Abstract
Background
Oxaliplatin-based adjuvant chemotherapy has been applied as standard treatment for high risk stages II and III colon cancer in many countries. There was no comprehensive report of oxaliplatin use in Indonesia. This research aimed to evaluate the short-term survival of patients with colon cancer treated with such strategy and the prognostic factors.
Methods
Medical records of patients with colon cancer receiving oxaliplatin-containing adjuvant chemotherapy were retrospectively reviewed. Demography, clinicopathological, and treatment data were collected. Two-year overall survival (OS) and disease-free survival (DFS) were calculated using Kaplan-Meier method and survival predictors were estimated using Cox proportional hazard models.
Results
Data of 81 patients had been included with a median follow-up of 25.2 months. The estimated OS and DFS at 2 years were 75.8% and 72.7%. In multivariate analyses, the Eastern Cooperative Oncology Group (ECOG) 2 performance status [hazard ratio (HR) =2.967; 95% confidence interval (CI), 1.265 to 6.957; P=0.012], T4 stage (HR =2.669; 95% CI, 1.087 to 6.557; P=0.032), and less cycles of chemotherapy administration (HR =3.280; 95% CI, 1.333 to 8.070; P=0.010) were significant independent factors for an increased risk of death. Cases with moderately to poorly differentiated tumors had significantly worse DFS compared with those with well differentiated tumors (HR =3.503; 95% CI, 1.403 to 8.744; P=0.007).
Conclusions
Colon cancer patients receiving oxaliplatin-based adjuvant regimens in our clinical practice had 2-year OS rate of 75.8% and 2-year DFS rate of 72.7%. ECOG 2 performance status, T4 stage, and less cycles of chemotherapy administration significantly predicted a poor OS and moderately to poorly histological grade significantly predicted a poor DFS.
Keywords: Oxaliplatin, adjuvant chemotherapy, colon cancer, short-term survival
Introduction
Colorectal cancer is the third leading cancer in the world, with 1.4 million new cases diagnosed in 2012 (1). In Indonesia, according to the registration of the International Agency for Research on Cancer of the World Health Organization, colon cancer is the second leading cause of death in men and third leading cause of death in women. Data from Dr. Sardjito Hospital Yogyakarta Indonesia showed that almost 75% of cases firstly presented with non-metastatic disease (2).
Surgery is the primary curative treatment for localized colon cancer. Nevertheless, recurrence rate of the malignancy is still relatively high and many patients progress to metastatic disease, which is associated with decreased survival. Adjuvant chemotherapy for high risk stages II and III colon cancer is recommended to eliminate micrometastases and, thus, improve patient’s survival after surgical resection (3). Multiple randomized trials demonstrated that incorporating oxaliplatin into the standard 5-fluorouracil (5-FU)/leucovorin (LV) adjuvant treatment improved patient care. The European Multicenter International Study of Oxaliplatin/Infusional 5-FU/LV (FOLFOX 4) in the Adjuvant Treatment of Colon Cancer (MOSAIC) study randomized 2,246 stages II and III colon cancer patients to receive 5-FU/LV regimens or the same regimen plus oxaliplatin. The study showed a significant improved 3-year disease-free survival (DFS) (78.2%) in the group assigned for combination treatment compared with the group assigned for 5-FU/LV alone (72.9%) (P=0.020) (4). The National Surgical Adjuvant Breast and Bowel Project (NSABP C-07) study also showed a significant improved 3- and 4-year DFS in a group receiving the addition of oxaliplatin to 5-FU/LV compared with patients in the group of 5-FU/LV alone. Another multicenter study involved 1,886 patients with stage III colon cancer and showed an increased 3-year DFS in patients receiving capecitabine and oxaliplatin versus 5-FU/LV (5). Since then, the oxaliplatin-containing regimens have been recommended by the international guidelines (6-8) for patients with high risk stages II and III colon cancer. Furthermore, adverse effects of oxaliplatin include myelotoxicity, gastrointestinal symptom, and neuropathy. The latter is relevant to clinical issues and generally associated with accumulative doses (9,10). In the MOSAIC study, as much as 92.1% of patients in the oxaliplatin/5-FU/LV group experienced peripheral neuropathy during treatment although the side effect only occurred as grade 1. Grades 2–3 neuropathy happened in 44% patients and 1.1% cases still had peripheral neurosensory symptoms after 18 months (4,11).
Many demography and clinicopathological variables have been observed as predictors for overall survival (OS) and DFS in patients with colon cancer receiving adjuvant chemotherapy. These included age (12), sex (13,14), disease stage (11,15), performance status (16), tumor invasion (12,13,15,17-19), lymph nodes removed (15,18,20-22), and histological grade (18). Multiple reports showed that treatment characteristics such as the type of regimen (23,24), time to start treatment after surgical resection (25,26), number of cycles of chemotherapy administration (3,4,27), and dose of oxaliplatin used (5,13) also predicted the patients’ survival.
