Abstract
Background
Yunnan Baiyao capsule (YBC), a marketed herbal medicine in mainland China, is widely used to control bleeding. This study’s aim was to determine the occurrence of YBC-related adverse drug reactions (ADRs) among users of the medicine.
Methods
This hospital-intensive monitoring study was conducted in 163 hospitals across China. Consumers who used YBC (Z53020799) between June 2015 and December 2016 were included. By face-to-face interview or telephone, the circumstances and experiences of their adverse events (AEs), during drug taking and 14 days after drug withdrawal, were recorded at follow-up and later encoded by International Conference on Harmonisation (ICH) 1997. The Naranjo Adverse Reaction Probability Scale (APS) was used to determine the likelihood of ADRs.
Results
A total of 31,556 participants were included (follow-up rate 99.40%). AEs occurred in 742 participants, of which 561 were reported as “not related with drug use” by their physician-in-charge. Based on the remaining 181 cases, the overall ADR incidence was 1.17% (intention to treat) and 0.58% (per protocol), with abnormal findings mainly concentrated in the digestive system, skin and respiratory system. The top 5 frequently reported reactions were nausea and vomiting (0.1785%, 56 cases of 31,367 participants), functional diarrhea (0.1180%, 37 of 31,367 participants), stomach discomfort (0.0893%, 28 of 31,367 participants), rash (0.0574%, 18 of 31,367 participants) and gastro-esophageal reflux (0.0383%, 12 of 31,367 participants). Among them, functional diarrhea and stomach discomfort were judged as definite ADRs of YBC.
Conclusions
In this large study, treatment of YBC was found to be associated with ADRs with an incidence of 1.17%, although most were relatively mild and not considered to be life-threatening.
Keywords: Adverse drug reaction, hospital intensive monitoring, Yunan Baiyao capsule (YBC)
Introduction
The Yunnan Baiyao capsule (YBC), a famous traditional Chinese medicine formula in China, was created in 1902, and has a long, over 100-year history. The YBC medicine, the formula of which is a state-protected secret, is available in the market. It is especially effective for hemorrhage, hemostasis, pain relief and apocatastasis (1), and is widely used in the departments such as orthopedics, respiratory care, gastroenterology and gynecology.
Apart from its therapeutic effect, the associated risk is a critical issue. For listed drugs, a comprehensive safety assessment which focuses on the adverse event (AE) and adverse drug reaction (ADR) should be the prerequisite. According to the dispensatory of YBC, the possible ADRs included urticaria, abdominal pain, chest tightness, perturbation, nausea, vomiting and other events. Reported ADRs, including those for local pain or numbness (2-4), rash (5), and allergic shock (6-8), can be searched for in the CNKI and Wanfang databases. This can be seen as a resource of a spontaneous report system (SRS), although inevitable defects, randomness, underreporting, and inability to calculate of incidence, are still a problem.
Hospital intensive monitoring (HIM) can explore more information about this drug and will contribute significantly to the ADR profile (9). AEs or ADRs are both reported by patients and physicians (10). The pooled data from the detailed records of AEs and ADRs in monitoring sites can be used for calculating the incidence of YBC-related ADRs, and subsequently analyzed to discover more information about drug safety.
For the traditional medicinal formulas bequeathed to us from the past, the challenge is how to assure the safety and quality of these herbal products for the consumer (11). The China Food and Drug Administration (CFDA) attaches significant importance for detecting, reporting and surveilling of the drug reactions for medicines during the manufacturing process. As this is an often used Chinese patent drug, achieving a more comprehensive understanding about the ADRs is a worthy endeavor. Therefore, a cross-sectional HIM of YBC was conducted at 163 hospitals in China, in which this patent drug was prescribed, to provide thorough and reliable data and ultimately formulate the ADR profile.
Methods
Ethical approval
This study was granted ethical approval by the Ethics Committee of the Peking Union Medical College Hospital, China Academy of Medical Sciences (No. HS-874).
Study setting
This HIM study was set in departments of 163 hospitals, located in 20 provinces in China, and was conducted from June 2015 to December 2016.
Inclusion criteria for participants
Data from in- and out-patients were all collected, on the condition that they were prescribed and took the CYB, without limitation on their age, disease, frequency and dosage of drug taken.
Data collection
Data was collected partly by chart review which included information on demographics, background disease, therapeutic schedule and prescriptions. Patients were followed-up by telephone consultation in the 14 days after drug withdrawal. During the treatment period when YBCs were given, any discomfort or symptoms were encouraged to be reported on the initiative of the patients, and recorded as AEs by the researchers. The information about AE profiles contained the types of AE, occurrence time, relief time, coping method and its outcome. All these were recorded in the Case Report Form and transferred into the AE Dairy Card if AE was detected. AEs and ADRs were coded according to the Medical Dictionary for Regulatory Activities (MedDRA) (12) and then transferred into the electronic data capture (EDC) system.
