Abstract
Background
Even when resectable pancreatic cancer (PC) is associated with a dismal prognosis. Initial presentation varies according with primary tumor location. Aim of this systematic review and meta-analysis was to evaluate the prognosis associated with site (head versus body/tail) in patients with PC.
Methods
We searched PubMed, Cochrane Library, SCOPUS, Web of Science, EMBASE, Google Scholar, LILACS, and CINAHL databases from inception to March 2018. Studies reporting information on the independent prognostic role of site in PC and comparing overall survival (OS) in head versus body/tail tumors were selected. Data were aggregated using hazard ratios (HRs) for OS of head versus body/tail PC according to fixed- or random-effect model.
Results
A total of 93 studies including 254,429 patients were identified. Long-term prognosis of head was better than body/tail cancers (HR =0.96, 95% CI: 0.92–0.99; P=0.02). A pooled HR of 0.95 (95% CI: 0.92–0.99, P=0.02) from multivariate analysis only (n=77 publications) showed that head site was an independent prognostic factor for survival.
Conclusions
Primary tumor location in the head of the pancreas at the time of diagnosis is a predictor of better survival. Such indicator should be acknowledged when designing future studies, in particular in the operable and neoadjuvant setting.
Keywords: Pancreatic cancer (PC), head, tail, survival, meta-analysis
Introduction
Pancreatic cancer (PC) is a very lethal disease associated with very poor 5-year survival rates generally not exceeding 10 percent (1). Ductal adenocarcinoma is by far the most common histologic subtype, accounting for about 85 percent of all pancreatic tumors.
Surgery is the only potentially curative option, with adjuvant chemotherapy adding a modest survival benefit (2). However, relapse after pancreatectomy is frequent and has a dramatic impact on final outcome.
According to primary tumor location initial presentation may vary. The vast majority of PCs involve the head of the organ, while 20 to 25 percent are located in the body/tail (3). Therefore, patients with tumors originating in the head often present with jaundice whereas pain and weight loss are typical symptoms of body/tail cancers. Pancreatic head tumors usually cause progressive jaundice with secondary hyperbilirubinemia due to the obstruction of the common bile duct. Accompanying symptoms are represented by pruritus, dark urine, and pale stools. Hyperbilirubinemia is characteristically of the cholestatic type, with a predominant increase in its conjugated fraction.
Several studies have suggested that the anatomic location of pancreatic tumors represent a potential determinant of survival (4-6). Thus, we performed a systematic review of the literature currently published on this topic and a meta-analysis of the available studies with the aim to demonstrate possible clinically meaningful differences in outcome of PCs located in the head, compared with those of the body and tail.
Methods
This systematic review was conducted according to PRISMA guidelines and the Cochrane Handbook for Systematic Reviews of Interventions. Due to the nature of the study, it did not require any ethics approval.
Search strategy
An electronic search of PubMed, the Cochrane Library, SCOPUS, Web of Science, EMBASE, LILACS, and CINAHL from inception to April 2018 was performed in order to identify all the eligible publications. Searches used the following keywords: (“Pancreatic Neoplasms”[Mesh] OR “pancreatic cancer” OR “pancreatic carcinoma” OR “adenocarcinoma of the pancreas”) AND (head and tail) AND survival. Additionally, a manual selection of any potential eligible studies was carried out with the related articles function. The references of all selected articles were analyzed to identify other relevant publications.
Study selection and data extraction
The following criteria for eligibility among studies were identified for selecting the articles: (I) site of PC was reported (head vs. tail or vs body/tail if site was not splitted); (II) survival information [overall survival (OS), progression-free survival (PFS) or disease-free survival (DFS)] at specific follow-up was reported in the article as hazard ratio (HR) according to univariate or multivariate Cox regression analysis, after primary tumor location was significantly associated with outcome in univariate analysis; (III) articles were written in English language; (IV) when several articles were published by the same authors or group, the most updated article was considered. Exclusion criteria were: (I) no information on OS or PFS provided; (II) letters to editor/commentary, reviews, and articles published in a book or papers published in a non-English language; (III) clinical studies presenting odds ratios or risk ratios as measure of effect; and (IV) studies including non-adenocarcinoma histologies or other gastrointestinal carcinomas.
Two authors (FP and GT) conducted the search and independently identified the relevant studies, and the selection of an article was reached by consensus with a third author (MG). The following information was extracted from each article by the two authors: author/year of publication, country, patient number, type of study, stages (I–III vs. IV), adjuvant or palliative chemotherapy exposure (rate), survival data [reported as HRs with 95% confidence interval (95% CI)].
Statistical analysis
For analysis of OS and PFS, HRs were aggregated to provide a pooled value. In this analysis, all HRs with 95% CIs obtained from uni- or multi-variate analysis, and available in the articles were combined, to obtain a prognostic information on the location of the primary tumor (cancers of the head vs the tail of pancreas), independent of other clinicopathological covariates. Sensitivity analysis was performed according to race (Asian vs. non-Asian origin participants), the number of patients > vs. < of the median number), stage (I–III vs. IV), year of publication (<2006 vs. 2006–2016), quality (high vs. low-quality papers) and type of study (retrospective vs. prospective). To explore the impact of inter-study variability in the inclusion of different stages of PCs, we also conducted a multivariate random-effect model meta-regression of OS adjusted for the proportion of patients that received surgery. Data were entered into the Comprehensive Meta-Analysis software v 3.3.070 (November 20th, 2014) and RevMan v 5.3. The Cochran’s test was used to assess the heterogeneity of included studies. For heterogeneity tests, P value <0.05 was considered to indicate significance. If the test of heterogeneity was significant (P<0.05 or I2 >50%), the random-effect model was used to pool the estimate across studies with the Der Simonian-Laird method. Otherwise, the fixed-effect model was used. By convention, an observed HR of <1 implied better survival for the pancreatic head cancers.