In Indonesia, oxaliplatin-based adjuvant regimens have been applied and covered by government insurance since 2007. However, their efficacy and effects on patients’ survival in clinical practice have not been comprehensively reported in the national level. Currently, no report has recorded its safety profile either. In fact, local cancer patients may present with more diverse features compared with the patient populations in the international literature. There are also variations in the local settings in applying oxaliplatin doses regarding its toxicity characteristics. Therefore, this study aimed to retrospectively analyse clinical outcomes and survival rate of colon cancer patients receiving combination of oxaliplatin and 5-FU/LV or capecitabine in an adjuvant setting. We also reported demography, clinicopathological, and treatment variables that predicted patients’ survival and the safety assessment.
Methods
Study design and subjects
This is a retrospective review of resectable patients who were diagnosed with colon cancer and started post-surgical adjuvant chemotherapy between January 2007 and May 2015. The primary colon tumor location was defined as the region from the caecum to the sigmoid colon. Data of cases with high risk stages II and III who underwent curative resection and received adjuvant 5-FU/LV plus oxaliplatin (FOLFOX) or capecitabine plus oxaliplatin (CAPOX) were obtained from medical record at Dr. Sardjito Hospital Yogyakarta Indonesia. Patients’ data that only contained minimal information were excluded.
General demography, clinicopathological, and treatment data of all subjects were collected for categorization and analyses. Age was defined as younger (≤50 years) and older (>50 years) (12). Sex included male and female. Clinical data was comprised of disease stage (II versus III), general performance status as defined by the Eastern Cooperative Oncology Group (ECOG) (28) (ECOG 1 versus ECOG 2), tumor invasion (T2–3 versus T4), lymph nodes removed (<10 versus ≥10) (11,13), and histological grade (well differentiated tumors versus moderately to poorly differentiated tumors). Treatment records included adjuvant chemotherapy used (FOLFOX versus CAPOX) and time to start treatment after surgical resection (<7 weeks versus ≥7 weeks) (25). Number of cycles for both regimens was categorized as less cycles (FOLFOX <10 cycles, CAPOX <6 cycles) versus more recommended cycles (FOLFOX ≥10 cycles, CAPOX ≥6 cycles) (3,4,27). For the CAPOX regimen, dose of oxaliplatin used was stratified as less than appropriate (85–115 mg) and appropriate (120–135 mg) (5,12). Adverse events were recorded including hematology toxicities and peripheral neuropathy. All patients were followed through May 2016. The study obtained ethics approval by the Ethics Committee of Faculty of Medicine, Public Health, and Nursing/Dr. Sardjito Hospital Yogyakarta (number of the approval: KE/FK/209/EC). All participants gave informed consent before taking part in the study.
The primary outcomes were 2-year or short-term OS and DFS. OS was defined as the time from date of pathological diagnosis to death from any cause or lost from observation. DFS was defined as the time from date of pathological diagnosis to either colon cancer recurrence, death, or lost from observation, whichever occurred first. Secondary outcomes were analyses of covariates that may predict the survival outcome.
Statistical analyses
Rates of OS and DFS were calculated using the Kaplan-Meier technique. The log rank test was conducted to analyse the difference between survival curves. Univariate and multivariate Cox proportional hazard models were done to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). The data was processed by the computer program Statistical Product and Service Solutions (SPSS) version 20.0 for Windows (IBM Corp. Armonk, New York, USA). A P value <0.05 was considered statistically significant.
Results
Patient characteristics
A total of 96 patients’ data had been included at the study initiation. Fifteen patients were excluded due to minimal information on their records. Thus, only 81 patients’ data were finally reviewed. Table 1 displayed an overview of patients included in this study. Median age of the cohort was 55 years (range, 30–78 years). The study population was dominated by cases aged >50 years old (63%), females (53.1%), patients with ECOG 1 performance status (64.2%), cases with high risk stage II (61.7%), cases with T2–3 tumors (60.5%), and cases with well differentiated tumors (45.7%).