Criteria for identification of AEs, SAEs and ADRs
AE after YBC treatment was defined as any type of symptom, disease or syndrome that can have an influence on a healthy state, including any abnormity from laboratory or other examinations during the observation period. Among them, a serious adverse event (SAE) was defined as any untoward medical occurrence which could include death, life-threatening conditions, requirement of inpatient hospitalization or prolongation of existing hospitalization, persistent or significant disability/incapacity, congenital anomaly/birth defects, or requirement of intervention to prevent permanent impairment or damage (13).
ADR assessment was attained by group decision. In this process, 5 representatives of the doctors-in-charge and 5 traditional Chinese medicine (TCM) specialists with a senior professional post, discussed, in meeting, the cause of AEs and assessed the probability of ADRs. In the data set of AEs, unlikely related AEs were initially excluded, and then the remaining likely related ADRs were classified according to the Naranjo Adverse Reaction Probability Scale (APS) (14), and applied as a judgment tool to assess the causality in 4 grades: “definite”, “probable”, “possible” or “doubtful” of potential ADRs. The Naranjo scale questions and judgment standard of causality categories are displayed in Table S1.
Statistical analysis
The data was imported into SAS 9.1 (SAS Inc., Beijing University of Chinese Medicine version). Descriptive analysis was undertaken for every item by number and percentage. For the incidence of the likely related ADRs, intention-to-treat (ITT) and per-protocol (PP) analyses were both calculated.
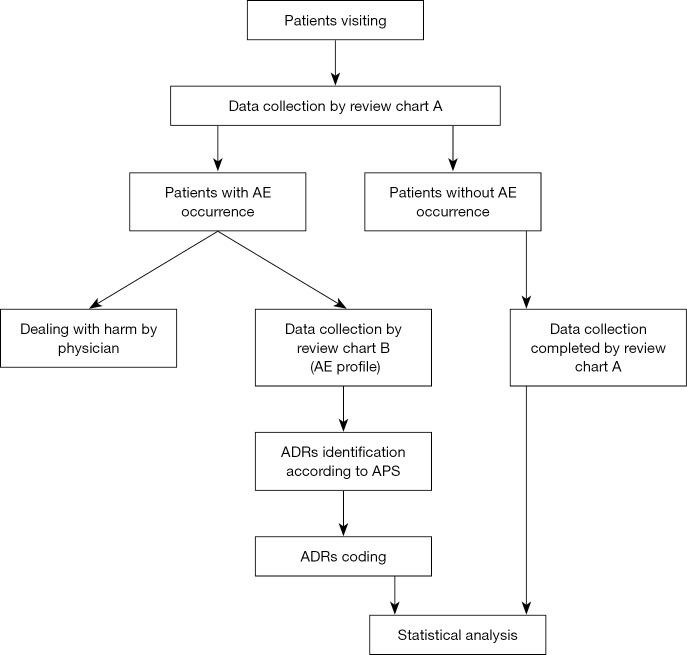
The study process is displayed in the flow chart (Figure 1).
Figure 1.
Flow chart of the study process of YBC intensive monitoring. YBC, Yunnan Baiyao capsules.
Results
A total of 31,556 patients were enrolled from these 163 hospitals in this study. During the follow-up, 188 patients in total had missing values in their record, and 31,368 remained in the full analysis set. Characteristics of all patients are displayed in Table 1.
Table 1. Characteristics of patients using Yunnan Baiyao capsule from 163 hospitals in China.
| Characteristic | Number | Percentage (%) |
|---|---|---|
| Sex | ||
| Total (missing) | 31,459 [97] | 100.00 |
| Male | 18,339 | 58.29 |
| Female | 13,120 | 41.71 |
| Age | ||
| Total (missing) | 31,427 [129] | – |
| Mean ± SD | 45.7±16.2 | – |
| Median (interquartile range) | 45.1 (34.0–56.5) | – |
| Education level | ||
| Total (missing) | 31,454 [102] | 100.00 |
| Elementary school or below | 8,747 | 27.81 |
| Junior school | 9,757 | 31.02 |
| High school | 8,998 | 28.61 |
| College | 3,854 | 12.25 |
| Postgraduate | 98 | 0.31 |
| Living area | ||
| Total (missing) | 31,459 [97] | 100.00 |
| Urban | 15,321 | 48.70 |
| Rural | 16,138 | 51.30 |
| Smoking | ||
| Total (missing) | 31,480 [76] | 100.00 |
| No | 26,931 | 85.55 |
| Yes | 3,980 | 12.64 |
| Unclear | 569 | 1.81 |
| Drinking | ||
| Total (missing) | 31,480 [76] | 100.00 |
| No drinking | 21,506 | 68.32 |
| <5 years | 8,676 | 27.56 |
| ≥5 years or with alcohol ≥40 g/day | 1,064 | 3.38 |
| ≥10 years or with alcohol ≥80 g/day | 234 | 0.74 |
| Allergy | ||
| Total (missing) | 31,480 [76] | 100.00 |
| No | 30,310 | 96.28 |
| Yes | 566 | 1.80 |
| Unclear | 604 | 1.92 |
| Family members’ allergies | ||
| Total (missing) | 31,480 [76] | 100.00 |
| No | 30,462 | 96.77 |
| Yes | 199 | 0.63 |
| Unclear | 819 | 2.60 |
| ADRs experienced previously | ||
| Total (missing) | 31,480 [76] | 100.00 |
| No | 30,742 | 97.66 |
| Yes | 122 | 0.39 |
| Unclear | 616 | 1.96 |
SD, standard deviation; ADR, adverse drug reaction.