We used the Newcastle-Ottawa Scale (NOS) for risk of bias assessment (7). Studies with scores of at least 7 were considered as having a low risk of bias. We assessed that follow-up was adequate if the median length was more than 5 years for early stages PC and more than 3 years for stage IV PC.
We finally investigated the publication bias for OS meta-analysis with funnel plots and with the Begg-Mazumdar Kendall’s tau and Egger’s bias test (8,9). Finally, in the presence of publication bias for the primary analysis, we conducted a trim-and-fill-adjusted analysis to remove the most extreme small studies from the positive side of the funnel plot, and recalculated the effect size at each iteration, until the funnel plot was symmetric about the (new) effect size.
Results
A total of 1,760 potentially relevant citations were reviewed (Figure 1). Among them, 23 reported OS data as risk ratios, odds ratio, or did not report 95% CI for inclusion in the final analysis. Ultimately, 93 studies (5,6,10-32) published from 1996 to 2018 (33-57), that reported the prognostic value of PC site were analyzed (58-82). The total number of patients included was 254,429 ranging from 25 to 52,759 patients per study (median, 209) (82-100). The major characteristics are shown in online table: http://jgo.amegroups.com/public/system/jgo/supp-jgo.2018.12.08.pdf.
Figure 1.
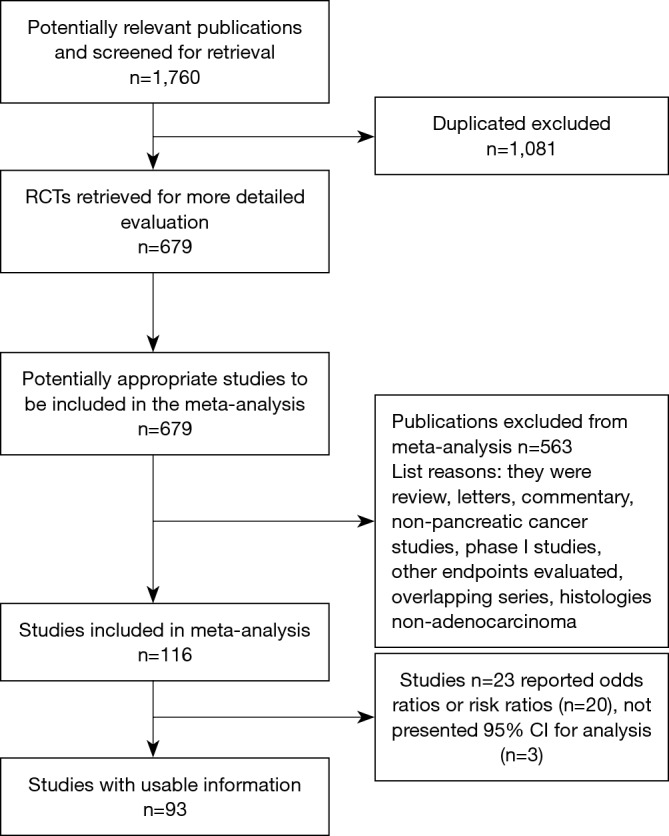
Overview of trials search and selection.
In n=80 publications, a retrospective analysis of PC patients was presented; n=12 papers reported a prospective cohort series and one was a case-control study. According to race, the majority of patients were of Asian origin (n=55); the remaining n=38 publications included Caucasian subjects. Stages were mixed (I–IV) with n=11 studies including only locally advanced or metastatic disease. Surgery rate ranged from 0 to 100% (median 64%) with data not available in n=2 publications. Data about adjuvant chemotherapy was available in n=39 papers (median delivery rate 42%). In n=15 publications data about chemotherapy was not provided, in n=15 it was offered for advanced disease. The quality of paper expressed by the NOS scale ranged from 5 to 8, with 56% including studies of sufficient to high quality (mean NOS scale scores: 5.77).
Meta-analysis of OS
Because the heterogeneity test showed a high level of heterogeneity (I2 =68%, P<0.001) between the studies, a random-effects model was used for the analysis. Overall prognosis of head was better than body/tail cancers (HR =0.96, 95% CI: 0.92–0.99; P=0.02; Figure 2). A pooled HR of 0.95 (95% CI: 0.92–0.99, P=0.02) from multivariate analysis only (n=77 publications) showed that head site was an independent prognostic factor for survival.
Figure 2.

Overall survival according to site of pancreatic cancer.
Meta-analysis of PFS
Data of PFS was available in n=13 studies with high heterogeneity (I2 =64%, P<0.001), so a random-effects model was used for the analysis. PC of the head was associated with a similar PFS of tail cancers (HR =0.99; 95% CI: 0.84–1.16; P=0.91) (Figure 3).
Figure 3.
Disease-free survival according to site of pancreatic cancer.
Subgroup analysis
The subgroup analysis performed according to the number of patients (> or < of the calculated median number), showed that in largest studies (>182 subjects), the effect size was similar to general population: HR =0.93 (95% CI: 0.87–0.99; P=0.03) but different from smallest studies were effect size was not significant (HR =0.97, 95% CI: 0.91–1.04; P=0.5).
Analysis according to race (Asian vs. non-Asian) led to a similar effect on OS for head cancer (HR =0.92, 95% CI: 0.81–1.04 and HR =0.96, 95% CI: 0.90–1.02, P=0.2).
Both studies with prospective (HR =1.00; 95% CI: 0.94–1.09; P=0.6) and retrospective design (HR =0.95, 95% CI: 0.90–1.00; P=0.05) gave similar results. Results were instead different according to quality of the study, with significant results for those with NOS score ≥7 vs. <7 (HR =0.91; 95% CI: 0.88–0.93 and HR =0.93; 95% CI: 0.84–1.00, P<0.001 and P=0.2, respectively).
Results remained significant only considering studies published from 2007 and 2018 (HR =0.95, 95% CI: 0.91–0.99; P=0.02); conversely, in older studies, the prognostic effect of site was not significant. Studies that included only stage IV or locally advanced inoperable patients (n=13) showed a similar mortality of head and body/tail PCs (HR =1.01, 95% CI: 0.91–1.12). In studies where disease stages I–III were at least 90%, PC of the head had a trend to better OS than body/tail (HR =0.91; 95% CI: 0.82–1.00; P=0.07).