Table 1. Patient characteristics of study population (n=81).
| Characteristic | Frequency (%) |
|---|---|
| Age | |
| ≤50 years | 30 (37.0) |
| >50 years | 51 (63.0) |
| Sex | |
| Male | 38 (46.9) |
| Female | 43 (53.1) |
| Performance status | |
| ECOG 1 | 52 (64.2) |
| ECOG 2 | 29 (35.8) |
| Clinical stage | |
| II (high risk) | 50 (61.7) |
| III | 31 (38.3) |
| T stage (tumor invasion) | |
| T2–3 | 49 (60.5) |
| T4 | 28 (34.6) |
| Missing | 4 (4.9) |
| Nodes removed | |
| <10 | 58 (71.6) |
| ≥10 | 8 (9.9) |
| Missing | 15 (18.5) |
| Histological grade | |
| Well differentiated tumor | 37 (45.7) |
| Moderately-poorly differentiated tumor | 29 (35.8) |
| Missing | 15 (18.5) |
| Adjuvant chemotherapy regimen | |
| FOLFOX | 11 (13.6) |
| CAPOX | 70 (86.4) |
| Time to start of treatment | |
| ≥7 weeks | 31 (38.3) |
| <7 weeks | 45 (55.6) |
| Missing | 5 (6.2) |
| Cycles of regimen | |
| FOLFOX (n=11) | |
| <10 cycles | 6 (54.5) |
| ≥10 cycles | 5 (45.5) |
| CAPOX (n=70) | |
| <6 cycles | 11 (15.7) |
| ≥6 cycles | 58 (82.9) |
| Missing | 1 (1.4) |
| Oxaliplatin dose used | |
| FOLFOX (n=11) | |
| 85 mg | 11 [100] |
| CAPOX (n=70) | |
| 85–115 mg | 16 (22.9) |
| 120–140 mg | 54 (77.1) |
The vast majority of patients (86.4%) were offered the CAPOX regimen. There were 45 (55.6%) patients who started adjuvant chemotherapy less than 7 weeks after surgical resection and 31 (38.3%) cases who started treatment in 7 weeks or more. The oxaliplatin dose applied in patients receiving FOLFOX regimen was 85 mg. The oxaliplatin dose used in CAPOX regimen varied from 85–115 mg (22.9%) to 120–140 mg (77.1%). The proportion of cases in the group receiving FOLFOX chemotherapy who completed ≥10 cycles of treatment was similar to those who completed less cycles. Contrarily, most of the patients (82.9%) in the group receiving CAPOX regimen completed ≥6 cycles and only 11 patients (15.7%) completed less cycles.
Survival outcomes, survival predictors, and disease progressivity
Median duration of follow up was 25.2 months (range, 1.8–111.8 months). The rate of 2-year OS was 77% and 2-year DFS was 73.5%. In the OS study, univariate analyses demonstrated that ECOG 2 performance status, T4 stage, and less cycles of chemotherapy were associated with increased risk of death (Table 2). In the multivariate analyses, ECOG 2 performance status (HR =2.967; 95% CI, 1.265 to 6.957; P=0.012), T4 stage (HR =2.669; 95% CI, 1.087 to 6.557; P=0.032) and completed less cycles of chemotherapy (HR =3.280; 95% CI, 1.333 to 8.070; P=0.010) were significant independent prognostic factors for poor short-term OS (Table 3, Figures 1-3). In the univariate analyses of DFS, factors which were significantly associated with poor clinical outcomes included stage III disease, moderately-poorly differentiated histological grade, and less cycles of chemotherapy (Table 4). Further multivariate analyses revealed that moderately-poorly differentiated histological grade was the only significant independent prognostic factor for short-term DFS (HR =3.503; 95% CI, 1.403 to 8.744; P=0.007) (Table 5, Figure 4).
Table 2. Results of Cox regression univariate analyses for overall survival.
| Factor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Younger age (≤50 years) | 0.887 | 0.381–2.063 | 0.781 |
| Male sex | 1.912 | 1.810–4.513 | 0.319 |
| ECOG 2 performance status | 2.925 | 1.313–6.517 | 0.009 |
| Stage III disease | 2.039 | 0.926–4.490 | 0.077 |
| T4 stage | 3.213 | 1.357–7.608 | 0.008 |
| Less lymph nodes removed | 0.396 | 0.142–1.102 | 0.076 |
| Moderately-poorly differentiated tumor | 2.194 | 0.798–6.026 | 0.128 |
| FOLFOX regimen | 0.501 | 0.118–2.130 | 0.349 |
| Longer time to start of treatment (>7 weeks) | 1.466 | 0.629–3.418 | 0.376 |
| Less cycles of chemotherapy | 3.418 | 1.530–7.917 | 0.003 |
| Less dose of oxaliplatin | 0.653 | 0.193–2.208 | 0.493 |
CI, confidence interval.
Table 3. Results of Cox regression multivariate analyses for overall survival.
| Factor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| ECOG 2 performance status | 2.967 | 1.265–6.957 | 0.012 |
| T4 stage | 2.669 | 1.087–6.557 | 0.032 |
| Less cycles of chemotherapy | 3.280 | 1.333–8.070 | 0.010 |
CI, confidence interval; ECOG, the Eastern Cooperative Oncology Group.
