Abstract
The “cerebrospinal fluid (CSF) circulation theory” of CSF flowing unidirectionally and circulating through the ventricles and subarachnoid space in a downward or upward fashion has been widely recognized. In this review, observations of CSF motion using different magnetic resonance imaging (MRI) techniques are described, findings that are shared among these techniques are extracted, and CSF motion, as we currently understand it based on the results from the quantitative analysis of CSF motion, is discussed, along with a discussion of slower water molecule motion in the perivascular, paravascular, and brain parenchyma. Today, a shared consensus regarding CSF motion is being formed, as follows: CSF motion is not a circulatory flow, but a combination of various directions of flow in the ventricles and subarachnoid space, and the acceleration of CSF motion differs depending on the CSF space. It is now necessary to revise the currently held concept that CSF flows unidirectionally. Currently, water molecule motion in the order of centimeters per second can be detected with various MRI techniques. Thus, we need new MRI techniques with high-velocity sensitivity, such as in the order of 10 μm/s, to determine water molecule movement in the vessel wall, paravascular space, and brain parenchyma. In this paper, the authors review the previous and current concepts of CSF motion in the central nervous system using various MRI techniques.
Keywords: cerebrospinal fluid, magnetic resonance imaging, perivascular space, paravascular space, glymphatic system
Introduction
According to the historical medical literature, cerebrospinal fluid (CSF) was first described by a Venetian physician, Massa, who depicted the presence of fluid within the ventricles.1,2) Later, Cotugno3) described the presence of a similar fluid also at the surface of the spinal cord. The term “CSF” that is used today was first coined by Magendie,4) and it has since been used by many researchers and clinicians to describe the fluid that is widely distributed throughout the subarachnoid space and ventricular system (CSF space) at the surfaces and within the brain and spinal cord.
Cerebrospinal fluid is constantly in motion, maintaining communication among the brain, spinal cord, nervous system, and lymphatic and vascular systems.5–12) In so doing, CSF has physical significance (buffer and buoyancy effects against external forces; transmitting vascular pulsation; buffer function of excess brain pulsation), as well as physiological significance (heat produced by neural activity; draining unnecessary substances from brain parenchyma; substance exchange among the brain parenchyma, spinal cord, and nervous system), thereby playing a crucial role in facilitating maintenance of nervous system function.13–21) Thus, for CSF to fully display these functions, it cannot stagnate as fluid in the CSF space and must always be moving. Until recently, the dynamics of CSF had been described as “CSF flow” or “CSF circulation.” Cathelin,22) a French physician, is considered to be the first person to use the term “CSF circulation”, and Cushing23) later called it the “third circulation,” distinguishing CSF circulation from the circulation of blood, lymph, and interstitial fluid (ISF). To visualize this CSF circulation, tracers such as air,24) oil,25) radioisotope,26) and/or contrast material27) were injected into the ventricles or the subarachnoid space to observe the bolus movement of the tracer over time. By following the movement of the tracer, a concept termed “CSF flow,” analogous to unidirectional river flow, emerged. Specifically in “CSF flow,” CSF in the ventricles seemingly descends in the caudal direction within the ventricles, ascends in the cephalic direction from the lower back area once it flows through the surface of the spinal cord, and mobilizes from the basal cistern to the Sylvian fissure laterally, ultimately reaching the convexity of the cerebrum.26) Conveniently, the arachnoid granulations and villi are present around the major venous sinuses in this convexity of the cerebrum, and Key and Retzius9) demonstrated that dye administered into the subarachnoid space accumulates at these arachnoid granulations and villi. Based on their observation, it was believed that CSF is absorbed at this site to return to the bloodstream and is secreted again from the choroid plexus, completing the CSF circulation. However, according to the traditional studies, Sweet et al.28) concluded that the choroid plexuses do not appear to be necessary for the exchange of water between blood and CSF by studies with heavy water in normal human subjects in 1950. Hassin29) proposed that CSF does not circulate, it acts as tissue fluid, it is not secreted by the choroid plexus, and it is not absorbed through the arachnoid granulation on pathological studies. Later, Milhorat30) emphasized that the removal of substances from the brain parenchyma through the lymphatic role of the CSF becomes truly important. As a result, the currently held theory of bulk flow in the CSF space of CSF from the production site of the choroid plexus to the site of absorption at the arachnoid granulations or villi has been criticized.
With the emergence of magnetic resonance imaging (MRI),31–37) findings that encourage a revision in the concept of “CSF flow” and “CSF circulation” have been discovered.38) Every MRI method understandably has advantages and disadvantages based on its unique principle of imaging. However, it would be possible to determine the essence of CSF motion by taking findings that are shared among the imaging methods and aggregating them as the greatest shared factors. In this review, shared findings that were obtained through observing CSF motion in the CSF space using various types of MRI techniques are described, and the physiological significance of such findings is summarized.
Analysis of CSF Motion in the Ventricular System
CSF motion in the Sylvian aqueduct and foramen of Monro
Because of its luminal structure within the brain and because anatomically it is in the center of the cranium, many CSF motion studies focused on the Sylvian aqueduct. Feinberg et al.32) used velocity density imaging to identify the CSF velocity through the Sylvian aqueduct; this study showed bi-directional CSF motion at the Sylvian fissure. Presumably, this was the first study to use MRI techniques to document CSF motion in the intracranial cavity. On phase contrast (PC) imaging, volumetric, almost sinusoidal, CSF motion through the Sylvian aqueduct during one cardiac cycle was shown by Bradley et al.39) They also stated that CSF motion in the systolic phase was toward the caudal direction, and during diastole, CSF motion was in the cephalic direction.39,40) Following this research, many investigators published similar research results using PC,41–47) time-resolved three-dimensional phase contrast (3DPC),48–51) echo-planar imaging (EPI),52) and time-spatial labeling inversion pulse (Time-SLIP).53,54) Investigation of dynamic improved motion-sensitized driven-equilibrium steady-state free precession (dynamic iMSDE SSFP) showed turbulent CSF motion that surged up from the Sylvian aqueduct to the third ventricle.37) Currently, real-time imaging of cardiac and respiratory components of CSF velocity using simultaneous multi-slice PC EPI shows inspiration phase CSF directed superiorly into the ventricles and the foramen magnum, and it is reversed in the expiration phase, giving bi-directional respiratory motion.55)
Changing the viewpoint to the foramen of Monro, there has been less investigation of the foramen of Monro than of the Sylvian aqueduct. However, when we review the investigations of the foramen of Monro, they reached the same conclusion that CSF shows bi-directional motion, and cardiac-related CSF motion was observed.42,48) Surprisingly, Time-SLIP and dynamic iMSDE SSFP demonstrated blow-up CSF motion from the third ventricle through the foramen of Monro.37,54) To summarize the results, at the foramen of Monro and the Sylvian aqueduct, which connect each ventricular system, CSF shows bi-directional motion. Figures 1–3 show typical CSF motion in volunteers with different MRI sequences.
