Abstract
Wild germplasm with elevated antioxidants are a useful resource for using directly and in a breeding program. In a study with 136 wild clones and two cranberry cultivars, phenolic, flavonoid and anthocyanin contents varied 2.79, 2.70 and 17.46 times, respectively. The antioxidant activity ranged from 1.17 ± 0.01 to 2.53 ± 0.05 mg/g and varied 2.16 times. Seventy-five of wild clones and the cultivar Franklin were grouped into five distinct classes by molecular structure analysis using inter simple sequence repeat (ISSR), expressed sequence tag-simple sequence repeat (EST-SSR) and EST-polymerase chain reaction (PCR) markers. Grouping with DNA markers did not coincide with that of based on antioxidant properties. Present study indicates that genetic diversity analysis combined with antioxidant properties of wild germplasm play a significant role for conservation and in selecting diverse genotypes for future berry crop improvement.
Keyword: Plant biology
1. Introduction
Cranberries (Vaccinium macrocarpon Aiton; 2n = 2x = 24) are a low-growing, evergreen, perennial woody vine and a health promoting berry crop native to North America (Vander Kloet, 1988). They produce edible red fruits rich in vitamins (A, C, B1, B2, B6 and E), fiber, sugars, minerals and organic acids (Borges et al., 2010). The high phenolic compounds present in cranberries with strong antioxidant properties prevent a number of human diseases, including cancer and cardiovascular disease (Mckay and Blumberg, 2007; Deyhim et al., 2007; Neto et al., 2008; Kalgaonkar et al., 2010). They possess therapeutic activities such as anti-bacterial (Leitao et al., 2005), anti-carcinogenic (Sun and Hai Liu, 2006), anti-viral (Weiss et al., 2005), anti-mutagenic (Vattem et al., 2006), anti-angiogenic (Roy et al., 2002), anti-tumorigenic (Seeram et al., 2004) and prevention of cardiovascular disease (McKay and Blumberg, 2007), stomach and oral ulcers (Weiss et al., 2002, 2004) and of urinary tract infection (Howell, 2007). Clinical trials showed a prompt improvement of endothelial function and a quick decrease of urinary tract infection after cranberry juice consumption (Blumberg et al., 2013).
Cranberries are highly praised for producing nutritious, healthy and edible fruits that are consumed fresh (5%) or in processed forms including juices (60%) and sauces, dried fruit and ingredients (35%) (Zuo et al., 2002). The natural spreading of cranberries extents from the Canadian province Newfoundland and Labrador west through the Great Lakes region to western Ontario and Minnesota in Canada and south at higher elevations in the Appalachian Mountains to North Carolina and Tennessee of the United States (Vander Kloet, 1988). Although USA and Canada are two leading countries for most of commercial cranberry production, countries like Azerbaijan, Belarus, Bulgaria, Chile, Germany, Latvia, Romania, the Former Yugoslav Republic of Macedonia, Tunisia and Ukraine are also producing cranberries (FAO, 2018).
Genotype identification for high antioxidant properties and genetic variation are increasingly essential in cranberry for germplasm characterization, breeding purposes and proprietary-rights protection (Debnath et al., 2012). Plant germplasm carries genetic information for the plant's hereditary makeup. Knowledge on wild species is required for their best use in a breeding program as they may possess a whole set of desirable features required for production and improvement (Debnath, 2016). DNA-based markers including inter simple sequence repeat (ISSR), expressed sequence tag - simple sequence repeat (EST-SSR) and EST - polymerase chain reaction (PCR) markers allows direct comparison of different genetic materials independent of environmental influences (Weising et al., 1995). Although ISSR markers are dominant in nature (cannot identify heterozygotes), they are randomly distributed throughout the genome, cost less and do not require sequence data for primer construction (Reddy et al., 2002). EST-SSR and EST-PCR are highly reproducible co-dominant functional markers. ESTs are produced from single-pass sequencing of randomly picked cDNA clones (Adams et al., 1991). They are derived from transcripts and target specific genes (Varshney et al., 2005).
An increased demand for developing cranberry cultivars with high antioxidant activities step up the need for selecting superior wild-growing cranberries for cultivation in Canada. We developed a program to improve cranberry cultivars at St. John's Research and Development Centre of Agriculture and Agri-Food Canada in Newfoundland and Labrador (Debnath, 2007; An et al., 2015). The diverse wild clones and cultivars collected under this program and their assessment for antioxidant properties and genetic diversity using molecular markers will contribute significantly in berry crop germplasm management and improvement. Although reports on antioxidant properties are available with a limited number of cranberry genotypes (Abeywickrama et al., 2016; Viskelis et al., 2009; Lu et al., 2017; Oszmiański et al., 2017, 2018), none is with wild diverse germplasm. Reports on linkage between molecular markers and antioxidant properties of cranberries are lacking. Biochemical analysis for antioxidant properties along with structure and genetic relationship at molecular level in a wide wild cranberry germplasm has not been reported before.
The present study aimed to determine the phenolic compounds in 136 Canadian wild cranberry clones and two cultivars, and to evaluate their antioxidant capacity and biodiversity for antioxidant properties. Seventy-five wild clones and one cultivar were also analyzed at molecular level for structure analysis using three types of markers. Biochemical and molecular data were used to select promising cranberry clones for possible use in a berry improvement program.
