network (NCCN) gastric cancer guidelines (14). All the patients were in stable condition without acute exacerbation, and the diagnostic criteria based on the consensus of Chinese experts according to standardization of TCM syndrome. There were no other organ metastasis and recurrence during the trial periods. All patients were willing to accept the additional TCM treatment based on chemotherapy. We excluded the patients with heart disease, hepatic disease or kidney disease, or mental disorder and those who could not comply with the added demands of an N-of-1 trial. Patients with anemia and infection were also excluded, for which a blood transfusion, erythropoietin therapy and anti-infection therapy in the previous 2 weeks was used.
Interventions
Concomitant decoction treatments were used after the 3-week platinum chemotherapy. Treatment decoction (A) and controlled decoction (B) based on the ancient literature and experience were prescribed by the physician who experts on TCM for 15 years. Compared with the controlled decoction, there were additional Astragalus mongholicus and Semen Cuscutae in the treatment decoction. Detailed decoction prescription records were presented: decoction A—Radix Pseudostellariae 45 g, Rhizoma Atractylodis Macrocephalae 45 g, Rhizoma Zingiberis 45 g, Radix Glycyrrhizae Preparata 45 g, Rhizoma Pinellinae Praeparata 15 g, Fructus Amomi 15 g, Rhiizoma Dioscoreae 60 g, Astragalus mongholicus 120 g, Semen Cuscutae 60 g, Lignum Millettiae 60 g; decoction B—Radix Pseudostellariae 45 g, Rhizoma Atractylodis Macrocephalae 45 g, Rhizoma Zingiberis 45 g, Radix Glycyrrhizae Preparata 45 g, Rhizoma Pinellinae Praeparata 15 g, Fructus Amomi 15 g, Rhiizoma Dioscoreae 60 g, Lignum Millettiae 60 g.
If the participants had insisted the trial with severe heartburn, the physician added Evodia Rutaecarpa 15 g and Sepium 20 g into the decoction.
Directions
Pieces of TCM which had passed quality inspection in line with the national norms were provided by the hospital pharmacy herbs’ store. Pieces of TCM were wrapped in nonwoven cloth bag, soaked in 2 liter water for 1 hour, and decocted 1 time for 60 min in a TCM decocting machine. The herbal decoctions were taken by one decoction a day and divided into 2 doses (100 mL once) as a dietary supplement.
Randomization and blinding
A total of 30 sets of random numbers were generated by www.randomizer.org. Every odd number was designated as decoction A and even number was designated as decoction B. The random orders of decoctions A and B were allocated by opaque envelope. We estimated to recruit 8 participants in the feasibility study and use 24 sets. Other 6 sets were spare. Blinding was maintained by use of identical-looking dispensing bottles and bag in which decoction A or B was compounded by the same pharmacy store, dispensed to the patient. The decoctions A and B had no differences in appearance, dosage form, color, specification, and so forth. This method successfully kept the physicians, patients, and outcome assessors blinded.
Outcomes
Leukocyte count
Chemotherapy-induced leukopenia has been expressed by leukocyte count, which is routinely determined in peripheral venous samples usually one or two days before the period of adjuvant chemotherapy.
QoL score
The researchers chose a revised Chinese version of the Quality of Life Questionnaire (SF-36) to measure QoL, which is a 36-item, self-administered questionnaire with ratings on a four-point scale (1= not at all and 4= extremely). The questionnaire contains: 10 physical function (PF) questions, 6 general health (GH) questions, 5 mental health (MH) questions, 4 role physical (RP) questions, 3 role emotion (RE) questions, 4 vitality (VT) questions, 2 body pain (BP) questions, and 2 social function (SF) questions that cover disease and treatment-related symptoms as well as the emotional consequences of stomach cancer (15). Based on linear conversion, the final score is between 0–100 for each domain [converted score = (real crude score/total maximum score) × 100]. A high score represents high QoL in gastric cancer patients. Generally, the boundary level of Cronbach’s â is 0.65, which suggests an acceptable reliability, while the number of 0.70 or above suggests a good reliability (16). The higher the score, the more improvement the symptoms get. Researchers did the surveying at the admission and the end of trial.
Physician RS
Physician with 15 years TCM experience rated the TCM symptoms (such as pulse condition, tongue condition and complexion). We observed all participants after taken decoction A and then gave the comprehensive conclusion by the same physician.
Data analysis
All statistical analyses were performed using SAS. A population estimate of effect by aggregating individual N-of-1 results was used. The results of all complete cycles were combined to produce a posterior probability of the overall difference between the A decoction cycles and the placebo cycles. A P value of less than 0.05 was considered statistically significant for each test.
Ethics
The study was approved by Ethics Committee of the First Hospital of Lanzhou University (LDYY LL2014-0068). All participants provided informed consent.