Figure 1.
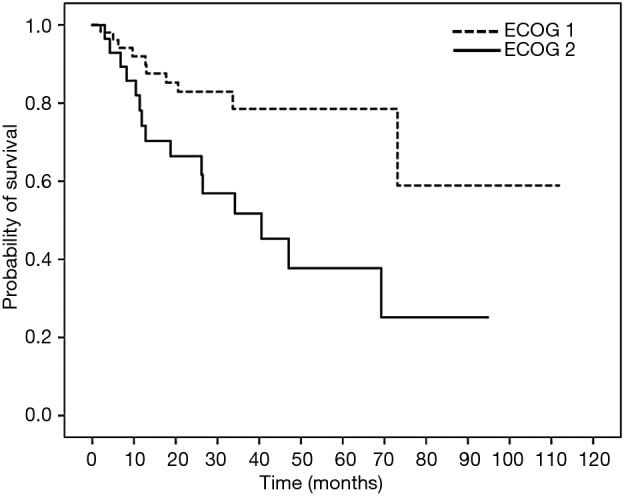
Survival curve of 2-year overall survival (OS) in patients with colon cancer treated with oxaliplatin-containing adjuvant chemotherapy based on ECOG performance status. Patients with ECOG 1 and ECOG 2 performance status had 2-year OS of 82.9% and 66.4% (HR =2.967; 95% CI, 1.265–6.957; P=0.012). ECOG, the Eastern Cooperative Oncology Group.
Figure 2.
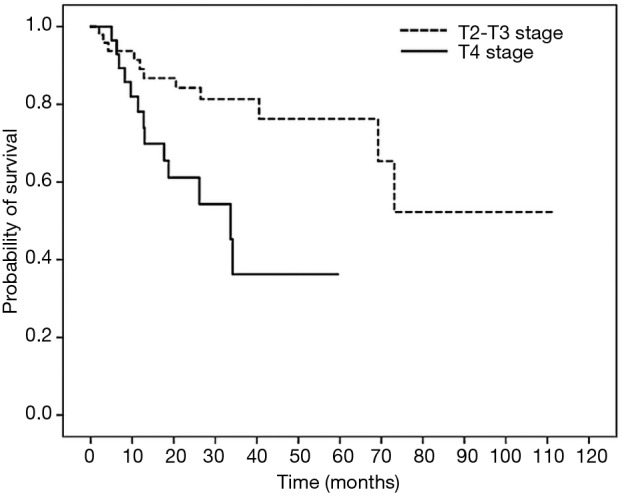
Survival curve of 2-year overall survival (OS) in patients with colon cancer treated with oxaliplatin-containing adjuvant chemotherapy based on T stage. Patients with T2–3 and T4 stage had 2-year OS of 84.2% and 61.1% (HR =2.669; 95% CI, 1.087–6.557; P=0.032).
Figure 3.
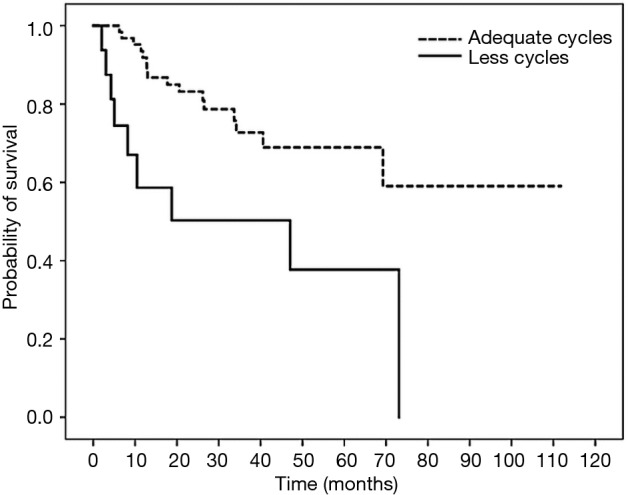
Survival curve of 2-year overall survival (OS) in patients with colon cancer treated with oxaliplatin-containing adjuvant chemotherapy based on total number of chemotherapy cycles. Patients who completed less cycles and adequate cycles of chemotherapy had 2-year OS of 83.2% and 50.3% (HR =3.280; 95% CI, 1.333–8.070; P=0.010).