The medication use of Yunnan Baiyao capsules is displayed in Table 2, with a median usage time of 15 days and a dosage of 7.7±1.2 capsules per day.
Table 2. The distribution of the medication use of Yunnan Baiyao capsules.
| Characteristics | Number | Percentage (%) |
|---|---|---|
| Use for the first time | ||
| Total (missing) | 31,506 [50] | 100.00 |
| No | 3,192 | 10.13 |
| Yes | 25,935 | 82.32 |
| Unclear | 2,379 | 7.55 |
| Administration route | ||
| Total (missing) | 31,506 [50] | 100.00 |
| Oral | 31,225 | 99.11 |
| External | 53 | 0.17 |
| Others | 13 | 0.04 |
| Oral and external | 214 | 0.68 |
| Oral and others | 1 | 0.00 |
| Dosage (number of capsules) | ||
| Total (missing) | 31,496 [60] | 100.00 |
| Mean ± SD | 7.7±1.2 | – |
| Median | 8.0 | – |
| Quartile range | 8.0–8.0 | – |
| Range | 0.5–24.0 | – |
| Medication time (days) | ||
| Total (missing) | 31,215 [341] | 100.00 |
| Mean ± SD | 16.4±9.3 | – |
| Median | 15.0 | – |
| Quartile | 10.0–18.0 | – |
| Range | 1.0–167.0 | – |
SD, standard deviation.
The primary diseases treated by YBC were mostly hemorrhage, with the majority of them resulting from injury of bone or soft tissue (47.05%). The hemorrhage or disease distribution of patients is displayed in Table 3.
Table 3. Hemorrhage of disease distribution of patients using Yunnan Baiyao capsule.
| Types of hemorrhage or disease | n (%) |
|---|---|
| Soft tissue damage | 8,107 (25.73) |
| Closed fracture | 6,716 (21.32) |
| Upper gastrointestinal hemorrhage | 6,163 (19.56) |
| Hemoptysis | 261 (0.83) |
| Lower gastrointestinal hemorrhage | 1,592 (5.05) |
| Hematochezia | 149 (0.47) |
| Colporrhagia | 1,348 (4.28) |
| Skin | 263 (0.83) |
| Other regions | 6,907 (21.92) |
| Total [missing] | 31,506 [50] (100.00) |
AEs incidence
Of the 31,556 patients monitored, AEs occurred in 747 cases (2.35%), with 772 occurrences. The most frequent occurrence of AEs was nasopharyngitis (92 cases and 0.89% incidence), followed by fever (76 cases and 0.84% incidence) and diarrhea (61 cases and 0.79% incidence). The frequency and incidence of AE occurrence for patients using Yunnan Baiyao capsule is displayed in Table 4.
Table 4. AE occurrence for patients using Yunnan Baiyao capsules.