2. Material and methods
2.1. Plant materials
One hundred and thirty six wild cranberry (Vaccinium macrocarpon Aiton) clones (Table 1) collected in 2001 from Newfoundland and Labrador (NL), Prince Edward Island (PE), Nova Scotia (NS) and New Brunswick (NB) in Canada and the cultivars ‘Franklin’ and ‘Bergman’ were maintained in a greenhouse under controlled condition at 20 ± 2 °C with a maximum PPF of 90 μmol m-2 s−1 and 85% RH for more than 14 years (Debnath, 2007; An et al., 2015). Each clone was an individual genotype and was selected based on good vigour with deep red large berries and good berry yield per plant, and apparently free from insects and diseases. The cultivars ‘Franklin’ and Bergman were derived from the crosses ‘Early Black’ × ‘Howes’ and ‘Early Black’ × ‘Searles’, respectively (Galletta and Ballington, 1996). The plants were grown in 6 L (25-cm in diameter and 18 cm deep) plastic pots (East-Chem Inc., Mount Pearl, NL, Canada) containing 2 peat: 1 perlite (vol/vol). Normal cultural practices were used and the winter dormancy requirements were fulfilled through maintaining the plants at or below 6 °C for at least three months (Debnath, 2007; An et al., 2015; Abeywickrama et al., 2016).
Table 1.
Wild cranberry clones collected from Newfoundland and Labrador (NL), Nova Scotia (NS), New Brunswick (NB) and Prince Edward Island (PE) in Canada.
| Clone | Province | Community | Latitude (N) | Longitude (W) |
|---|---|---|---|---|
| NL1 | NL | Cape Spear | 47°31′ | 52°37′ |
| NL2 | NL | Flatrock | 47°42′ | 52°42′ |
| NL3, 59, 87, 88, | NL | Logy Bay | 47°37′ | 52°40′ |
| NL4, 57 | NL | Portugal Cove | 47°37′ | 52°51′ |
| NL5 – 37, 60–86 | NL | Bell Island | 47°38′ | 52°56′ |
| NL38 | NL | Mobile | 47°14′ | 52°50′ |
| NL39 | NL | Biscay Bay | 46°44′ | 53°17′ |
| NL40 | NL | Ferryland | 47°02′ | 52°52′ |
| NL41 | NL | Frenchman's Cove | 46°54′ | 52°55′ |
| NL42 | NL | Freshwater | 47°50′ | 52°55′ |
| NL43 | NL | Peters River | 46°45′ | 53°36′ |
| NL44, 92, 93, 94 | NL | Port Kirwan | 46°58′ | 52°54′ |
| NL45 – 48 | NL | Soldiers Pond | 47°20′ | 53°04′ |
| NL49 – 51, 89 | NL | New Melbourne | 48°03′ | 53°09′ |
| NL52 | NL | St. Brides | 46°55′ | 54°10′ |
| NL53 | NL | Lamaline | 46°51′ | 55°48′ |
| NL54, 55, 95, 96 | NL | Lord's Cove | 46°52′ | 55°40′ |
| NL56 | NL | Searston | 47°50′ | 59°19′ |
| NL58 | NL | Blackhead | 47°31′ | 52°39′ |
| NL90, 91 | NL | Old Perlican | 48°05′ | 53°00′ |
| NL97 | NL | Corbin | 46°58′ | 55°14′ |
| PE1 – 4, 11, 12 | PE | Blooming Point | 46°23′ | 62°58′ |
| PE5 – 16 | PE | Harrington | 46°21′ | 63°10′ |
| NB1 – 4, 9–12 | NB | Clifton | 47°43′ | 65°22′ |
| NB5 – 19 | NB | Little Shemogue | 46°06′ | 64°01′ |
| NS1 – 4 | NS | Canso | 45°20′ | 60°59′ |
2.2. Preparation of fruit extracts
During 2015 growing season, ripe fruits were harvested from all genotypes at maturity based on external colour and size uniformity and stored at – 80 °C until analysis. The extracts were obtained according to the methods described by Goyali et al. (2015). Briefly, berries from each genotype was homogenized with 80% aqueous acetone containing 0.2% formic acid and following centrifugation at 13,000 rpm for 15 min at 4 °C (Allegra 64R; Beckman Coulter Inc., Palo Alto, CA, USA), the supernatant was diluted with same solvent to make the fruit extract of 25 mg/ml working concentration. The extract was used to determine total phenolic, flavonoid and anthocyanin contents and antioxidant activity. Analyses were carried out at least three times for each sample and mean values were used for analysis.
2.3. Total phenolic content (TPC)
Photometric method using Folin-Ciocalteu reagent (Singleton and Rossi, 1965) was used to determine TPC in the cranberry fruit extracts following Goyali et al. (2015). In brief, 100 μL of Folin-Ciocalteu reagent was mixed to 100 μL fruit. After 5 min, 200 μL 20% saturated (w/v) sodium carbonate was added to it followed by addition of 1.5 mL distilled water. The mixture was incubated in dark for 35 min at room temperature and centrifuged at 4000 × g for 10 min in Allegra 64R. The samples and standard (gallic acid) were analyzed with a UV spectrophotometer (Libra S32 PC, Biochrom Ltd. Cambridge, UK). The absorbance values were read after 3 min at 725 nm and the results were expressed as milligrams of gallic acid equivalents per gram of fresh fruit weight (mg GAE/g fw).