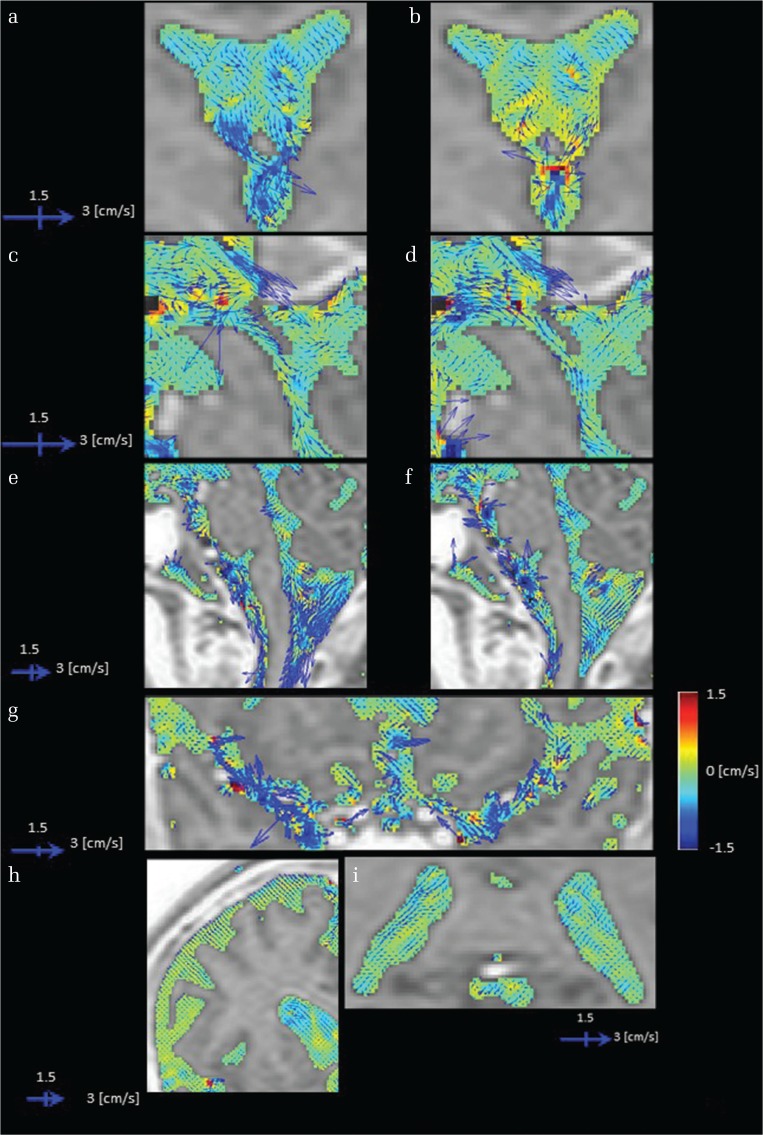
Fig. 1.
Images of cerebrospinal fluid (CSF) velocity with 3DPC in a healthy volunteer. CSF velocity in each imaging plane is represented by the vector length and direction, while the velocity orthogonal to the imaging plane is shown by color coding. The vectors and color scales are as displayed. Sites with irregular motion show long vectors that point in various directions with colors that also change. In addition, blood flow-derived velocity components (non-CSF components) arising from inside the blood vessels were subtracted and removed from the images. Rotational motion within the anterior horn as a vector (a), and the antero-posterior direction as changes on a color scale (b). A motion that ascends toward the lateral and the third ventricle (b and d) and conversely descends toward the third and fourth ventricle (a and c). Irregular motions are evident in the third ventricle based on rotational motion expressed as long vectors and the large change in color display that represents motion orthogonal to the imaging plane (a–d). In the fourth ventricle, augmented motion is observed (e and f). A gentle motion is observed in the trigone (i). A strong motion is shown to strengthen toward the subarachnoid space of the upper cervical spine (e and f). An active CSF motion proximal to the Sylvian fissure that attenuates as it transmits laterally (g), although motion is gradually attenuated toward the distal Sylvian fissure. An augmented motion in a limited area near the vascular structure (g). A suppressed motion seen in convexity (h).
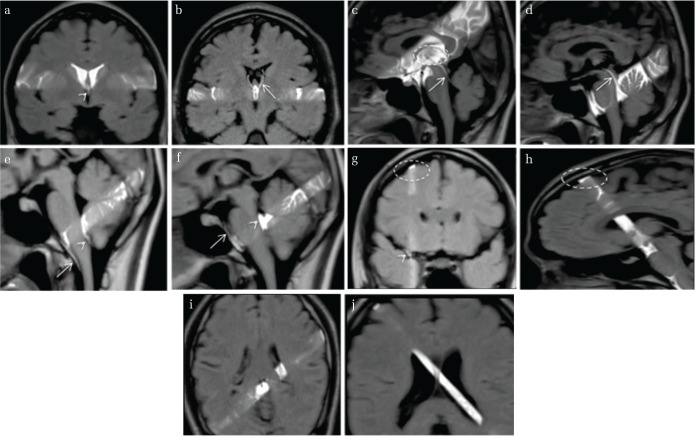
Fig. 2.
Cerebrospinal fluid (CSF) motion in a healthy individual visualized using dynamic improved motion-sensitized driven-equilibrium steady-state free precession (dynamic iMSDE SSFP). In dynamic iMSDE SSFP, the dark regions on the grayscale images indicate vigorous CSF motion. The image contrast is achieved by the signal attenuation induced by irregular motions in each site compared with the surrounding site where CSF moves relatively mildly. With dynamic iMSDE SSFP, irregular motion is observed at the anterior horn, but not at the posterior half of the lateral ventricles (a and b). A motion that sprayed upward from the third ventricle to the anterior horn is visualized (a and b). Augmented motion at the Sylvian aqueduct is observed (b). Turbulent motions are evident in the third and fourth ventricles (b and c). Increased turbulent motion around the brainstem is observed (c and d). A turbulent motion proximal to the Sylvian fissure that attenuates as it transmits laterally (e). Although this augmented motion gradually attenuates toward the distal part of the Sylvian fissure, it also shows limited turbulence near the vascular structure (e). However, motion that attenuates toward the convexity of the cerebrum is not subsequently affected by additional driving forces and is visualized to maintain a suppressed movement (e). Depressed turbulent motion in the convexity is observed (a). Suppressed motion is observed in the trigone (f and g).The axial image shown in (f) confirms irregular motion propagated from the foramen of Monro at the anterior horn, but not at the posterior half of the lateral ventricles.
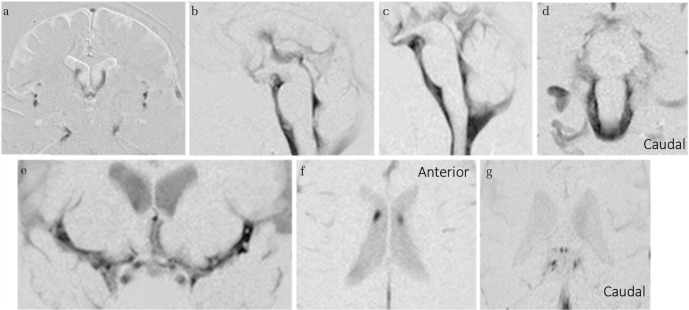
Fig. 3.
Cerebrospinal fluid (CSF) motion in a healthy individual visualized using time-spatial labeling inversion pulse (Time-SLIP). Time-SLIP enables observation of the movement of labeled CSF, shown in white on the image slab, in the first 1–6 s after starting the imaging. Conversely, unlabeled CSF flowing into the specified slab is visualized in black. Motion of labeled (white band) CSF to the third ventricle at the anterior horn and conversely to the anterior horn at the third ventricle is observed (a and b). CSF motion that ascends toward the third ventricle (d) and conversely descends toward the fourth ventricle (c). Irregular motions (black unlabeled turbulent wake in the white labeled area) are evident in the third ventricle (c circle). Motion in both caudal and cephalic directions in the fourth ventricle and the ventral surface of the brainstem (e and f). An image showing (g) lateral movement in the Sylvian fissure (arrow), but no movement is observed in the convexity of the cerebrum (circle). A sagittal image visualizes CSF that appears to be stagnant, with no movement at all (h circle). Labeling around the choroid plexus does not show marked CSF movement (i and j).