| Categories by coding | Frequency (cases) | Incidence (ITT) (%) | Incidence (PP) (%) | Proportion (%) |
|---|---|---|---|---|
| Nasopharyngitis | 92 | 0.89 | 0.29 | 11.92 |
| Fever | 76 | 0.84 | 0.24 | 9.84 |
| Diarrhea | 61 | 0.79 | 0.19 | 7.90 |
| Nausea | 52 | 0.76 | 0.17 | 6.74 |
| Dizziness | 37 | 0.71 | 0.12 | 4.79 |
| Rash | 34 | 0.70 | 0.11 | 4.40 |
| Wound | 33 | 0.70 | 0.11 | 4.27 |
| Epigastric discomfort | 31 | 0.69 | 0.10 | 4.02 |
| Flatulence | 26 | 0.68 | 0.08 | 3.37 |
| Vomiting | 26 | 0.68 | 0.08 | 3.37 |
| Cough | 22 | 0.67 | 0.07 | 2.85 |
| Epigastric pain | 21 | 0.66 | 0.07 | 2.72 |
| Upper respiratory infection | 15 | 0.64 | 0.05 | 1.94 |
| Allergic dermatitis | 13 | 0.64 | 0.04 | 1.68 |
| Chest discomfort | 12 | 0.63 | 0.04 | 1.55 |
| Constipation | 12 | 0.63 | 0.04 | 1.55 |
| Headache | 12 | 0.63 | 0.04 | 1.55 |
| Itching | 12 | 0.63 | 0.04 | 1.55 |
| Abdominal pain | 10 | 0.63 | 0.03 | 1.30 |
| Dyspepsia | 9 | 0.62 | 0.03 | 1.17 |
| Parulis | 9 | 0.62 | 0.03 | 1.17 |
| Oral ulcer | 8 | 0.62 | 0.03 | 1.04 |
| Palpitation | 7 | 0.62 | 0.02 | 0.91 |
| Urticaria | 7 | 0.62 | 0.02 | 0.91 |
| Loss of appetite | 6 | 0.61 | 0.02 | 0.78 |
| Abdominal discomfort | 5 | 0.61 | 0.02 | 0.65 |
| Drug hypersensitivity | 5 | 0.61 | 0.02 | 0.65 |
| Gastroesophageal reflux | 5 | 0.61 | 0.02 | 0.65 |
| Uncomfortable | 5 | 0.61 | 0.02 | 0.65 |
| Asthenia | 4 | 0.61 | 0.01 | 0.52 |
| Gastrointestinal diseases | 4 | 0.61 | 0.01 | 0.52 |
| Pain | 4 | 0.61 | 0.01 | 0.52 |
| Postoperative hemorrhage | 4 | 0.61 | 0.01 | 0.52 |
| Running nose | 4 | 0.61 | 0.01 | 0.52 |
| Thermal burn | 4 | 0.61 | 0.01 | 0.52 |
| Bleeding | 3 | 0.61 | 0.01 | 0.39 |
| Insomnia | 3 | 0.61 | 0.01 | 0.39 |
| Joint pain | 3 | 0.61 | 0.01 | 0.39 |
| Dry throat | 2 | 0.60 | 0.01 | 0.26 |
| Eczema | 2 | 0.60 | 0.01 | 0.26 |
| Erythropoiesis | 2 | 0.60 | 0.01 | 0.26 |
| Hypoglycemia | 2 | 0.60 | 0.01 | 0.26 |
| Phlebitis | 2 | 0.60 | 0.01 | 0.26 |
| Shortness of breath | 2 | 0.60 | 0.01 | 0.26 |
| Stuffy nose | 2 | 0.60 | 0.01 | 0.26 |
| Acne | 1 | 0.60 | 0.00 | 0.13 |
| Acute gastroenteritis | 1 | 0.60 | 0.00 | 0.13 |
| Allergic rhinitis | 1 | 0.60 | 0.00 | 0.13 |
| Amaurosis | 1 | 0.60 | 0.00 | 0.13 |
| Amygdalitis | 1 | 0.60 | 0.00 | 0.13 |
| Allergic shock | 1 | 0.60 | 0.00 | 0.13 |
| Ankle sprains | 1 | 0.60 | 0.00 | 0.13 |
| Chronic gastritis | 1 | 0.60 | 0.00 | 0.13 |
| Decubitus ulcer | 1 | 0.60 | 0.00 | 0.13 |
| Drowsiness | 1 | 0.60 | 0.00 | 0.13 |
| Dry mouth | 1 | 0.60 | 0.00 | 0.13 |
| Dysphonia | 1 | 0.60 | 0.00 | 0.13 |
| Dysuria | 1 | 0.60 | 0.00 | 0.13 |
| Epistaxis | 1 | 0.60 | 0.00 | 0.13 |
| Erythema | 1 | 0.60 | 0.00 | 0.13 |
| Eye hyperaemia | 1 | 0.60 | 0.00 | 0.13 |
| Eyelid edema | 1 | 0.60 | 0.00 | 0.13 |
| Flush | 1 | 0.60 | 0.00 | 0.13 |
| Gastrointestinal disorder | 1 | 0.60 | 0.00 | 0.13 |
| Gum swelling | 1 | 0.60 | 0.00 | 0.13 |
| Hemoptysis | 1 | 0.60 | 0.00 | 0.13 |
| Hemorrhoid bleeding | 1 | 0.60 | 0.00 | 0.13 |
| High blood pressure | 1 | 0.60 | 0.00 | 0.13 |
| Hypertrophy of tonsil | 1 | 0.60 | 0.00 | 0.13 |
| Hyperuricemia | 1 | 0.60 | 0.00 | 0.13 |
| Hypoesthesia | 1 | 0.60 | 0.00 | 0.13 |
| Internal fixation of fracture | 1 | 0.60 | 0.00 | 0.13 |
| Numb lips | 1 | 0.60 | 0.00 | 0.13 |
| Melena | 1 | 0.60 | 0.00 | 0.13 |
| Menorrhagia | 1 | 0.60 | 0.00 | 0.13 |
| Menstruation delay | 1 | 0.60 | 0.00 | 0.13 |
| Slow micturition | 1 | 0.60 | 0.00 | 0.13 |