Results
Participants recruitment
At last, six patients who underwent chemotherapy after gastrectomy were included in this feasibility study. Eight patients with stable condition after gastrectomy attended the feasibility trial to observe the effectiveness of the intervention decoction (Figure 2). After the administration of the decoction for eight months, the adherence rate was 75%. The 6 participants between August 2014 and March 2015 completed 16 cycles: 4 (50%) participants completed three cycle, and 2 (25%) completed two cycles. The rest 2 patients cannot put up with the side effect like nausea and then withdrew the trial at the 1st period before collecting any data (Table 1).
Figure 2.
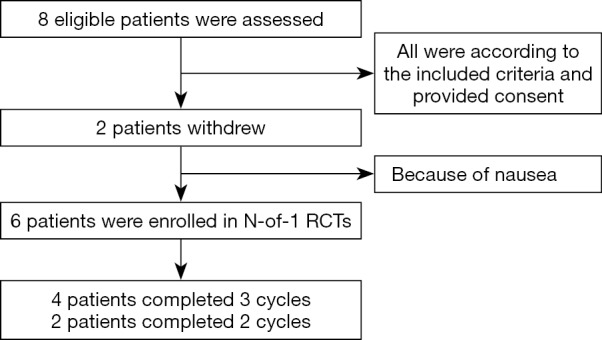
The flow chart of the participants including process of the feasibility study.
Table 1. Baseline information of participants.
| Participant | Decoction order** | Outcomes | Completed cycles | Completed rate (%) |
|---|---|---|---|---|
| 1 | AB-AB-BA | Leukocyte; QoL; RS | 3 | 100 |
| 2 | BA-AB-AB | Leukocyte; QoL; RS | 3 | 100 |
| 3 | AB-AB-AB | Leukocyte; QoL; RS | 3 | 100 |
| 4 | AB-AB-BA | Leukocyte; QoL; RS | 3 | 100 |
| 5 | BA-BA | Leukocyte; QoL; RS | 2 | 67 |
| 6 | AB-BA | Leukocyte; QoL; RS | 2 | 67 |
**, decoction order. QoL, quality of life; RS, rated symptom.
The enrolled participants included 4 males and 2 females, aged 45–65 years, and numbered as participant 1–6. All of the participants accepted radical-operation with D2 lymphadenectomy. Three (50%) participants’ pathological result reported GNor poorly differentiated adenocarcinoma, 2 (33.3%) reported GNor, moderate differentiated adenocarcinoma and 1 (16.7%) reported gastric mucosa, adenocarcinoma (Table 2). All the participants showed various symptoms degrees of improvement, but no significant differences were found between individualized herbal decoction and control decoction on symptoms.
Table 2. Baseline characteristic of participants.
| Characteristics | Numerical value |
|---|---|
| Years, mean (SD) | 52 (10.38) |
| Male, n (%) | 4 (66.7%) |
| Surgical procedures, n (%) | |
| Radical-operation with D 2 lymphadenectomy | 6 (100%) |
| Postoperative pathologic results | |
| GNor, poorly differentiated adenocarcinoma, infiltrative type | 3 (50.0%) |
| GNor, moderate differentiated adenocarcinoma | 2 (33.3%) |
| Gastric mucosa, adenocarcinoma | 1 (16.7%) |
Individual effectiveness assessment
Leukocyte
The leukocyte was measured at the beginning of the first chemotherapy and end of each period. Participants 1, 3 and 4 had significant difference between intervention and control decoction (Table 3). There were 16 cycles compared between intervention and control decoction (2.28, 95% CI: 1.24–5.47), P<0.0001.
Table 3. The leukocyte of each end of periods.
| Participant | Leukocyte (109/L) | ||||||
|---|---|---|---|---|---|---|---|
| Admission | 1st period end | 2nd period end | 3rd period end | 4th period end | 5th period end | 6th period end | |
| 1 | 3.90 | 4.83 (A) | 3.53 (B) | 3.83 (A) | 3.11 (B) | 4.01 (B) | 4.64 (A) |
| 2 | 6.46 | 4.71 (B) | 8.51 (A) | 4.52 (A) | 4.24 (B) | 3.15 (A) | 2.61 (B) |
| 3 | 3.68 | 3.87 (A) | 3.56 (B) | 4.03 (A) | 3.56 (B) | 4.91 (A) | 4.01 (B) |
| 4 | 11.21 | 6.39 (A) | 4.30 (B) | 4.95 (A) | 3.48 (B) | 3.26 (B) | 4.52 (A) |
| 5 | 5.85 | 5.03 (B) | 8.20 (A) | 7.66 (B) | 7.86 (A) | ||
| 6 | 9.29 | 11.95 (A) | 6.39 (B) | 7.61 (B) | 8.1 (A) | ||
QoL score
Each participant who completed 2–3 cycles accepted surveying of QoL score. The result reported the score after the trial is a little higher than before, t=3.87, P=0.01 (Table 4).