CSF motion in the ventricles
When understanding CSF motion in the ventricles, it is easy to understand the physical perspective, not only directional CSF motion and velocity imaging in the ventricles, but also by grasping the acceleration of the CSF in the ventricles. There have been few studies focusing on the quantitative analysis of physical variables.50,56) In Fig. 4, representative previous results show the acceleration in various parts of the intracranial cavities.56) Quantitative analysis of CSF acceleration in the ventricular system showed that CSF acceleration was greater in the third and fourth ventricles than in the lateral ventricle. Imaging analysis using 3DPC, Time-SLIP, and dynamic iMSDE SSFP showed increased CSF velocity, and marked turbulent motion in the third and fourth ventricles was noted by several researchers.37,42,49,54,57,58) Thus, both imaging and quantitative evaluations resulted in the same conclusion that the third and fourth ventricles are in a hyperdynamic state. The third ventricle is anatomically located at the center of CSF movement in the ventricular system; it is small and has an important function in CSF motion in the ventricular system, and it is caught between the two thalami. O’Connell59) proposed that capillary flow into the brain parenchyma during the systolic phase causes expansion of the brain parenchyma. Thus, squeezing of both sides of the thalami at the third ventricle was thought to be the driving force of CSF pulsation; presumably this squeezing leads to acceleration of CSF motion in the third ventricle, and, for this reason, the third ventricle acts as a CSF pump. Another approach to CSF motion using MRI described by Sunohara et al.60,61) determined the correlation between the velocity waveforms, as well as the delay time, and found that the CSF motion of the ventral surface of the brainstem is correlated with that of the third and fourth ventricles. It may be possible to surmise from these findings that the origin of the CSF motion in the third ventricle is pulsation of the arteries. However, the velocity propagation of the pulsation is extremely fast compared with the lateral ventricle, and different results are also obtained depending on the location of reference, indicating the need for additional studies. CSF motions of the fourth ventricle and ventral surface of the brainstem are also highly correlated,60–62) likely due to the CSF communication between the fourth ventricle and the subarachnoid space around the brainstem that is directly connected through the foramen of Magendie and the foramen of Luschka.
Fig. 4.
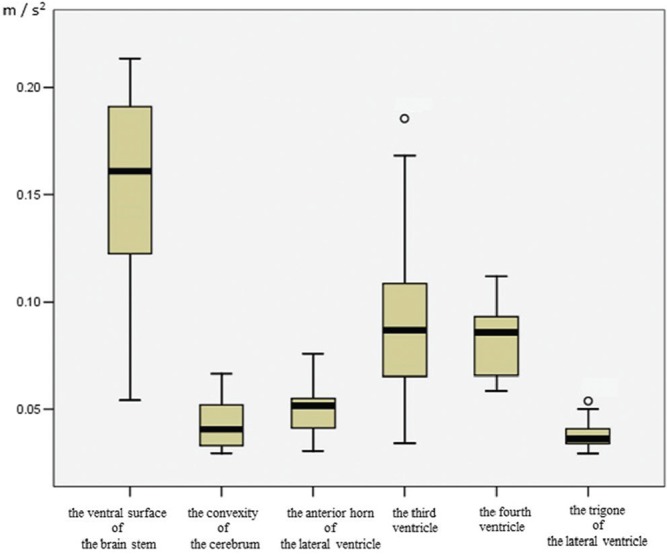
Quantitative value of CSF acceleration in the ventricular system and subarachnoid space of healthy volunteers. Healthy volunteers (age range 28–73 years, male = 6, female = 6). Acceleration of CSF is shown as the rate of velocity change per unit time. From a fluid mechanics perspective, sites with small volumes, such as the subarachnoid space at the ventral surface of the brainstem, are considered to have increased fluid velocity compared with sites with large volumes, such as the trigone; however, because acceleration is less affected than velocity by the volume of the site in which the fluid is present, it is excellent for fluid mechanics analysis that compares sites with different volumes. Circles indicate outliers. Reprinted from Takizawa et al.56) (Fig. 4). The quantitative value indicating augmented acceleration in the anterior horn is increased compared with that of the trigone. High CSF acceleration in the third and fourth ventricles. CSF motion demonstrates a gentle CSF acceleration at the trigone compared with other ventricles, consistent with results from other imaging (Figs. 1–3) evaluations. CSF acceleration corroborates the finding that CSF motion in the subarachnoid space from the ventral surface of the brainstem is elevated, whereas acceleration at the convexity of the cerebrum is suppressed.
Quantitative analysis of CSF motion shown in Fig. 4 demonstrated a gentle CSF acceleration at the trigone compared with other ventricles,56) consistent with results from 3DPC imaging evaluations.48,56) Focusing on the ventricular system, although it has been classically described that CSF pulsation originates from the choroid plexus,63) it has been reported that the choroid plexus of the trigone at the very least does not undergo a large enough volumetric change to become a driving force of CSF.48,56) This finding is accepted by clinicians, because this pulsation in the choroid plexus has also been questioned when observed under neuroendoscopic examination.64) On the other hand, CSF motion in the lateral ventricles, far from the basal cistern, has a poor correlation with the CSF motion in the ventral surface of the brainstem,60,62) and, in particular, the CSF motion in the trigone of the lateral ventricles, farthest away from the basal cistern, has a lower correlation with the CSF motion in the ventral surface of the brainstem.61) Figures 1–3 show typical CSF motion in volunteers with different MRI sequences.
CSF motion in the subarachnoid space
Cerebrospinal fluid motion in the subarachnoid space of the ventral surface of the brainstem is quite vigorous on 3DPC, Time-SLIP, and dynamic iMSDE SSFP.37,48,50,54,65) Because a bony structure (the clivus) is present at the front of the subarachnoid space on the ventral surface of the brainstem, and the vertebro-basilar artery is traveling in the subarachnoid space on the ventral surface of the brainstem over a long distance, which extends along the perpendicular direction inside the subarachnoid space, and is surrounded by CSF, the anatomical structure is set up such that the heartbeat easily propagates through arteries to the subarachnoid space.48) On the upper cervical spine, the venous plexus of the epidural space pulsates in response to intrathoracic pressure changes, and this vigorous CSF motion related to respiration may be spread in the ventral surface of the brainstem and propagate in the upward direction, thereby causing CSF motion to become active, with both arterial and respiratory elements complementing each other. These results are consistent with those of other studies that have used EPI and showed that respiration and cardiac pulsation both affect CSF movement at the cervical level.66,67)
Within the Sylvian fissure, vigorous CSF motion in the proximal Sylvian fissure decreased laterally, and anatomically, arachnoid trabeculae exist in a complex manner along with the vascular system.68,69) It is postulated that, due to these structures, CSF motion becomes buffered as it moves from the center toward the distal part of the cranial cavity.37,48) At the areas distal to the Sylvian fissure, which is part of the subarachnoid space, and at the convexity of the cerebrum, slow CSF motion is predominantly observed; however, it has been reported that CSF motion increases, although in a limited manner, around the vascular structure within this area.56) It is evident from this finding that there is irregularity in the movement of CSF around the vascular structure within the subarachnoid space.
At the convexity of the cerebrum far from the basal cistern, CSF appeared to be stagnant on Time-SLIP,70) and minimal CSF motion was observed on dynamic iMSDE SSFP and 3DPC.37,48,50,56) With Time-SLIP, it is possible to observe CSF motion for 1–6 s after applying a radio frequency pulse to the region of interest,54) and it appeared that CSF was stagnant at the convexity of the cerebrum during these 1–6 s. 3DPC showed decreased velocity and acceleration of CSF at the convexity of the cerebrum.48,50,56) When the shared findings of the 3DPC, dynamic iMSDE SSFP, and Time-SLIP methods were extracted, the movement of CSF appeared to be quite suppressed at the convexity of the cerebrum. These findings, specifically that CSF motion was attenuated as it passed through the basal cistern and transmitted to the convexity of the cerebrum, are consistent with results demonstrated in many other articles.37,48,50,54,56) Figures 1–3 show typical CSF motion in volunteers with different MRI sequences. The quantitative analysis and imaging analysis of CSF motion described above showed the mutual findings listed in Table 1.
Table 1.
Shared findings of CSF movement in the cranial cavity
| In general | Moves, not a circulatory flow. |
| Moves, unstable very complex motion. | |
| Moves, repeats acceleration and deceleration, not only a simple dispersion. | |
| Related to cardiac gate, respiratory cycle, and daily human activities. | |
| Subarachnoid space | Augmented at ventral surface of the brainstem. |
| Strengthened at the center of the cranial cavity, weakened toward the distal part. | |
| Suppressed in the convexity. | |
| Ventricular system | Augmented in the third and fourth ventricles. |
| Suppressed in the trigone. |
CSF: cerebrospinal fluid.
Representative typical MRI studies to describe CSF motion
Every MRI method understandably has advantages and disadvantages based on its unique principle of imaging. However, it would be possible to determine the essence of CSF motion by taking findings that are mutual between each imaging method and aggregating them as the greatest common factors. In this review, the shared findings that were obtained through observing CSF motion in the CSF space using typical MRI techniques are described, and the physiological significance of such findings is described. The characteristics of each imaging technique discussed in this review are presented in Table 2.