Table 4. Results of Cox regression univariate analyses for disease-free survival.
| Factor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Younger age (≤50 years) | 1.292 | 0.651–2.562 | 0.464 |
| Male sex | 1.822 | 0.887–3.740 | 0.102 |
| ECOG 2 performance status | 1.509 | 0.766–2.973 | 0.235 |
| Stage III disease | 2.006 | 1.019–3.947 | 0.044 |
| T4 stage | 1.942 | 0.945–3.991 | 0.071 |
| Less nodes removed | 0.643 | 0.240–1.677 | 0.358 |
| Moderately-poorly differentiated tumor | 3.571 | 1.476–8.640 | 0.005 |
| FOLFOX regimen | 0.717 | 0.252–2.042 | 0.533 |
| Longer time to start of treatment (>7 weeks) | 1.073 | 0.519–2.218 | 0.849 |
| Less cycles of chemotherapy | 2.567 | 1.216–5.421 | 0.013 |
| Less dose of oxaliplatin | 0.437 | 0.059–3.230 | 0.417 |
CI, confidence interval; ECOG, the Eastern Cooperative Oncology Group.
Table 5. Results of Cox regression multivariate analyses for disease-free survival.
| Factor | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Stage III disease | 1.349 | 0.557–3.265 | 0.507 |
| Moderately-poorly differentiated tumor | 3.503 | 1.403–8.744 | 0.007 |
| Less cycles of chemotherapy | 2.560 | 0.967–6.781 | 0.059 |
CI, confidence interval.
Figure 4.
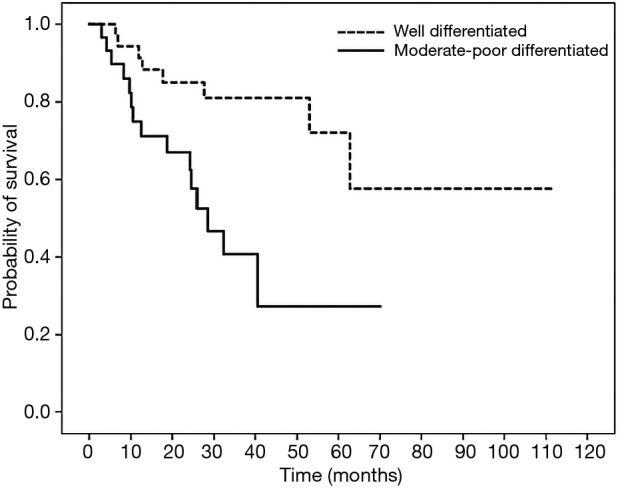
Survival curve of 2-year disease-free survival (DFS) in patients with colon cancer treated with oxaliplatin-containing adjuvant chemotherapy based on histological grade. Patients with well differentiated and moderately-poorly differentiated tumors had 2-year DFS of 85.1% and 66.9% (HR =3.503; 95% CI, 1.403–8.744; P=0.007).
A total of 27 patients experienced metastasis and recurrence during the follow-up period. Seventeen patients died during the study follow-up. Locoregional spread was the most common type of recurrence (17; 63.0%). Distant metastatic sites included lung (5; 18.5%), liver (3; 11.1%), brain (1; 3.7%) and peritoneum (1; 3.7%).
Chemotherapy side effects
Record of incidence of neuropathy in the present study was obtained in 25 patients (30.9%), including 22 patients in the XELOX group and 3 patients in the FOLFOX group. Overall, neutropenia occurred in 21 patients, with 2 patients (9.5%) of grade 1, 15 patients (71.4%) of grade 2, 3 patients (14.3%) of grade 3, and 1 patient (4.7%) of grade 4 toxicities. Thrombocytopenia occurred in 44 patients, with 13 patients (29.5%) of grade 1, 18 patients (40.9%) of grade 2, 11 patients (25%) of grade 3, and 2 patients (4.5%) of grade 4 toxicities. Eighteen patients experienced anemia, with 5 patients (27.8%) of grade 1, 12 patients (66.7%) of grade 2, and 1 patient (5.6%) of grade 3 toxicities.
Discussion
Adjuvant chemotherapy is an important element in the treatment for patients with selected stages II and III colon cancer. The addition of oxaliplatin to the fluoropyrimidine-based regimens has been shown by multiple reports to increase DFS and OS in such patient populations. By analysing an actual practice in the use of oxaliplatin-containing regimens, the present study became the first investigation on the efficacy, survival benefit, and safety profile of the adjuvant treatment in Indonesia. Its findings will provide a basis for further exploration in the clinical practice of treating colon cancer throughout the country.