The funnel plot (P=0.45; Figure 4) and Egger test (P=0.19) did not indicate the existence of obvious publication bias.
Figure 4.

Funnel plot for publication bias.
Discussion
Treatment of PC is one of the biggest challenges in oncology. Surgical resection is the only chance for cure, but unfortunately, because of the late presentation of the disease, only 15 to 20 percent of patients are candidates for pancreatectomy. As a result, even after surgery, long-term prognosis remains very disappointing, with 5-year survival rates of about 30% after margin-negative (R0) pancreaticoduodenectomy for node-negative and 10% for node-positive disease (101).
Recent studies and meta-analyses have reported in tumors different from pancreas (e.g., colorectal and gastric) that the anatomic site of origin may have a significant impact on prognosis (102,103). This meta-analysis aimed at investigating possible differences in outcome between PCs arising in the head compared to body and tail.
Results of our study demonstrate that, although not particularly deep, a significant difference in prognosis exists. Specifically, patients with PC located in the head have a 5% reduced risk of death as compared with subjects affected by tumors arising in the body/tail.
Major reasons for such different prognosis probably rely on the lack of early symptoms at the time of initial presentation. Because ductal adenocarcinomas involving the body or tail of the pancreas usually do not cause obstruction of the intrapancreatic portion of the common bile duct, early diagnosis is rare, therefore the majority have locally advanced or metastatic disease at the time of first diagnosis. Moreover, painless jaundice is a relatively early sign, and tumors arising from the pancreatic head have been reported to be associated with a relatively more favorable prognosis compared with those that present with pain and obstructive jaundice (104,105). Jaundice secondary to cancers of the body/tail frequently occurs at late disease stages and may be due to the presence of liver metastases. This observation is strengthened by the results of our subgroup analysis performed according to stages. In fact, although not statistically significant, a positive trend towards a survival benefit in favor of PC of the head has been found in patients diagnosed in stages I to III.
Beyond these clear differences in clinical presentation, the two entities probably retain distinct molecular features which may be responsible for a different biological behavior.
In this regard, very recently, a retrospective study tried to shed light on molecular heterogeneity according to tumor location in the pancreas. Specifically, by performing genomic analyses (whole genome and RNA sequencing) on 421 PC cases, authors were able to demonstrate that patients with tumors of the body and tail had significantly worse survival than those with pancreatic head tumors (12.1 vs. 22.0 months; P=0.001) (106). Primary tumor location in the body and tail was associated with the squamous subtype of PC. Body and tail PCs were also shown to be enriched for gene programs involved in tumor invasion and epithelial-to-mesenchymal transition, as well as features of poor antitumor immune response. Such aggressive behavior may therefore explain the worse prognosis associated with body and tail tumors. It still remains to be elucidated if these molecular alterations are present from the outset or develop at definite time points during tumor progression.
Ultimately, further results coming from our subgroup analysis conducted according to the year of publication, revealed a larger and significant OS benefit for pancreatic head cancers in more recent studies (from 2007 and 2017) compared to older ones. This probably reflects a general improvement over the years in surgical skills and imaging techniques which made it possible to reduce post-operative complications and enhance the possibilities of achieving earlier diagnoses, respectively. Historically, pancreaticoduodenectomy has always represented a complex surgical procedure associated with high perioperative morbidity and mortality rates. However, perioperative mortality drastically declined over the last few decades reaching in modern series rates of less than 4 percent (107-113). The main reason for this improved outcome is probably the increase in the proportion of patients undergoing surgery at higher-volume hepatobiliary centers. Centralization of pancreatic surgery can definitely improve outcomes as reported by a recent meta-analysis of 14 studies (114) in which a significant association between hospital volume and postoperative mortality (odds ratio 0.32, 95% CI: 0.16–0.64), and between hospital volume and survival (HR =0.79, 95% CI: 0.70–0.89) was demonstrated.
Our paper has some intrinsic limitations. First, only retrospective studies were included. Thus, since the indication and outcome after surgery depend on local surgeons and center preference, morbidity and mortality after resection were not standardized. Second, while in some studies location of cancer into the body and tail were aggregated and compared to head PC, in other tail cancers only were separated and compared with head PCs. Finally, a high heterogeneity was observed and, although the random effects model takes into account such heterogeneity among studies, conclusions should be interpreted with caution.