Table 2.
Characteristics of each imaging technique presented in this review
| Time-SLIP | 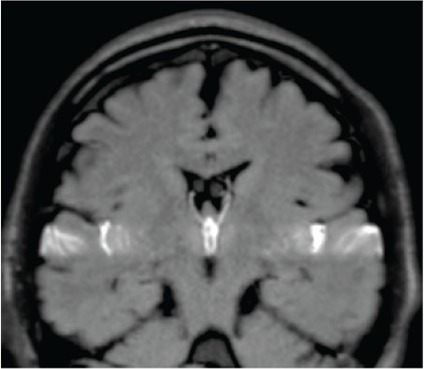 |
Directly observes the signal intensity change due to transference of water protons from certain slab-like regions, in which the proton spins are excited sometime before (about 1–6 s). |
| Dynamic iMSDE SSFP | 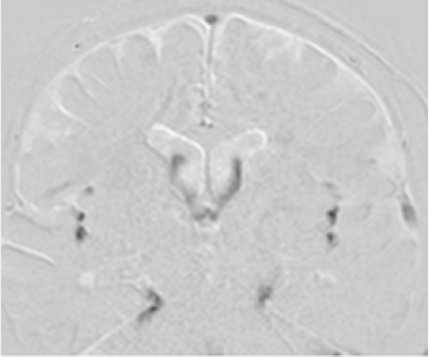 |
Detects and visualizes irregular movement of water protons as signal attenuation induced by phase dispersion in each voxel. |
| 3DPC | 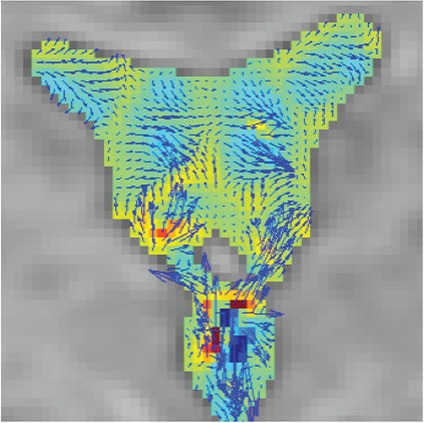 |
Quantifies and visualizes time-resolved CSF velocity in 3D space, and thus enables characterization of CSF motion in a quantitative manner. |
Time-SLIP: time-spatial labeling inversion pulse, Dynamic iMSDE SSFP: dynamic improved motion-sensitized driven-equilibrium steady-state free precession, 3DPC: time-resolved three-dimensional phase contrast.
Cardiac- or respiratory-related CSF motion
Recent studies that examined CSF motion linked to the changes in intrathoracic pressure from respiration were reviewed. Time-SLIP, dynamic iMSDE SSFP, and EPI visualize CSF motion that contains both cardiac pulsatile and respiratory elements because the images are taken during free breathing; however, it is not feasible to separate cardiac pulse- and respiratory-related CSF motion for visualization or to conduct a quantitative evaluation of CSF motion.52,54,66,67,71) On the other hand, 3DPC takes images with the peripheral arterial pulse and ECG or chest wall movements as a trigger33,72) while visualizing CSF motion that contains both cardiac pulsatile and respiratory elements; however, there is also a risk that the part of the respiratory aspect that is longer than the cardiac cycle is not taken into account.70,71) Measurement of respiratory fluctuation-induced distance of CSF movement was therefore attempted, where quantitative analysis of the distance of CSF moving in the cephalic and caudal directions was performed.71) Additionally, Kao et al.73) and Chen et al.55) used an EPI velocity phase contrast technique to perform frequency analysis of CSF motion associated with heartbeat and respiratory fluctuations. Furthermore, frequency analyses of CSF motion have recently been attempted by some researchers, visualizing CSF motion during free breathing or controlled breathing with the PC method and analyzing the results with various methods.62,74,75) These researchers recently published their success in separating cardiac pulsatile and respiratory components of CSF motion.62,75) A frequency analysis, albeit not evaluated quantitatively, concluded that the respiratory element is more dominant than the cardiac pulsatile element in CSF motion.76) On the other hand, some have concluded that CSF motion is greatly impacted by cardiac pulsation, and that the large movements of CSF are affected by respiratory elements; in other words, cardiac pulsation affects the basic pulsation of CSF, and respiration affects the large pulsations.62,75) Traditional pressure wave research by Hamit et al.77) has shown that arterial blood pressure is an important factor in maintaining the static pressure of the CSF. However, strong dynamic changes in the pressure of the CSF are affected mainly through venous channels.77) Wszedybyl-Winklewska et al.78) showed that increased inspiratory resistance is associated with large swings in the heart-generated dynamic relationship between blood pressure and subarachnoid oscillations in healthy subjects. In particular, if CSF motion reaches a far distance due to respiratory fluctuations, it would indicate that such movements greatly impact substance mobilization, and this would be an essential study that gives significance to the relationship between respiration and CSF motion.78) All these studies concern the frequency analysis of CSF motion that started around 2015, and future developments and progress are anticipated.
Approach to CSF motion
There are two approaches to study CSF that mobilizes in the cranium and spinal canal: 1) understanding physiological CSF motion in healthy individuals, and 2) pathological elucidation of a condition that induces CSF motion abnormalities. In this review, findings that were shared among the different types of imaging methods were extracted, and the physiological aspects of CSF motion in healthy individuals are described. For the pathological elucidation of various types of disorders, a more sophisticated analysis has become feasible today by combining multiple imaging techniques, based on their specific characteristics, as described above.
Future direction: need detection of slower water molecule motion
The main sources of ISF are blood and CSF.10) With respect to the pathway of CSF flow into and out of the brain parenchyma, many researchers have been focusing on perivascular movement,10,11,79,80) paravascular movement,7,10,81–84) and dural lymphatic drainage.84–89) The most significant exchange between vessels and brain parenchyma happens at the capillary level.10) ISF re-enters the basement membrane at the capillary level, and the ISF is eliminated along the tunica media and tunica adventitia of the major cerebral arteries.90–93) Mathematical models indicate perivascular transport of ISF by the reflection (reverse direction to the flow of blood; reverse direction to the major pulse wave) motion that follows each vascular pulsation.90) Research from the group of Carare and Weller91,94,95) has suggested an alternative route along the vasculature. They injected tracer into the basal ganglia, and the tracer distributed in the brain parenchyma, but the tracer was also simultaneously present in the laminin in the basement membranes of capillaries and in the basement membranes in the tunica media of arteries.91) Additionally, amyloid beta has almost the same distribution as tracers that are draining from the brain parenchyma along basement membranes in the walls of capillaries and arteries, not around the venous channels.91,96) Mestre et al.97) performed a quantitative analysis of CSF perivascular flow in live mice. Their research showed that the CSF moves into the brain parenchyma through the pariarterial (perivascular space around the artery) area and drains from the brain parenchyma through the same vessel wall route. The radioactive tracer appears in the intracranial arteries and disappears at the wall of the carotid artery in the neck,93) and this phenomenon strongly suggests that ISF is eliminated in the artery wall to drain into the cervical lymphatic system,92) completing the perivascular ISF drainage system.