Our results showed that 2-year OS and DFS rates of cases receiving oxaliplatin-containing adjuvant chemotherapy were lower compared to the findings of MOSAIC study that demonstrated 3-year OS of 87.7% and 3-year DFS of 78.2% (4). Results of Kuebler et al. [2007] sing the FLOX regimen was also higher than our results demonstrating 5-year OS of 80.2% and 3-year DFS of 71.8% (3). Improvement in OS and DFS using the XELOX regimen on stage III colon cancer was also supported by another study observing a 5-year OS of 77.6% and a 3-year DFS of 70.9% (5). Furthermore, locoregional spread was the common recurrence pattern in the local patients, which is contrary with previous reports showing the liver as the common site of metastasis due to its location and vascularization (29,30). Dissimilarity of our results with others might be explained by clinical practice in our local setting that showed a general lack of lymph nodes removal. Domination of locoregional spread is relevant to the fact that the proportion of total patients undergoing resection with >10 lymph nodes removal was only 9.9%. Our previous observation found that around 25% of patients with colon cancer who visited our hospital underwent surgical resection in non-A-type hospitals. Our hospital, Dr. Sardjito Hospital, is classified by the Ministry of Health Republic of Indonesia as an A-type hospital or a national referral center. We demonstrated that patients having their cancer resected in non-A-type hospitals had lower number of lymph nodes removed and lower survival than patients having their cancer resected in A-type hospitals, although significant differences in outcomes were not achieved (Khuzairi et al., 2018, manuscript submitted). In line with the issue, a meta-analysis demonstrated that inappropriate evaluation on lymph nodes was associated with the presence of undetected tumors or micro-metastasis and increased loco-regional recurrence or systemic disease (31). This pattern also may justify why short term survival rates of the local patients were lower compared to those of previous reports. Whether locoregional spread is naturally affected by inappropriate practice of surgical or pathological care, or by other aspects, is still a subject for further investigation. Moreover, a multidisciplinary team involving all related disciplines needs to be better applied in our local hospital in order to achieve higher quality of care and survival rate improvement of patients with colon cancer.
Among various demography, clinicopathological, and treatment characteristics, performance status of ECOG 2, T4 tumor invasion, and less cycles of chemotherapy significantly predicted the short-term OS, while histological grade significantly predicted short-term DFS in the local patients. Adachi et al. also observed that performance status of WHO ≥2 was associated with a lower OS in patients with colorectal cancer (16). Association of T4 tumor invasion with lower OS in our study was consistent with the report of Wuxiao et al. [2015] and An et al. [2015]. The latter showed the association of T stage not only with OS as our results, but also with DFS (17,18). Number of chemotherapy cycles generally predict survival of patients with colon cancer receiving adjuvant treatment (3,4,27). Multiple studies showed that a minimum number of 7 (20) to 10 cycles regimen (3,4) are needed to provide a benefit for patients’ survival. Our univariate analyses revealed that less cycles of chemotherapy were associated with worse OS and DFS. However, the association with lower DFS lost statistical significance with multivariate analyses, although marginal significance was still reached. Our results showing that tumors with poor differentiated histology were associated with an increased probability of disease progressivity did not support previous report (15), although Wuxiao et al. [2015] confirmed its association with OS (18).
The local cohort consisted of cases with high risk stages II and III colon cancer. According to the American Joint Committee 7th editions stage II referred to patients that had undergone complete resection of histology proven T3 or T4, N0, M0 colon cancer and stage III referred to patients that had undergone complete resection of histology proven any T, N1 or N2, M0 colon cancer (32). Patients with stage III disease clearly showed worse DFS compared to those with high risk stage II tumors and this finding supported studies addressing the role of disease stage in predicting survivals (11,15). Nevertheless, by multivariate analyses, the extent of disease lost its influence on patients’ survival. In fact, our patients with stage II disease were generally considered as high risk stage II. This is regarding the fact that pathology report is commonly incomplete (unpublished data), so that the TNM review might become under or over assessed.
In our study population, age, sex, number of lymph nodes removed, and time to start chemotherapy after surgical resection did not predict patients’ survival. However, our results on similar survival rates of patients receiving CAPOX and FOLFOX were consistent with previous reports showing similar 3-year OS and DFS for both regimens (33,34).
The incidence of neuropathy in patients receiving oxaliplatin-containing regimens was only available in 30.9% of the local cohort, indicating an under-reporting practice. As a consequence, this result was much lower than the report of the MOSAIC study showing an incidence of 92.1%. Other studies using CAPOX regimen also demonstrated a much higher incidence of neuropathy than ours (78.0%) (4,5).
Limitations of this study included the use of retrospective methodology which might introduce bias due to unrecorded confounders and incomplete data. This implicates a need for improvement in the recording system of the medical records in the future. Furthermore, median follow-up of patients was shorter than previous reports. However, since this is the first observation on the use of oxaliplatin combined with the fluoropyrimidine-based regimen in Indonesia, it refined the basis of rational therapy for adjuvant chemotherapy in the region. It also laid the groundwork for improvement of the standard care in the local hospitals as well as in others to benefit patients with localized and locally advanced colon cancer.