Table 4. the compared QoL score between before and after the trial.
| Participant | Before or After | Physical health score | Mental health score | Converted score | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PF | RP | BP | GH | VT | SF | RE | MH | ||||
| 1 | Before | 20 | 4 | 4 | 10 | 15 | 4 | 1 | 13 | 71 | |
| After | 28 | 4 | 8 | 12 | 11 | 5 | 3 | 13 | 84 | ||
| 2 | Before | 25 | 0 | 8 | 5 | 10 | 4 | 1 | 11 | 64 | |
| After | 29 | 2 | 7 | 11 | 11 | 5 | 3 | 16 | 84 | ||
| 3 | Before | 27 | 4 | 8 | 12 | 10 | 5 | 1 | 11 | 78 | |
| After | 28 | 4 | 6 | 17 | 11 | 4 | 4 | 13 | 87 | ||
| 4 | Before | 24 | 4 | 8 | 7 | 10 | 4 | 1 | 13 | 71 | |
| After | 24 | 4 | 9 | 7 | 10 | 4 | 2 | 13 | 73 | ||
| 5 | Before | 26 | 4 | 8 | 5 | 10 | 6 | 1 | 11 | 71 | |
| After | 26 | 4 | 8 | 8 | 13 | 6 | 1 | 10 | 76 | ||
| 6 | Before | 25 | 4 | 8 | 4 | 11 | 4 | 2 | 12 | 70 | |
| After | 27 | 4 | 8 | 10 | 11 | 7 | 1 | 13 | 81 | ||
QoL, quality of life; PF, physical function; GH, general health; MH, mental health; RP, role physical; RE, role emotion; VT, vitality; BP, body pain; SF, social function.
Physician RS
The blockage point on the tongue of 2 participants significantly improved after taken decoction A, 3 participants slightly improved and there was no improvement in the other 1 participant. The force-limiting deep pulse existed in 2 participants, 1 was slippery and others’ pulse improved to be fortis after taken decoction A. 2 participants looked flushing, 3 looked gloomy and 1 looked pallid (Table 5).
Table 5. Condition of physician rated symptom.
| Participant | Tongue | Pulse | Complexion |
|---|---|---|---|
| 1 | Significantly improved | Fortis | Gloomy |
| 2 | Not improved | Slippery | Gloomy |
| 3 | Slightly improved | Fortis | Flushing |
| 4 | Significantly improved | Force-limiting | Gloomy |
| 5 | Slightly improved | Fortis | Flushing |
| 6 | Slightly improved | Force-limiting | Pallid |
Discussion
From the results, it could be seen the feasibility of deriving a population estimate of effect using N-of-1 trial methodology in TCM field and the participants actively cooperated better. Herbal treatment decoction with Astragalus mongholicus and Semen cuscutae had effect on leukocyte and QoL score in patients with chemotherapy of advanced gastric cancer, supporting our previous expected results showing that Astragalus mongholicus and Semen cuscutae can really improve quality in advanced gastric cancer or end-of-life populations. No obvious side effects occurred during and after the six periods. We had proved long-term effects of syndrome differentiation treatment based on the decoction.
The therapeutic principle of TCM in the treatment of advanced gastric cancer includes strengthening and consolidating the body resistance, tonifying middle-Jiao and Qi, and effecting a permanent cure; its mechanisms of action have not been fully investigated. Generally speaking, TCM trials has not been properly designed as placebo-controlled and randomization. It is really difficult but necessary to find a reasonable and effective trial method to make decision based on the evidence. Parallel RCT has been not applicable in TCM because of different single patients’ symptoms, different body constitution, different desire on treatment, and fully compliance with the ethical principles, so there is few high quality TCM evidence based on well-designed clinical RCT. However, N-of-1 RCTs provide the better way to get the high quality evidence in the present Chinese cultural background.
In the feasibility study, a form of compromise on single participant was adopted; individual decoction which had been proved effective and safety by physicians’ experience was used as positive based on chemotherapy. It is very important and special that participants can possess the development and treatment themselves, and the participants were prone to receive the trial.
They are potential pitfalls with the TCM N-of-1 trial design that the test intervention must have a rapid onset and a rapid efficacy withdrawal when the period is completed, and it is difficult to determine the process of Chinese herbal medicine metabolism and the validity of patients self-rated measurement method. It means the washout period cannot be determined before carrying out the trial and cannot be calculated in the protocol. In the feasibility trial, we obtained the washout period of the individual decoction without violating the trial protocol.