| Numbness after administration contact | 1 | 0.60 | 0.00 | 0.13 |
| Oliguria | 1 | 0.60 | 0.00 | 0.13 |
| Oropharyngeal pain | 1 | 0.60 | 0.00 | 0.13 |
| Otitis media | 1 | 0.60 | 0.00 | 0.13 |
| Panmetatarsal pain syndrome | 1 | 0.60 | 0.00 | 0.13 |
| Peripheral edema | 1 | 0.60 | 0.00 | 0.13 |
| Physical pain | 1 | 0.60 | 0.00 | 0.13 |
| Postoperative wound infection | 1 | 0.60 | 0.00 | 0.13 |
| Purulent sputum | 1 | 0.60 | 0.00 | 0.13 |
| Radial fracture | 1 | 0.60 | 0.00 | 0.13 |
| Red skin | 1 | 0.60 | 0.00 | 0.13 |
| Scapulohumeral periarthritis | 1 | 0.60 | 0.00 | 0.13 |
| Petechia | 1 | 0.60 | 0.00 | 0.13 |
| Skin and subcutaneous tissue disease | 1 | 0.60 | 0.00 | 0.13 |
| Skin disease | 1 | 0.60 | 0.00 | 0.13 |
| Sneezing | 1 | 0.60 | 0.00 | 0.13 |
| Sore eyes | 1 | 0.60 | 0.00 | 0.13 |
| Stomach bleeding | 1 | 0.60 | 0.00 | 0.13 |
| Tachycardia | 1 | 0.60 | 0.00 | 0.13 |
| Tailbone pain | 1 | 0.60 | 0.00 | 0.13 |
| Throat discomfort | 1 | 0.60 | 0.00 | 0.13 |
| Throat pain | 1 | 0.60 | 0.00 | 0.13 |
| Tinnitus | 1 | 0.60 | 0.00 | 0.13 |
| Toothache | 1 | 0.60 | 0.00 | 0.13 |
| Unstable blood pressure | 1 | 0.60 | 0.00 | 0.13 |
| Urinary retention | 1 | 0.60 | 0.00 | 0.13 |
| Urine erythrocyte positive | 1 | 0.60 | 0.00 | 0.13 |
| Urogenital hemorrhage | 1 | 0.60 | 0.00 | 0.13 |
| Vaginal bleeding | 1 | 0.60 | 0.00 | 0.13 |
| Wound infection | 1 | 0.60 | 0.00 | 0.13 |
| Total | 772 | 3.04 | 2.46 | 100.00 |
AE, adverse event; PP, per-protocol; ITT, intention-to-treat.
SAE incidence
A total of 11 SAEs occurred. They were 5 cases of bleeding resulting from operation, 2 cases of bone fracture, 2 cases of postoperative wound infection, 1 case of decubitus ulcer and 1 case of allergic shock resulting from radiocontrast agent.
ADR occurrence
According to the APS scale categories, of the 742 AEs, 525 cases were assessed as unlikely to be related with the ingestion of Yunnan Baiyao capsule, and could be explained by other causes. They were acute upper respiratory infection (86 cases), fever by infection (67 cases), fatigue after operation (30 cases), dyspepsia after operation (30 cases), wound (26 cases) and others. The remaining 217 cases of the 31,367 patients (0.69%) were the likely YDC-related ADRs. Frequency and incidence of the likely related ADRs for patients using Yunnan Baiyao capsule are displayed in Table 5.
Table 5. The frequency and incidence of the likely related ADRs for patients using Yunnan Baiyao capsule.
| Likely ADRs | Frequency | Incidence (PP, %) | Incidence (ITT, %) |
|---|---|---|---|
| Nausea and vomiting | 56 | 0.1785 | 0.7764 |
| Functional diarrhea | 37 | 0.1180 | 0.7162 |
| Stomach discomfort | 28 | 0.0893 | 0.6877 |
| Rash | 18 | 0.0574 | 0.6560 |
| Gastro-esophageal reflux disease | 12 | 0.0383 | 0.6370 |
| Allergic dermatitis | 10 | 0.0319 | 0.6306 |
| Epigastric pain | 9 | 0.0287 | 0.6275 |
| Chest distress | 8 | 0.0255 | 0.6243 |
| Flatulence | 7 | 0.0223 | 0.6211 |
| Pruritus | 6 | 0.0191 | 0.6179 |
| Palpitation | 5 | 0.0159 | 0.6148 |
| Anorexia | 4 | 0.0128 | 0.6116 |
| Lower abdomen pain | 4 | 0.0128 | 0.6116 |
| Dizziness | 4 | 0.0128 | 0.6116 |
| Fever | 2 | 0.0064 | 0.6053 |
| Toxic effect of alcohol | 2 | 0.0064 | 0.6053 |
| Eczema | 2 | 0.0064 | 0.6053 |
| Shortness of breath | 1 | 0.0032 | 0.6021 |
| Halitosis | 1 | 0.0032 | 0.6021 |
| Asthenia | 1 | 0.0032 | 0.6021 |
| Total | 217 | 0.6918 | 1.2866 |
ADR, adverse drug reaction; PP, per-protocol; ITT, intention-to-treat.