Conclusions
Our review on the clinical practice showed that oxaliplatin-containing regimen in an adjuvant chemotherapy in patients with high risk stages II and III colon cancer provided a short-term OS of 75.8% and DFS of 72.7%. Locoregional spread was the common recurrence observed and the incidence of neuropathy-induced chemotherapy was lower than the previous studies due to under recording. We identified that performance status, T4 tumors, and less chemotherapy cycles independently predicted the short-term OS, while histological grade was the only independent predictor for short-term DFS.
Acknowledgements
The authors are grateful to Ibnu Purwanto and Mardiah Suci Hardianti who provided and cared for study patients. The authors also thank the staff of “Klinik Bahasa”, Office for Research and Publication, Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Indonesia, for proofreading the text.
Funding: Study program of Y Wardhani was supported by Ministry of Research, Technology, and Higher Education, Republic of Indonesia (administrative number: 774.5/E.4.2/2011).
Ethical Statement: The study obtained ethics approval by the Ethics Committee of Faculty of Medicine, Public Health, and Nursing/Dr. Sardjito Hospital Yogyakarta (number of the approval: KE/FK/209/EC). All participants gave informed consent before taking part in the study.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. 10.1002/ijc.29210 [DOI] [PubMed] [Google Scholar]
- 2.Jogja Cancer Registry-Report of Dr Sardjito Hospital Based Cancer Registry. Sleman, DIY: Fakultas Kedokteran Kesehatan Masyarakat dan Keperawatan Universitas Gadjah Mada; 2008-2014 [cited 2018 may]. Available online: http://canreg.fk.ugm.ac.id/home-eng/
- 3.Kuebler JP, Wieand HS, O'Connell MJ, et al. Oxaliplatin combined with weekly bolus fluorouracil and leucovorin as surgical adjuvant chemotherapy for stage II and III colon cancer: results from NSABP C-07. J Clin Oncol 2007;25:2198-204. 10.1200/JCO.2006.08.2974 [DOI] [PubMed] [Google Scholar]
- 4.André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol 2009;27:3109-16. 10.1200/JCO.2008.20.6771 [DOI] [PubMed] [Google Scholar]
- 5.Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol 2011;29:1465-71. 10.1200/JCO.2010.33.6297 [DOI] [PubMed] [Google Scholar]
- 6.Benson AB, 3rd, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology recommendations on adjuvant chemotherapy for stage II colon cancer. J Clin Oncol 2004;22:3408-19. 10.1200/JCO.2004.05.063 [DOI] [PubMed] [Google Scholar]
- 7.Schmoll HJ, Van Cutsem E, Stein A, et al. ESMO consensus guidelines for management of patients with colon and rectal cancer. A personalized approach to clinical decision making. Ann Oncol 2012;23:2479-516. 10.1093/annonc/mds236 [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Foundation. NCCN guidelines for patient: colon cancer. NCCN 2017;1. [Google Scholar]
- 9.Maindrault-Goebel F, Louvet C, Andre T, et al. Oxaliplatin added to the simplified bimonthly leucovorin and 5-fluorouracil regimen as second-line therapy for metastatic colorectal cancer (FOLFOX6). GERCOR. Eur J Cancer 1999;35:1338-42. 10.1016/S0959-8049(99)00149-5 [DOI] [PubMed] [Google Scholar]
- 10.Zafar SY, Marcello JE, Wheeler JL, et al. Treatment-related toxicity and supportive care in metastatic colorectal cancer. J Support Oncol 2010;8:15-20. [PubMed] [Google Scholar]
- 11.André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med 2004;350:2343-51. 10.1056/NEJMoa032709 [DOI] [PubMed] [Google Scholar]
- 12.Kumar A, Kennecke HF, Renouf DJ, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer 2015;121:527-34. 10.1002/cncr.29072 [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Peixoto RD, Kennecke HF, et al. Effect of adjuvant FOLFOX chemotherapy duration on outcomes of patients with stage III colon cancer. Clin Colorectal Cancer 2015;14:262-8.e1. 10.1016/j.clcc.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 14.Quirt JS, Nanji S, Wei X, et al. Is there a sex effect in colon cancer? Disease characteristics, management, and outcomes in routine clinical practice. Curr Oncol 2017;24:e15-23. 10.3747/co.24.3410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsikitis VL, Larson DW, Huebner M, et al. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer 2014;14:336. 10.1186/1471-2407-14-336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adachi T, Hinoi T, Kinugawa Y, et al. Lower body mass index predicts worse cancer-specific prognosis in octogenarians with colorectal cancer. J Gastroenterol 2016;51:779-87. 10.1007/s00535-015-1147-z [DOI] [PubMed] [Google Scholar]