The small participants might be not representative of the broader population, even we did combined analysis. However, in the feasibility study there was significant difference in the leukocyte and QoL score between the two interventions, which can be used in a larger-scale trial and then combine much more cycles to obtain generalize effectiveness. The utility of the feasibility N-of-1 trials is to make effective and positive palliative care in these including participants and deliver relatively valuable recommendation for future research. We also know the reasons of participants withdrew, which can be prevented in further trials to produce high quality TCM evidence.
Conclusions
N-of-1 trials are a potentially powerful method for more informed, individualized decision making and closer to TCM clinical practice and had good compliance. The methods in this study were feasible and could be further improved. Better designing and more series analysis would serve the benefit of individual patients care.
Acknowledgements
The trial was funded by Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province.
Ethical Statement: The study was approved by Ethics Committee of the First Hospital of Lanzhou University (No. LDYY LL2014-0068) and written informed consent was obtained from all patients.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Venerito M, Nardone G, Selgrad M, et al. Gastric cancer--epidemiologic and clinical aspects. Helicobacter 2014;19 Suppl 1:32-7. 10.1111/hel.12164 [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Zhao AG, Li ZY, et al. Survival benefit of traditional Chinese herbal medicine (a herbal formula for invigorating spleen) for patients with advanced gastric cancer. Integr Cancer Ther 2013;12:414-22. 10.1177/1534735412450512 [DOI] [PubMed] [Google Scholar]
- 4.Liang F, Li L, Wang M, et al. Molecular network and chemical fragment-based characteristics of medicinal herbs with cold and hot properties from Chinese medicine. J Ethnopharmacol 2013;148:770-9. 10.1016/j.jep.2013.04.055 [DOI] [PubMed] [Google Scholar]
- 5.Tao W, Luo X, Cui B, et al. Practice of traditional Chinese medicine for psycho-behavioral intervention improves quality of life in cancer patients: A systematic review and meta-analysis. Oncotarget 2015;6:39725-39. 10.18632/oncotarget.5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang WR, Chen WJ, Yu CT, et al. Effects of acupressure on fatigue of lung cancer patients undergoing chemotherapy: an experimental pilot study. Complement Ther Med 2014;22:581-91. 10.1016/j.ctim.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Qi F, Li A, Inagaki Y, et al. Chinese herbal medicines as adjuvant treatment during chemo- or radio-therapy for cancer. Biosci Trends 2010;4:297-307. [PubMed] [Google Scholar]
- 8.Tian Y, Li X, Li H, et al. Astragalus mongholicus regulate the Toll-like-receptor 4 meditated signal transduction of dendritic cells to restrain stomach cancer cells. Afr J Tradit Complement Altern Med 2014;11:92-6. 10.4314/ajtcam.v11i3.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liao JC, Chang WT, Lee MS, et al. Antinociceptive and anti-inflammatory activities of Cuscuta chinensis seeds in mice. Am J Chin Med 2014;42:223-42. 10.1142/S0192415X14500153 [DOI] [PubMed] [Google Scholar]
- 10.Chan HY, Chui YY, Chan CW, et al. Exploring the influence of Traditional Chinese Medicine on self-care among Chinese cancer patients. Eur J Oncol Nurs 2014;18:445-51. 10.1016/j.ejon.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 11.Liu JP, Zhang M, Yang M. The application of the design of N-of-1 randomized controlled trials in research of traditional Chinese medicine. Chinese Journal of Information on Traditional Chinese Medicine 2002;9:66-8. [Google Scholar]
- 12.Li J, Tian J, Ma B, et al. N-of-1 trials in China. Complement Ther Med 2013;21:190-4. 10.1016/j.ctim.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Guyatt G, Sackett D, Adachi J, et al. A clinician's guide for conducting randomized trials in individual patients. CMAJ 1988;139:497-503. [PMC free article] [PubMed] [Google Scholar]
- 14.Ajani JA, Bentrem DJ, Besh S, et al. National Comprehensive Cancer Network. Gastric cancer, version 2.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:531-46. 10.6004/jnccn.2013.0070 [DOI] [PubMed] [Google Scholar]
- 15.Garland SN, Pelletier G, Lawe A, et al. Prospective evaluation of the reliability, validity, and minimally important difference of the functional assessment of cancer therapy-gastric (FACT-Ga) quality-of-life instrument. Cancer 2011;117:1302-12. 10.1002/cncr.25556 [DOI] [PubMed] [Google Scholar]
- 16.Li Lu, Hongmei W, Yi S. Development and psychometric tests of a Chinese version of the SF-36 health survey scales. Zhonghua Yu Fang Yi Xue Za Zhi 2002;36:109-13. [PubMed] [Google Scholar]