2.4. Total flavonoid content (TFC)
Total flavonoid content was determined using aluminium chloride colorimetric assay (Zhishen et al., 1999) following (Goyali et al., 2015). An aliquot (500 μL) of sample extract was added to 2mL distilled water and 150 μL of 5% (w/v) sodium nitrate to which 150 μL of 10% (w/v) aluminium chloride was added after 5 min. After 6 min of incubation at room temperature, 1 M sodium hydroxide solution (1 mL) was added to the mixture. The mixture was diluted with 1.2 mL distilled water and the absorbance was measured at 510 nm. Catechin solution (1 mg/mL) with ≥98% purity was used for standard curve calibration and the total flavonoid content was calculated as milligrams of catechin equivalents per gram of fresh fruit weight (mg CE/g fw) (Abeywickrama et al., 2016).
2.5. Total monomeric anthocyanin contents (TAC)
The total monomeric anthocyanin content on the extract was estimated by the pH-differential method of Lee et al. (2005) following Abeywickrama et al. (2016). Absorbance was read at 520 nm and 700 nm. The total TAC was expressed as milligrams of cyanidin-3-glucoside equivalent per gram of fresh fruit weight (mg C3GE/g fw) and was calculated using the following formula:
Where, A (Absorbance) = (Aλ520 – Aλ700) pH 1.0 – (Aλ520 – Aλ700) pH 4.5; MW = molecular weight of cyanidin-3-glucoside (C15 H11 O6, 449.2); DF = dilution factor; ε = molar extinction coefficient for cyanidin-3-glucoside (26,900); and W = sample weight.
2.6. Antioxidant activity
The radical scavenging activity of fruit extract for all genotypes was carried out using the method described by Hatano et al. (1988) with 2,2-diphenyl-1-picrylhydrazyl (DPPH), an artificial stabilized free radical, following Goyali et al. (2015). In brief, 1.7 ml of 60 μM DPPH solution was mixed with 100 μl of berry extract or gallic acid (standard solution) and after 20 min of incubation at room temperature in dark, absorbance was recorded at 517 nm. The DPPH scavenging activity was calculated as a percentage of inhibition of DPPH radicals using the following formula (Mishra et al., 2012):
Where, A is optical density of the blank and B is optical density of the fruit extract. Total antioxidant activity of sample was measured within the linear relationship of concentration vs. optical density decrease and presented as gallic acid equivalent (GAE) in milligram per gram of fresh fruit weight (fw).
2.7. DNA extraction, PCR amplification and electrophoresis
Genomic DNA was extracted from fresh young leaf tissues of 75 wild clones (NL 1-56, PE 1-10, NB 1- 8, NS1) and the cultivar Franklin using the Wizard® Genomic DNA Purification Kit (Promega, Madison, WI) with manufacturer's instruction following An et al. (2015) and quantified with a Ultrospec 2000 Spectrophotometer (Pharmacia Biotech, Cambridge, UK). Twelve ISSR (UBC 801, 808–810, 816, 817, 826, 827, 835, 867, 890, and 891), 10 EST-PCR (CA21, 54, 227, 231, 1029, 1423, and 1590; NA27, 353, and 1068) and five EST-SSR primers (CA421 and 794; NA800, 961, and 1040) that worked well with cranberries (An et al., 2015) were used. While EST primers were purchased from Integrated DNA Technologies, Inc., Coralville, IA, USA; the ISSR markers were obtained from the University of British Columbia Biotechnology Laboratory, Vancouver, BC, Canada. PCR amplification and electrophoresis were done following An et al. (2015). Briefly, for all marker types, the 25 mL reaction mixture contained 10 ng DNA template, 200 mM of each dNTP, PCR buffer [50 mM KCl, 1.5 mM MgCl2, 10 mM TrisHCl pH 8.3, and 0.001% (wt/vol) gelatin], 0.05 U Taq DNA polymerase, 25 pmol of each primer/primer pair, and PCR grade distilled water (Sigma Chemical Co., Oakville, ON, Canada). The DNA amplification was performed in a PTC-100® Programmable Thermal Controller (MJ Research, Watertown, MA, USA). The PCR cycle consisted of an initial denaturation at 94 °C for 10 min, followed by 45 cycles at 94 °C for 1 min (denaturation), 1 min at annealing temperatures of each primer, 2 min at 72 °C (elongation) and a final extension at 72 °C for 10 min before holding the sample at 4 °C for analysis.
2.8. Statistical analysis
Data for phenolic, flavonoid, and monomeric anthocyanin contents and antioxidant activity are presented as mean values ±standard deviation (SD) of at least three independent determinations. The results were statistically evaluated by variance analysis (ANOVA) using the SAS statistical software package (version 9.4; SAS Institute, Cary, NC) and Tukey's Test was used to compare treatment means at a critical difference (P) of ≤0.05.
Biodiversity and relationship among genotypes for biochemical data were studied by cluster analysis using the Numerical Taxonomy and Multivariate Analysis System (NTSYS)-pc software (version 2.1, Exeter Software, Setauket, New York) (Rohlf, 1998). To compute a similarity distance matrix, data were transformed with the STAND procedure where mean values were subtracted from the original values followed by division with SD. Standardized values were used in the NTSYS-pc SIMINT procedure to get a matrix of distances among all genotype pairs with the average taxonomic distance. The Unweighted Pair-Group Mean Average (UPGMA) (Sokal and Michener, 1958), estimated by DIST, was used for grouping the genotypes by the clustering method and a dendrogram was constructed from the Sequential, Agglomerative, Hierarchical, and Nested (SAHN) clustering method in UPGMA (Rohlf, 1998).
