1. Introduction
Despite the advances made in cancer treatment, there are subsets of patients who do not respond to conventional chemotherapy treatment paradigms or who have disease-related relapse. Recently researchers have focused on the role that the immune system plays in cancer control. While previous conceptions of cancer were based on the proliferation of a single, clonal, disordered cell, an important hallmark of cancer is now accepted to be the evasion of cancer cells from immune destruction [1,2]. It is now appreciated that the interaction between cancer cells and immune cells within the microenvironment is the basis for cancer cell escape from immune surveillance. In an effort to address this issue, cancer immunotherapy has emerged as a treatment modality for various malignancies. Cancer immunotherapy is based on generating strategies to exploit the mechanisms that govern the interplay between cancer cells and immune cells within the microenvironment. This mini-review will provide background into the discovery of important biomarkers in current major cancer immunotherapy modalities including immune checkpoint blockade and chimeric antigen receptor (CAR) T cell therapy. Additionally, we will provide an overview of existing cutting-edge methodologies used in biomarker discovery, highlight the advantages of utilizing each method, and discuss current and future directions for biomarker discovery.
2. Immune Checkpoint Therapy
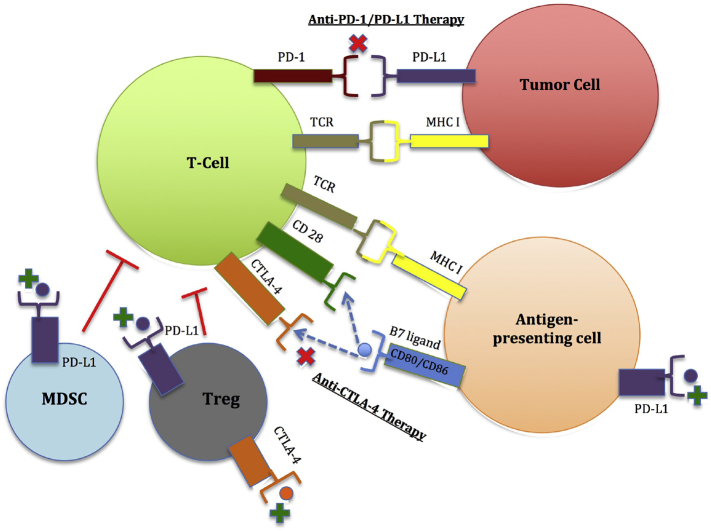
Immune checkpoint molecules function to prevent autoimmunity and tissue damage during pathogenic infection. These molecules are inhibitory receptors expressed on the surfaces of T cells and tumor cells, and mediate the functional interaction between these cells [3]. In a process referred to as adaptive immune resistance, engagement of immune checkpoint molecules on T cells by tumor cells suppresses the cytotoxic capacity of T cells and enables tumor cells to escape cytotoxicity [4,5]. Extrinsic T cell immune-inhibition involves the secretion of inhibitory molecules such as TGF-β, IL-10, and indoleamine 2,3-dioxyenase (IDO). This process decreases cytotoxic T lymphocyte function, and decreases the recruitment of anti-inflammatory cells, regulatory T cells (Treg) and myeloid derived suppressor cells (MDSC) [6,7]. Evidence has emerged that cancers can be further categorized into two distinct tumor types: immunologically-ignorant and immunologically-responsive tumors [7]. Immunologically-ignorant tumors have low mutation load, are immune tolerant against self-antigens, and lack of infiltrating T cells [6]. Immunologically-responsive tumors, on the other hand, have a plethora of infiltrating T cells which in turn reflects intrinsic T cell immune-inhibition and extrinsic tumor-related T cell immunosuppression [8]. The process of T cell immune-inhibition is mediated through immune checkpoint molecule activation. These immune checkpoint molecules include cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), programmed cell death 1 (PD-1), T cell immunoglobulin mucin-3 (Tim-3) and lymphocyte-activation gene 3 (LAG-3) [6,9,10]. This review will focus on the CTLA-4 and PD-1/PD-L1 checkpoints given their advanced clinical development and relevance. TIGIT (T cell immunoreceptor with Ig and ITIM domains) is an inhibitory immune checkpoint molecule that has recently emerged in the field of immunotherapy. TIGIT is expressed on immune cells including regulatory T cells (Tregs) and natural killer (NK) cells [[11], [12], [13], [14]]. An increased TIGIT/CD226 expression ratio on Tregs has been associated with reduced cytokine production and poor survival in multiple cancer models, including acute myeloid leukemia (AML), glioblastoma multiforme (GBM), and melanoma [[11], [12], [13], [14]]. Table 1 provides a summary of the biomarkers studied that are associated with clinical response in immune checkpoint blockade of both CTLA-4 and PD-1. Fig. 1 provides an overview regarding the mechanisms involved in regulating the functional interaction between immune cells and tumor cells. Table 2 provides a summary of the cancer immunotherapies approved by the United States Food and Drug Administration (FDA). Table 3 provides a summary of the cutting-edge technologies that are currently being utilized in the discovery and validation of immunotherapeutic biomarkers.
Table 1.
Summary of biomarkers associated with cancer immunotherapy biomarkers.
| Biomarkers | Clinical correlation | References |
|---|---|---|
| T cell immunoreceptor with Ig and ITIM domains (TIGIT) |
|
|
| CTLA-4 blockade | ||
| Tumor infiltrating lymphocytes (TIL) |
|
|
| ||
| Absolute lymphocyte count (ALC) and absolute neutrophil count (ANC) |
|
|
| ||
| ||
| Inducible co-stimulator (ICOS) |
|
|
| T cell repertoire (TCR) |
|
|
| Tumor associated antigens (TAA) |
|
|
| ||
| Myeloid derived suppressor cells (MDSC) |
|
|
| Regulatory T cells (Treg) |
|
|
| ||
| ||
| ||
| Indoleamine 2,3-Dioxygenase (IDO) |
|
|
| Microbiome profile |
|
|
| PD-1/PD-L1 blockade | ||
| PD-L1 expression |
|
|
| ||
| ||
| ALC and ANC |
|
|
| TIL |
|
|
| ||
| Peripheral blood markers |
|
|
| ||
| ||
| ||
| IDO |
|
|
| ||
| Mutational load |
|
|
| ||
| ||
| Mismatch repair deficiency (mmrd) |
|
|
| ||
| TCR |
|
|
| Microbiome profile |
|
|
| ||
| ||
| ||
| ||
| ||
| ||
| Human leukocyte antigen class I (HLA—I) genotype |
|
|
| ||
| ||
| ||
| ||
| ||
| Mutational load and increased neoantigen (neoAg) frequency |
|
|
| NeoAg-reactive CD4+ and CD8+ T cells |
|
|
| NK cell frequency |
|
|
| Ki-67 Expression on PD-1+ CD8 T Cells |
|
|
| Signatures of T cell dysfunction and exclusion (TIDE) |
|
|
| ||
| Anti-CD19 chimeric antigen receptor (CAR) T cell therapy | ||
| Polyfunctional CAR T Cells |
|
|
| IL-6/STAT3 |
|
|
| Upregulation of programs involved in effector differentiation, glycolysis, exhaustion, and apoptosis |
|
|
Fig. 1.
Mechanisms of immune checkpoint regulation.
CTLA-4 and PD-1/PD-L1 are immune checkpoint molecules present on the surfaces of activated T cells. CTLA-4 competes for B7 ligands (CD80 and CD86) with CD28, a costimulatory molecule, and attenuate T cell proliferation and activation. When PD-1 binds to its corresponding ligand, PD-L1, on the tumor cell surface, this results in T cell exhaustion. PD-L1 expression induced on antigen-presenting cells may also suppress T-cell responses by binding to CD80 on T cells. Myeloid derived suppressor cells (MDSC) and regulatory T cells (Tregs) are key immunosuppressive cells of the immune system that promote cancer progression to limit antitumor T cell immunity through a number of contact-dependent and independent mechanisms. CTLA-4 expressed on Tregs is crucial for their suppressive activity. PD-L1+ MDSCs and PDL1+ Tregs are likely another major source of PD-L1 that inhibits T cell activation and function.
Table 2.
Summary of United States food and drug administration (FDA) approvals for cancer immunotherapy.
| Immunotherapy | Clinical indication |
|---|---|
| Anti-CTLA-4 therapy |
|
| - Ipilimumab (Yervoy) | |
| Anti-PD-1 therapy |
|
| - Nivolumab (Opdivo) |
|
| |
| |
| |
| |
| |
| |
| |
| |
| |
| Anti-PD-1 therapy |
|
| - Pembrolizumab (Keytruda) |
|
| |
| |
| |
| |
| |
| |
| |
| |
| Anti-PD-L1 therapy |
|
| - Atezolizumab (Tecentriq) | |
| |
| |
| |
| Anti-PD-L1 therapy |
|
| - Avelumab (Bavencio) |
|
| Anti-PD-L1 therapy |
|
| - Durvalumab (Imfinzi) | |
| CAR T Cell therapy |
|
| - Tisagenlecleucel (Kymriah) | |
| |
|
Table 3.
Summary of technologies used to discover biomarkers in cancer immunotherapy.
| Technology | Indications |
|---|---|
| Whole exome sequencing |
|
| |
| Gene expression technology |
|
| |
| |
| Epigenomics |
|
| |
| Proteomics |
|
| |
| |
| Flow cytometry |
|
| |
| Mass cytometry |
|
| |
| |
| B and T cell immunosequencing |
|
| |
| Multiplexed and multicolored immunohistochemistry |
|
| |
| |
| |
| |
| Radiomics |
|
3. Biomarkers for Cytotoxic T Lymphocyte-Associated Antigen 4 (CTLA-4) Checkpoint Therapy
CTLA-4, the first intrinsic immune checkpoint molecule discovered in 1987, is expressed exclusively on T cells, regulates T cell activation, and inhibits CD28 activity [15]. CTLA-4 blockade results in helper T cell-dependent enhancement of Treg suppressive function [3]. Further investigations into the function of CTLA-4 led to the discovery that CTLA-4 checkpoint blockade eliminated tumors and in 2011 subsequent approval of ipilimumab by the United States Food and Drug Administration (FDA) for the treatment of advanced melanoma [16].