In conclusion, our study confirms that primary tumor location in the head of the pancreas at the time of diagnosis is a significant predictor of better survival. Although prognosis of patients with PC remains poor, such indicator deserves to be acknowledged when designing future trials, particularly in the operable and neoadjuvant setting. Hopefully the constant progresses in the field of precision medicine will allow oncologists to identify the exact molecular profile of the single patient which better correlates with long-term outcome. This will be fundamental to spare patients from high morbidity surgical procedures and select those who will benefit most from adjuvant treatments.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017;389:1011-24. 10.1016/S0140-6736(16)32409-6 [DOI] [PubMed] [Google Scholar]
- 3.Modolell I, Guarner L, Malagelada JR. Vagaries of clinical presentation of pancreatic and biliary tract cancer. Ann Oncol 1999;10 Suppl 4:82-4. 10.1093/annonc/10.suppl_4.S82 [DOI] [PubMed] [Google Scholar]
- 4.Lau MK, Davila JA, Shaib YH. Incidence and Survival of Pancreatic Head and Body and Tail Cancers. Pancreas 2010;39:458-62. 10.1097/MPA.0b013e3181bd6489 [DOI] [PubMed] [Google Scholar]
- 5.Watanabe I, Sasaki S, Konishi M, et al. Onset symptoms and tumor locations as prognostic factors of pancreatic cancer. Pancreas 2004;28:160-5. 10.1097/00006676-200403000-00007 [DOI] [PubMed] [Google Scholar]
- 6.Artinyan A, Soriano PA, Prendergast C, et al. The anatomic location of pancreatic cancer is a prognostic factor for survival. HPB (Oxford) 2008;10:371-6. 10.1080/13651820802291233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Ottawa Hosp Res Inst 2013:1-4. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
- 8.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 9.Stuck AE, Rubenstein LZ, Wieland D. Bias in meta-analysis detected by a simple, graphical test. Asymmetry detected in funnel plot was probably due to true heterogeneity. BMJ 1998;316:469; author reply 470-1. 10.1136/bmj.316.7129.469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnachellum RP, Cariou M, Nousbaum JB, et al. Pancreatic Adenocarcinoma in the Finistère Area, France, between 2002 and 2011 (1002 Cases): Population Characteristics, Treatment and Survival. Pancreas 2016;45:953-60. 10.1097/MPA.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 11.Barugola G, Partelli S, Crippa S, et al. Outcomes after resection of locally advanced or borderline resectable pancreatic cancer after neoadjuvant therapy. Am J Surg 2012;203:132-9. 10.1016/j.amjsurg.2011.03.008 [DOI] [PubMed] [Google Scholar]
- 12.Bednar F, Zenati MS, Steve J, et al. Analysis of Predictors of Resection and Survival in Locally Advanced Stage III Pancreatic Cancer: Does the Nature of Chemotherapy Regimen Influence Outcomes? Ann Surg Oncol 2017;24:1406-13. 10.1245/s10434-016-5707-0 [DOI] [PubMed] [Google Scholar]
- 13.Ben QW, Wang JC, Liu J, et al. Positive expression of L1-CAM is associated with perineural invasion and poor outcome in pancreatic ductal adenocarcinoma. Ann Surg Oncol 2010;17:2213-21. 10.1245/s10434-010-0955-x [DOI] [PubMed] [Google Scholar]
- 14.Chadha AS, Liu G, Chen HC, et al. Does Unintentional Splenic Radiation Predict Outcomes After Pancreatic Cancer Radiation Therapy? Int J Radiat Oncol Biol Phys 2017;97:323-32. 10.1016/j.ijrobp.2016.10.046 [DOI] [PubMed] [Google Scholar]
- 15.Chakraborty S, Singh S. Surgical resection improves survival in pancreatic cancer patients without vascular invasion- a population based study. Ann Gastroenterol 2013;26:346-52. [PMC free article] [PubMed] [Google Scholar]
- 16.Chang DK, Johns AL, Merrett ND, et al. Margin Clearance and Outcome in Resected Pancreatic Cancer. J Clin Oncol 2009;27:2855-62. 10.1200/JCO.2008.20.5104 [DOI] [PubMed] [Google Scholar]
- 17.Chang JS, Choi SH, Lee Y, et al. Clinical usefulness of18F-fluorodeoxyglucose-positron emission tomography in patients with locally advanced pancreatic cancer planned to undergo concurrent chemoradiation therapy. Int J Radiat Oncol Biol Phys 2014;90:126-33. 10.1016/j.ijrobp.2014.05.030 [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Shi Y, Yan H, et al. Timing of chemotherapy-induced neutropenia: the prognostic factor in advanced pancreatic cancer patients treated with gemcitabine / gemcitabine-based chemotherapy. Oncotarget 2017;8:66593-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chung KH, Ryu JK, Lee BS, et al. Early decrement of serum carbohydrate antigen 19-9 predicts favorable outcome in advanced pancreatic cancer. J Gastroenterol Hepatol 2016;31:506-12. 10.1111/jgh.13075 [DOI] [PubMed] [Google Scholar]
- 20.Cui X, Li Z, Piao J, et al. Mortalin expression in pancreatic cancer and its clinical and prognostic significance. Hum Pathol 2017;64:171-8. 10.1016/j.humpath.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 21.Eloubeidi MA, Desmond RA, Wilcox CM, et al. Prognostic factors for survival in pancreatic cancer: a population-based study. Am J Surg 2006;192:322-9. 10.1016/j.amjsurg.2006.02.017 [DOI] [PubMed] [Google Scholar]
- 22.Enewold L, Harlan LC, Tucker T, et al. Pancreatic Cancer in the USA: Persistence of Undertreatment and Poor Outcome. J Gastrointest Cancer 2015;46:9-20. 10.1007/s12029-014-9668-x [DOI] [PubMed] [Google Scholar]
- 23.Fujimoto K, Koizumi S, Nishimura S, et al. Prognostic significance of serum CA19-9 levels in resected pancreatic cancer. J Hepatobiliary Pancreat Surg 1996;3:66-70. 10.1007/BF01212783 [DOI] [Google Scholar]