2.9. Structured diversity with molecular markers
Band scoring was done for the presence or absence of each locus and all bands from one primer pair were treated as alleles and one locus (An et al., 2015). Cluster numbers (K) were identified using the Bayesian approach for the model-based program with STRUCTURE version 2.3.4 (Pritchard et al., 2000). It was assumed that each K population followed Hardy–Weinberg equilibrium and all loci were independent. The runs were finished with 100,000 burns in iterations and 100,000 subsequent Monte Carlo Markov Chain (MCMC) runs with K, ranging from 1 to 10. The consistency of results was confirmed by running ten replicates for each assumed K value. A website based program, STRUCTURE HARVESTER (http://taylor0.biology.ucla.edu/structureHarvester/#) was used to evaluate the results (Earl and Vonholdt, 2012). The optimal value for K was identified by L(K) (Pritchard et al., 2000) and delta K (DK) (Evanno et al., 2005) methods. In the former case, K value is considered true when posterior possibilities of K is advancing to plateaus (or continues rising slightly) and has high difference between runs (Rosenberg et al., 2001). In later case, data on the second order rate of change of the likelihood, DK had a clear peak in the graph at true K value (Evanno et al., 2005).
3. Results and discussion
3.1. Total phenolic content (TPC)
TPC using Folin–Ciocalteu reagent is well accepted but the reagent reacts with other reducing compounds and thus it can be used conditionally (Kraujalytė et al., 2015). Relevant results are presented in Tables 2 and 3. TPC data were expressed as mg GAE/g fw. Highly significant variation was noticed for TPC among the genotypes (p < 0.05), it varied 2.79 times among the clones collected from four provinces and the two cultivars (Table 2). Among all genotypes, the highest TPC values were found for NS1 (9.15 ± 0.29 mg) followed by NB2, NB1 and NL69 (8.94 ± 0.26, 8.64 ± 0.28 and 8.53 ± 0.29 mg, respectively) whereas the value for NL95 was the lowest (3.28 ± 0.09 mg) and it was followed by PE13 (3.90 ± 0.29 mg) (Table 3). The four clones collected from NS had higher average TPC followed by two cultivars (Table 2). Among four provinces, variation for TPC was the highest among NL clones (2.60-fold difference between the highest and the lowest values) and it was followed by the clones collected from NB (2.07 times), PE (1.76 times) and NS (1.45 times). In NL clones, TPC values ranged from 3.28 ± 0.09 mg for NL95 to 8.53 ± 0.29 mg for NL69 and it was followed by NL 46 (8.36 ± 0.03 mg), NL92 (8.30 ± 0.09 mg) and NL81 (8.23 ± 0.03 mg). Among the PE clones, lowest TPC was observed in the berries of PE13 (3.90 ± 0.29 mg) while PE11 had the highest TPC (6.86 ± 0.20 mg) and it was followed by PE10 (6.44 ± 0.05 mg), PE16 (6.44 ± 0.10 mg) and PE4 (6.36 ± 0.23 mg). The TPC for 19 NB wild collections varied from 4.31 ± 0.17 mg for NB8 to 8.94 ± 0.26 mg for NB2 which was followed by NB1 (8.64 ± 0.28 mg). In NS clones, maximum TPC was observed in NS1 and it was followed by NS4 (8.35 ± 0.12 mg), NS3 (7.86 ± 0.08 mg) and NS2 (6.29 ± 0.36 mg). The cultivar Franklin was superior to Bergman for TPC (8.08 ± 0.04 mg and 5.67 ± 0.14 mg, respectively). It is interesting noting that the TPC values of nine wild clones namely NL11 (8.85 ± 0.08 mg), NL46 (8.36 ± 0.03 mg), NL69 (8.53 ± 0.29 mg), NL81 (8.23 ± 0.03 mg), NL92 (8.30 ± 0.09 mg), NB1 (8.64 ± 0.28 mg), NB2 (8.94 ± 0.26 mg), NS1 (9.15 ± 0.29 mg) and NS4 (8.35 ± 0.12 mg) were higher than those of the two cultivars (Table 3). These wild clones are valuable resources for using in improving TPC in a cranberry breeding program. These results are in comparable with those reported in blackberry (8.50 mg/g fw), red raspberry (3.57 mg/g fw), strawberry (6.21 mg/g fw), blueberry (3.05 mg/g fw) and cherry (3.14 mg/g fw) (de Souza et al., 2014). TPC reported previously in four cranberry cultivars (1.84–2.21 mg/g fw) was less than our results that might be due to differences for genotypes and growing condition (Viskelis et al., 2009). Working with four wild clones and a cultivar Pilgrim, Abeywickrama et al. (2016) reported higher amounts of phenolics in Pilgrim than the wild clones. Variation for TPC was also noticed among six cranberry cultivars where the cultivars Franklin and Howes exhibited 1.6 and 1.5 times, respectively more TPC than the cultivar Red Star (Oszmiański et al., 2017). It has been reported that the presence of carbohydrates and/or amino acids in the crude extracts could interfere with TPC estimation when the Folin−Ciocalteu assay is followed (Zielinski and Kozlowska, 2000) although this method is well accepted and has been used by various authors for TPC determination in berry crops (Goyali et al., 2015; Kraujalytė et al., 2015; Abeywickrama et al., 2016). Current study indicates that total phenolic contents in cranberry vary widely among wild clones and cultivars although a number of other factors including pre- and post-harvest practices, environmental conditions and maturity stage at harvest have significant role in the accumulation of total phenolics (Manganaris et al., 2014).
