Abstract
Background
Definitive concurrent chemoradiation is the current standard of care for all stage I anal canal squamous cell carcinoma. Local excision as primary treatment for selected stage I lesions has been reported in the literature but is not currently recommended by major guidelines. We herein compared the oncologic outcomes of patients with stage I anal canal squamous cell carcinoma treated with local excision alone versus chemoradiation to determine if there are any significant differences in outcomes including disease free survival, overall survival (OS) and local failure rate.
Methods
A retrospective review of all patients treated for stage I anal canal squamous cell carcinoma between 1990 and 2016 was conducted. Data collected included baseline demographics, staging studies, pathology, treatment received, relapse pattern and survival.
Results
A total of 57 patients were treated for stage I anal canal squamous cell carcinoma between 1990 and 2016; 13 were treated with local excision alone and 44 were treated with chemoradiation therapy. Baseline characteristics in both cohorts of patients were comparable. Median follow-up duration of the local excision and the chemoradiation cohorts were 106 and 70 months, respectively. Of the 13 patients in local excision cohort, two patients had disease recurrence, at 21 and 97 months from the diagnosis. Both patients were long term survivors with salvage treatment. In chemoradiation cohort, 1 out of 44 patients had a local recurrence at 1 year who underwent curative resection. Five-year progression free survival (PFS) of subjects in local excision cohort and chemoradiation cohort were 91% and 83%, respectively (P=0.57).
Conclusions
Local excision as primary treatment may be safe and effective for a selected group of stage I anal canal squamous cell carcinoma patients.
Keywords: Anal canal, squamous cell carcinoma, local excision, chemoradiation
Introduction
Squamous cell carcinoma of anal canal (ACSCC) is an uncommon cancer which comprises 0.5 percent of all new cancer cases diagnosed in the United States and 8,580 new cases are expected to be diagnosed in 2018 (1). Incidence of ACSCC, however, has shown a gradual uptrend in western countries during the last several decades, up to 2.2% per year (1,2). Definitive concurrent radiation and chemotherapy (CRT) has evolved as the standard of care for all patients with localized and locally advanced ACSCC, including patients with stage I cancer. National Cancer Center Network (NCCN) and European Society For Medical Oncology (ESMO) guidelines recommend CRT as the only treatment option for all patients with stage I ACSCC, irrespective of the size of the primary tumor (3,4). However, the randomized clinical trial data (5-12) that led to the current treatment guidelines are predominantly derived from patients with stages II and III disease. A number of randomized phase III trials did not include stage I patients and the trials which included stage I patients had less than 15% of patients with stage I disease (13). Therefore, uncertainty exists if these data can be generalized to all patients with stage I disease and whether CRT is necessarily needed in all such patients, as CRT is associated with considerable short and long term toxicities which can cause significant morbidities and decline in quality of life . There may be a subset of patients within the stage I group who could be treated with local excision alone, as is typically performed for stage I anal margin squamous cell carcinoma. Historical data supports feasibility of local excision as the primary curative treatment in patients with small localized stage I tumors (14,15). However, there is limited literature to date looking at oncologic outcomes of stage I ACSCC with local excision as compared to CRT. Therefore, we sought to evaluate oncologic outcomes of stage I ACSCC patients who were treated with local excision alone compared to those treated with CRT.
Methods
The cancer registry of Mayo Clinic was searched for patients of stage I ACSCC who were treated between 1990 and 2016 at the three sites of Mayo Clinic (Rochester, MN; Scottsdale, AZ; and Jacksonville, FL). Patients were grouped into two cohorts based on treatment: patients who were treated with local excision alone (LE cohort) and patients treated with CRT (CRT cohort). Patients were excluded from the analysis if the anatomic location of the cancer could not be accurately determined from the chart review or if the patient had anal margin lesion. CRT consisted of 2 cycles of combination chemotherapy with 5-fluorouracil (5-FU) and mitomycin-C (MMC) administered concurrently with radiation treatment, typically 45–50.4 Gy in 25–28 fractions over a 5–6-week period. Data were collected by retrospective chart review on patients who were treated between January, 1990 and January, 2016 which included patient demographics, diagnostic and staging evaluation, pathology at the time of diagnosis and surgical excision, treatment and oncologic outcomes (data collection cut-off date was March 31, 2018). Pathology slides of all patients in the LE cohort were reviewed by collaborating pathologists for verification. After local excision, patients were typically followed at 3–6 months intervals with physical examination and anoscopy.