According to the Naranjo APS guideline (14), the 217 cases of ADRs assessed as “definite” were functional diarrhea and stomach discomfort. The “probable” included nausea and vomiting, gastro-esophageal reflux disease, functional diarrhea, epigastric pain, stomach discomfort, flatulence, anorexia, allergic dermatitis, chest distress, halitosis, and palpitation. The “possible” included nausea and vomiting, stomach discomfort, functional diarrhea, rash, gastro-esophageal reflux disease, dizziness, allergic dermatitis, lower abdomen pain, chest distress, epigastric pain, palpitations, asthenia, anorexia, and shortness of breath. The “doubtful” included functional diarrhea, nausea and vomiting, rash, stomach discomfort, pruritus, allergic dermatitis, epigastric pain, flatulence, chest distress, toxic effect of alcohol, eczema, fever, anorexia, lower abdominal pain, and gastro-esophageal reflux disease. Grade of the likely related ADRs for patients using Yunnan Baiyao capsule is displayed in Table 6.
Table 6. Grade of the likely related ADRs for patients using Yunnan Baiyao capsule.
| Grade by APS scale | ADRs | Frequency |
|---|---|---|
| Definite (2 cases) | Functional diarrhea | 1 |
| Stomach discomfort | 1 | |
| Probable (22 cases) | Nausea and vomiting | 5 |
| Gastro-esophageal reflux disease | 3 | |
| Functional diarrhea | 2 | |
| Epigastric pain | 2 | |
| Stomach discomfort | 2 | |
| Flatulence | 2 | |
| Anorexia | 2 | |
| Allergic dermatitis | 1 | |
| Chest distress | 1 | |
| Halitosis | 1 | |
| Palpitation | 1 | |
| Possible (99 cases) | Nausea and vomiting | 34 |
| Stomach discomfort | 17 | |
| Functional diarrhea | 10 | |
| Rash | 9 | |
| Gastro-esophageal reflux disease | 8 | |
| Dizziness | 4 | |
| Allergic dermatitis | 4 | |
| Lower abdominal pain | 3 | |
| Chest distress | 3 | |
| Epigastric pain | 2 | |
| Palpitation | 2 | |
| Asthenia | 1 | |
| Anorexia | 1 | |
| Shortness of breath | 1 | |
| Doubtful (94 cases) | Functional diarrhea | 24 |
| Nausea and vomiting | 17 | |
| Rash | 9 | |
| Stomach discomfort | 8 | |
| Pruritus | 6 | |
| Allergic dermatitis | 5 | |
| Epigastric pain | 5 | |
| Flatulence | 5 | |
| Chest distress | 4 | |
| Toxic effect of alcohol | 2 | |
| Eczema | 2 | |
| Fever | 2 | |
| Anorexia | 1 | |
| Lower abdominal pain | 1 | |
| Gastro-esophageal reflux disease | 1 | |
| Transfusion reaction | 1 | |
| Infectious diarrhea | 1 |
APS, Adverse Reaction Probability Scale; ADR, adverse drug reaction.
Discussion
This hospital-based monitoring study, covering 163 hospitals in China, found a total of 217 cases of likely ADRs which were associated with the treatment of YBC amongst 31,556 participants. Of these, only 2 cases (functional diarrhea and stomach discomfort) were assessed as definite YBC-related ADRs. No serious ADRs occurred. This demonstrates that YBCs were well-tolerated and only had ADRs for a few consumers, with most of them being mild.
One other frequently used hemostatic drug, recombinant human thrombin, showed, in pooled analysis, an AE incidence of 10.1% to 47.4%, which included postoperative incision site pain, procedural pain, nausea, constipation, pyrexia and so on (15); fibrinogen concentrate in published data showed a 6.18% of ADR rate, which included possible hypersensitive reactions, thromboembolic events, virus transmission and so on (16). Another example includes Vitamin K, which is commonly used in the treatment and prevention of hemorrhagic disease. Although occurring rarely, intravenous vitamin K may induce anaphylactoid reactions (132 cases reported) (17). Compared to these drugs, YBC has shown a relatively low incidence rate of ADRs.
From the data demonstrated in this hospital-intensive monitoring study, most (61.29%) of the likely related ADRs that occurred could be attributed to gastrointestinal symptoms, which involved nausea and vomiting, functional diarrhea, stomach discomfort and gastro-esophageal reflux disease. Thus, we inferred that gastrointestinal symptoms might occur for a small portion of the consumers after taking YBC.
As a Chinese state-protected herbal prescription, the formula component and production process of YBC remains elusive and secreted. What we are creditably informed of from YDC medical inserts is that Borneol, Panax Notoginseng, and Radix Aconiti are part of the formula (1).