- 24.Furukawa D, Chijiwa T, Matsuyama M, et al. Plasma membrane expression of ZNF185 is a prognostic factor in pancreatic ductal carcinoma. Oncol Lett 2017;14:3633-40. 10.3892/ol.2017.6633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ganti AK, Potti A, Koch M, et al. Predictive value of clinical features at initial presentation in pancreatic adenocarcinoma: A Series of 308 Cases. Med Oncol 2002;19:233-7. 10.1385/MO:19:4:233 [DOI] [PubMed] [Google Scholar]
- 26.Gobbi PG, Bergonzi M, Comelli M, et al. The prognostic role of time to diagnosis and presenting symptoms in patients with pancreatic cancer. Cancer Epidemiol 2013;37:186-90. 10.1016/j.canep.2012.12.002 [DOI] [PubMed] [Google Scholar]
- 27.Gong Z, Holly EA, Bracci PM. Survival in population-based pancreatic cancer patients: San Francisco Bay Area, 1995-1999. Am J Epidemiol 2011;174:1373-81. 10.1093/aje/kwr267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu YL, Lan C, Pei H, et al. Applicative Value of Serum CA19-9, CEA, CA125 and CA242 in Diagnosis and Prognosis for Patients with Pancreatic Cancer Treated by Concurrent Chemoradiotherapy. Asian Pac J Cancer Prev 2015;16:6569-73. 10.7314/APJCP.2015.16.15.6569 [DOI] [PubMed] [Google Scholar]
- 29.Gundewar C, Sasor A, Hilmersson KS, et al. The role of SPARC expression in pancreatic cancer progression and patient survival. Scand J Gastroenterol 2015;50:1170-4. 10.3109/00365521.2015.1024281 [DOI] [PubMed] [Google Scholar]
- 30.Hang J, Xue P, Yang H, et al. Pretreatment C-reactive protein to albumin ratio for predicting overall survival in advanced pancreatic cancer patients. Sci Rep 2017;7:2993. 10.1038/s41598-017-03153-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayman TJ, Strom T, Springett GM, et al. Outcomes of resected pancreatic cancer in patients age ≥70. J Gastrointest Oncol 2015;6:498-504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herman JM, Chang DT, Goodman KA, et al. Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 2015;121:1128-37. 10.1002/cncr.29161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hirabayashi K, Imoto A, Yamada M, et al. Positive Intraoperative Peritoneal Lavage Cytology is a Negative Prognostic Factor in Pancreatic Ductal Adenocarcinoma: A Retrospective Single-Center Study. Front Oncol 2015;5:182. 10.3389/fonc.2015.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hori S, Shimada K, Ino Y, et al. Macroscopic features predict outcome in patients with pancreatic ductal adenocarcinoma. Virchows Arch 2016;469:621-34. 10.1007/s00428-016-2026-6 [DOI] [PubMed] [Google Scholar]
- 35.Hu H, Hang JJ, Han T, et al. The M2 phenotype of tumor-associated macrophages in the stroma confers a poor prognosis in pancreatic cancer. Tumour Biol 2016;37:8657-64. 10.1007/s13277-015-4741-z [DOI] [PubMed] [Google Scholar]
- 36.Hur C, Tramontano AC, Dowling EC, et al. Early Pancreatic Ductal Adenocarcinoma Survival Is Dependent on Size: Positive Implications for Future Targeted Screening. Pancreas 2016;45:1062-6. 10.1097/MPA.0000000000000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inoue D, Ozaka M, Matsuyama M, et al. Prognostic value of neutrophil-lymphocyte ratio and level of C-reactive protein in a large cohort of pancreatic cancer patients: A retrospective study in a single institute in Japan. Jpn J Clin Oncol 2015;45:61-6. 10.1093/jjco/hyu159 [DOI] [PubMed] [Google Scholar]
- 38.Jiang H, He C, Geng S, et al. RhoT1 and Smad4 are correlated with lymph node metastasis and overall survival in pancreatic cancer. PLoS One 2012;7:e42234. 10.1371/journal.pone.0042234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanda M, Fujii T, Takami H, et al. The combination of the serum carbohydrate antigen 19-9 and carcinoembryonic antigen is a simple and accurate predictor of mortality in pancreatic cancer patients. Surg Today 2014;44:1692-701. 10.1007/s00595-013-0752-9 [DOI] [PubMed] [Google Scholar]
- 40.Kim HW, Lee JC, Paik KH, et al. Initial Metastatic Site as a Prognostic Factor in Patients With Stage IV Pancreatic Ductal Adenocarcinoma. Medicine (Baltimore) 2015;94:e1012. 10.1097/MD.0000000000001012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Komoto M, Nakata B, Amano R, et al. HER2 overexpression correlates with survival after curative resection of pancreatic cancer. Cancer Sci 2009;100:1243-7. 10.1111/j.1349-7006.2009.01176.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kondo N, Murakami Y, Uemura K, et al. Prognostic impact of perioperative serum CA 19-9 levels in patients with resectable pancreatic cancer. Ann Surg Oncol 2010;17:2321-9. 10.1245/s10434-010-1033-0 [DOI] [PubMed] [Google Scholar]
- 43.Kondo S, Ueno H, Hashimoto J, et al. Circulating endothelial cells and other angiogenesis factors in pancreatic carcinoma patients receiving gemcitabine chemotherapy. BMC Cancer 2012;12:268. 10.1186/1471-2407-12-268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kooby DA, Gillespie TW, Liu Y, et al. Impact of adjuvant radiotherapy on survival after pancreatic cancer resection: An appraisal of data from the national cancer data base. Ann Surg Oncol 2013;20:3634-42. 10.1245/s10434-013-3047-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosuge T, Kiuchi T, Mukai K, et al. A multicenter randomized controlled trial to evaluate the effect of adjuvant cisplatin and 5-fluorouracil therapy after curative resection in cases of pancreatic cancer. Jpn J Clin Oncol 2006;36:159-65. 10.1093/jjco/hyi234 [DOI] [PubMed] [Google Scholar]