Table 2.
Total phenolic (TPC), flavonoid (TFC) and anthocyanin contents (TAC) and DPPH scavenging activity of groups of wild cranberry clones collected from four Canadian provinces: Newfoundland and Labrador (NL), Nova Scotia (NS), New Brunswick (NB) and Prince Edward Island (PE), and cultivars (CV) Franklin and Bergman. GAE = gallic acid equivalents, CE = catechin equivalents, C3GE = cyanidin-3-glucoside equivalents, fw = fresh fruit weight.
| Wild Clones (clone/cultivar no.) | TPC (mg GAE/g fw) | TFC (mg CE/g fw) | TAC (mg C3GE/g fw) | DPPH scavenging activity (mg GAE/g fw) | |
|---|---|---|---|---|---|
| NL (97) | Mean ± SD | 6.03 ± 0.98 | 4.77 ± 0.94 | 1.00 ± 0.27 | 1.78 ± 0.17 |
| Min - Max | 3.28–8.53 | 2.78–7.51 | 0.13–1.72 | 1.17–2.17 | |
| PE (16) | Mean ± SD | 5.32 ± 0.88 | 4.16 ± 0.70 | 0.97 ± 0.21 | 1.66 ± 0.22 |
| Min - Max | 3.90–6.86 | 3.14–5.56 | 0.54–1.34 | 1.24–2.08 | |
| NB (19) | Mean ± SD | 6.41 ± 1.26 | 4.82 ± 1.14 | 1.15 ± 0.42 | 1.77 ± 0.18 |
| Min - Max | 4.31–8.94 | 3.31–7.27 | 0.53–1.92 | 1.43–2.10 | |
| NS (4) | Mean ± SD | 7.91 ± 1.21 | 6.00 ± 1.29 | 1.67 ± 0.29 | 2.16 ± 0.30 |
| Min - Max | 6.29–9.15 | 4.24–7.13 | 1.35–2.01 | 1.79–2.53 | |
| CV(2) | Mean ± SD | 6.87 ± 1.70 | 4.94 ± 1.24 | 1.46 ± 1.14 | 1.95 ± 0.45 |
| Min - Max | 5.67–8.08 | 4.06–5.81 | 0.65–2.27 | 1.63–2.27 | |
| All (138) | Mean ± SD | 6.07 ± 1.09 | 4.75 ± 0.98 | 1.04 ± 0.33 | 1.95 ± 0.20 |
| Min - Max | 3.28–9.15 | 2.78–7.51 | 0.13–2.27 | 1.17–2.53 | |
Values are means ± SD values of at least three replicates.
Table 3.
Total phenolic (TPC), flavonoid (TFC) and anthocyanin contents (TAC) and DPPH scavenging activity of individual wild cranberry clones collected from Canadian provinces: Newfoundland and Labrador (NL), Nova Scotia (NS), New Brunswick (NB) and Prince Edward Island (PE), and two cultivars, Franklin (FRA) and Bergman (BRG). GAE = gallic acid equivalents, CE = catechin equivalents, C3GE = cyanidin-3-glucoside equivalents, fw = fresh fruit weight.
| Clone/cultivar | TPC (mg GAE/g fw) | TFC (mg CE/g fw | TAC (mg C3GE/g fw) | DPPH scavenging activity (mg GAE/g fw) |
|---|---|---|---|---|
| NL1 | 7.63 ± 1.91 | 5.96 ± 1.47 | 0.73 ± 0.09 | 1.95 ± 0.01 |
| NL2 | 5.23 ± 0.30 | 3.86 ± 0.05 | 1.18 ± 0.04 | 1.57 ± 0.05 |
| NL3 | 5.29 ± 0.14 | 4.01 ± 0.03 | 0.76 ± 0.01 | 1.61 ± 0.01 |
| NL4 | 5.59 ± 0.35 | 3.97 ± 0.32 | 0.98 ± 0.08 | 1.60 ± 0.05 |
| NL5 | 6.72 ± 1.77 | 5.09 ± 1.00 | 1.02 ± 0.11 | 1.74 ± 0.18 |
| NL6 | 6.61 ± 0.34 | 5.52 ± 0.57 | 0.76 ± 0.14 | 1.83 ± 0.14 |