Since the material Borneol tastes spicy and bitter, and has a cool characteristic (18), we inferred that it might have a stimulating effect on gastrointestinal mucosa, which could lead to gastrointestinal symptoms such as nausea, vomiting, diarrhea and stomach discomfort. Although camphor, which is an aromatic product and a derivative of Borneol, has been described in a past individual case report as a causal agent in epileptic seizure (19-21), in this study, nothing of this sort was found. In fact, in 2015, the Research Institute for Fragrance Materials (RIFM) performed an overall assessment of this herb and concluded that it was safe under the limits of dosage (22). As for Panax Notoginseng, critical appraisal of clinical studies revealed minimal risk such as headache, sleep and gastrointestinal disorder (23-25) but all the ADRs occurred were not associated with it. Finally, previous studies showed the Aconitum had a cardiotoxicity effect leading to arrhythmia (26-28). In this intensive monitoring study, we did observe cardiovascular symptoms including 8 cases with chest distress and 5 cases with palpitation. Nevertheless, the event rate was low (0.04%).
One of the limitations of this study is that it may contain confounding factors that might have had an influence on the results. Even though the study covered 163 hospitals, hospitalization of different departments in different hospitals might not be balanced; the majority of the participants were in orthopedics departments. Many underlying diseases which were more likely to have an ADR appearance accounted for a limited proportion in the monitoring, and thus might lead to Berkson’s bias. However, from another aspect, the Orthopedics Department, which receives wound patients, might thus be the department consuming the most YBCs. The lack of a control group which led to difficulty in inferring causality is another limitation of this study, and we used the APS score to classify the grade of the likely related ADRs.
Conclusions
Based on the data of this hospital-intensive monitoring study from 163 centers in China, we inferred that although the YBC possibly induced ADRs, most of them were relatively mild or non-serious. Among them, gastro esophageal reactions were the most common. The incidence of ADRs attributable to YBC was 1.1693%. Overall, this large-scale hospitalized survey found that YBC demonstrated a relatively high drug safety.
Table S1. The Naranjo scale questions and judgment standard.
| Questions | Yes | No | Don’t know |
|---|---|---|---|
| 1. Are there previous conclusive reports on this reaction? | +1 | 0 | 0 |
| 2. Did the adverse event appear after the suspected drug was administered? | +2 | −1 | 0 |
| 3. Did the adverse reaction improve when the drug was discontinued or a specific antagonist was administered? | +1 | 0 | |
| 4. Did the adverse reaction reappear when the drug was re-administered? | +2 | −1 | 0 |
| 5. Are there alternative causes (other than the drug) that could on their own have caused the reaction? | −1 | +2 | 0 |
| 6. Did the reaction reappear when a placebo was given? | −1 | +1 | 0 |
| 7. Was the drug detected in the blood (or other fluids) in concentrations known to be toxic? | +1 | 0 | 0 |
| 8. Was the reaction more severe when the dose was increased, or less severe when the dose was decreased? | +1 | 0 | 0 |
| 9. Did the patient have a similar reaction to the same or similar drugs in any previous exposure? | +1 | 0 | 0 |
| 10. Was the adverse event confirmed by any objective evidence? | +1 | 0 | 0 |
Scoring: ≥9: definite ADR; 5–8: probable ADR; 1–4: possible ADR; 0: doubtful ADR. ADR, adverse drug reaction.
Acknowledgements
Funding: This study was funded by the Yunnan Province Science and Technology Development. The funding supported the electronic data system contribution and labor cost of the physicians who followed up the patients. However, the company was not involved in the interpretation of data and manuscript writing. B Li was supported by National Natural Science Foundation of China (No. 81774146) and Beijing NOVA Programme (No. xxjh2015A093).
Ethical Statement: This study was granted ethical approval by the Ethics Committee of Peking Union Medical College Hospital, China Academy of Medical Science (No. HS-874).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Yang B, Xu ZQ, Zhang H, et al. The efficacy of Yunnan Baiyao on haemostasis and antiulcer: a systematic review and meta-analysis of randomized controlled trials. Int J Clin Exp Med 2014;7:461-82. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang HT. A case of anaphylaxis caused by Yunnan Baiyao Powder. Chinese Journal of Misdiagnosis 2007;3:664. [Google Scholar]
- 3.Sun YM, Lin BY. A case of anaphylaxis caused by Yunnan Baiyao. Strait Pharmaceutical Journal 1997;2:51. [Google Scholar]
- 4.Rong ZH. A case report of anaphylaxis caused by Yunnan Baiyao Capsule. Chinese Journal of Family Planning 2005;3:188-9. [Google Scholar]
- 5.Zhang HY, Wei LH. A case of drug rash caused by Yunnan Baiyao. Di Yi Jun Yi Da Xue Xue Bao 1999;1:21. [Google Scholar]
- 6.Tang YW. A case of allergic shock caused by external use of Yunnan Baiyao. People's Military Surgeon 2000;7:433. [Google Scholar]
- 7.Fang CF, Zhu Y, Zuo HL, et al. Hemolytic reaction caused by Yunnan Baiyao. Chinese Patent Drug 1995;4:52.