- 17.An MS, Yoo JH, Kim KH, et al. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J Surg Oncol 2015;13:64. 10.1186/s12957-015-0488-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wuxiao ZJ, Zhou HY, Wang KF, et al. A prognostic model to predict survival in stage III colon cancer patients based on histological grade, preoperative carcinoembryonic antigen level and the neutrophil lymphocyte ratio. Asian Pac J Cancer Prev 2015;16:747-51. 10.7314/APJCP.2015.16.2.747 [DOI] [PubMed] [Google Scholar]
- 19.Perron L, Daigle JM, Vandal N, et al. Characteristics affecting survival after locally advanced colorectal cancer in Quebec. Curr Oncol 2015;22:e485-92. 10.3747/co.22.2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsai HL, Lu CY, Hsieh JS, et al. The prognostic significance of total lymph node harvest in patients with T2-4N0M0 colorectal cancer. J Gastrointest Surg 2007;11:660-5. 10.1007/s11605-007-0119-x [DOI] [PubMed] [Google Scholar]
- 21.Isik A, Peker K, Firat D, et al. Importance of metastatic lymph node ratio in non-metastatic, lymph node-invaded colon cancer: a clinical trial. Med Sci Monit 2014;20:1369-75. 10.12659/MSM.890804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hogan J, Chang KH, Duff G, et al. Lymphovascular invasion: a comprehensive appraisal in colon and rectal adenocarcinoma. Dis Colon Rectum 2015;58:547-55. 10.1097/DCR.0000000000000361 [DOI] [PubMed] [Google Scholar]
- 23.Mamo A, Easaw J, Ibnshamsah F, et al. Retrospective analysis of the effect of CAPOX and mFOLFOX6 dose intensity on survival in colorectal patients in the adjuvant setting. Curr Oncol 2016;23:171-7. 10.3747/co.23.3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pectasides D, Karavasilis V, Papaxoinis G, et al. Randomized phase III clinical trial comparing the combination of capecitabine and oxaliplatin (CAPOX) with the combination of 5-fluorouracil, leucovorin and oxaliplatin (modified FOLFOX6) as adjuvant therapy in patients with operated high-risk stage II or stage III colorectal cancer. BMC Cancer 2015;15:384. 10.1186/s12885-015-1406-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bos AC, van Erning FN, van Gestel YR, et al. Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer. Eur J Cancer 2015;51:2553-61. 10.1016/j.ejca.2015.08.016 [DOI] [PubMed] [Google Scholar]
- 26.Jeong IS, Yoo JH, Seo SH, et al. Association between time (initiation and length) and oncological outcomes for the patients with colon cancer treated with adjuvant chemotherapy. Indian J Surg 2015;77:1252-7. 10.1007/s12262-015-1270-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai YJ, Lin JK, Chen WS, et al. Adjuvant FOLFOX treatment for stage III colon cancer: how many cycles are enough? Springerplus 2016;5:1318. 10.1186/s40064-016-2976-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sargent DJ. Novel endpoints in phase III clinical trials. Clin Adv Hematol Oncol 2005;3:21-3. [PubMed] [Google Scholar]
- 29.Ohlsson B, Tranberg KG, Lundstedt C, et al. Detection of hepatic metastases in colorectal cancer: a prospective study of laboratory and imaging methods. Eur J Surg 1993;159:275-81. [PubMed] [Google Scholar]
- 30.Sadahiro S, Suzuki T, Tanaka A, et al. Hematogenous metastatic patterns of curatively resected colon cancer were different from those of stage IV and autopsy cases. Jpn J Clin Oncol 2013;43:444-7. 10.1093/jjco/hyt002 [DOI] [PubMed] [Google Scholar]
- 31.Chang GJ, Rodriguez-Bigas MA, Skibber JM, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 2007;99:433-41. 10.1093/jnci/djk092 [DOI] [PubMed] [Google Scholar]
- 32.Edge S, Byrd DR, Compton CC, et al. AJCC cancer staging manual. Springer 2010;7. [Google Scholar]
- 33.Xu F, Rimm AA, Fu P, et al. The impact of delayed chemotherapy on its completion and survival outcomes in stage II colon cancer patients. PLoS One 2014;9:e107993. 10.1371/journal.pone.0107993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon C, Ashley S, Smith IE. Does timing of adjuvant chemotherapy for early breast cancer influence survival? J Clin Oncol 2003;21:3792-7. 10.1200/JCO.2003.01.073 [DOI] [PubMed] [Google Scholar]