| NL7 | 5.78 ± 0.26 | 4.74 ± 0.54 | 0.81 ± 0.26 | 1.81 ± 0.14 |
| NL8 | 4.34 ± 0.11 | 3.36 ± 0.01 | 0.92 ± 0.03 | 1.52 ± 0.02 |
| NL9 | 4.27 ± 0.43 | 3.57 ± 0.35 | 0.70 ± 0.13 | 1.50 ± 0.08 |
| NL10 | 5.61 ± 0.31 | 4.21 ± 0.06 | 1.30 ± 0.03 | 1.72 ± 0.01 |
| NL11 | 8.85 ± 0.08 | 6.86 ± 0.08 | 1.49 ± 0.02 | 2.06 ± 0.03 |
| NL12 | 6.13 ± 0.47 | 5.08 ± 0.04 | 1.00 ± 0.01 | 1.92 ± 0.01 |
| NL13 | 7.62 ± 0.30 | 4.86 ± 0.01 | 1.03 ± 0.04 | 1.66 ± 0.02 |
| NL14 | 6.20 ± 0.02 | 5.46 ± 0.05 | 0.98 ± 0.01 | 1.80 ± 0.01 |
| NL15 | 5.73 ± 0.52 | 4.68 ± 0.29 | 0.90 ± 0.11 | 1.78 ± 0.15 |
| NL16 | 6.36 ± 0.26 | 4.46 ± 0.04 | 0.53 ± 0.03 | 1.58 ± 0.01 |
| NL17 | 5.79 ± 0.08 | 4.78 ± 0.08 | 0.95 ± 0.04 | 1.78 ± 0.02 |
| NL18 | 5.34 ± 0.30 | 3.94 ± 0.20 | 1.11 ± 0.14 | 1.76 ± 0.05 |
| NL19 | 7.04 ± 0.20 | 5.87 ± 0.01 | 0.77 ± 0.03 | 1.90 ± 0.02 |
| NL20 | 6.22 ± 1.49 | 5.01 ± 1.20 | 1.18 ± 0.24 | 1.75 ± 0.24 |
| NL21 | 7.27 ± 0.23 | 6.57 ± 0.26 | 0.81 ± 0.16 | 1.94 ± 0.09 |
| NL22 | 4.67 ± 0.27 | 3.42 ± 0.04 | 0.96 ± 0.03 | 1.68 ± 0.01 |
| NL23 | 6.76 ± 0.68 | 5.52 ± 0.69 | 1.16 ± 0.08 | 1.86 ± 0.15 |
| NL24 | 4.71 ± 0.04 | 3.42 ± 0.01 | 1.18 ± 0.01 | 1.40 ± 0.01 |
| NL25 | 7.93 ± 0.11 | 6.66 ± 0.04 | 1.48 ± 0.01 | 2.14 ± 0.02 |
| NL26 | 5.55 ± 0.20 | 4.66 ± 0.04 | 0.87 ± 0.02 | 1.80 ± 0.00 |
| NL27 | 5.45 ± 1.02 | 4.40 ± 0.88 | 1.22 ± 0.18 | 1.66 ± 0.25 |
| NL28 | 6.77 ± 1.13 | 5.39 ± 1.39 | 1.15 ± 0.30 | 1.90 ± 0.08 |
| NL29 | 5.73 ± 0.15 | 4.44 ± 0.02 | 1.18 ± 0.02 | 1.64 ± 0.01 |
| NL30 | 6.38 ± 0.05 | 5.67 ± 0.12 | 1.15 ± 0.03 | 1.83 ± 0.01 |
| NL31 | 6.03 ± 0.28 | 5.16 ± 0.02 | 0.99 ± 0.01 | 1.75 ± 0.04 |
| NL32 | 5.53 ± 0.03 | 4.37 ± 0.02 | 0.98 ± 0.03 | 1.85 ± 0.03 |
| NL33 | 7.51 ± 0.50 | 6.30 ± 0.20 | 1.20 ± 0.08 | 1.98 ± 0.12 |
| NL34 | 6.27 ± 0.37 | 5.47 ± 0.14 | 1.25 ± 0.02 | 1.84 ± 0.01 |
| NL35 | 4.94 ± 0.03 | 4.05 ± 0.01 | 0.45 ± 0.01 | 1.57 ± 0.01 |
| NL36 | 5.26 ± 0.09 | 4.22 ± 0.06 | 0.90 ± 0.02 | 1.82 ± 0.00 |
| NL37 | 6.27 ± 0.41 | 4.85 ± 0.70 | 1.20 ± 0.13 | 1.81 ± 0.04 |
| NL38 | 4.74 ± 0.83 | 3.94 ± 0.99 | 0.92 ± 0.08 | 1.57 ± 0.15 |
| NL39 | 6.89 ± 1.28 | 5.34 ± 0.94 | 1.27 ± 0.29 | 1.91 ± 0.21 |
| NL40 | 6.12 ± 0.32 | 4.24 ± 0.01 | 0.87 ± 0.03 | 1.77 ± 0.02 |
| NL41 | 6.45 ± 0.23 | 4.50 ± 0.02 | 1.49 ± 0.00 | 1.94 ± 0.01 |
| NL42 | 5.61 ± 0.20 | 4.24 ± 0.01 | 1.09 ± 0.01 | 1.71 ± 0.01 |
| NL43 | 6.30 ± 0.15 | 4.69 ± 0.04 | 1.22 ± 0.02 | 2.06 ± 0.05 |
| NL44 | 6.06 ± 0.43 | 4.78 ± 0.05 | 1.13 ± 0.01 | 1.90 ± 0.00 |
| NL45 | 6.26 ± 0.96 | 4.73 ± 0.27 | 1.20 ± 0.04 | 1.94 ± 0.11 |