Descriptive statistics were used to characterize the cohorts. Continuous variables were compared using Mann Whitney U Test while categorical variables were compared using the Chi-square test. Progression free survival (PFS) and overall survival (OS) were evaluated using Kaplan-Meier survival curves, and differences in survival were tested using log-rank tests. PFS was calculated from date of diagnosis to progression or death or was censored at date of last follow up. Multivariable analysis was performed using Cox proportional hazard regression model. The two-sided P value of 0.05 was used for statistical significance. All statistical analyses were done using JMP and R software. This study was approved by the Mayo Clinic’s Institutional Review Board under protocol # 17-001373.
Results
The LE and CRT cohorts included 13 and 44 patients, respectively. The median age of LE cohort was 64 years (range, 36–89 years). Table 1 summarizes the characteristics and outcomes of the patients in LE cohort. The majority of patients in LE cohort were women (n=12; 92%). Median tumor size was 0.6 cm (range, 0.1–1.8 cm). The reasons for not receiving CRT were small size of lesion (n=10) and co-morbid conditions (n=3). Among the 44 patients in the CRT cohort, there were 7 male (16%) and 37 female (84%) patients and median age was 58 years (range, 40–79 years). The median tumor size was 1.5 cm (range, 0.1–2 cm). Table 2 describes the detailed pathological characteristics of the patients in LE cohort.
Table 1. Clinicopathological characteristics and outcome of patients with stage I anal canal squamous cell carcinoma treated with local excision alone (LE cohort).
| Patient | Age at diagnosis (years) | Gender | Race | Tumor size (cm) | Reason for local excision alone | Recurrence | PFS (months) | OS (months) | Comment |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 36 | F | W | 0.1 | Small size | No | 138 | 138 | Alive at the time of analysis |
| 2 | 79 | F | W | 0.7 | Co-morbidities | No | 106 | 106 | Expired from unrelated cause |
| 3 | 56 | M | W | 0.6 | Small size | No | 108 | 108 | Alive at the time of analysis |
| 4 | 65 | F | B | 1.8 | Co-morbidity | No | 64 | 64 | Subject had concomitant diagnosis of metastatic colon cancer. Alive at the time of analysis |
| 5 | 81 | F | W | 1.0 | Small size | No | 106 | 106 | Expired from unrelated cause |
| 6 | 71 | F | W | 0.2 | Small size | No | 180 | 180 | Expired from unrelated cause |
| 7 | 63 | F | W | 0.8 | Small size | No | 60 | 60 | Alive at the time of analysis |
| 8 | 51 | F | W | 0.2 | Small size | No | 97 | 97 | Alive at the time of analysis |
| 9 | 64 | F | W | 0.2 | Small size | Yes | 21 | 123 | Salvaged with CRT. Alive at the time of analysis |
| 10 | 89 | F | W | 1.2 | Co-morbidity | No | 14 | 14 | Alive at the time of analysis |
| 11 | 64 | F | W | 0.1, 2 foci | Small size | No | 60 | 60 | Alive at the time of analysis |
| 12 | 56 | F | W | 0.7 | Small size | No | 161 | 161 | Alive at the time of analysis |
| 13 | 58 | F | W | 0.3 | Small size | Yes | 97 | 264 | Salvaged with CRT. Alive at the time of analysis |
M, male; F, female; W, White; B, Black; PFS, progression free survival; OS, overall survival; CRT, chemoradiation.