- 8.Hu TT, Xu T. Senvens-Johnson caused by Yunnan Baiyao Capsule: A report of serious case. Herald of Medicine 2016;35:1386-7. [Google Scholar]
- 9.Bian ZX, Tian HY, Gao L, et al. Improving reporting of adverse events and adverse drug reactions following injections of Chinese materia medica. J Evid Based Med 2010;3:5-10. 10.1111/j.1756-5391.2010.01055.x [DOI] [PubMed] [Google Scholar]
- 10.Doshi MS, Patel PP, Shah SP, et al. Intensive monitoring of adverse drug reactions in hospitalized patients of two medical units at a tertiary care teaching hospital. J Pharmacol Pharmacother 2012;3:308-13. 10.4103/0976-500X.103687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu SH, Chuang WC, Lam W, et al. Safety surveillance of traditional Chinese medicine: current and future. Drug Saf 2015;38:117-28. 10.1007/s40264-014-0250-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International Conference on Harmonisation (ICH). MedDRA(r) Term Selection: Points to Consider: ICH-Endorsed Guide for MedDRA Users. Available online: https://www.meddra.org/
- 13.Baber N. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use (ICH). Br J Clin Pharmacol 1994;37:401-4. 10.1111/j.1365-2125.1994.tb05705.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239-45. 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 15.Singla NK, Foster KN, Alexander WA, et al. Safety and immunogenicity of recombinant human thrombin: a pooled analysis of results from 10 clinical trials. Pharmacotherapy 2012;32:998-1005. 10.1002/phar.1128 [DOI] [PubMed] [Google Scholar]
- 16.Solomon C, Gröner A, Ye J, et al. Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data. Thromb Haemost 2015;113:759-71. 10.1160/TH14-06-0514 [DOI] [PubMed] [Google Scholar]
- 17.Fiore LD, Scola MA, Cantillon CE, et al. Anaphylactoid Reactions to Vitamin K. J Thromb Thrombolysis 2001;11:175-83. 10.1023/A:1011237019082 [DOI] [PubMed] [Google Scholar]
- 18.National Pharmacopoeia Committee. Pharmacopoeia of People’s Republic of China. Beijing: Chemical Industry Press, 2015. [Google Scholar]
- 19.Agarwal A, Malhotra HS. Camphor ingestion: an unusual cause of seizure. J Assoc Physicians India 2008;56:123-4. [PubMed] [Google Scholar]
- 20.Goel A, Aggarwal P. Camphor--a lesser-known killer. South Med J 2007;100:134. 10.1097/01.smj.0000254202.13518.f3 [DOI] [PubMed] [Google Scholar]
- 21.Khine H, Weiss D, Graber N, et al. A cluster of children with seizures caused by camphor poisoning. Pediatrics 2009;123:1269-72. 10.1542/peds.2008-2097 [DOI] [PubMed] [Google Scholar]
- 22.Api AM, Belsito D, Bhatia S, et al. RIFM fragrance ingredient safety assessment, borneol, CAS registry number 507-70-0. Food Chem Toxicol 2015;82 Suppl:S81-8. 10.1016/j.fct.2015.04.012 [DOI] [PubMed] [Google Scholar]
- 23.Coon JT, Ernst E. Panax ginseng: a systematic review of adverse effects and drug interactions. Drug Saf 2002;25:323-44. 10.2165/00002018-200225050-00003 [DOI] [PubMed] [Google Scholar]
- 24.Izzo AA, Hoon-Kim S, Radhakrishnan R, et al. A Critical Approach to Evaluating Clinical Efficacy, Adverse Events and Drug Interactions of Herbal Remedies. Phytother Res 2016;30:691-700. 10.1002/ptr.5591 [DOI] [PubMed] [Google Scholar]
- 25.Seely D, Dugoua JJ, Perri D, et al. Safety and efficacy of panax ginseng during pregnancy and lactation. Can J Clin Pharmacol 2008;15:e87-94. [PubMed] [Google Scholar]
- 26.Sheth S, Tan EC, Tan HH, et al. Herb-induced cardiotoxicity from accidental aconitine overdose. Singapore Med J 2015;56:e116-9. 10.11622/smedj.2015114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Wang H, Zhang A, et al. Metabolomicsstudy on the toxicity of aconite root and its processed products using ultra-performance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition approach and ingenuity pathways analysis. J Proteome Res 2012;11:1284-301. 10.1021/pr200963e [DOI] [PubMed] [Google Scholar]
- 28.Cai Y, Gao Y, Tan G, et al. Myocardial lipidomics profiling delineate the toxicity of traditional Chinese medicine Aconiti Lateralis radix praeparata. J Ethnopharmacol 2013;147:349-56. 10.1016/j.jep.2013.03.017 [DOI] [PubMed] [Google Scholar]