3.1. Tumor Infiltrating Lymphocytes (TIL)
In a study of lung tumors, Ruffini, et al. reported that the presence of lymphocytes within tumor could serve as a favorable prognostic biomarker of immunotherapeutic blockade [17,18]. In patients with metastatic melanoma treated with anti-CTLA-4 therapy, TIL increase at baseline and at 3 weeks after anti-CTLA-4 therapy was associated with clinical response [17,18]. Granzyme B, a surrogate of CD8 effector function, was increased in metastatic melanoma patients who were found to have a positive response to anti-CTLA-4 [17]. Further discussion into TIL as a biomarker in cancer immunotherapy will be discussed in the sections on PD-1 biomarkers and immunosequencing technology.
3.2. Absolute Lymphocyte Count (ALC) and Absolute Neutrophil Count (ANC)
The mechanism of ipilimumab in treating cancer involves removing the “brake” on antigen specific T cell proliferation. Since ipilimumab increases T cell proliferation, quantifying the ALC and the ANC could be potential biomarkers. ALC in particular contains a heterogeneous lymphocyte population. A prospective study of 720 patients with metastatic melanoma receiving ipilimumab therapy revealed that ANC levels greater than or equal to 7500 were associated with decreased OS and PFS [18]. Zaragoza, et al. found that patients with high neutrophil-to-lymphocyte ratios (NLR) prior to anti-CTLA-4 therapy was associated with poor prognosis [20]. This is consistent with previous reports associating high NLR to poor clinical outcomes in patients with other malignancies [18,21]. Concurrently neutrophilia and eosinophilia was noted in patients who suffered complications of colitis related to their ipilimumab therapy [18,21]. Yet NLR can potentially be a prognostic biomarker prior to immune checkpoint blockade therapy. It should be noted that at least one study done by Schindler, et al. found that an isolated increased ALC in metastatic melanoma patients receiving anti-CTLA-4 therapy found that ALC was not predictive of clinical response [22].
3.3. Inducible Co-Stimulator (ICOS)
ICOS is expressed on the cell surface of activated T cells and is involved with both T cell expansion and survival [6,18,19,21]. In bladder cancer, breast cancer, and mesothelioma patients treated with anti-CTLA-4 therapy, there was a noted increase in CD4+ICOS+ T cell expression [6]. These patients were also observed to have an increased frequency of CD4+ICOS+ T cells following 12 weeks of treatment with subsequent improved clinical response and OS [6]. Increased frequency of CD4+ICOS+ T cell expression in conjunction with anti-CTLA-4 therapy may be a potential biomarker for monitoring clinical response [18,19,21].
3.4. T Cell Repertoire (TCR)
The human T-cell receptor (TCR) repertoire maintains pathogenic control and autoimmunity. Schindler, et al. performed a retrospective review of metastatic melanoma patients treated with ipilimumab that revealed an association between high response rate in those patients who displayed a more diverse TCR repertoire [22]. Conversely a study of both metastatic melanoma and prostate cancer patients on ipilimumab therapy found that those patients with increased TCR diversity had decreased rates of overall survival compared with those patients who were found to have stable T cell clonotypes [21]. Another study of metastatic melanoma patients treated with anti-CTLA-4 therapy revealed an expanded CD8+ T cell response, but this response was not associated with improved clinical responses [18]. Tumeh, et al. observed that metastatic melanoma patients treated with anti-PD-1 therapy had less TCR repertoire diversity in pretreatment tumor samples. After the treatment course, this patient subset had increased TCR repertoire diversity and clonal expansion [5]. Given the varying results, additional studies are needed for validation of TCR as biomarker for immune checkpoint therapy.
3.5. Tumor Associated Antigens (TAA)
Tumor cells express a diverse array of tumor associated antigens (TAAs) [6]. Anti-CTLA-4 therapy increases the production of TAA-specific antibodies. Anti-CTLA-4 therapy also increases the response of antigen-specific CD4+ and CD8+ T cell response in patients with melanoma, ovarian and prostate cancer [23,24]. Melanoma patients in particular have high positivity for cancer-testis antigen NY-ESO-1. Melanoma patients treated with anti-CTLA-4 therapy who were positive for NY-ESO-1 were reported to have improved clinical outcomes compared to their counterparts who were negative for NY-ESO-1 [25]. Possibly confounding this finding is the fact that the sample size for the aforementioned patient cohort was small and received different doses of anti-CTLA-4 therapy. Therefore, additional investigation needs to be performed before NY-ESO-1 can be validated as a biomarker for CTLA-4 blockade efficacy.
Melan-A has also emerged as another potential biomarker for anti-CTLA-4 therapy. Studies investigating Melan-A discovered that this TAA is increased in melanoma and prostate cancer patients with improved clinical response to anti-CTLA-4 therapy [26]. Concurrently these patient subsets were also found to have improved overall survival. Van Allen, et al. published a study of melanoma patients treated with ipilimumab and discovered that the presence of granzyme A and perforin, two cytolytic factors in the tumor microenvironment, were also associated with improved clinical response [27]. The conclusion reached from this study was that anti-CTLA-4 therapy stimulates immune cell response against mutant genes. Patients with prostate cancer also treated with anti-CTLA-4 therapy were also observed to have enhanced TAA response [18,21]. Further prospective studies investigating TAA response in the setting of cancer immunotherapy will need to be done to validate TAAs as a potential biomarker for anti-CTLA-4 therapy [18,21].
3.6. Myeloid Derived Suppressor Cells (MDSC)
MDSC are a heterogeneous cell population composed of both precursor and progenitor myeloid cells. MDSCs also function as antigen-presenting cells (APC) [6]. This cell population has been discovered in patients with various solid tumor malignancies including breast cancer, head and neck squamous cell carcinoma, non-small cell lung cancer (NSCLC), and pancreatic cancer [28]. Additionally, MDSC produce molecules that function in immunosuppression including arginase 1 (ARG1), IL-10, and transforming growth factor-beta (TGF- β) [6]. MDSC have emerged as a biomarker in cancer immunotherapy due to the role that CD14+/HLA−DRlow/− cells play in lymphocyte suppression. Poschke, et al. showed that elevation in MDSC activity is associated with increased melanoma disease activity [29]. Additionally studies have found that anti-CTLA-4 therapy decreases MDSC frequency and that decreased MDSC frequency is associated with improved survival outcomes [[30], [31], [32]].
3.7. Regulatory T Cells (Treg)
Regulatory T cells (Tregs) have an increased frequency in peripheral blood samples collected from cancer patients. T cells expressing FOXP3, previously considered a surrogate Treg marker, were associated with improved clinical response especially in breast and colorectal malignancies [33]. Subsequent studies discovered that the accumulation of both MDSC and Treg cells in patients undergoing cancer immunotherapy is linked to accumulation of CD4+FOXP3+CD25hi cells [33]. Soluble CD25 can function to capture IL-2, a cytokine involved in T cell activation, and decrease anti-CTLA-4 antibody efficacy [18,34]. In patients with malignant melanoma, soluble CD25 is associated with poorer clinical outcome [18,34]. Additionally, accumulation of CD4+FOXP3+CD25hi T cells is associated with antitumor immune suppression and poor clinical prognosis [18,34]. Therefore, since FOXP3 expression alone is not a reliable biomarker for Treg activity, additional investigations have been performed to validate Treg response as a biomarker in cancer immunotherapy [35].
Since Treg expression becomes altered in patients with cancer undergoing immunotherapy treatment, the focus has shifted to investigating induced regulatory T cells (iTregs) as immunotherapeutic biomarkers. iTregs are the predominant in situ Treg subset [36]. Examples of overexpressed Tregs in various malignancies include CD39, latency-associated peptide (LAP), glycoprotein A repetitions predominant (GARP), and CTLA-4 [6]. Tregs constitutively express CTLA-4 and have high therapeutic potential [18,21]. Studies investigating the role of Tregs in anti-CLTA-4 therapy found decreased circulating Tregs following treatment. Other Treg studies performed had results that were not consistent with this observation [6,18]. Another confounding issue in validating Treg biomarkers is distinguishing between different types of Tregs. For example, researchers have focused on Kruppel-like factor 2 (KLF2), a transcription factor necessary for the development of iTregs [6,36]. There remains no consensus on which Treg subset should be monitored in cancer immunotherapy, though consideration has been given to focus on a single subset, such as the CD4+CD39+CD25+, to follow disease-related changes with clinical response to immunotherapy treatment [6,36].
3.8. Indoleamine 2,3-Dioxygenase (IDO)
Indoleamine 2,3-dioxygenase (IDO) is an enzyme that breaks down tryptophan [18]. This breakdown process contributes to an immunosuppressive tumor microenvironment which results in T cell suppression and enhanced activation and recruitment of Tregs and MDSCs that further suppress T cell activity against the tumor cells. In animal studies, IDO expression induces resistance to anti-CTLA-4 [18]. Hamid, et al. found that high expression of IDO at baseline was associated with improved clinical efficacy of anti-CTLA-4 therapy in metastatic melanoma [37]. Further discussion on the inhibition of IDO as an synergistic anticancer strategy with anti-PD-1 therapy is discussed in the IDO subsection of the PD-1 biomarkers section.
3.9. Microbiome Profile
Recent studies have looked into the previous unknown role of the gut microbiome profile in influencing immune checkpoint immunotherapy. In animal model studies of anti-CTLA antibodies in solid tumor models, when animals stored in pathogen-free environments received broad-spectrum antibiotic treatment, CTLA-4 antibodies were observed to not have any effect in influencing tumor growth [21,38]. When these animal models received feedings containing B. fragilis or Bifidobacterium, these models exhibited improvement in dendritic cell function resulting in improved T cell activation [38]. TIL and splenic CD4 + T cells from animal models treated with broad-spectrum antibiotics showed less production and proliferation of cytokines when treated with anti-CTLA-4 antibodies [21,38]. When models were colonized with Bacteroides species received anti-CTLA-4 therapy, there was restoration in anti-tumor response [21,38]. Furthermore, melanoma animal models transplanted with fecal Bacteroides species had improved clinical response when treated with anti-CTLA-4 therapy [21,38]. Future directions for the study of the interplay of the gut microbiome profile in patients receiving cancer immunotherapy could help to understand how changes in the gut microbiome may effect clinical response to cancer immunotherapy.