Table 2. Pathological characteristics of patients with anal canal squamous cell carcinoma treated with local excision alone (LE cohort).
| Patient | Size of the tumor (cm) | Differentiation | Margin involvement by carcinoma | Lymphovascular invasion | Comment |
|---|---|---|---|---|---|
| 1 | 0.1 | Moderately differentiated | See comment | Absent | Margin could not be assessed |
| 2 | 0.7 | Poorly differentiated | Negative | Absent | Margins negative for invasive or in situ carcinoma, 6 mm clearance |
| 3 | 0.6 | Well differentiated | Negative | Absent | Margin focally involved by carcinoma—in situ |
| 4 | 1.8 | Well differentiated | Negative | Absent | Margin focally involved by carcinoma—in situ |
| 5 | 1.0 | Moderately differentiated | Positive | Absent | Invasive carcinoma present at margin on first resection, follow-up resection led to negative margins (>1 cm clearance) |
| 6 | 0.2 | Moderately differentiated | Negative | Absent | Margins negative for invasive or in situ carcinoma, 4 mm clearance |
| 7 | 0.8 | Moderately differentiated | Negative | Absent | Invasive carcinoma within 1 mm of margin |
| 8 | 0.2 | Well differentiated | Negative | Absent | Margins negative for in situ and invasive carcinoma, 5 mm clearance |
| 9 | 0.2 | Well differentiated | Negative | Absent | Margins negative for invasive carcinoma, but carcinoma in situ present at edges of tissue fragments—unclear whether this represents true margin (specimen fragmented). In situ carcinoma at margin could not be excluded |
| 10 | 1.2 | Well differentiated | Positive | Absent | Invasive carcinoma transected at deep and lateral margins |
| 11 | 0.1, 2 foci | Well differentiated | Negative | Absent | Margin negative for invasive carcinoma. In situ carcinoma extends to margin |
| 12 | 0.7 | Well differentiated | Negative | Absent | In situ carcinoma 0.5 mm from margin |
| 13 | 0.3 | Poorly differentiated | Negative | Present | Margins negative for invasive or in situ carcinoma, 7 mm clearance |
Baseline characteristics of the patients in LE and CRT cohorts were compared. Patients in the LE cohort had significantly smaller tumors compared to patients in the CRT cohort (median tumor size of 0.6 vs. 1.5 cm, P<0.001). However, there was no statistically significant difference noted in the median age (64 versus 58 years, P=0.25) or sex (92% versus 84%, P=0.45) between the two cohorts.
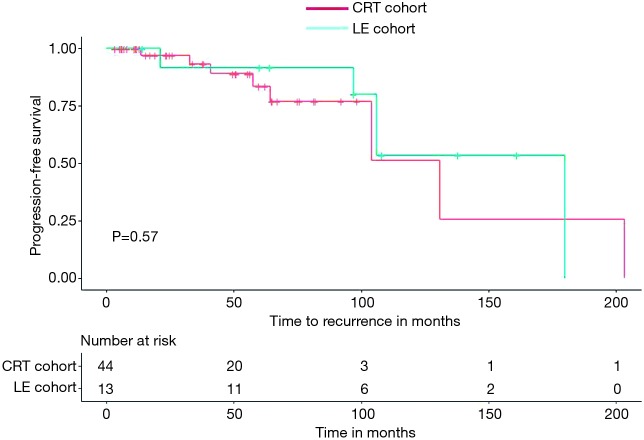
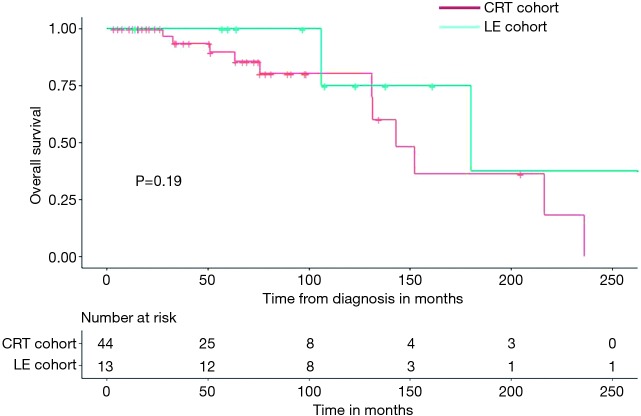
Median follow up duration for the LE cohort was 106 months (range, 14–264 months). Median OS for the LE cohort was 180 months (range, 14–264 months) and median PFS was not reached. The median follow up duration was 70 months for the CRT cohort, median OS was 143 months (range, 3.4–236 months) and median PFS was not reached. There were no differences in PFS and OS in the two groups (Figures 1,2).
Figure 1.