Many studies have shown the radioactive tracer activity recognized at the cervical lymph node after injection of tracer into the brain parenchyma.98–100) The discovery of lymphatic structure in the dura mater provides new insights into how ISF and CSF reach the cervical lymphatic system6,85) other than the perineural space. However, the black box between the brain and lymphatic structure in the dura mater is still far from being completely understood. We are still missing the connecting bridge between the CSF spaces and/or brain parenchyma and the meningeal lymphatic system,87) which cleans up the unnecessary substances and heat produced by neural activity. The term glymphatic system has appeared as a bridge between water clearance and the lymphatic system and has attracted a great deal of attention by many researchers. The glymphatic system was proposed by Iliff et al. This concept was the basis for in vivo two-proton imaging of fluorescent tracers.8) The glymphatic system shows that CSF moves into the brain parenchyma along paravascular spaces that surround penetrating arteries (Virchow–Robin space);101,102) CSF entering from the CSF space into brain parenchyma pass through aquaporin-4 (AQP4), which are water-selective channels that regulate osmotically driven water transport through the cell membrane that is present on the astrocyte endfeet and ependymal cells; mixed CSF and ISF moves by the mechanism of convective solute transport in the brain parenchyma; and parenchymal ISF is eliminated by the paravenous (perivascular space around the vein) drainage pathway.7,103,104) An early study by Rennels et al.105) reported that apparent convective tracer influx may be facilitated by transmission of the pulsations of the cerebral arteries to the microvasculature, and that fluid circulation through the central nervous system occurs through paravascular pathways. Bedussi et al.81) showed that the paravascular space extends from the CSF space into the brain parenchyma and provides the possibility for unnecessary substance removal that could be facilitated by a mixing action generated by pressure pulsation in the CSF space. Ohashi et al.106) showed strong contrast enhancement around the vein of Labbe after intravenous administration of gadolinium, and, at the same time, Naganawa et al.107) showed that intravenously administered gadolinium leaks from the cortical veins into the surrounding subarachnoid space. Today, many researchers are trying to identify the routes by which CSF enters into and drains from brain parenchyma, but the theory of the glymphatic system is not yet established.
Smith et al.,108) writing against the glymphatic system, showed that AQP4 deletion does not impair transfer of solutes from CSF into the brain parenchyma; movement of fluorescence of different sizes through the brain parenchyma is consistent with their diffusion coefficients; and local movement of solute in the brain parenchyma is not impaired immediately after cardiorespiratory arrest. These results do not support the glymphatic clearance mechanism that transfer of water molecules from CSF to ISF requires AQP4-dependent convection in the brain parenchyma.108) Thus, the precise mechanism of drainage from where interstitial fluid mixes with CSF remains controversial.109) The water molecule movement of the brain parenchyma is also unresolved. A computational model by Asgari et al.110) showed that arterial pulsation may lead to fast paravascular water molecule transport by dispersion, and that glymphatic water molecule transport does not require bulk flow. Faghih and Sharp109) showed that the glymphatic circulation driven by steady pressure is implausible, given current estimates of anatomical and fluid dynamics. Jin et al.111) discussed, using their mathematical model, that significant convective transport requires a sustained pressure difference of several mmHg between the para-arterial and paravenous fluid, and it is not affected by pulsatile pressure fluctuations; diffusion (without convection) in the extracellular space is adequate to account for experimental transport studies in brain parenchyma. Therefore, their modeling results do not support a physiologically important role for local parenchymal convective flow in solute transport through brain extracellular space.111) A traditional anatomical study showed that the final route of mixed ICF and CSF from brain parenchyma though the paravenous space is not obviously developed compared with the para-arterial space, which was seen in a classical pathological study in human spacemen.112) Recently, Abbott et al.113) presented an excellent review, a comprehensive re-evaluation of the previously proposed glymphatic concepts in favor of a new system that better considers basic cerebrovascular physiology and fluid transport considerations. Currently, research on the glymphatic system versus the perivascular system remains a major debate. The discovery of the glymphatic system was done under non-physiological conditions such as tracer injected into the cisterna magna and ventricular infusion, and two-proton imaging detected a limited surface of the cortex through two small cranial windows. In the studies of slow water molecule motion, tracer studies were mostly used. It should be noted that the injection of tracers into the cranium is very sensitive to pressure and volume disturbances, and excess injection speed and/or volume of the tracers may lead to non-physiological conditions in the cranium.10) Thus, a method for monitoring water movement in the brain other than tracer studies is required. Therefore, use of MRI is desired to identify the slow water movement in the central nervous system as a non-tracer study.
In the present review, the detection of water protons by MRI in the order of centimeters per second was presented. Thus, imaging techniques with higher velocity sensitivity, such as several tens of micrometers per second are needed. They include diffusion tensor imaging analysis along the perivascular space,114) brain surface motion imaging,115) double diffusion encoding oscillatory gradient spin technique,116) microscopic diffusional kurtosis imaging with symmetrized double diffusion encoding EPI,117) q-space imaging,84,118,119) and ultra-fast magnetic resonance encephalography.120) Such techniques will greatly improve our knowledge in the near future.
Conclusion
The classical concepts of “CSF flow” and “CSF circulation” should be amended. Furthermore, the expression “CSF motion” is appropriate from a physics perspective when evaluating the various directionalities and dynamics of CSF in the space where it exists. Moreover, CSF repeats acceleration and deceleration not only through simple dispersion of water, but also through pressure gradients and rotation, resulting in very complex motions with the addition of movements associated with activities of daily living. Furthermore, it is postulated that CSF mixes with newly produced CSF and is absorbed near the production site at times or after it is transported far from the production site through respiratory fluctuation or human movements, thereby maintaining homeostasis of the central nervous system. As described above, CSF constantly maintains its movement, acting as a mediator of draining metabolites and metabolic heat generated by neural activities. When CSF is stagnant, the CSF space simply becomes a “garbage sink” and increases the concentration of CSF protein, which restricts CSF motion in the CSF space.
In future, detection of slower water molecule motion in the perivascular and/or paravascular and brain parenchyma spaces are needed. Much more work remains using imaging techniques with higher velocity sensitivity, such as several tens of micrometers per second.
The conclusion of this review is an aggregation of the greatest shared factors obtained through various MRI techniques that ascertain the movement of protons of water molecules in the order of centimeters per second regarding CSF motion in the CSF space, providing an understanding of CSF motion at the present time (2018) that can be accepted by many researchers.
Acknowledgments
The authors are grateful to Mr. Tomohiko Horie and Nao Kajihara, Department of Diagnostic Imaging, Tokai University Hospital for technical assistance with MR imaging.