4. Biomarkers for PD-1/PD-L1 Checkpoint Therapy
PD-1 plays a role in inhibiting T cell activity in pro-inflammatory states and limiting autoimmunity [3]. When PD-1 receptors on T lymphocytes are activated and bound to its associated ligands, PD-L1 and PD-L2, this immune checkpoint works to inhibit T cell function. The PD-1/PD-L1 axis regulates T cell activation, prevents bystander tissue damage in pro-inflammatory states, and provides the mechanism for tumor cells to evade immune surveillance in the tumor microenvironment [6]. Following promising results in early clinical trials, the FDA approved nivolumab and pembrolizumab for patients with advanced melanoma in 2014 and in 2015 approved these therapies for patients with metastatic squamous and non-squamous NSCLC. Subsequently following this approval, a number of other anti PD-1 and anti PD-L1 antibodies have been approved for therapeutic purposes.
4.1. PD-L1 Expression
With identification of and increased understanding regarding the PD-1/PD-L1 pathway, investigations sought to validate PD-L1 expression in tumor cells as a potential surrogate biomarker in patients receiving treatment with anti-PD-1 therapy. The premise behind this concept was that elevated tumor cell expression of PD-L1 correlates with immune evasion and results in poorer prognosis in patients treated with cancer immunotherapy. This association was supported by the results of the KEYNOTE-001 trial [18,21]. A meta-analysis of close to 1500 patients receiving treatment with anti-PD-1 therapy (where two times as many patients with low or no PD-L1 expression tumor expression had positive clinical response compared to those with tumors with PD-L1 overexpression), revealed that this correlation did not hold true for all cancer types [39,40]. Despite the approval of anti-PD-1 for a variety of solid tumor conditions, the studies to date support that PD-L1 overexpression in tumor cells may be a prognostic biomarker, but not a predictive biomarker [18,21].
The incongruence with this observation may be attributable to different factors. PD-L1 expression may be influenced by tumor-infiltrating T cells producing IFN- γ, which in turn resulted in favorable clinical outcomes [18,21]. Despite various immunohistochemistry staining techniques utilized, there is no standard protocol for analyzing PD-L1 expression [18,21]. PD-L1 heterogeneity reflects a dynamic process wherein a tumor may not express PD-L1 at baseline but may have increased expression in inflammatory states or during metastatic disease [18,21].
Despite issues with PD-L1 immunohistochemistry, malignancies with increased PD-L1 expression exhibited improved response rate, progression-free survival, and overall survival. For example, studies of melanoma patients undergoing treatment with nivolumab revealed that patients with PD-L1 expression had over twice the response rate and OS compared to their counterparts without PD-L1 expression [41]. Similar data was seen in melanoma patients with PD-L1 expression treated with combination nivolumab and ipilimumab immunotherapy [41]. In patients with NSCLC, 16 studies to date have been performed of which the majority of the studies showed higher response rates in patients with high PD-L1 expression in NSCLC tumors, although some studies reported no association between PD-L1 expression and response to anti-PD-1 therapy [42]. Multiple factors affect the generalizability of PD-L1 expression as a predictive biomarker that highlights the need for standardized and validated IHC assays [41,42].
A reported mechanism of resistance to anti-PD-1/anti-PD-L1 therapy pertains to adoptive immune resistance where tumor cells escape T cell destruction via IFN- γ signaling which in turn results in PD-L1 expression [43,44]. JAK kinases play an essential role in downstream signaling when exposed to IFN- γ. Whole exome sequencing performed on tumors from patients who initially had response to anti-PD-1 therapy but subsequently developed treatment-related resistance revealed JAK1/JAK2 mutations [44]. Loss-of-function mutations in the JAK1/2 signaling pathway inhibit antitumor activity and results in the activation of T cells to attack cancer cells [43]. During anti-PD-1 therapy, JAK1/2 mutations prevent PD-L1 expression upon IFN- γ exposure, thereby inhibiting the mechanism of anti-PD-1/PD-L1 therapy [44]. Manguso, et al. utilized in vivo CRISPR screening with melanoma mouse models highlighting that deletion of IFN- γ receptors and JAK1, JAK2, and STAT1 resulted in resistance to anti-PD-1 therapy [45]. This suggests that the JAK/STAT pathway may mediate tumor cell escape from response to immune checkpoint blockade.
4.2. Tumor Infiltrating Lymphocytes (TIL)
Melanoma patients with high baseline TIL who received anti-PD-1 therapy are more likely to have positive clinical response to treatment [13]. Increased granzyme B activity in metastatic melanoma patients treated with anti-PD-1 therapy was also associated with a positive reponse [13]. Interestingly TIL was increased during both chemotherapy and radiation therapy. This observation may be driven by augmented by activation of CD8+ T cells and IFN-γ production during treatment with these modalities, which subsequently stimulates PD-L1 expression [13].
4.3. ALC and ANC
There have not been extensive studies investigating the predictive or prognostic value of ALC or ANC in anti-PD-1 therapy [14]. Lin, et al. performed a study of patients with intrahepatic cholangiocarcinoma treated with anti-PD-1 therapy and found that patients with increased NLR had an increased percentage of positive PD-1 T cells, but a decreased percentage of IFN- γ positive T cells [46]. More investigations are needed to study the association of ALC and ANC in patients treated with anti-PD-1 therapy.
4.4. Peripheral Blood Markers
Studies have shown that PD-1/PD-L1 blockade resulted in augmented effector T-cell proliferation. Additionally, Yuan, et al. reported that the blockade of the PD-1/PD-L1 axis activates production of inducible T-cell alpha chemo-attractant (ITAC), IFN-γ, and IL-18 [6]. Based on the studies performed to date, it remains unclear whether there is any correlation between expression of the aforementioned peripheral blood markers and clinical response in patients receiving immunotherapy. Increased IFN-γ was associated with positive clinical response in melanoma patients treated with anti-PD-1 therapy, though this finding was not supported in NSCLC or renal cell carcinoma patients who also received anti-PD-1 therapy [47,48].
Another potential peripheral blood biomarker is circulating monocytes. In single-cell analyses of patients with metastatic melanoma treated with anti-PD-1 therapy, the patients with clinical response exhibited classical monocytes (CD14+CD16−) with higher expression of ICAM-1 and HLA-DR. [49] This finding suggests that monocytes sustain the development of improved anti-tumor immune response during anti-PD-1 therapy [49]. Additional studies of melanoma patients treated with anti-PD-1 therapy revealed that patients with poor clinical response had deregulated intermediate (CD14+CD16+) and non-classical monocytes (CD14−CD16+) characterized by decreased expression of HLA-DR and inflammatory markers [49].
4.5. Indoleamine 2,3-Dioxygenase (IDO)
Some studies performed have revealed that certain subsets of patients with solid tumors exhibiting IDO overexpression respond well to anti-PD-1 therapy. A study of melanoma patients treated with anti-PD-1 therapy had elevated levels of both IFN-γ and IDO, expressed by tumor cells in the presence of IFN-γ [48]. Increases in IDO expression may indicate tumor-reactive T cells presence within the tumor microenvironment. Investigations into other patient cohorts, such as those patients with NSCLC or RCC, did not yield similar findings [48].
Another avenue of ongoing investigation is exploring the efficacy of utilizing combination therapy with anti-PD-1 therapy and anti-IDO-1 therapy. The phase I/II ECHO-202/KEYNOTE-037 trial utilized combination therapy to treat patients with a variety of malignancies including metastatic melanoma, head and neck squamous cell carcinoma, urothelial carcinoma, and renal cell carcinoma. Improved clinical efficacy was observed in 29 of 53 (55%) patients, including 7 patients who had complete response [50,51]. The median progression-free survival (PFS) for patients receiving combination therapy was 22.8 months [50]. Despite the optimism resulting from these respective clinical trials, the recent phase III double blind ECHO-301/KEYNOTE-252 study of 706 patients with unresectable or metastatic melanoma concluded that the combination of anti-PD-1 and anti-IDO-1 therapies did not show improved PFS in this patient cohort compared to anti-PD-1 therapy alone [52]. Further studies are needed to validate IDO as biomarker in cancer immunotherapy.
4.6. Mutational Load
Mutational load is associated with the number of somatic mutations in tumor cells. This concept is based on the higher number of mutations present. Tumor cells with high mutational load can augment CD4+ and CD8+ T cells specific for neoantigens [6]. PD-1/PD-L1 checkpoint blockade enhances endogenous immunity against mutated neoantigen-specific CD4+ and CD8+ T cells.
Investigations into anti-PD-1 therapy reveal a correlation between mutational load and treatment response. Patients with NSCLC identified with high mutational load showed clinical benefit to treatment with anti-PD-1 therapy [6,18,21]. Rosenberg, et al., based on a study of patients treated with anti-PD-1 therapy in bladder cancer, established two predictive factors: the molecular subtype of the tumor according to The Cancer Genome Atlas, and mutational load [53]. Rooney, et al. found a correlation between tumor cytolytic activity (cytolytic activity defined by increased perforin/ granzyme B levels) and mutational load in eight types of solid tumors including colorectal and lung cancer [54]. Tumeh, et al. discovered that melanoma patients who with improved clinical response following anti-PD-1 therapy had an increased amount of CD8+ T cells and TCR oligoclonality [5,6]. Tumor cells with a high mutational load could serve as a biomarker for PD-1/PD-L1 checkpoint blockade immunotherapy at diagnosis and during evaluations of disease-related relapse.