Kaplan-Meier curve of progression free survival of stage I anal squamous cell carcinoma patients treated with local excision alone (LE cohort, n=13) versus patients treated with definitive chemoradiation (CRT cohort, n=44).
Figure 2.
Kaplan-Meier curve of overall survival of stage I anal squamous cell carcinoma patients treated with local excision alone (LE cohort, n=13) versus patients treated with definitive chemoradiation (CRT cohort, n=44).
There were only 2 patients (15%) who had disease recurrence in LE cohort, one at 21 months in the anal canal (patient #9) and the other at 97 months in the anal canal and retroperitoneal lymph nodes (patient #13). Of the 44 patients in CRT cohort, one patient (2%) had a local relapse at 1 year after CRT and was salvaged with surgery. There were no statistically significant differences in local failure rates in the two groups (P=0.33).
Five-year PFS of subjects in LE cohort and CRT cohort were 91% and 83%, respectively by Kaplan-Meier survival analysis (Figures 1,2) and the difference was not statistically significant (P=0.57). Five-year OS of subjects in LE cohort and CRT cohort were 100% and 85% respectively by Kaplan-Meier survival analysis, which was also did not meet statistical significance (P=0.19). In multivariable Cox proportional hazard regression model, including tumor size and age at diagnosis as co-variates, no statistically significant differences were noted in OS (HR 0.32, 95% CI: 0.01–5.78, P=0.44) or PFS (HR 0.94, 95% CI: 0.09–9.44) between the LE and CRT cohorts.
In LE cohort, there was no death within 5 years of diagnosis. Five patients in CRT cohort died within 5 years of diagnosis—2 deaths were unrelated and 3 deaths were suspected to be related to treatment (1 death from infection and bleeding associated with chemotherapy and 2 deaths secondary to pulmonary hypertension likely related to MMC therapy). There were 3 deaths in LE cohort during follow up period, all unrelated to ACSCC.
Among the 2 patients in LE cohort who had disease relapse, patient #13 had poorly differentiated histology and presence of lympho-vascular invasion. The other patient with relapse did not have any apparent high risk pathological features (patient #9). Patient #9 was salvaged with CRT and remained alive and disease free 123 months out from the diagnosis. Patient #13 was treated with CRT followed by 1 cycle of 5-FU plus MMC and 2 cycles of 5-FU and cisplatin. This patient is alive and disease free 167 months out from the relapse and 264 months out from the diagnosis.
Of the patients in LE cohort who had at least 5 years of follow up after diagnosis (12 of 13), 11 patients (83%) were alive and disease free at 5 years. None of the patients in LE cohort developed long term bowel toxicities from the surgery.
Discussion
Prior to establishment of definitive CRT as the standard of care for localized and locally advanced squamous cell carcinoma of the anal canal, abdominoperineal resection was the primary treatment which produced 5-year OS rates of approximately 70%, but resulted in a permanent colostomy (16,17). First report of complete pathological response in 2 out of 3 patients with concurrent radiation and chemotherapy consisting of 5-FU and MMC by Nigro and colleagues from Wayne State University (18) in 1974 opened the door for non-surgical treatment of localized ACSCC. A follow up series of 28 patients (19) reported 80% complete pathological response rate when patients received the same protocol as neoadjuvant chemoradiation followed surgery. A subsequent study evaluated definitive CRT with 5-FU/MMC on a series of 45 patients which reported an impressive complete response rate of 84% (20). After this publication, a number of phase III randomized clinical trials were conducted to validate the role of definitive CRT in ACSCC. These trials have established definitive CRT with 5-FU and MMC as the standard of care for localized and locally advanced ACSCC which achieves a locoregional control rate of 68–84%, a colostomy free survival rate of 47–75% and a 5-year OS of 56–79% (21). Based on these trial results, current major treatment guidelines, including NCCN and ESMO guidelines, recommend CRT as the only treatment option for all stage I patients with ACSCC in absence of a treatment contraindication. However, these aforementioned trials, upon which this recommendation is made, included very few patients with stage I disease. RTOG 98-11 (9) and EORTC (7) trials did not include any patient with stage I disease. In UKCCCR trial (22), only 12% patients accrued in CRT arm had stage I disease and ACT II trial had approximately 10% stage I patients in each study arms (10). Most of the trials with stage I patients did not report outcomes of the stage I patients separately. Northover et al. of UKCCCR trial group reported a significant advantage with CRT compared to radiation alone in terms of local failure rate in T1, N0 patients (P=0.0352, RR 0.35, 95% CI: 0.12–0.97) (11). Since large phase III randomized trials enrolled primarily stage II and III patients, applicability of these trial data for all stage I patients remain uncertain. Efficacy of CRT in eradicating local disease is well established but it is possible that a subgroup of stage I patients with small tumor volume do not necessarily need CRT if it can be completely removed with LE. Thus offering CRT to all patients of stage I disease may potentially overtreat a group of patients.