Footnotes
Conflicts of Interest Disclosure
The authors report no conflict interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1).Massa N: Liber Introductorius Anatomiae. 1st edition Venetio Italy, Francesco Bindoni et Masseo Pasini, 1536 [Google Scholar]
- 2).Herbowski L: The maze of the cerebrospinal fluid discovery. Anat Res Int 2013: 8, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3).Cotugno D: De ischiade nervosa commentarius. Viennae, Apud Rudolphum Gräffer, 1770 [Google Scholar]
- 4).Magendie F: Recherches physiologiques et cliniques sur le liquide céphalo-rachidien ou cérébro-spinal. Paris, Méquignon-Marvis Fils, 1842 [Google Scholar]
- 5).Abbott NJ: Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45: 545–552, 2004 [DOI] [PubMed] [Google Scholar]
- 6).Louveau A, Smirnov I, Keyes TJ, et al. : Structural and functional features of central nervous system lymphatic vessels. Nature 523: 337–341, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Iliff JJ, Wang M, Liao Y, et al. : A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med 4: 147ra111, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8).Iliff JJ, Goldman SA, Nedergaard M: Implications of the discovery of brain lymphatic pathways. Lancet Neurol 14: 977–979, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Key A, Retzius G: Studien in der Anatomie des Nervensystems und des Bindegewebes. Stockholm, Norstedt & Söner, 1876 [Google Scholar]
- 10).Bakker EN, Bacskai BJ, Arbel-Ornath M, et al. : Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol 36: 181–194, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Dobson H, Sharp MM, Cumpsty R, et al. : The perivascular pathways for influx of cerebrospinal fluid are most efficient in the midbrain. Clin Sci (Lond) 131: 2745–2752, 2017 [DOI] [PubMed] [Google Scholar]
- 12).Plog BA, Nedergaard M: The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol 13: 379–394, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13).Johanson C: Choroid plexus–cerebrospinal fluid circulatory dynamics: impact on brain growth, metabolism, and repair. In Conn PM. (ed) Neuroscience in Medicine, Totowa, Humana Press, 2008, pp. 173–200 [Google Scholar]
- 14).Matsumae M, Sato O, Hirayama A, et al. : Research into the physiology of cerebrospinal fluid reaches a new horizon: intimate exchange between cerebrospinal fluid and interstitial fluid may contribute to maintenance of homeostasis in the central nervous system. Neurol Med Chir (Tokyo) 56: 416–441, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Nakada T: Virchow-Robin space and aquaporin-4: new insights on an old friend. Croat Med J 55: 328–336, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16).Zhu DC, Xenos M, Linninger AA, Penn RD: Dynamics of lateral ventricle and cerebrospinal fluid in normal and hydrocephalic brains. J Magn Reson Imaging 24: 756–770, 2006 [DOI] [PubMed] [Google Scholar]
- 17).Miyati T, Mase M, Kasai H, et al. : Noninvasive MRI assessment of intracranial compliance in idiopathic normal pressure hydrocephalus. J Magn Reson Imaging 26: 274–278, 2007 [DOI] [PubMed] [Google Scholar]
- 18).Min KJ, Yoon SH, Kang JK: New understanding of the role of cerebrospinal fluid: offsetting of arterial and brain pulsation and self-dissipation of cerebrospinal fluid pulsatile flow energy. Med Hypotheses 76: 884–886, 2011 [DOI] [PubMed] [Google Scholar]
- 19).Lee HS, Yoon SH: Hypothesis for lateral ventricular dilatation in communicating hydrocephalus: new understanding of the Monro-Kellie hypothesis in the aspect of cardiac energy transfer through arterial blood flow. Med Hypotheses 72: 174–177, 2009 [DOI] [PubMed] [Google Scholar]
- 20).Greitz D: Cerebrospinal fluid circulation and associated intracranial dynamics. A radiologic investigation using MR imaging and radionuclide cisternography. Acta Radiol Suppl 386: 1–23, 1993 [PubMed] [Google Scholar]
- 21).Linninger AA, Xenos M, Sweetman B, Ponkshe S, Guo X, Penn R: A mathematical model of blood, cerebrospinal fluid and brain dynamics. J Math Biol 59: 729–759, 2009 [DOI] [PubMed] [Google Scholar]
- 22).Cathelin F: La Circulation du Liquide Cephalo-Rachidien. Paris, Librairie J. B. Bailliere et Fils, 1912 [Google Scholar]
- 23).Cushing H: The third circulation and its channels. Lancet 209: 851–857, 1925 [Google Scholar]
- 24).Evans WA: An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neur Psych 47: 931–937, 1942 [Google Scholar]
- 25).Du Boulay GH: Pulsatile movements in the CSF pathways. Br J Radiol 39: 255–262, 1966 [DOI] [PubMed] [Google Scholar]
- 26).Di Chiro G: Observations on the circulation of the cerebrospinal fluid. Acta Radiol Diagn (Stockh) 5: 988–1002, 1966 [DOI] [PubMed] [Google Scholar]
- 27).Greitz T, Hindmarsh T: Computer assisted tomography of intracranial CSF circulation using a water-soluble contrast medium. Acta Radiol Diagn (Stockh) 15: 497–507, 1974 [DOI] [PubMed] [Google Scholar]
- 28).Sweet WH, Selverstone B, Soloway S, Stetten D: Studies of formation, flow and absorption of cerebrospinal fluid. II. Studies with heavy water in the normal man. Surg Forum 376–381, 1950 [PubMed] [Google Scholar]
- 29).Hassin GB: So called circulation of the cerebrospinal fluid; chairman’s address. J Am Med Assoc 101: 821–823, 1933 [Google Scholar]
- 30).Milhorat TH: The third circulation revisited. J Neurosurg 42: 628–645, 1975 [DOI] [PubMed] [Google Scholar]
- 31).Pelc NJ, Herfkens RJ, Shimakawa A, Enzmann DR: Phase contrast cine magnetic resonance imaging. Magn Reson Q 7: 229–254, 1991 [PubMed] [Google Scholar]
- 32).Feinberg DA, Mark AS: Human brain motion and cerebrospinal fluid circulation demonstrated with MR velocity imaging. Radiology 163: 793–799, 1987 [DOI] [PubMed] [Google Scholar]
- 33).Enzmann DR, Pelc NJ: Normal flow patterns of intracranial and spinal cerebrospinal fluid defined with phase-contrast cine MR imaging. Radiology 178: 467–474, 1991 [DOI] [PubMed] [Google Scholar]
- 34).Miyazaki M, Sugiura S, Tateishi F, Wada H, Kassai Y, Abe H: Non-contrast-enhanced MR angiography using 3D ECG-synchronized half-Fourier fast spin echo. J Magn Reson Imaging 12: 776–783, 2000 [DOI] [PubMed] [Google Scholar]
- 35).van Uijen CM, den Boef JH: Driven-equilibrium radiofrequency pulses in NMR imaging. Magn Reson Med 1: 502–507, 1984 [DOI] [PubMed] [Google Scholar]
- 36).Yoneyama M, Nakamura M, Takahara T, et al. : Improvement of T1 contrast in whole-brain black-blood imaging using motion-sensitized driven-equilibrium prepared 3D turbo spin echo (3D MSDE-TSE). Magn Reson Med Sci 13: 61–65, 2014 [DOI] [PubMed] [Google Scholar]
- 37).Horie T, Kajihara N, Matsumae M, et al. : Magnetic resonance imaging technique for visualization of irregular cerebrospinal fluid motion in the ventricular system and subarachnoid space. World Neurosurg 97: 523–531, 2017 [DOI] [PubMed] [Google Scholar]
- 38).Stivaros SM, Jackson A: Changing concepts of cerebrospinal fluid hydrodynamics: role of phase-contrast magnetic resonance imaging and implications for cerebral microvascular disease. Neurotherapeutics 4: 511–522, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39).Bradley WG: CSF flow in the brain in the context of normal pressure hydrocephalus. AJNR Am J Neuroradiol 36: 831–838, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40).Bradley WG: Magnetic resonance imaging of normal pressure hydrocephalus. Semin Ultrasound CT MR 37: 120–128, 2016 [DOI] [PubMed] [Google Scholar]
- 41).Daouk J, Chaarani B, Zmudka J, et al. : Relationship between cerebrospinal fluid flow, ventricles morphology, and DTI properties in internal capsules: differences between Alzheimer’s disease and normal-pressure hydrocephalus. Acta Radiol 55: 992–999, 2014 [DOI] [PubMed] [Google Scholar]
- 42).Kurtcuoglu V, Soellinger M, Summers P, et al. : Computational investigation of subject-specific cerebrospinal fluid flow in the third ventricle and aqueduct of Sylvius. J Biomech 40: 1235–1245, 2007 [DOI] [PubMed] [Google Scholar]
- 43).Nowoslawska E, Gwizdala D, Baranska D, et al. : The oscillatory flow of the cerebrospinal fluid in the Sylvian aqueduct and the prepontine cistern measured with phase contrast MRI in children with hydrocephalus-a preliminary report. Childs Nerv Syst 34: 845–851, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44).Lindstrøm EK, Ringstad G, Mardal KA, Eide PK: Cerebrospinal fluid volumetric net flow rate and direction in idiopathic normal pressure hydrocephalus. Neuroimage Clin 20: 731–741, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45).Greitz D, Franck A, Nordell B: On the pulsatile nature of intracranial and spinal CSF-circulation demonstrated by MR imaging. Acta Radiol. 34: 321–328, 1993 [PubMed] [Google Scholar]