The phase 2 CheckMate 568 trial which assessed the efficacy of combining nivolumab with ipilimumab in NSCLC determined that a tumor mutational load of at least 10 mutations per megabase was predictive of patients who would respond to this therapy despite their PD-L1 expression level [55]. In the phase 3 CheckMate 227 trial that assessed progression-free survival in NSCLC patients who received the combination of nivolumab with ipilimumab, NSCLC patients who had a high mutational load had significantly higher progression-free survival rates across all patient subgroups, 42.6% compared to 13.2% respectively with standard chemotherapy [55]. In patients with high mutational load, but low PD-L1 expression, such as with patients with small cell lung cancer (SCLC), the combination of nivolumab and ipilimumab appears to have improved clinical efficacy as opposed to nivolumab monotherapy [55,56]. In the CheckMate 032 study, progression-free survival and overall survival rates with combination immunotherapy were higher in the patient subset with high tumor mutational load (21.2% and 30.0% for nivolumab monotherapy and nivolumab plus ipilimumab, respectively) compared with the low or medium tumor mutational burden groups [56]. Therefore, within the context of immunotherapy, high mutational load could be a predictive biomarker.
4.7. Mismatch Repair Deficiency (MMRD)
Le, et al. studied patients with hereditary non-polyposis colorectal cancer (HNPCC) who received treatment with anti-PD-1 therapy and found that mismatch repair deficiency could serve as a predictive biomarker for positive clinical resposne [57]. The mechanism behind MMRD is that the greater amount of mutations not resolved by DNA mismatch repair would increase the immunogenicity of HNPCC tumor cells [57]. MMR-deficient colorectal cancers have increased cytotoxic T cell infiltration, indicating a robust immune response. Lee, et al. analyzed MMRD as a predictive biomarker in multiple tumor types and proposed that testing for MMRD and microsatellite instability (MSI) will become the standard of care in any malignancy where MMRD is discovered [58]. In 2017, pembrolizumab received FDA approval for the treatment of malignancies with high MMRD or high MSI. This was the first FDA approval of a medication based on molecular aberration rather than cell type.
4.8. Microbiome Profile
Animal models of melanoma tumor cells treated with anti-PD-1 therapy and either B. fragilis or Bifidobacterium were observed to have augmented functionality of dendritic cells [59]. Another study by Routy, et al. of animal models with MCA-205 sarcoma and RET melanoma who were either untreated or treated with broad-spectrum antibiotics revealed that the antibiotic treatment compromised antitumor effects in the group who received anti-PD-1 therapy [59]. These results were similar to the studies of NSCLC patients treated with broad-spectrum antibiotics who had decreased progression-free and overall survival [59].
Routy, et al. also looked at the composition of gut microbiota in NSCLC and RCC who responded to anti-PD-1 therapy versus those patients who were non-responders. The study found that the commensal that was associated with favorable clinical outcome was A. muciniphila [59]. Gopalakrishnan et al. observed that melanoma patients treated with anti-PD-1 therapy had higher concentrations of Ruminococcaceae/Faecalibacterium which in turn enhanced immune surveillance and the functionality of effector T cells within the tumor microenvironment [60]. This same study also highlighted that those patients deemed to have an unfavorable gut microbiota (defined as a high concentration of Bacteroidales species) had impaired systemic and anti-tumor immune responses [60]. These findings from these studies underscore the therapeutic potential in modulating the gut microbiome during cancer immunotherapy. To further this point, Matson, et al. found that commensal microbiota composition in patients treated with anti-PD-1 therapy was associated with improved efficacy [61]. Additionally, the commensal microbiota associated with improved clinical response to anti-PD-1 therapy included Enterococcus faecium, Collinsella aerofaciens, Bifidobacterium adolescentis, Klebsiella pneumoniae, Veillonella parvula, Parabacteroides merdae, Lactobacillus sp., and Bifidobacterium longum [61]. Conversely, Ruminococcus obeum and Roseburia intestinalis were associated with poor clinical response to anti-PD-1 therapy [61]. This study also proposed that increased beneficial bacteria coupled with a lower frequency of bacteria with negative impact would be a stronger indicator of positive clinical response in cancer immunotherapy [61].
4.9. Human Leukocyte Antigen Class I (HLA—I) Genotype
The human leukocyte antigen class I (HLA-I) genotype plays a role in the immune system's response to cancer [62]. The efficacy of both anti-CTLA-4 and anti-PD-1 therapies depend on the HLA class I–dependent immune activity [[63], [64], [65]]. Chowell, et al. studied 1500 patients with advanced melanoma and NSCLC receiving cancer immunotherapy at Memorial Sloan Kettering to analyze HLA-I variation at HLA-A, HLA—B, and HLA-C [62]. Heterozygosity at HLA-I loci was associated with improved survival outcomes in comparison to homozygosity at one or more HLA-I genes [62]. Homozygosity at HLA—B, and loss of heterozygosity (LOH) at HLA-I genes was associated with decreased overall survival [62]. The possible mechanism for this may involve increased cell surface expression of HLA-B expression and greater binding affinity of HLA-B alleles to a diverse array of peptides [66,67]. Chowell, et al. also found that HLA-I homozygosity and low mutational load were also associated with decreased survival compared with patients who were heterozygous at each class I locus and had tumors with high mutational load [62]. HLA-I homozygosity and LOH at HLA-I represent genetic barriers to cancer immunotherapy.
With regard to the impact of HLA supertype on overall survival, melanoma patients undergoing either anti-CTLA-4 or anti-PD-1 therapy who were found to have B44 superfamily alleles had improved survival. Conversely patients with B62 alleles had significantly decreased overall survial [63]. The B44 superfamily alleles are influenced by a variety of HLA subtypes including HLA-B*18:01, HLA-B*44:02, HLA-B*44:03, HLA-B*44:05, and HLA-B*50:01 [63]. B62 is activated by HLA-B*15:01, which in turn impairs neoantigen recognition within the T cell receptor [63]. The positive clinical response associated with B44 alleles could serve as platform for continued investigations and immunotherapy development [62].
4.10. Neoantigens (NeoAgs)
Endogenous mutated cancer proteins, referred to as neoantigens (neoAgs), are present on the surfaces of tumor cells [68]. Neoantigens enable immune cells to distinguish themselves from tumor cells and are targets for immunotherapy. Previous studies identified neoAgs in several malignancies including cholangiocarcinoma, leukemia, melanoma, NSCLC, and ovarian cancer [[69], [70], [71], [72], [73], [74]]. In these studies where patients received either anti-CTLA-4 or anti-PD-1 therapy, the mutational load and increased neoAg frequency correlated with clinical response [[68], [69], [70], [71], [72], [73], [74]]. This finding is also similar to what has been observed in studies which have identified neoAg-reactive CD4+ and CD8+ T cells that have correlated the presence of these cells with improved clinical outcomes [[68], [69], [70], [71], [72], [73], [74]]. The findings from these studies point towards the emerging role of neoAg identification in cancer immunotherapy.
4.11. NK Cell Frequency
While anti-PD-1 immunotherapy has been successful in the treatment of certain patient subsets with cancer, there are patients who do not respond to this treatment modality. This lack of treatment response suggests the presence of immune cell-tumor interaction outside of the activity of cytotoxic T cells that impacts immune cell response to immunotherapy. Natural killer (NK) cells are cytotoxic lymphocytes that mediate immune response through chemokine and cytokine release [75]. Increased NK cell frequency has been reported to be a good prognostic factor in patients with solid tumors including metastatic prostate cancer, colorectal carcinoma, and melanoma [[75], [76], [77]]. Another function of NK cells within the tumor microenvironment is the recruitment of dendritic cells, specifically conventional type I dendritic cells (cDC1) [76]. cDC1s promote antitumor immunity via T cell recruitment and IL-12 secretion which in turn stimulates the productions of TILs [76]. A decrease in the number of cDC1s has been associated with poor prognosis in patients receiving immunotherapy [76]. Studies by Böttcher, et al. concurrently showed that NK cells or the associated XCR1 ligands can recruit cDC1s to the tumor microenvironment which in turn would elicit anti-tumor response and possibly make the tumor more responsive to immune checkpoint blockade [76]. FLT3L, another important cytokine involved in the anti-tumor response, has been linked to increased NK cell frequency [77]. To further support this finding, Barry, KC, et al. found that inhibition of CD96, an inhibitory receptor that is found on both NK cells and T cells, increases NK cell frequency and works synergistically with anti-PD-1 and anti-CTLA-4 immunotherapy [77].
4.12. Ki-67 Expression on PD-1+ CD8 T Cells
In patients undergoing therapy with immune checkpoint blockade, Ki-67 has emerged as a surrogate biomarker for T cell proliferation. Tregs have the highest expression of Ki-67 [78]. Additionally, studies have shown that CD8 T cells that are Ki-67+ and PD-1+ also have a high expression of granzyme B, which highlights the potential cytotoxicity of these cells [78]. Furthermore, a study of NSCLC patients receiving anti-PD-1 therapy revealed that PD-1+ Ki-67+ CD8 T cells had lower expression of Bcl-2, an anti-apoptotic protein, along with increased expression of ICOS and costimulatory molecules CD27 and CD28 [79]. In prospective studies of patients with metastatic melanoma and NSCLC undergoing anti-PD-1 therapy, patients who were reported to have a positive post-treatment response were also found to have increased Ki-67 expression on PD-1+ CD8 T cells [78,79]. While more validation, especially in other solid tumor pathologies, is needed to confirm Ki-67 as a surrogate biomarker for CD8 response, these studies highlight that early Ki-67 expression on peripheral PD-1+ CD8 T-cell anti-PD-1 therapy may be associated with positive treatment response.