Doubt surrounding the recommendation that all patients of stage I ACSCC need CRT prevails widely in the community as highlighted by two recent NCDB (National Cancer Database) analyses (13,23). These analyses reported widespread use of LE alone in the treatment of stage I ACSCC, 22.4% in one analysis and 35% in the other. Importantly, both of these analyses reported that LE alone was not associated with an overall decreased survival compared to treatment with CRT. Multivariable modeling by Kole et al. (23) showed that patients who were significantly less likely to receive CRT as compared to LE included those aged ≥70 years versus 50 to 69 years, male sex, treatment at an academic versus non-academic facility, tumor size <1 cm versus 1 to 2 cm and low versus intermediate/high tumor grade. In addition, the analysis by Chai et al. (13) reported a significant increase in use of local excision alone over time for the management of T1N0 ACSCC. In our series, we compared OS and PFS between the LE and the CRT cohorts which did not show any statistically significant differences.
In carefully selected patients (patients with small anal canal lesion without invasion into underlying muscular layer) where local excision is feasible with negative margins and without risk of long term impairment of anal sphincter function, LE alone may be adequate. High rates of local control and survival rate with local excision alone has been described in some series in the literature (14,16,24,25). Table 3 summarizes studies in which ACSCC patients were treated with local excision alone. One study reported outcome with LE as primary treatment in 13 patients (25). In this series, 2 (15%) of the 13 patients had local recurrence, but both patient originally had presented with stage II disease. In our series, 2 out of 13 patients who had LE alone had recurrence with a median follow up of 106 months. The patient who had a recurrence after 97 months from initial diagnosis most likely had a second primary rather than a local recurrence given the fact that most recurrences take place within first 2 years after treatment (6). This patient was successfully treated with CRT and systemic chemotherapy.
Table 3. Reported series of anal canal squamous cell carcinoma treated with local excision alone.
| Study | Number of subjects | Local recurrence rate (%) | Survival | Comment |
|---|---|---|---|---|
| Alfa-Wali (24) | 15 | 0 | 100% (median follow up of 4 years) | HIV positive |
| Klas (14) | 7 | 23 | 5-year survival—60% | – |
| Faynsod (25) | 5 | 0 | Not reported | Mean follow up 40 months |
| Boman (16) | 13 | 7 | 100% 5-year overall survival | – |
| Frost (17) | 20 | 60 | 66% 5-year survival | Mean tumor size 2.3 cm |
| Our series | 13 | 15 | 100% 5-year overall survival | – |
CRT is associated with potentially severe short and long term toxicities (26). Major acute toxicities of CRT which include hematologic toxicities (neutropenia, thrombocytopenia), infection, dermatitis and gastrointestinal toxicities (stomatitis, diarrhea, nausea and vomiting) can cause significant morbidity and rarely death. Toxicities associated with MMC (thrombocytopenia, leukopenia, pulmonary toxicity, nephrotoxicity, and hemolytic-uremic syndrome) are well documented (26). In UKCCCR trial (5), there were six deaths which were attributed to chemotherapy with 5-FU + MMC. Acute toxicities could be particularly disabling in elderly patients. In RTOG-9811 trial (9), the CRT arm with 5-FU/MMC was associated with 74% acute grade 3 and 4 non-hematologic toxicity and 61% acute grade 3 and 4 hematologic toxicity. Although radiation techniques (such as intensity modulated radiotherapy) and supportive care (such as widespread availability of growth factors to prevent and treat neutropenia) to address the acute gastrointestinal and hematologic toxic effects have improved, more than 30% of patients still experience severe toxic effects during treatment with CRT (27). In the CRT cohort described in this report, there were 3 deaths suspected to be related to chemotherapy and severe pulmonary hypertension related to MMC toxicity was suspected in 2 patients. Late complications (26) of CRT include anal stenosis, bowel frequency, fecal incontinence, rectal bleeding, vaginal stenosis, sexual dysfunction, urinary complications (frequency, both diurnal and nocturnal, incontinence, urgency and dysuria) which can be disabling for some patients. RTOG 98-11trial reported 23% late combined grade 3 and 4 toxicity in radiation plus 5-FU/MMC arm (9). In some reported series, 6 to 12 percent of patients required a colostomy because of bowel related late complications in absence of disease recurrence (28,29). In our study, no patients in the LE cohort developed long term bowel toxicities. Thus, local excision, when feasible, could potentially spare patients of short and long term toxicities of CRT.