- 46).Naidich TP, Altman NR, Gonzalez-Arias SM: Phase contrast cine magnetic resonance imaging: normal cerebrospinal fluid oscillation and applications to hydrocephalus. Neurosurg Clin N Am 4: 677–705, 1993 [PubMed] [Google Scholar]
- 47).Bradley WG, Kortman KE, Burgoyne B: Flowing cerebrospinal fluid in normal and hydrocephalic states: appearance on MR images. Radiology 159: 611–616, 1986 [DOI] [PubMed] [Google Scholar]
- 48).Matsumae M, Hirayama A, Atsumi H, Yatsushiro S, Kuroda K: Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging. J Neurosurg 120: 218–227, 2014 [DOI] [PubMed] [Google Scholar]
- 49).Hirayama A, Matsumae M, Yatsushiro S, Abdulla A, Atsumi H, Kuroda K: Visualization of pulsatile CSF motion around membrane-like structures with both 4D velocity mapping and Time-SLIP technique. Magn Reson Med Sci 14: 263–273, 2015 [DOI] [PubMed] [Google Scholar]
- 50).Hayashi N, Matsumae M, Yatsushiro S, Hirayama A, Abdullah A, Kuroda K: Quantitative analysis of cerebrospinal fluid pressure gradients in healthy volunteers and patients with normal pressure hydrocephalus. Neurol Med Chir (Tokyo) 55: 657–662, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51).Yatsushiro S, Hirayama A, Matsumae M, Kuroda K: Visualization of pulsatile CSF motion separated by membrane-like structure based on four-dimensional phase-contrast (4D-PC) velocity mapping. Conf Proc IEEE Eng Med Biol Soc 2013: 6470–6473, 2013 [DOI] [PubMed] [Google Scholar]
- 52).Klose U, Strik C, Kiefer C, Grodd W: Detection of a relation between respiration and CSF pulsation with an echoplanar technique. J Magn Reson Imaging 11: 438–444, 2000 [DOI] [PubMed] [Google Scholar]
- 53).Shibukawa S, Miyati T, Niwa T, et al. : Time-spatial labeling inversion pulse (Time-SLIP) with pencil beam pulse: a selective labeling technique for observing cerebrospinal fluid flow dynamics. Magn Reson Med Sci 17: 259–264, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54).Yamada S, Miyazaki M, Kanazawa H, et al. : Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology 249: 644–652, 2008 [DOI] [PubMed] [Google Scholar]
- 55).Chen L, Beckett A, Verma A, Feinberg DA: Dynamics of respiratory and cardiac CSF motion revealed with real-time simultaneous multi-slice EPI velocity phase contrast imaging. Neuroimage 122: 281–287, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56).Takizawa K, Matsumae M, Hayashi N, et al. : The choroid plexus of the lateral ventricle as the origin of CSF pulsation is questionable. Neurol Med Chir (Tokyo) 58: 23–31, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57).Stadlbauer A, Salomonowitz E, Brenneis C, et al. : Magnetic resonance velocity mapping of 3D cerebrospinal fluid flow dynamics in hydrocephalus: preliminary results. Eur Radiol 22: 232–242, 2012 [DOI] [PubMed] [Google Scholar]
- 58).Sweetman B, Linninger AA: Cerebrospinal fluid flow dynamics in the central nervous system. Ann Biomed Eng 39: 484–496, 2011 [DOI] [PubMed] [Google Scholar]
- 59).O’Connell JE: The vascular factor in intracranial pressure and the maintenace of the cerebrospinal fluid circulation Brain 66: 204–228, 1943 [Google Scholar]
- 60).Sunohara S, Yatsushiro S, Takizawa K, Matsumae M, Kajihara N, Kuroda K: Investigation of driving forces of cerebrospinal fluid motion by power and frequency mapping based on asynchronous phase contrast technique. Conf Proc IEEE Eng Med Biol Soc 2016: 1232–1235, 2016 [DOI] [PubMed] [Google Scholar]
- 61).Yatsushiro S, Sunohara S, Hayashi N, et al. : Cardiac-driven pulsatile motion of intracranial cerebrospinal fluid visualized based on a correlation mapping technique. Magn Reson Med Sci 17: 151–160, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62).Yatsushiro S, Sunohara S, Takizawa K, Matsumae M, Kajihara N, Kuroda K: Characterization of cardiac- and respiratory-driven cerebrospinal fluid motions using correlation mapping with asynchronous 2-dimensional phase contrast technique. Conf Proc IEEE Eng Med Biol Soc 2016: 3867–3870, 2016 [DOI] [PubMed] [Google Scholar]
- 63).Bering EA: Choroid plexus and arterial pulsation of cerebrospinal fluid; demonstration of the choroid plexuses as a cerebrospinal fluid pump. AMA Arch Neurol Psychiatry 73: 165–172, 1955 [DOI] [PubMed] [Google Scholar]
- 64).Picard N: Regarding Bering’s hypothesis on choroid plexus pulsatility. J Neurosurg 122: 479–480, 2015 [DOI] [PubMed] [Google Scholar]
- 65).Linninger AA, Sweetman B, Penn R: Normal and hydrocephalic brain dynamics: the role of reduced cerebrospinal fluid reabsorption in ventricular enlargement. Ann Biomed Eng 37: 1434–1447, 2009 [DOI] [PubMed] [Google Scholar]
- 66).Daouk J, Bouzerar R, Baledent O: Heart rate and respiration influence on macroscopic blood and CSF flows. Acta Radiol 58: 977–982, 2017 [DOI] [PubMed] [Google Scholar]
- 67).Friese S, Hamhaber U, Erb M, Kueker W, Klose U: The influence of pulse and respiration on spinal cerebrospinal fluid pulsation. Invest Radiol 39: 120–130, 2004 [DOI] [PubMed] [Google Scholar]
- 68).Mortazavi MM, Quadri SA, Khan MA, et al. : Subarachnoid trabeculae: a comprehensive review of their embryology, histology, morphology and surgical significance. World Neurosurg 111: 279–290, 2018 [DOI] [PubMed] [Google Scholar]
- 69).Barshes N, Demopoulos A, Engelhard HH: Anatomy and physiology of the leptomeninges and CSF space. In Abrey LE, Chamberlain MC, Engelhard HH. (eds) Leptomeningeal Metastases, Springer, USA, 2005, pp. 1–16 [DOI] [PubMed] [Google Scholar]
- 70).Yamada S, Tsuchiya K, Bradley WG, et al. : Current and emerging MR imaging techniques for the diagnosis and management of CSF flow disorders: a review of phase-contrast and time-spatial labeling inversion pulse. AJNR Am J Neuroradiol 36: 623–630, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71).Yamada S, Miyazaki M, Yamashita Y, et al. : Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS 10: 36, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72).Mauer UM, Freude G, Danz B, Kunz U: Cardiac-gated phase-contrast magnetic resonance imaging of cerebrospinal fluid flow in the diagnosis of idiopathic syringomyelia. Neurosurgery 63: 1139–1144, 2008 [DOI] [PubMed] [Google Scholar]
- 73).Kao YH, Guo WY, Liou AJ, Hsiao YH, Chou CC: The respiratory modulation of intracranial cerebrospinal fluid pulsation observed on dynamic echo planar images. Magn Reson Imaging 26: 198–205, 2008 [DOI] [PubMed] [Google Scholar]
- 74).Yildiz S, Thyagaraj S, Jin N, et al. : Quantifying the influence of respiration and cardiac pulsations on cerebrospinal fluid dynamics using real-time phase-contrast MRI. J Magn Reson Imaging 46: 431–439, 2017 [DOI] [PubMed] [Google Scholar]
- 75).Takizawa K, Matsumae M, Sunohara S, Yatsushiro S, Kuroda K: Characterization of cardiac- and respiratory-driven cerebrospinal fluid motion based on asynchronous phase-contrast magnetic resonance imaging in volunteers. Fluids Barriers CNS 14: 25, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76).Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gärtner J, Frahm J: Inspiration is the major regulator of human CSF flow. J Neurosci 35: 2485–2491, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77).Hamit HF, Beall AC, Debakey ME: Hemodynamic influences upon brain and cerebrospinal fluid pulsations and pressures. J Trauma 5: 174–184, 1965 [DOI] [PubMed] [Google Scholar]