4.13. Signatures of T Cell Dysfunction and Exclusion
In an effort to further define tumor cell escape within the microenvironment, Jiang, P, et al. developed Tumor Immune Dysfunction and Exclusion (TIDE) [80]. TIDE is a computational modality that models two primary mechanisms of tumor immune evasion: T cell dysfunction in tumors with increased cytotoxic T lymphocytes and impaired T cell infiltration in tumors with decreased levels of cytotoxic T lymphocytes [80]. In an analysis of patients with melanoma, the TIDE modality correlated the T cell dysfunction signature with tumor expression data to predict that melanoma patients with high correlation to T cell dysfunction would not respond to either anti-PD-1 or anti-CTLA-4 immunotherapy [80]. Conversely, in patients with malignancies with low expression of cytotoxic T lymphocytes, these patients may have positive treatment-related response to immune checkpoint blockade [80]. The utilization of the TIDE modality was helpful in identifying SERPINB9, a regulatory gene encoding for serine protease that inactivates granzyme B and is experimentally found to be highly expressed in patients who did not respond to immunotherapy [80]. Therefore, SERPINB9 may be a potential predictive biomarker for patients with malignancies resistant to immune checkpoint blockade.
5. Biomarkers for Anti-CD19 Chimeric Antigen Receptor (CAR) T Cell Therapy
Adoptive CAR T cell immunotherapy is an emerging treatment modality being utilized in therapeutic protocols for a variety of malignancies. CAR T cells are genetically engineered autologous T cells that express chimeric antigen receptors against B-lineage antigen CD19 [[81], [82], [83]]. This antigen is expressed on tumor cells and the use of this CAR T cell therapy has been applied in the treatment of diffuse large B-cell lymphoma (DLBCL) and B-cell precursor acute lymphocytic leukemia (B-ALL) [[84], [85], [86]]. Studies investigating the efficacy of CAR T cell therapy have resulted in remission rates between 60 and 90% in both adult and pediatric patients with relapsed and refractory B-ALL [[85], [86], [87], [88]]. CAR T cell therapy has also been used to treat other malignancies though the remission rates reported have been mixed. The variability in response rates to CAR T cell therapy may be due to varying pre-conditioning regimens, and production and administration of the CAR T cells [81]. Ongoing investigative efforts have focused on studying the functional attributes of these cells using high-resolution single-cell analysis to develop more efficacious and safer therapies [81].
The development of biomarkers to assess CAR T cell therapy is based on the use of multiplexed single-cell analyses. Current evidence suggests that polyfunctional CAR-T cells may be a surrogate biomarker used to assess treatment efficacy [81,89]. Studies analyzing the CAR T cell polyfunctionality have focused on Melan-A recognized by T cell 1 (or MART-1) specific TCR-engineered T cells. Studies looking into MART-1 specific TCR-engineered T cells reveal that TNF-α+IFN-γ+ polyfunctional T cell delayed disease-related relapse [90]. Further in vitro analysis of CAR T cell polyfunctionality highlighted that polyfunctionality was a better predictor of clinical response than CAR T cell cytotoxicity [81,90]. Fraietta, et al. studied into biomarkers for responders in chronic lymphocytic leukemia (CLL) patients receiving CAR T cell therapy and identified that increased expression of memory-related genes including IL-6/STAT3 signatures can serve as a surrogate biomarker for complete response to therapy [91]. In this patient subset, highly functional CAR T cells produced STAT3-related cytokines, and serum IL-6 levels correlated with CAR T cell expansion [91]. Blockade of IL-6/STAT3 diminished CAR T cell proliferation [91]. Furthermore, CD27+PD-1−CD8+ CAR T cells with increased expression of IL-6 receptors correlated with clinical response [91]. Upregulation of cellular programs involved in effector differentiation, glycolysis, exhaustion, and apoptosis were associated with no response to CAR T cell therapy [91]. Patients with sustained clinical remission had increased frequency of CD27+CD45RO−CD8+ T cells before CAR T cell generation [91]. These findings highlight the potential of identifying biomarkers to determine which patients may potentially benefit from CAR T cell therapy.
6. Cutting-Edge Technologies for Biomarker Discovery
6.1. Whole Exome Sequencing
The identification and clinical application of biomarkers for cancer immunotherapy requires several steps of validation including utilizing standardized tissue banking and studies incorporating large-scale, randomized, controlled clinical trials. Matsushita et al. and Castle et al. highlighted the use of cancer exome analysis to identify neoantigens recognized by CD8+ T cells [92,93]. Multiple computational tools, such as EBcall, JointSNVMix, MuTect, SomaticSniper, Strelka, and VarScan 2, have been utilized to identify and compare specific tumor antigens in order to increase the accuracy of somatic single nucleotide variant (sSNV) calling [94,95]. Additionally investigations have revealed that autologous T cells do not recognize all neoantigens. This variability of neoantigen discovery has created an avenue for the development of high-throughput technologies such as in vitro T cell culture protocols, MHC multimer flow staining, and TCR gene capture. These technologies work to filter whole exome data and to assess the diversity of the neoantigen specific T cell response [[96], [97], [98], [99]].
6.2. Gene Expression Technology
Gene expression technology is a high-throughput tool used in the identification of biomarkers in cancer immunotherapy. This technology uses a single experiment to analyze multiple cell types. Gene expression technology can also identify intrinsic and extrinsic immunosuppressive molecules that in turn may serve as potential biomarkers and targets of immune checkpoint blockade [6]. This tool can analyze various cell types within the tumor microenvironment including tumor-associated macrophages, Th2 cells, and Tregs and can identify expression profiles associated with these cell types. Yuan, et al. report that the optimal application of gene expression technology involves incorporating utilities from other technologies including gene expression analysis, flow cytometry staining, B and T cell receptor deep sequencing, and multiplex immunohistochemistry (IHC) [6].
6.3. Epigenomic Technology
Epigenomics pertains to the investigation of cellular gene expression by analyzing DNA methylation patterns and histone modifications. These epigenomic components can potentially serve as reversible targets for cancer immunotherapy [100]. These components also include instructions in identifying different cell types. The functional interaction of these components is instructive in identifying the status of gene expression, chromatin organization, and cellular identity. DNA methylation and histone modifications also enhance the complexity of epigenetic regulation of gene expression, which in turn contributes to cellular identity and function [101]. The information from these components can deepen the understanding of cell-cell interaction in the tumor microenvironment [101,102]. Epigenomics allows for a significantly broader range of acceptable sample conditions collected by clinical sites to account for the inherent stability of DNA markers [[101], [102], [103]]. While epigenetic therapy has intersected with cancer immunotherapy in the treatment of different tumor types, additional investigations will help to validate the application of epigenomics as a potential tool to identify immunotherapeutic biomarkers.
6.4. Proteomic Technology
Proteomics is a tool that has been used to identify biomarkers and monitoring their clinical response to cancer therapy. In the past, proteomics was limited to the analysis of just a few proteins at any given timepoint. With the development of high throughput technologies, proteomics now allows for simultaneous analysis of a multitude of proteins, including chemokines, cytokines, and soluble factors [104]. The application of proteomics has been the basis of several clinical studies, including IL-2 immunotherapy.
Immunoproteomics, an extension of proteomics, pertains to the investigation of immune proteins and peptides. The components of immunoproteomics include serologic proteome analysis (SERPA), serological analysis of recombinant cDNA expression libraries (SEREX), and protein microarray. These tools can identify TAAs and their associated antibodies [6,104,105]. SEREX, for example, was utilized in discovering NY-ESO-1 in sera from patients with different types of cancer [[106], [107], [108]]. These tools are impacted by assay preparation and specificity [6]. With ongoing modifications of proteomic microarray assays, immunoproteomics can be used to identify proteins and their binding properties, analyze post-translational modifications, and subsequently identify potential immunotherapeutic biomarkers [6]. The advantages of utilizing protein microarray technologies include the need for less sample volume for testing, improved sensitivity and specificity, and improved high-dimensional data generation [6]. Utilizing high-dimensional data generated from protein microarray provides a more specific representation of the immunologic processes occurring within the tumor microenvironment and clinical response of tumors to cancer immunotherapy.
6.5. Flow Cytometry and Mass Cytometry (CyTOF)
Flow cytometry is a bioinformatics tool that characterizes the function of cells by exploring protein expression, cell subset frequency, cell function, immunophenotype, and ploidy [[109], [110], [111]]. This tool is also invaluable in investigating intracellular pathway activity which in turn provides more information regarding cell-cell interaction within the tumor microenvironment and how the microenvironment is influenced by immunotherapy [[109], [110], [111]]. Flow cytometry allows for investigations of large single cell populations utilizing parallel probes. This methodology subsequently allows for the analysis of function and phenotype of rare cell types [6]. One notable disadvantage with flow cytometry technology is that simultaneous biomarker analysis is limited by fluorescence spectral overlap as computational analysis and gating beyond the number of fluorophores allowed for in the apparatus increases in complexity as additional parameters are included [112].
During this same time period, a new single-cell analysis technology emerged to address the limitations of flow cytometry. Mass cytometry (Cytometry by Time of Flight, CyTOF) increases the number of deployable isotopes, novel nano-crystal configurations, and computational tools [113]. Mass cytometry uses heavy metal ion probes linked with chelation polymers which subsequently leads to a mass spectrometry readout allowing for the simultaneous detection of more unique markers [113,114]. The limitations of this technology include slow collection speed (about 300 events/s), reduced cell recovery (typically recovery of 30% of viable cells), and high expense [113]. These limitations are mitigated by utilizing a single tube for antibody staining as opposed to creating an antibody panel consisting of several tubes [6]. Mass cytometry can analyze complex tissue types investigate intracellular pathways. Mass cytometry has previously been utilized to study epidermal growth factor receptor (EGFR) signaling, epithelial-mesenchymal transition, the Wnt pathway, apoptosis, survival, proliferation, DNA damage response, cell cycle, metabolism, embryonic stem cells and induced pluripotent stem cells [113]. The mass cytometry technology can be expanded to measure immune cell phenotypes and functions in tumor biopsies that can be used to identify prognostic biomarkers to assess a patient's clinical response to cancer immunotherapy.