- 46.Kurata M, Honda G, Murakami Y, et al. Retrospective Study of the Correlation Between Pathological Tumor Size and Survival After Curative Resection of T3 Pancreatic Adenocarcinoma: Proposal for Reclassification of the Tumor Extending Beyond the Pancreas Based on Tumor Size. World J Surg 2017;41:2867-75. 10.1007/s00268-017-4077-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuroda T, Kumagi T, Yokota T, et al. Improvement of long-term outcomes in pancreatic cancer and its associated factors within the gemcitabine era: A collaborative retrospective multicenter clinical review of 1,082 patients. BMC Gastroenterol 2013;13:134. 10.1186/1471-230X-13-134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee C, He H, Jiang Y, et al. Elevated expression of tumor miR-222 in pancreatic cancer is associated with Ki67 and poor prognosis. Med Oncol 2013;30:700. 10.1007/s12032-013-0700-y [DOI] [PubMed] [Google Scholar]
- 49.Lee SH, Yoon SH, Lee HS, et al. Can metformin change the prognosis of pancreatic cancer? Retrospective study for pancreatic cancer patients with pre-existing diabetes mellitus type 2. Dig Liver Dis 2016;48:435-40. 10.1016/j.dld.2015.12.006 [DOI] [PubMed] [Google Scholar]
- 50.Lian S, Zhai X, Wang X, et al. Elevated expression of growth-regulated oncogene-alpha in tumor and stromal cells predicts unfavorable prognosis in pancreatic cancer. Medicine (Baltimore) 2016;95:e4328. 10.1097/MD.0000000000004328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu L, Zhao G, Wu W, et al. Low intratumoral regulatory T cells and high peritumoral CD8+ T cells relate to long-term survival in patients with pancreatic ductal adenocarcinoma after pancreatectomy. Cancer Immunol Immunother 2016;65:73-82. 10.1007/s00262-015-1775-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu P, Zhu Y, Liu L. Elevated serum CA72-4 levels predict poor prognosis in pancreatic adenocarcinoma after intensity-modulated radiation therapy. Oncotarget 2015;6:9592-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lorgis V, Chauffert B, Gentil J, et al. Influcence of localization of primary tumor on effectiveness of 5-fluorouracil/leucovorin combined with irinotecan and oxaliplatin (FOLFIRINOX) in patients with metastatic pancreatic adenocarcinoma: A retrospective study. Anticancer Res 2012;32:4125-30. [PubMed] [Google Scholar]
- 54.Luo J, Xiao L, Wu C, et al. The Incidence and Survival Rate of Population-Based Pancreatic Cancer Patients: Shanghai Cancer Registry 2004-2009. PLoS One 2013;8:e76052. 10.1371/journal.pone.0076052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marchegiani G, Andrianello S, Malleo G, et al. Does size matter in pancreatic cancer?: Reappraisal of tumour dimension as a predictor of outcome beyond the TNM. Ann Surg 2017;266:142-8. 10.1097/SLA.0000000000001837 [DOI] [PubMed] [Google Scholar]
- 56.Mayo SC, Nathan H, Cameron JL, et al. Conditional survival in patients with pancreatic ductal adenocarcinoma resected with curative intent. Cancer 2012;118:2674-81. 10.1002/cncr.26553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mellon EA, Springett GM, Hoffe SE, et al. Adjuvant radiotherapy and lymph node dissection in pancreatic cancer treated with surgery and chemotherapy. Cancer 2014;120:1171-7. 10.1002/cncr.28543 [DOI] [PubMed] [Google Scholar]
- 58.Merchant NB, Rymer J, Koehler EAS, et al. Adjuvant Chemoradiation Therapy for Pancreatic Adenocarcinoma: Who Really Benefits? J Am Coll Surg 2009;208:829-38. 10.1016/j.jamcollsurg.2008.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Moghanaki D, Mick R, Furth EE, et al. Resection status, age and nodal involvement determine survival among patients receiving adjuvant chemoradiotherapy in pancreatic adenocarcinoma. JOP 2011;12:438-44. [PubMed] [Google Scholar]
- 60.Moon HJ, An JY, Heo JS, et al. Predicting survival after surgical resection for pancreatic ductal adenocarcinoma. Pancreas 2006;32:37-43. 10.1097/01.mpa.0000194609.24606.4b [DOI] [PubMed] [Google Scholar]
- 61.Morganti AG, Falconi M, Van Stiphout RGPM, et al. Multi-institutional pooled analysis on adjuvant chemoradiation in pancreatic cancer. Int J Radiat Oncol Biol Phys 2014;90:911-7. 10.1016/j.ijrobp.2014.07.024 [DOI] [PubMed] [Google Scholar]
- 62.Nagai K, Doi R, Katagiri F, et al. Prognostic value of metastin expression in human pancreatic cancer. J Exp Clin Cancer Res 2009;28:9. 10.1186/1756-9966-28-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nakata B, Fukunaga S, Noda E, et al. Chemokine receptor CCR7 expression correlates with lymph node metastasis in pancreatic cancer. Oncology 2008;74:69-75. 10.1159/000139126 [DOI] [PubMed] [Google Scholar]
- 64.Ninomiya G, Fujii T, Yamada S, et al. Clinical impact of sarcopenia on prognosis in pancreatic ductal adenocarcinoma: A retrospective cohort study. Int J Surg 2017;39:45-51. 10.1016/j.ijsu.2017.01.075 [DOI] [PubMed] [Google Scholar]
- 65.Ogura T, Yamao K, Hara K, et al. Prognostic value of K-ras mutation status and subtypes in endoscopic ultrasound-guided fine-needle aspiration specimens from patients with unresectable pancreatic cancer. J Gastroenterol 2013;48:640-6. 10.1007/s00535-012-0664-2 [DOI] [PubMed] [Google Scholar]
- 66.Oguro S, Shimada K, Ino Y, et al. Pancreatic intraglandular metastasis predicts poorer outcome in postoperative patients with pancreatic ductal carcinoma. Am J Surg Pathol 2013;37:1030-8. 10.1097/PAS.0b013e3182834d22 [DOI] [PubMed] [Google Scholar]
- 67.Paiella S, Malleo G, Cataldo I, et al. Radiofrequency ablation for locally advanced pancreatic cancer: SMAD4 analysis segregates a responsive subgroup of patients. Langenbecks Arch Surg 2018;403:213-20. [DOI] [PubMed] [Google Scholar]
- 68.Park JJ, Hajj C, Reyngold M, et al. Stereotactic body radiation vs. intensity-modulated radiation for unresectable pancreatic cancer. Acta Oncol 2017;56:1746-53. 10.1080/0284186X.2017.1342863 [DOI] [PubMed] [Google Scholar]