It is important to recognize that only a minority of stage I patients (13 out of 57 patients, or 23%) were treated with LE, reflecting a selection bias for this approach versus CRT. Furthermore, patients treated with LE had significantly small tumors versus those treated with CRT (median size 0.6 vs. 1.5 cm, P<001). Specifically, patients with small tumors which can be excised with a negative margin without disturbing the integrity of anal sphincter were deemed suitable candidates for this approach. This approach would also require a compliant patient and close surveillance at regular intervals, so that recurrences can be dealt with promptly.
Based on our data, it appears that LE alone might be a safe and effective treatment option for a highly selected group of stage I ACSCC patients. However, further study is needed to confirm this result before this approach can be widely adopted.
Acknowledgements
None.
Ethical Statement: The study was approved by the Mayo Clinic’s Institutional Review Board (IRB) under protocol # 17-001373, “Local excision for patients with stage I anal canal squamous cell carcinoma can be curative.” This retrospective study was consent exempt as per the IRB and therefore no patient informed consent was obtained.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Surveillance, Epidemiology, and End Results Program. Stat fact sheets: anal cancer. Available online: http://seer.cancer.gov/statfacts/html/anus.html, accessed on August 1, 2018.
- 2.van der Zee RP, Richel O, de Vries HJ, et al. The increasing incidence of anal cancer: can it be explained by trends in risk groups? Neth J Med 2013;71:401-11. [PubMed] [Google Scholar]
- 3.Benson AB III VA, et al. NCCN Clinical Practice Guidelines in Oncology: Anal Carcinoma, Version 1. 2018.
- 4.Glynne-Jones R, Nilsson PJ, Aschele C, et al. Anal cancer: ESMO-ESSO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25:iii10-20. 10.1093/annonc/mdu159 [DOI] [PubMed] [Google Scholar]
- 5.Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54. 10.1016/S0140-6736(96)03409-5 [DOI] [PubMed] [Google Scholar]
- 6.Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21. 10.1001/jama.299.16.1914 [DOI] [PubMed] [Google Scholar]
- 7.Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. 10.1200/JCO.1997.15.5.2040 [DOI] [PubMed] [Google Scholar]
- 8.Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39. 10.1200/JCO.1996.14.9.2527 [DOI] [PubMed] [Google Scholar]
- 9.Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344-51. 10.1200/JCO.2012.43.8085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.James RD, Glynne-Jones R, Meadows HM, et al. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomised, phase 3, open-label, 2 x 2 factorial trial. Lancet Oncol 2013;14:516-24. 10.1016/S1470-2045(13)70086-X [DOI] [PubMed] [Google Scholar]
- 11.Northover J, Meadows H, Ryan C, et al. Combined radiotherapy and chemotherapy for anal cancer. Lancet 1997;349:205-6. 10.1016/S0140-6736(05)60945-2 [DOI] [PubMed] [Google Scholar]
- 12.Peiffert D, Tournier-Rangeard L, Gerard JP, et al. Induction chemotherapy and dose intensification of the radiation boost in locally advanced anal canal carcinoma: final analysis of the randomized UNICANCER ACCORD 03 trial. J Clin Oncol 2012;30:1941-8. 10.1200/JCO.2011.35.4837 [DOI] [PubMed] [Google Scholar]