6.6. B and T Cell Immunosequencing
Immunosequencing is a high-throughput tool developed to investigate B or T cell receptor (BCR or TCR) sequences from a single sample [[115], [116], [117]]. Immunosequencing encodes functional immune receptors that initially exist in germline DNA as unique segments. Immunosequencing quantifies every B or T cell in a sample high sensitivity and precision. Immunosequencing also provides insights into the mechanisms of immunotherapy, measurements of immune system dynamics, and the potential for identifying prognostic biomarkers [6]. Tumeh, et al. applied immunosequencing to assess TIL clonality from stage II DNA mismatch repair-proficient colon cancer patients and observed that patients with below-median clonality and TIL were at increased risk for disease-related recurrence [5]. Assessment of TIL can also be applied to predict a patient's response to immunotherapy. When Tumeh, et al. applied assessment of TIL in melanoma patients being treated with anti-PD-1 therapy, patients exhibiting TIL beneath the median number and level of clonality were less likely to have clinical response to therapy [5,118,119]. These findings support the idea that TIL activation is involved in the mechanism of immune checkpoint molecule inhibition. Therefore, utilization of immunosequencing to further investigate TIL could potentially validate this measure as a predictive and prognostic biomarker.
6.7. Multiplexed Multicolored Immunohistochemistry (IHC)
Multiplexed IHC technologies are being used to identify the presence of multiple biomarkers on a single tissue sample or a collection of different tissue samples. This technology detects the location of proteins within the microenvironment by utilizing immune-labeling with specific antibodies [120]. IHC utilizes antibody panels specific for a tumor subtype while maintaining optimal cell morphology [6]. Multiplexed and multicolored systems are then utilized in order to ascertain the spatial relationships of the proteins within the microenvironment [6,120].
Multiplexed IHC characterizes the spatial relationships in tumors between stromal and immune cells. Multiplexed imaging samples uses morphological structures and cellular to identify cells and their intracellular compartments. Imaging analysis from multiplexed IHC includes information regarding the sample's phenotype, positivity/negativity counts, H-scoring, density measurements, and spatial point pattern analyses [120]. When applied to the study of biomarkers in cancer immunotherapy, multiplexed IHC has been used in the investigation of FOXP3+ Tregs, which are associated with poor clinical response to therapy [120]. Multiplexed IHC analysis of CD3, CD4, CD8, CD25, FOXP3, and Ki-67 could also provide more information regarding the role and function of Tregs within the context of anti-CTLA-4 therapy [120]. In a study of melanoma patients receiving anti-PD-1 therapy, multiplexed IHC has illustrated the density of CD8+ T cell infiltrates which in turn could potentially be applied as a predictive biomarker in the surveillance of patients undergoing anti-PD-1 therapy.
The multiplex staining bleaching methods include multi-epitope-ligand cartography, sequential immunoperoxidase labeling and erasing, multiOmyx platform, and CO-detection by indexing. The multiplex staining bleaching methods work by using bleaching procedures to study formalin-fixed paraffin-embedded (FFPE) tissue samples, which is then repeated several times to identify multiple antigens in a single tissue sample [120]. Multi-epitope-ligand cartography allows for co-localization and detection of a large number of proteins with high functional resolution, though is limited by cost, longer sampling time, and imaging being limited to a single microscopic medium-to-high power field [120]. Sequential immunoperoxidase labeling and erasing is compatible with antibodies from the same species and allows for analysis of multiple antigens, but is limited by a maximum of 5 antibody labels per section [120]. MultiOmyx platforms allow for the analysis of up to 60 biomarkers per slide, but are limited by longer sampling time [120]. CO-detection also allows for the analysis of multiple markers and eliminates autofluorescence, but is also limited by sampling time and has limited use with FFPE [120].
Multiplex signal amplification techniques allow for the simultaneous detection of multiple biomarkers. The multiplex signal amplification techniques include multiplex modified hapten-based, tyramide signal amplification, and nanocrystal quantum dots. The examples of mass spectrometry imaging include mass cytometry (discussed earlier), multiplexed ion beam imaging, and matrix-assisted laser desorption/ionization. Multiplex modified hapten-based is a fast technique (approximately 2 h) and utilizes a cocktail of markers, but only utilizes 4 markers per slide [120,121]. Tyramide signal amplification is compatible with primary antibodies from the same species, but is limited by 7 markers per slide. Nanocrystal quantum dots, similar to CO-detection, eliminates autofluorescence, but is the limited number of nanocrystals that possess the proper chemistry to attach themselves to their targeted molecule [122].
Mass spectrometry imaging (MSI) is a technique used to visualize the spatial distribution of chemical compositions. The MSI modalities include mass cytometry (discussed previously), multiplexed ion beam imaging, and matrix-assisted laser desorption/ionization. Multiplexed ion beam imaging, similar to mass cytometry in function, allows for simultaneous labeling of up to 100 antibodies with metals, but like mass cytometry is limited by sampling time and small area of sampling [120]. Matrix-assisted laser desorption/ionization can identify the presence of multiple proteins, peptides, and small molecules within biological tissues without having to pre-select antibodies or other detection-biasing reagents, but is limited by a relatively low sensitivity and the inability to quantitatively compare signals from different antigen molecules due to differences in ionization characteristics [120].
Multiplexed and multicolored IHC can further our understanding of the cellular interactions in the microenvironment. The knowledge gained from this can in turn be used to identify potential immunotherapeutic biomarkers.
6.8. Radiomics
Radiomics is a new modality that is being utilized to discover new biomarkers in cancer immunotherapy. The radiomics biomarker, or radiomics signature, is comprised of contrast-enhanced CT images and RNA-seq genomic data acquired from biopsies of patients with metastatic solid tumors to quantify tumor infiltration of CD8 cells [123]. As a corollary to the MOSCATO trial conducted in France from 2012 to 2016, patients with solid tumor malignancies receiving treatment with either anti-PD-1 therapy or anti-PD-L1 therapy were assessed utilizing the aforementioned modalities to determine a radiomic score [123]. Those patients who received a high radiomic score, or high CD8 score, were associated with positive treatment response at 3-and 6-months post treatment and higher rates of overall survival [123]. There are currently 27 ongoing clinical trials in patients receiving anti-PD-1/anti-PD-L1 treatment that utilize this methodology [123]. The use of radiomics continues to grow in prospective studies as radiomics offers an efficient, cost-effective, non-invasive, and reliable alternative to assess for predictive biomarkers in cancer immunotherapy.
7. Conclusion
Immune checkpoint molecules and understanding the implications for therapeutic checkpoint blockade underscore the importance of learning more about tumor immunology, the interaction of immune cells and tumor cells within the microenvironment, and the role that tumor neoantigens play in promoting tumor growth and exploiting neoantigens for therapeutic potential. To date therapeutic interventions targeting immune checkpoint molecule blockade has shown promising results in treating various malignancies including melanoma, non-small cell lung carcinoma, bladder cancer, and Hodgkin's lymphoma. Concurrently there are still avenues for continued investigation including, but not limited to understanding which patients are ideal candidates for immune checkpoint molecule blockade therapy, treatment-specific biomarkers to monitor treatment response, the utility of monotherapy checkpoint molecule blockade versus combination therapy (for example incorporating into the treatment plan the use of additional checkpoint inhibitors, adjuvant chemotherapy, or adjuvant radiation therapy), and the appropriate management of treatment-related side effects. Further research into tumor immunology will substantiate our understanding of immune checkpoint molecules and functional interactions of immune cells within the tumor microenvironment with the hope of identifying biomarkers with specific clinical correlation and developing more efficacious and safe therapies.
Acknowledgments
This research was in part supported by National Institutes of Health grant CA149669 and CA208354, Centers of Cancer Nanotechnology Excellence (CCNE) (U54CA199091), PCF Challenge Award, OCRP Clinical Development Award, Northwestern University RHLCCC Flow Cytometry Facility, a Cancer Center Support Grant (NCI CA060553).
The authors have no conflicting financial interests to disclose.