- 78).Wszedybyl-Winklewska M, Wolf J, Swierblewska E, et al. : Increased inspiratory resistance affects the dynamic relationship between blood pressure changes and subarachnoid space width oscillations. PLoS One 12: e0179503, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79).Hadaczek P, Yamashita Y, Mirek H, et al. : The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 14: 69–78, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80).Hladky SB, Barrand MA: Elimination of substances from the brain parenchyma: efflux via perivascular pathways and via the blood-brain barrier. Fluids Barriers CNS 15: 30, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81).Bedussi B, van der Wel NN, de Vos J, et al. : Paravascular channels, cisterns, and the subarachnoid space in the rat brain: a single compartment with preferential pathways. J Cereb Blood Flow Metab 37: 1374–1385, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82).Kress BT, Iliff JJ, Xia M, et al. : Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 76: 845–861, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83).Myllylä T, Harju M, Korhonen V, Bykov A, Kiviniemi V, Meglinski I: Assessment of the dynamics of human glymphatic system by near-infrared spectroscopy. J Biophotonics 11: e201700123, 2018 [DOI] [PubMed] [Google Scholar]
- 84).Komlosh ME, Benjamini D, Williamson NW, Horkay F, Hutchinson EB, Basser PJ: A novel MRI phantom to study interstitial fluid transport in the glymphatic system. Magn Reson Imaging 56: 181–186, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85).Aspelund A, Antila S, Proulx ST, et al. : A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med 212: 991–999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86).Louveau A, Da Mesquita S, Kipnis J: Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron 91: 957–973, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87).Raper D, Louveau A, Kipnis J: How do meningeal lymphatic vessels drain the CNS? Trends Neurosci 39: 581–586, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88).Da Mesquita S, Fu Z, Kipnis J: The meningeal lymphatic system: a new player in neurophysiology. Neuron 100: 375–388, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89).Louveau A, Herz J, Alme MN, et al. : CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci 21: 1380–1391, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90).Schley D, Carare-Nnadi R, Please CP, Perry VH, Weller RO: Mechanisms to explain the reverse perivascular transport of solutes out of the brain. J Theor Biol 238: 962–974, 2006 [DOI] [PubMed] [Google Scholar]
- 91).Carare RO, Bernardes-Silva M, Newman TA, et al. : Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol 34: 131–144, 2008 [DOI] [PubMed] [Google Scholar]
- 92).Weller RO, Djuanda E, Yow HY, Carare RO: Lymphatic drainage of the brain and the pathophysiology of neurological disease. Acta Neuropathol 117: 1–14, 2009 [DOI] [PubMed] [Google Scholar]
- 93).Szentistványi I, Patlak CS, Ellis RA, Cserr HF: Drainage of interstitial fluid from different regions of rat brain. Am J Physiol 246: F835–F844, 1984 [DOI] [PubMed] [Google Scholar]
- 94).Carare RO, Hawkes CA, Weller RO: Afferent and efferent immunological pathways of the brain. Anatomy, function and failure. Brain Behav Immun 36: 9–14, 2014 [DOI] [PubMed] [Google Scholar]
- 95).Weller RO, Subash M, Preston SD, Mazanti I, Carare RO: Perivascular drainage of amyloid-beta peptides from the brain and its failure in cerebral amyloid angiopathy and Alzheimer’s disease. Brain Pathol 18: 253–266, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96).Preston SD, Steart PV, Wilkinson A, Nicoll JA, Weller RO: Capillary and arterial cerebral amyloid angiopathy in Alzheimer’s disease: defining the perivascular route for the elimination of amyloid beta from the human brain. Neuropathol Appl Neurobiol 29: 106–117, 2003 [DOI] [PubMed] [Google Scholar]
- 97).Mestre H, Tithof J, Du T, et al. : Flow of cerebrospinal fluid is driven by arterial pulsations and is reduced in hypertension. Nat Commun 9: 4878, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98).Cserr HF: Convection of brain interstitial fluid and drainage into cerebrospinal fluid and deep cervical lymph. Wiss Z Karl Marx Univ Leipzig Math Naturwiss R 36: 127–130, 1987 [Google Scholar]
- 99).Földi M, Csillik B, Zoltán OT: Lymphatic drainage of the brain. Experientia 24: 1283–1287, 1968 [DOI] [PubMed] [Google Scholar]
- 100).McComb JG, Hyman S, Weiss MH: Lymphatic drainage of cerebrospinal fluid in the cat. In Shapiro K, Marmarou A, Portnoy H. (eds) Hydrocephalus, New York, NY, USA, Raven Press, 1984, pp. 83–98 [Google Scholar]
- 101).Virchow R: Ueber die Erweiterung kleinerer Gefäfse. Arch Pathol Anat Physiol Klin Med 3: 427–462, 1851 [Google Scholar]
- 102).Robin C: Recherches sur quelques particularités de la structure des capillaires de l’encéphale. J Physiol Homme 2: 537–548, 1859 [Google Scholar]
- 103).Ringstad G, Valnes LM, Dale AM, et al. : Brain-wide glymphatic enhancement and clearance in humans assessed with MRI. JCI Insight 3: pii: 121537, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104).Rasmussen MK, Mestre H, Nedergaard M: The glymphatic pathway in neurological disorders. Lancet Neurol 17: 1016–1024, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105).Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA: Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res 326: 47–63, 1985 [DOI] [PubMed] [Google Scholar]
- 106).Ohashi T, Naganawa S, Ogawa E, Katagiri T, Kuno K: Signal intensity of the cerebrospinal fluid after intravenous administration of gadolinium-based contrast agents: strong contrast enhancement around the vein of labbe. Magn Reson Med Sci doi: 10.2463/mrms.mp.2018-0043 Epub 2018 November 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107).Naganawa S, Nakane T, Kawai H, Taoka T: Age dependence of gadolinium leakage from the cortical veins into the cerebrospinal fluid assessed with whole brain 3D-real inversion recovery MR imaging. Magn Reson Med Sci doi: 10.2463/mrms.mp.2018-0053 Epub 2018 November 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108).Smith AJ, Yao X, Dix JA, Jin BJ, Verkman AS: Test of the ‘glymphatic’ hypothesis demonstrates diffusive and aquaporin-4-independent solute transport in rodent brain parenchyma. Elife 6: pii: e27679, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109).Faghih MM, Sharp MK: Is bulk flow plausible in perivascular, paravascular and paravenous channels? Fluids Barriers CNS 15: 17, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110).Asgari M, de Zélicourt D, Kurtcuoglu V: Glymphatic solute transport does not require bulk flow. Sci Rep 6: 38635, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111).Jin BJ, Smith AJ, Verkman AS: Spatial model of convective solute transport in brain extracellular space does not support a “glymphatic” mechanism. J Gen Physiol 148: 489–501, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112).Zhang ET, Inman CB, Weller RO: Interrelationships of the pia mater and the perivascular (Virchow–Robin) spaces in the human cerebrum. J Anat 170: 111–123, 1990 [PMC free article] [PubMed] [Google Scholar]
- 113).Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG: The role of brain barriers in fluid movement in the CNS: is there a ‘glymphatic’ system? Acta Neuropathol 135: 387–407, 2018 [DOI] [PubMed] [Google Scholar]
- 114).Taoka T, Masutani Y, Kawai H, et al. : Evaluation of glymphatic system activity with the diffusion MR technique: diffusion tensor image analysis along the perivascular space (DTI-ALPS) in Alzheimer’s disease cases. Jpn J Radiol 35: 172–178, 2017 [DOI] [PubMed] [Google Scholar]
- 115).Taoka T, Yamada S, Yamatani Y, et al. : Brain surface motion imaging to predict adhesions between meningiomas and the brain surface. Neuroradiology 52: 1003–1010, 2010 [DOI] [PubMed] [Google Scholar]
- 116).Nielsen JS, Dyrby TB, Lundell H: Magnetic resonance temporal diffusion tensor spectroscopy of disordered anisotropic tissue. Sci Rep 8: 2930, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117).Ji Y, Paulsen J, Zhou IY, et al. : In vivo microscopic diffusional kurtosis imaging with symmetrized double diffusion encoding EPI. Magn Reson Med 81: 533–541, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118).Mohanty V, McKinnon ET, Helpern JA, Jensen JH: Comparison of cumulant expansion and q-space imaging estimates for diffusional kurtosis in brain. Magn Reson Imaging 48: 80–88, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119).King MD, Houseman J, Roussel SA, van Bruggen N, Williams SR, Gadian DG: q-Space imaging of the brain. Magn Reson Med 32: 707–713, 1994 [DOI] [PubMed] [Google Scholar]
- 120).Kiviniemi V, Wang X, Korhonen V, et al. : Ultra-fast magnetic resonance encephalography of physiological brain activity - Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36: 1033–1045, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]