- 69.Park SJ, Gu MJ, Lee DS, et al. EGFR expression in pancreatic intraepithelial neoplasia and ductal adenocarcinoma. Int J Clin Exp Pathol 2015;8:8298-304. [PMC free article] [PubMed] [Google Scholar]
- 70.Pollom EL, Alagappan M, Von Eyben R, et al. Single- versus multifraction stereotactic body radiation therapy for pancreatic adenocarcinoma: Outcomes and toxicity. Int J Radiat Oncol Biol Phys 2014;90:918-25. 10.1016/j.ijrobp.2014.06.066 [DOI] [PubMed] [Google Scholar]
- 71.Qi Q, Zhuang L, Shen Y, et al. A novel systemic inflammation response index (SIRI) for predicting the survival of patients with pancreatic cancer after chemotherapy. Cancer 2016;122:2158-67. 10.1002/cncr.30057 [DOI] [PubMed] [Google Scholar]
- 72.Qiu M, Qiu H, Jin Y, et al. Pathologic diagnosis of pancreatic adenocarcinoma in the United States: Its status and prognostic value. J Cancer 2016;7:694-701. 10.7150/jca.14185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Riall TS, Nealon WH, Goodwin JS, et al. Pancreatic Cancer in the General Population: Improvements in Survival Over the Last Decade. J Gastrointest Surg 2006;10:1212-23; discussion 1223-4. 10.1016/j.gassur.2006.08.010 [DOI] [PubMed] [Google Scholar]
- 74.Shin HC, Kim MJ, Gu MJ, et al. Significance of E-cadherin expression in pancreatic intraepithelial neoplasia and ductal adenocarcinoma. Int J Clin Exp Pathol 2016;9:4872-9. [PMC free article] [PubMed] [Google Scholar]
- 75.Satoi S, Murakami Y, Motoi F, et al. Reappraisal of Peritoneal Washing Cytology in 984 Patients with Pancreatic Ductal Adenocarcinoma Who Underwent Margin-Negative Resection. J Gastrointest Surg 2015;19:6-14; discussion 14. 10.1007/s11605-014-2637-7 [DOI] [PubMed] [Google Scholar]
- 76.Satoi S, Yamaue H, Kato K, et al. Role of adjuvant surgery for patients with initially unresectable pancreatic cancer with a long-term favorable response to non-surgical anti-cancer treatments: Results of a project study for pancreatic surgery by the Japanese Society of Hepato-Biliary-Pan. J Hepatobiliary Pancreat Sci 2013;20:590-600. 10.1007/s00534-013-0616-0 [DOI] [PubMed] [Google Scholar]
- 77.Sawaki A, Kanemitsu Y, Mizuno N, et al. Practical prognostic index for patients with metastatic pancreatic cancer treated with gemcitabine. J Gastroenterol Hepatol 2008;23:1292-7. 10.1111/j.1440-1746.2006.04734.x [DOI] [PubMed] [Google Scholar]
- 78.Sugawara A, Kunieda E. Effect of adjuvant radiotherapy on survival in resected pancreatic cancer: A propensity score surveillance, epidemiology, and end results database analysis. J Surg Oncol 2014;110:960-6. 10.1002/jso.23752 [DOI] [PubMed] [Google Scholar]
- 79.Suzuki R, Takagi T, Hikichi T, et al. Derived neutrophil/lymphocyte ratio predicts gemcitabine therapy outcome in unresectable pancreatic cancer. Oncol Lett 2016;11:3441-5. 10.3892/ol.2016.4381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tanaka T, Ikeda M, Okusaka T, et al. Prognostic factors in Japanese patients with advanced pancreatic cancer treated with single-agent gemcitabine as first-line therapy. Jpn J Clin Oncol 2008;38:755-61. 10.1093/jjco/hyn098 [DOI] [PubMed] [Google Scholar]
- 81.Tao L, Yuan C, Ma Z, et al. Surgical resection of a primary tumor improves survival of metastatic pancreatic cancer: A population-based study. Cancer Manag Res 2017;9:471-9. 10.2147/CMAR.S145722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tsavaris N, Kavantzas N, Tsigritis K, et al. Evaluation of DNA ploidy in relation with established prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. BMC Cancer 2009;9:264. 10.1186/1471-2407-9-264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tsavaris N, Kosmas C, Papadoniou N, et al. CEA and CA-19.9 serum tumor markers as prognostic factors in patients with locally advanced (unresectable) or metastatic pancreatic adenocarcinoma: a retrospective analysis. J Chemother 2009;21:673-80. Erratum in: J Chemother 2010;22:72. Kopteridis, P [corrected to Kopterides, P]. 10.1179/joc.2009.21.6.673 [DOI] [PubMed] [Google Scholar]
- 84.Wang DS, Luo HY, Qiu MZ, et al. Comparison of the prognostic values of various inflammation based factors in patients with pancreatic cancer. Med Oncol 2012;29:3092-100. 10.1007/s12032-012-0226-8 [DOI] [PubMed] [Google Scholar]
- 85.Wang HC, Meng QC, Shan ZZ, et al. Overexpression of Tbx3 Predicts Poor Prognosis of Patients with Resectable Pancreatic Carcinoma. Asian Pac J Cancer Prev 2015;16:1397-401. Erratum in: J Chemother 2010;22:72. Kopteridis, P [corrected to Kopterides, P]. 10.7314/APJCP.2015.16.4.1397 [DOI] [PubMed] [Google Scholar]
- 86.Wang Z, Ren ZG, Ma NY, et al. Intensity modulated radiotherapy for locally advanced and metastatic pancreatic cancer: a mono-institutional retrospective analysis. Radiat Oncol 2015;10:14. 10.1186/s13014-014-0312-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wentz SC, Zhao ZG, Shyr Y, et al. Lymph node ratio and preoperative CA 19-9 levels predict overall survival and recurrence-free survival in patients with resected pancreatic adenocarcinoma. World J Gastrointest Oncol 2012;4:207-15. 10.4251/wjgo.v4.i10.207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wild AT, Herman JM, Dholakia AS, et al. Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients with Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 2016;94:571-9. 10.1016/j.ijrobp.2015.11.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Worni M, Guller U, White RR, et al. Modest improvement in overall survival for patients with metastatic pancreatic cancer: A trend analysis using the surveillance, epidemiology, and end results registry from 1988 to 2008. Pancreas 2013;42:1157-63. 10.1097/MPA.0b013e318291fbc5 [DOI] [PubMed] [Google Scholar]