References
- 1.Beck B., Blanpain C. Unraveling cancer stem cell potential. Nat Rev Cancer. 2013;13(10):727–738. doi: 10.1038/nrc3597. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribas A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 2015;5(9):915–919. doi: 10.1158/2159-8290.CD-15-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tumeh P.C., Harview C.L., Yearley J.H. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yuan J., Hegde P.S., Clynes R. Novel technologies and emerging biomarkers for personalized cancer immunotherapy. J Immunother Cancer. 2016;4:3. doi: 10.1186/s40425-016-0107-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kelderman S., Schumacher T.N., Haanen J.B. Acquired and intrinsic resistance in cancer immunotherapy. Mol Oncol. 2014;8(6):1132–1139. doi: 10.1016/j.molonc.2014.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galon J., Costes A., Sanchez-Cabo F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 9.Gajewski T.F. Cancer immunotherapy. Mol Oncol. 2012;6(2):242–250. doi: 10.1016/j.molonc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin D.S., Ribas A. The evolution of checkpoint blockade as a cancer therapy: what's here, what's next? Curr Opin Immunol. 2015;33C:23–35. doi: 10.1016/j.coi.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 11.Hung A.L., Maxwell R., Theodros D. TIGIT and PD-1 dual checkpoint blockade enhances antitumor immunity and survival in GBM. Oncoimmunology. 2018;7(8) doi: 10.1080/2162402X.2018.1466769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chauvin J.-M., Pagliano O., Fourcade J., Sun Z., Wang H., Sander C. TIGIT and PD-1 impair tumor antigen-specific CD8+ T cells in melanoma patients. J Clin Invest. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kong Y., Zhu L., Schell T.D., Zhang J., Claxton D.F., Ehmann W.C. T-cell immunoglobulin and ITIM domain (TIGIT) associates with CD8+ T-cell exhaustion and poor clinical outcome in AML patients. Clin Cancer Res. 2016;22:3057–3066. doi: 10.1158/1078-0432.CCR-15-2626. [DOI] [PubMed] [Google Scholar]
- 14.Fourcade J., Sun Z., Chauvin J.M. CD226 opposes TIGIT to disrupt tregs in melanoma [published online ahead of print, 2018 Jul 25] JCI Insight. 2018;3(14) doi: 10.1172/jci.insight.121157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunet J.F., Denizot F., Luciani M.F. A new member of the immunoglobulin superfamily- CTLA-4. Nature. 1987;328(6127):267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 16.Leach D.R., Krummel M.F., Allison J.P. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271(5256):1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 17.Ruffini E., Asioli S., Filosso P.L. Clinical significance of tumor-infiltrating lymphocytes in lung neoplasms. Ann Thorac Surg. 2009;87:365–371. doi: 10.1016/j.athoracsur.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 18.Manson G., Norwood J., Marabelle A. 27(7) Oxford University Press (OUP); 2016. Biomarkers associated with checkpoint inhibitors. Annals of Oncology; pp. 1199–1206. [DOI] [PubMed] [Google Scholar]
- 19.Ng Tang D., Shen Y., Sun J. Increased frequency of ICOS+ CD4 T cells as a pharmacodynamic biomarker for anti-CTLA-4 therapy. Cancer Immunol Res. 2013;1(4):229–234. doi: 10.1158/2326-6066.CIR-13-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zaragoza J., Caille A., Beneton N. High neutrophil to lymphocyte ratio measured before starting ipilimumab treatment is associated with reduced overall survival in patients with melanoma. Br J Dermatol. 2016;174:146–151. doi: 10.1111/bjd.14155. [DOI] [PubMed] [Google Scholar]
- 21.Maleki Vareki S., Garrigós C., Duran I. Biomarkers of response to PD-1/PD-L1 inhibition. Crit Rev Oncol Hematol. 2017;116:116–124. doi: 10.1016/j.critrevonc.2017.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Schindler K., Harmankaya K., Postow M. ASCO annual meeting. 2013. Pretreatment levels of absolute and relative eosinophil count to improve overall survival (OS) in patients with metastatic melanoma under treatment with ipilimumab, an anti CTLA-4 antibody. [Google Scholar]
- 23.Fong L., Kwek S.S., O'Brien S. Potentiating endogenous antitumor immunity to prostate Cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 2009;69(2):609–615. doi: 10.1158/0008-5472.CAN-08-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kwek S.S., Dao V., Roy R. Diversity of antigen-specific responses induced in vivo with CTLA-4 blockade in prostate cancer patients. J Immunol. 2012;189(7):3759–3766. doi: 10.4049/jimmunol.1201529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuan J., Adamow M., Ginsberg B.A. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc Natl Acad Sci U S A. 2011;108(40):16723–16728. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weide B., Zelba H., Derhovanessian E. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clin Oncol. 2012;30(15):1835–1841. doi: 10.1200/JCO.2011.40.2271. [DOI] [PubMed] [Google Scholar]
- 27.Van Allen E.M., Miao D., Schilling B. Genomic correlates of response to CTLA4 blockade in metastatic melanoma. Science. 2015;350(6257):207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzoni A., Bronte V., Visintin A. Myeloid suppressor lines inhibit T cell responses by a NO-dependent mechanism. J Immunol. 2002;168(2):689–695. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 29.Poschke I., Mougiakakos D., Hansson J. Immature immunosuppressive CD14+HLA-DR−/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70(11):4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 30.Pico de Coana Y., Poschke I., Gentilcore G. Ipilimumab treatment results in an early decrease in the frequency of circulating granulocytic myeloid-derived suppressor cells as well as their Arginase1 production. Cancer Immunol Res. 2013;1(3):158–162. doi: 10.1158/2326-6066.CIR-13-0016. [DOI] [PubMed] [Google Scholar]
- 31.Kitano S., Postow M.A., Ziegler C.G. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res. 2014;2(8):812–821. doi: 10.1158/2326-6066.CIR-14-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer C., Cagnon L., Costa-Nunes C.M. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.deLeeuw R.J., Kost S.E., Kakal J.A., Nelson B.H. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18(11):3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 34.Hannani D., Vétizou M., Enot D. Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res. 2015;25(2):208–224. doi: 10.1038/cr.2015.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adeegbe D.O., Nishikawa H. Natural and induced T regulatory cells in cancer. Front Immunol. 2013;4:190. doi: 10.3389/fimmu.2013.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Whiteside T.L., Jackson E.K. Adenosine and prostaglandin e2 production by human inducible regulatory T cells in health and disease. Front Immunol. 2013;4:212. doi: 10.3389/fimmu.2013.00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamid O., Schmidt H., Nissan A. A prospective phase II trial exploring the association between tumor microenvironment biomarkers and clinical activity of ipilimumab in advanced melanoma. J Transl Med. 2011;9:204. doi: 10.1186/1479-5876-9-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vétizou M., Pitt J.M., Daillère R. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science. 2015;350(6264):1079–1084. doi: 10.1126/science.aad1329. (New York, NY) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herbst R.S., Soria J.-C., Kowanetz M. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Powles T., Eder J.P., Fine G.D. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–562. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 41.Patel S.P., Kurzrock R. PD-L1 Expression as a Predictive Biomarker in Cancer Immunotherapy. Mol Cancer Ther. 2015;14(4):847–856. doi: 10.1158/1535-7163.MCT-14-0983. April 1. [DOI] [PubMed] [Google Scholar]
- 42.Brody R., Zhang Y., Ballas M. PD-L1 expression in advanced NSCLC: insights into risk stratification and treatment selection from a systematic literature review. Lung Cancer. 2017 Oct;112:200–215. doi: 10.1016/j.lungcan.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 43.Shin D.S., Zaretsky J.M., Escuin-Ordinas H. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov. 2017;7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jenkins R.W., Barbie D.A., Flaherty K.T. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer. 2018;118(1):9–16. doi: 10.1038/bjc.2017.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manguso R.T., Pope H.W., Zimmer M.D. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature. 2017;547(7664):413–418. doi: 10.1038/nature23270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin G., Liu Y., Li S. Elevated neutrophil-to-lymphocyte ratio is an independent poor prognostic factor in patients with intrahepatic cholangiocarcinoma. Oncotarget. 2016;7:50963–50971. doi: 10.18632/oncotarget.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martens A., Wistuba-Hamprecht K., Foppen M.G. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. 2016;22(12):2908–2918. doi: 10.1158/1078-0432.CCR-15-2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.McNamara M.J., Hilgart-Martiszus I., Barragan Echenique D.M. Interferon-gamma production by peripheral lymphocytes predicts survival of tumor-bearing mice receiving dual PD-1/CTLA-4 blockade. Cancer Immunol Res. 2016;21:650–657. doi: 10.1158/2326-6066.CIR-16-0022. [DOI] [PubMed] [Google Scholar]
- 49.Krieg C., Nowicka M., Guglietta S. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. 2018;2:144–153. doi: 10.1038/nm.4466. [DOI] [PubMed] [Google Scholar]
- 50.Epacadostat shows value in two SCCHN trialsCancer Discov. 2017;7(9) doi: 10.1158/2159-8290.CD-NB2017-100. September 1. (OF2) [DOI] [PubMed] [Google Scholar]
- 51.Hamid O., Gajewski T.F., Frankel A.E. Proceedings from the 2017 ESMO Congress. 2017. Epacadostat plus pembrolizumab in patients with advanced melanoma: Phase 1 and 2 efficacy and safety results from ECHO-202/ KEYNOTE-037. September 8–12. (Madrid, Spain. Abstract 1214O) [Google Scholar]
- 52.Pembrolizumab combo fails in melanoma . 2018. OncLive. April 6. [Google Scholar]
- 53.Rosenberg J.E., Hoffman-Censits J., Powles T. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–1920. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rooney M.S., Shukla S.A., Wu C.J. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell. 2015;160:48–61. doi: 10.1016/j.cell.2014.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hellmann M.D., Ciuleanu T.E., Pluzanski A. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378:2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hellmann M.D., Callahan M.K., Awad M.M. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853–861. doi: 10.1016/j.ccell.2018.04.001. May 14. (e4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Le D.T., Uram J.N., Wang H. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lee V., Murphy A., Le D.T., Diaz L.A., Jr. Mismatch repair deficiency and response to immune checkpoint blockade. Oncologist. 2016;21:1200–1211. doi: 10.1634/theoncologist.2016-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Routy B., Le Chatelier E., Derosa L. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(6371):91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 60.Gopalakrishnan V., Spencer C.N., Nezi L. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359(6371):97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matson V., Fessler J., Bao R. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science. 2018;359(6371):104–108. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chowell D., Morris L.G.T., Grigg C.M. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science. 2018;359(6375):582–587. doi: 10.1126/science.aao4572. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gubin M.M., Zhang X., Schuster H. Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature. 2014;515:577–581. doi: 10.1038/nature13988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tran E., Ahmadzadeh M., Lu Y.C. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tran E., Robbins P.F., Lu Y.C. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375:2255–2262. doi: 10.1056/NEJMoa1609279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snary D., Barnstable C.J., Bodmer W.F. Molecular structure of human histocompatibility antigens: the HLA-C series. Eur J Immunol. 1977;7:580–585. doi: 10.1002/eji.1830070816. [DOI] [PubMed] [Google Scholar]