| NL46 | 8.36 ± 0.03 | 7.02 ± 0.04 | 1.28 ± 0.01 | 2.08 ± 0.02 |
| NL47 | 7.45 ± 0.25 | 6.64 ± 0.06 | 0.98 ± 0.13 | 2.03 ± 0.02 |
| NL48 | 5.92 ± 0.61 | 4.46 ± 0.18 | 1.09 ± 0.02 | 1.73 ± 0.04 |
| NL49 | 5.95 ± 0.65 | 4.84 ± 0.87 | 1.14 ± 0.07 | 1.90 ± 0.20 |
| NL50 | 6.20 ± 0.24 | 5.04 ± 0.01 | 0.67 ± 0.01 | 1.62 ± 0.03 |
| NL51 | 4.71 ± 0.08 | 2.78 ± 0.02 | 0.80 ± 0.01 | 1.42 ± 0.02 |
| NL52 | 6.35 ± 0.20 | 4.51 ± 0.24 | 0.87 ± 0.01 | 1.83 ± 0.04 |
| NL53 | 5.84 ± 2.04 | 4.41 ± 1.15 | 0.66 ± 0.42 | 1.72 ± 0.36 |
| NL54 | 6.73 ± 1.24 | 3.17 ± 0.87 | 1.31 ± 0.09 | 1.87 ± 0.06 |
| NL55 | 7.03 ± 1.13 | 5.50 ± 0.71 | 1.14 ± 0.10 | 1.95 ± 0.16 |
| NL56 | 4.33 ± 0.06 | 3.15 ± 0.03 | 0.77 ± 0.01 | 1.41 ± 0.02 |
| NL57 | 6.14 ± 0.06 | 4.87 ± 0.05 | 1.09 ± 0.01 | 1.86 ± 0.01 |
| NL58 | 5.18 ± 0.14 | 3.74 ± 0.03 | 0.64 ± 0.01 | 1.73 ± 0.00 |
| NL59 | 5.92 ± 0.71 | 4.66 ± 0.81 | 0.61 ± 0.33 | 1.65 ± 0.11 |
| NL60 | 6.56 ± 0.08 | 4.76 ± 0.03 | 1.25 ± 0.01 | 1.95 ± 0.03 |
| NL61 | 6.04 ± 0.09 | 4.96 ± 0.16 | 1.04 ± 0.04 | 1.80 ± 0.00 |
| NL62 | 7.14 ± 0.10 | 6.26 ± 0.08 | 0.99 ± 0.03 | 1.90 ± 0.00 |
| NL63 | 5.47 ± 0.19 | 3.94 ± 0.01 | 0.51 ± 0.29 | 1.60 ± 0.02 |
| NL64 | 4.68 ± 0.20 | 3.32 ± 0.03 | 1.20 ± 0.00 | 1.68 ± 0.02 |
| NL65 | 6.24 ± 0.36 | 4.55 ± 0.03 | 1.29 ± 0.01 | 1.69 ± 0.02 |
| NL66 | 5.55 ± 0.72 | 4.32 ± 0.60 | 0.95 ± 0.12 | 1.69 ± 0.02 |
| NL67 | 5.73 ± 0.24 | 5.05 ± 0.12 | 1.12 ± 0.01 | 1.77 ± 0.01 |
| NL68 | 6.47 ± 0.26 | 5.77 ± 0.04 | 1.00 ± 0.01 | 1.90 ± 0.04 |
| NL69 | 8.53 ± 0.29 | 6.27 ± 0.09 | 1.38 ± 0.01 | 2.06 ± 0.02 |
| NL70 | 5.26 ± 0.04 | 4.72 ± 0.04 | 0.63 ± 0.01 | 1.92 ± 0.03 |
| NL71 | 5.94 ± 0.50 | 4.49 ± 0.04 | 0.95 ± 0.06 | 1.91 ± 0.06 |
| NL72 | 5.82 ± 1.23 | 4.59 ± 1.35 | 1.02 ± 0.30 | 1.88 ± 0.35 |
| NL73 | 5.20 ± 0.11 | 3.47 ± 0.04 | 0.98 ± 0.00 | 1.71 ± 0.17 |
| NL74 | 6.26 ± 1.76 | 5.05 ± 1.51 | 1.11 ± 0.11 | 1.90 ± 0.39 |
| NL75 | 5.83 ± 0.39 | 4.43 ± 0.06 | 1.11 ± 0.18 | 1.81 ± 0.24 |
| NL76 | 4.55 ± 0.30 | 3.55 ± 0.03 | 0.68 ± 0.02 | 1.61 ± 0.02 |
| NL77 | 4.49 ± 0.10 | 3.56 ± 0.01 | 0.13 ± 0.01 | 1.33 ± 0.02 |
| NL78 | 5.43 ± 0.31 | 3.96 ± 0.01 | 0.84 ± 0.01 | 1.68 ± 0.05 |
| NL79 | 5.94 ± 1.16 | 4.54 ± 1.26 | 1.30 ± 0.26 | 1.80 ± 0.18 |
| NL80 | 5.71 ± 0.89 | 4.46 ± 0.38 | 0.51 ± 0.07 | 1.95 ± 0.15 |
| NL81 | 8.23 ± 0.03 | 6.21 ± 0.06 | 1.25 ± 0.05 | 2.02 ± 0.01 |
| NL82 | 5.65 ± 0.20 | 4.94 ± 0.01 | 0.94 ± 0.01 | 1.79 ± 0.02 |
| NL83 | 6.00 ± 0.16 | 5.27 ± 0.07 | 0.74 ± 0.12 | 1.78 ± 0.03 |
| NL84 | 5.43 ± 0.06 | 4.37 ± 0.17 | 0.91 ± 0.02 | 1.75 ± 0.06 |