- 13.Chai CY, Tran Cao HS, Awad S, et al. Management of Stage I Squamous Cell Carcinoma of the Anal Canal. JAMA Surg 2018;153:209-15. 10.1001/jamasurg.2017.3151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klas JV, Rothenberger DA, Wong WD, et al. Malignant tumors of the anal canal: the spectrum of disease, treatment, and outcomes. Cancer 1999;85:1686-93. [DOI] [PubMed] [Google Scholar]
- 15.Stearns MW, Jr, Urmacher C, Sternberg SS, et al. Cancer of the anal canal. Curr Probl Cancer 1980;4:1-44. 10.1016/S0147-0272(80)80015-8 [DOI] [PubMed] [Google Scholar]
- 16.Boman BM, Moertel CG, O'Connell MJ, et al. Carcinoma of the anal canal. A clinical and pathologic study of 188 cases. Cancer 1984;54:114-25. [DOI] [PubMed] [Google Scholar]
- 17.Frost DB, Richards PC, Montague ED, et al. Epidermoid cancer of the anorectum. Cancer 1984;53:1285-93. [DOI] [PubMed] [Google Scholar]
- 18.Nigro ND, Vaitkevicius VK, Considine B., Jr Combined therapy for cancer of the anal canal: a preliminary report. Dis Colon Rectum 1974;17:354-6. 10.1007/BF02586980 [DOI] [PubMed] [Google Scholar]
- 19.Nigro ND, Seydel HG, Considine B, et al. Combined preoperative radiation and chemotherapy for squamous cell carcinoma of the anal canal. Cancer 1983;51:1826-9. [DOI] [PubMed] [Google Scholar]
- 20.Leichman L, Nigro N, Vaitkevicius VK, et al. Cancer of the anal canal. Model for preoperative adjuvant combined modality therapy. Am J Med 1985;78:211-5. 10.1016/0002-9343(85)90428-0 [DOI] [PubMed] [Google Scholar]
- 21.Smith CA, Kachnic LA. Randomized Clinical Trials in Localized Anal Cancer. Surg Oncol Clin N Am 2017;26:705-18. 10.1016/j.soc.2017.05.009 [DOI] [PubMed] [Google Scholar]
- 22.Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123-8. 10.1038/sj.bjc.6605605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kole AJ, Stahl JM, Park HS, et al. Predictors of Nonadherence to NCCN Guideline Recommendations for the Management of Stage I Anal Canal Cancer. J Natl Compr Canc Netw 2017;15:355-62. 10.6004/jnccn.2017.0035 [DOI] [PubMed] [Google Scholar]
- 24.Alfa-Wali M, Dalla Pria A, Nelson M, et al. Surgical excision alone for stage T1 anal verge cancers in people living with HIV. Eur J Surg Oncol 2016;42:813-6. 10.1016/j.ejso.2016.02.253 [DOI] [PubMed] [Google Scholar]
- 25.Faynsod M, Vargas HI, Tolmos J, et al. Patterns of recurrence in anal canal carcinoma. Arch Surg 2000;135:1090-3; discussion 1094-5. 10.1001/archsurg.135.9.1090 [DOI] [PubMed] [Google Scholar]
- 26.Ludmir EB, Kachnic LA, Czito BG. Evolution and Management of Treatment-Related Toxicity in Anal Cancer. Surg Oncol Clin N Am 2017;26:91-113. 10.1016/j.soc.2016.07.004 [DOI] [PubMed] [Google Scholar]
- 27.Deshmukh AA, Zhao H, Das P, et al. Clinical and Economic Evaluation of Treatment Strategies for T1N0 Anal Canal Cancer. Am J Clin Oncol 2018;41:626-31. 10.1097/COC.0000000000000339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cummings BJ, Keane TJ, O'Sullivan B, et al. Epidermoid anal cancer: treatment by radiation alone or by radiation and 5-fluorouracil with and without mitomycin C. Int J Radiat Oncol Biol Phys 1991;21:1115-25. 10.1016/0360-3016(91)90265-6 [DOI] [PubMed] [Google Scholar]
- 29.Touboul E, Schlienger M, Buffat L, et al. Epidermoid carcinoma of the anal canal. Results of curative-intent radiation therapy in a series of 270 patients. Cancer 1994;73:1569-79. [DOI] [PubMed] [Google Scholar]