- 67.Marsh S.G., Parham P., Barber L.D. Academic Press; 1999. The HLA factsbook. [Google Scholar]
- 68.Bobisse S., Foukas P.G., Coukos G., Harari A. Neoantigen-based cancer immunotherapy. Ann Transl Med. 2016;4(14):262. doi: 10.21037/atm.2016.06.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robbins P.F., Lu Y.C., El-Gamil M. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat Med. 2013;19:747–752. doi: 10.1038/nm.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Rooij N., van Buuren M.M., Philips D. Tumor exome analysis reveals neoantigen-specific T-cell reactivity in an ipilimumab-responsive melanoma. J Clin Oncol. 2013;31:e439–e442. doi: 10.1200/JCO.2012.47.7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wick D.A., Webb J.R., Nielsen J.S. Surveillance of the tumor mutanome by T cells during progression from primary to recurrent ovarian cancer. Clin Cancer Res. 2014;20:1125–1134. doi: 10.1158/1078-0432.CCR-13-2147. [DOI] [PubMed] [Google Scholar]
- 72.Snyder A., Makarov V., Merghoub T. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Linnemann C., van Buuren M.M., Bies L. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat Med. 2015;21:81–85. doi: 10.1038/nm.3773. [DOI] [PubMed] [Google Scholar]
- 74.Rajasagi M., Shukla S.A., Fritsch E.F. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood. 2014;124:453–462. doi: 10.1182/blood-2014-04-567933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hsu J., Hodgins J.J., Marathe M. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128(10):4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Böttcher J.P., Bonavita E., Chakravarty P. NK cells stimulate recruitment of cDC1 into the tumor microenvironment promoting cancer immune control. Cell. 2018;172(5):1022–1037. doi: 10.1016/j.cell.2018.01.004. (e14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barry K.C., Hsu J., Broz M.L. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018 Aug;24(8):1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang A.C., Postow M.A., Orlowski R.J. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature. 2017;545(7652):60–65. doi: 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kamphorst A.O., Pillai R.N., Yang S. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A. 2017;114(19):4993–4998. doi: 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jiang P., Gu S., Pan D. Signatures of T cell dysfunction and exclusion predict cancer immunotherapy response. Nat Med. 2018 Oct;24(10):1550–1558. doi: 10.1038/s41591-018-0136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xue Q., Bettini E., Paczkowski P. Single-cell multiplexed cytokine profiling of CD19 CAR-T cells reveals a diverse landscape of polyfunctional antigen-specific response. J Immunother Cancer. 2017;5:85. doi: 10.1186/s40425-017-0293-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Restifo N.P., Dudley M.E., Rosenberg S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol. 2012;12:269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.June C.H., Maus M.V., Plesa G. Engineered T cells for cancer therapy. Cancer immunology. Immunotherapy. 2014;63(9):969–975. doi: 10.1007/s00262-014-1568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grupp S.A., Kalos M., Barrett D. Chimeric antigen receptor–modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee D.W., Kochenderfer J.N., Stetler-Stevenson M. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385:517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Davila M.L., Riviere I., Wang X. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224) doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maude S.L., Frey N., Shaw P.A. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kochenderfer J.N., Dudley M.E., Kassim S.H. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol. 2015;33(6):540–549. doi: 10.1200/JCO.2014.56.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kassim S.H. Toward an integrated model of product characterization for CAR-T cell therapy drug development efforts. Cell Gene Ther Insights. 2017;3:227–237. [Google Scholar]
- 90.Ma C., Cheung A.F., Chodon T. Multifunctional T-cell analyses to study response and progression in adoptive cell transfer immunotherapy. Cancer Discov. 2013;3(4):418–429. doi: 10.1158/2159-8290.CD-12-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fraietta J.A., Lacey S.F., Orlando E.J. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med. 2018;24(5):563–571. doi: 10.1038/s41591-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matsushita H., Vesely M.D., Koboldt D.C. Cancer exome analysis reveals a T-cell-dependent mechanism of cancer immunoediting. Nature. 2012;482(7385):400–404. doi: 10.1038/nature10755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Castle J.C., Kreiter S., Diekmann J. Exploiting the mutanome for tumor vaccination. Cancer Res. 2012;72(5):1081–1091. doi: 10.1158/0008-5472.CAN-11-3722. [DOI] [PubMed] [Google Scholar]
- 94.Wang Q., Jia P., Li F. Detecting somatic point mutations in cancer genome sequencing data: a comparison of mutation callers. Genome Med. 2013;5(10):91. doi: 10.1186/gm495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bao R., Huang L., Andrade J. Review of current methods, applications, and data management for the bioinformatics analysis of whole exome sequencing. Cancer Inf. 2014;13(Suppl. 2):67–82. doi: 10.4137/CIN.S13779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin Y., Gallardo H.F., Ku G.Y. Optimization and validation of a robust human T-cell culture method for monitoring phenotypic and polyfunctional antigen-specific CD4 and CD8 T-cell responses. Cytotherapy. 2009;11(7):912–922. doi: 10.3109/14653240903136987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Andersen R.S., Kvistborg P., Frosig T.M. Parallel detection of antigen-specific T cell responses by combinatorial encoding of MHC multimers. Nat Protoc. 2012;7(5):891–902. doi: 10.1038/nprot.2012.037. [DOI] [PubMed] [Google Scholar]
- 98.Linnemann C., Heemskerk B., Kvistborg P. High-throughput identification of antigen-specific TCRs by TCR gene capture. Nat Med. 2013;19(11):1534–1541. doi: 10.1038/nm.3359. [DOI] [PubMed] [Google Scholar]
- 99.Abbas A.R., Baldwin D., Ma Y. Immune response in silico (IRIS): immune-specific genes identified from a compendium of microarray expression data. Genes Immun. 2005;6(4):319–331. doi: 10.1038/sj.gene.6364173. [DOI] [PubMed] [Google Scholar]
- 100.Romanoski C.E., Glass C.K., Stunnenberg H.G. Epigenomics: roadmap for regulation. Nature. 2015;518(7539):314–316. doi: 10.1038/518314a. [DOI] [PubMed] [Google Scholar]
- 101.Chiappinelli K.B., Zahnow C.A., Ahuja N., Baylin S.B. Combining epigenetic and immune therapy to combat cancer. Cancer Res. 2016;76(7):1683–1689. doi: 10.1158/0008-5472.CAN-15-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma S., Kelly T.K., Jones P.A. Epigenetics in cancer. Carcinogenesis. 2010;31(1):27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Polak P., Karlic R., Koren A. Cell-of-origin chromatin organization shapes the mutational landscape of cancer. Nature. 2015;518(7539):360–364. doi: 10.1038/nature14221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Desmetz C., Cortijo C., Mange A., Solassol J. Humoral response to cancer as a tool for biomarker discovery. J Proteomics. 2009;72(6):982–988. doi: 10.1016/j.jprot.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Fulton K.M., Twine S.M. Immunoproteomics: current technology and applications. Methods Mol Biol. 2013;1061:21–57. doi: 10.1007/978-1-62703-589-7_2. [DOI] [PubMed] [Google Scholar]
- 106.Chen Y.T., Scanlan M.J., Sahin U. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc Natl Acad Sci U S A. 1997;94(5):1914–1918. doi: 10.1073/pnas.94.5.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Madoz-Gurpide J., Kuick R., Wang H. Integral protein microarrays for the identification of lung cancer antigens in sera that induce a humoral immune response. Mol Cell Proteomics. 2008;7(2):268–281. doi: 10.1074/mcp.M700366-MCP200. [DOI] [PubMed] [Google Scholar]
- 108.Bouwman K., Qiu J., Zhou H. Microarrays of tumor cell derived proteins uncover a distinct pattern of prostate cancer serum immunoreactivity. Proteomics. 2003;3(11):2200–2207. doi: 10.1002/pmic.200300611. [DOI] [PubMed] [Google Scholar]
- 109.Maecker H.T., McCoy J.P., Nussenblatt R. Standardizing immunophenotyping for the human immunology project. Nat Rev Immunol. 2012;12(3):191–200. doi: 10.1038/nri3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Streitz M., Miloud T., Kapinsky M. Standardization of whole blood immune phenotype monitoring for clinical trials: panels and methods from the ONE study. Transplant Res. 2013;2(1):17. doi: 10.1186/2047-1440-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.van Dongen J.J., Lhermitte L., Bottcher S. EuroFlow antibody panels for standardized n-dimensional flow cytometric immunophenotyping of normal, reactive and malignant leukocytes. Leukemia. 2012;26(9):1908–1975. doi: 10.1038/leu.2012.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chattopadhyay P.K., Price D.A., Harper T.F. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat Med. 2006;12(8):972–977. doi: 10.1038/nm1371. [DOI] [PubMed] [Google Scholar]
- 113.Bjornson Z.B., Nolan G.P., Fantl W.J. Single cell mass cytometry for analysis of immune system functional states. Curr Opin Immunol. 2013;25(4):10. doi: 10.1016/j.coi.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bendall S.C., Nolan G.P., Roederer M., Chattopadhyay P.K. A deep profiler's guide to cytometry. Trends Immunol. 2012;33(7):323–332. doi: 10.1016/j.it.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Robins H.S., Campregher P.V., Srivastava S.K. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. 2009;114(19):4099–4107. doi: 10.1182/blood-2009-04-217604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Freeman J.D., Warren R.L., Webb J.R. Profiling the T-cell receptor beta-chain repertoire by massively parallel sequencing. Genome Res. 2009;19(10):1817–1824. doi: 10.1101/gr.092924.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Robins H. Immunosequencing: applications of immune repertoire deep sequencing. Curr Opin Immunol. 2013;25(5):646–652. doi: 10.1016/j.coi.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 118.Cha E., Klinger M., Hou Y. Improved survival with T cell clonotype stability after anti-CTLA-4 treatment in cancer patients. Sci Transl Med. 2014;6(238) doi: 10.1126/scitranslmed.3008211. (238ra70) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robert L., Harview C., Emerson R. Distinct immunological mechanisms of CTLA-4 and PD-1 blockade revealed by analyzing TCR usage in blood lymphocytes. Oncoimmunology. 2014;3 doi: 10.4161/onci.29244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Parra E.R. Novel platforms of multiplexed immunofluorescence for study of paraffin tumor tissues. J Cancer Treat Diagn. 2018;2(1):43–53. [Google Scholar]
- 121.Levin M., Kron S.J., Schwartz D. Rapid 5-marker multiplex phenotyping of breast cancer subtypes & tumor-infiltrating leukocytes “in situ” in FFPE sections. Cancer Res. 2016;76 [Google Scholar]
- 122.Mansfield J.R., Gossage K.W., Hoyt C.C. Autofluorescence removal, multiplexing, and automated analysis methods for in-vivo fluorescence imaging. J Biomed Opt. 2005;10(4):41207. doi: 10.1117/1.2032458. [DOI] [PubMed] [Google Scholar]
- 123.Sun R., Limkin E.J., Vakalopoulou M. A radiomics approach to assess tumour-infiltrating CD8 cells and response to anti-PD-1 or anti-PD-L1 immunotherapy: an imaging biomarker, retrospective multicohort study. Lancet Oncol. 2018 Sep;19(9):1180–1191. doi: 10.1016/S1470-2045(18)30413-3. [DOI] [PubMed] [Google Scholar]