- 90.Wu M, Guo J, Guo L, et al. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol 2016;37:12525-33. 10.1007/s13277-016-5122-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wylie N, Adib R, Barbour AP, et al. Surgical management in patients with pancreatic cancer: A Queensland perspective. ANZ J Surg 2013;83:859-64. 10.1111/j.1445-2197.2012.06312.x [DOI] [PubMed] [Google Scholar]
- 92.Xu H, Chen X, Tao M, et al. B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer. Oncol Lett 2016;11:1841-6. 10.3892/ol.2016.4128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu HX, Liu L, Xiang JF, et al. Postoperative serum CEA and CA125 levels are supplementary to perioperative CA19-9 levels in predicting operative outcomes of pancreatic ductal adenocarcinoma. Surgery 2017;161:373-84. 10.1016/j.surg.2016.08.005 [DOI] [PubMed] [Google Scholar]
- 94.Xue P, Kanai M, Mori Y, et al. Neutrophil-to-lymphocyte ratio for predicting palliative chemotherapy outcomes in advanced pancreatic cancer patients. Cancer Med 2014;3:406-15. 10.1002/cam4.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yamada M, Sugiura T, Okamura Y, et al. Microscopic Venous Invasion in Pancreatic Cancer. Ann Surg Oncol 2018;25:1043-51. 10.1245/s10434-017-6324-2 [DOI] [PubMed] [Google Scholar]
- 96.Yamada M, Hirabayashi K, Kawanishi A, et al. Nectin-1 expression in cancer-associated fibroblasts is a predictor of poor prognosis for pancreatic ductal adenocarcinoma. Surg Today 2018;48:510-6. 10.1007/s00595-017-1618-3 [DOI] [PubMed] [Google Scholar]
- 97.Yamada S, Fujii T, Hirakawa A, et al. Lymph node ratio as parameter of regional lymph node involvement in pancreatic cancer. Langenbecks Arch Surg 2016;401:1143-52. 10.1007/s00423-016-1412-5 [DOI] [PubMed] [Google Scholar]
- 98.Zhang DX, Dai YD, Yuan SX, et al. Prognostic factors in patients with pancreatic cancer. Exp Ther Med 2012;3:423-32. 10.3892/etm.2011.412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhu Z, Zhou J, Zhang Z, et al. Decreased expression of lncRNA TMED11P is correlated with progression and prognosis in pancreatic ductal adenocarcinoma. Int J Clin Exp Pathol 2016;9:10550-6. [Google Scholar]
- 100.Nakamori S, Yashima K, Murakami Y, et al. Association of p53 gene mutations with short survival in pancreatic adenocarcinoma. Jpn J Cancer Res 1995;86:174-81. 10.1111/j.1349-7006.1995.tb03036.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Allen PJ, Kuk D, Castillo CF, et al. Multi-institutional Validation Study of the American Joint Commission on Cancer (8th Edition) Changes for T and N Staging in Patients With Pancreatic Adenocarcinoma. Ann Surg 2017;265:185-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Petrelli F, Ghidini M, Barni S, et al. Prognostic Role of Primary Tumor Location in Non-Metastatic Gastric Cancer: A Systematic Review and Meta-Analysis of 50 Studies. Ann Surg Oncol 2017;24:2655-68. 10.1245/s10434-017-5832-4 [DOI] [PubMed] [Google Scholar]
- 103.Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol 2016;3:211. 10.1001/jamaoncol.2016.4227 [DOI] [PubMed] [Google Scholar]
- 104.Manabe T, Miyashita T, Ohshio G, et al. Small carcinoma of the pancreas. Clinical and pathologic evaluation of 17 patients. Cancer 1988;62:135-41. [DOI] [PubMed] [Google Scholar]
- 105.Kalser MH, Barkin J, MacIntyre JM. Pancreatic cancer. Assessment of prognosis by clinical presentation. Cancer 1985;56:397-402. [DOI] [PubMed] [Google Scholar]
- 106.Dreyer SB, Jamieson NB, Upstill-Goddard R, et al. Defining the molecular pathology of pancreatic body and tail adenocarcinoma. Br J Surg 2018;105:e183-91. 10.1002/bjs.10772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Balcom JH, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: changing indications, older patients, and decreasing length of hospitalization. Arch Surg 2001;136:391-8. 10.1001/archsurg.136.4.391 [DOI] [PubMed] [Google Scholar]
- 108.Cameron JL, Pitt HA, Yeo CJ, et al. One hundred and forty-five consecutive pancreaticoduodenectomies without mortality. Ann Surg 1993;217:430-5; discussion 435-8. 10.1097/00000658-199305010-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Crist DW, Sitzmann J V, Cameron JL. Improved hospital morbidity, mortality, and survival after the Whipple procedure. Ann Surg 1987;206:358-65. 10.1097/00000658-198709000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kneuertz PJ, Pitt HA, Bilimoria KY, et al. Risk of morbidity and mortality following hepato-pancreato-biliary surgery. J Gastrointest Surg 2012;16:1727-35. 10.1007/s11605-012-1938-y [DOI] [PubMed] [Google Scholar]
- 111.Pellegrini CA, Heck CF, Raper S, et al. An analysis of the reduced morbidity and mortality rates after pancreaticoduodenectomy. Arch Surg 1989;124:778-81. 10.1001/archsurg.1989.01410070028006 [DOI] [PubMed] [Google Scholar]
- 112.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447-58. 10.1097/00000658-199004000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yeo CJ, Cameron JL, Sohn TA, et al. Six hundred fifty consecutive pancreaticoduodenectomies in the 1990s: pathology, complications, and outcomes. Ann Surg 1997;226:248-57; discussion 257-60. 10.1097/00000658-199709000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gooiker GA, van Gijn W, Wouters MW, et al. Systematic review and meta-analysis of the volume-outcome relationship in pancreatic surgery. Br J Surg 2011;98:485-94. 10.1002/bjs.7413 [DOI] [PubMed] [Google Scholar]