| NL85 | 6.06 ± 0.11 | 5.12 ± 0.33 | 0.94 ± 0.01 | 1.85 ± 0.03 |
| NL86 | 5.63 ± 0.19 | 4.96 ± 0.02 | 0.99 ± 0.02 | 1.69 ± 0.01 |
| NL87 | 6.08 ± 0.17 | 4.85 ± 0.15 | 0.88 ± 0.19 | 1.84 ± 0.06 |
| NL88 | 6.14 ± 0.52 | 5.03 ± 0.25 | 0.64 ± 0.13 | 1.79 ± 0.03 |
| NL89 | 6.71 ± 0.19 | 5.17 ± 0.34 | 1.35 ± 0.03 | 1.93 ± 0.08 |
| NL90 | 5.12 ± 0.08 | 3.61 ± 0.02 | 1.18 ± 0.08 | 1.72 ± 0.02 |
| NL91 | 5.50 ± 0.31 | 3.93 ± 0.04 | 1.07 ± 0.00 | 1.53 ± 0.01 |
| NL92 | 8.30 ± 0.09 | 7.51 ± 0.09 | 1.32 ± 0.02 | 2.17 ± 0.02 |
| NL93 | 7.80 ± 0.00 | 6.62 ± 0.02 | 1.72 ± 0.54 | 1.96 ± 0.01 |
| NL94 | 6.34 ± 0.59 | 4.82 ± 0.23 | 1.61 ± 0.03 | 1.99 ± 0.06 |
| NL95 | 3.28 ± 0.09 | 6.60 ± 0.14 | 0.46 ± 0.01 | 1.17 ± 0.01 |
| NL96 | 5.20 ± 0.02 | 4.16 ± 0.16 | 0.79 ± 0.01 | 1.54 ± 0.02 |
| NL97 | 6.00 ± 0.40 | 4.40 ± 0.32 | 1.09 ± 0.06 | 1.91 ± 0.04 |
| PE1 | 4.52 ± 0.21 | 3.53 ± 0.01 | 0.77 ± 0.02 | 1.59 ± 0.00 |
| PE2 | 4.75 ± 0.31 | 4.16 ± 0.01 | 0.80 ± 0.02 | 1.53 ± 0.01 |
| PE3 | 5.78 ± 0.05 | 4.62 ± 0.09 | 1.10 ± 0.00 | 1.77 ± 0.03 |
| PE4 | 6.36 ± 0.23 | 5.04 ± 0.16 | 0.88 ± 0.01 | 1.75 ± 0.03 |
| PE5 | 5.39 ± 0.53 | 4.14 ± 0.54 | 1.08 ± 0.10 | 1.71 ± 0.12 |
| PE6 | 4.93 ± 0.19 | 3.61 ± 0.07 | 1.11 ± 0.01 | 1.68 ± 0.03 |
| PE7 | 4.63 ± 0.19 | 3.14 ± 0.03 | 0.94 ± 0.01 | 1.44 ± 0.01 |
| PE8 | 5.71 ± 0.33 | 4.07 ± 0.04 | 1.22 ± 0.01 | 1.67 ± 0.03 |
| PE9 | 5.30 ± 0.07 | 3.83 ± 0.01 | 1.01 ± 0.02 | 1.72 ± 0.02 |
| PE10 | 6.44 ± 0.05 | 4.69 ± 0.02 | 1.19 ± 0.03 | 2.08 ± 0.06 |
| PE11 | 6.86 ± 0.20 | 5.56 ± 0.01 | 0.98 ± 0.00 | 1.94 ± 0.00 |
| PE12 | 5.29 ± 0.19 | 4.23 ± 0.09 | 0.93 ± 0.01 | 1.79 ± 0.01 |
| PE13 | 3.90 ± 0.29 | 3.25 ± 0.05 | 0.54 ± 0.00 | 1.24 ± 0.02 |
| PE14 | 4.31 ± 0.28 | 3.29 ± 0.02 | 0.75 ± 0.01 | 1.37 ± 0.01 |
| PE15 | 4.51 ± 0.05 | 4.57 ± 0.08 | 0.84 ± 0.01 | 1.44 ± 0.02 |
| PE16 | 6.44 ± 0.10 | 4.80 ± 0.30 | 1.34 ± 0.17 | 1.86 ± 0.02 |
| NB1 | 8.64 ± 0.28 | 5.57 ± 0.05 | 1.04 ± 0.06 | 1.83 ± 0.01 |
| NB2 | 8.94 ± 0.26 | 7.27 ± 0.02 | 1.61 ± 0.01 | 2.10 ± 0.01 |
| NB3 | 7.36 ± 0.44 | 6.30 ± 0.71 | 1.34 ± 0.09 | 1.93 ± 0.05 |
| NB4 | 7.20 ± 0.06 | 4.99 ± 0.07 | 0.99 ± 0.01 | 1.89 ± 0.00 |
| NB5 | 5.40 ± 0.13 | 3.76 ± 0.20 | 0.80 ± 0.13 | 1.57 ± 0.07 |
| NB6 | 6.21 ± 0.17 | 4.41 ± 0.02 | 1.92 ± 0.01 | 1.78 ± 0.01 |
| NB7 | 5.14 ± 0.20 | 3.60 ± 0.08 | 0.87 ± 0.03 | 1.62 ± 0.01 |
| NB8 | 4.31 ± 0.17 | 3.31 ± 0.04 | 0.53 ± 0.15 | 1.65 ± 0.02 |
| NB9 | 7.25 ± 2.15 | 6.32 ± 2.55 | 0.92 ± .037 | 1.81 ± 0.16 |
| NB10 | 6.52 ± 0.02 | 5.04 ± 0.01 | 1.11 ± 0.05 | 1.76 ± 0.03 |
| NB11 | 6.64 ± 0.19 | 5.74 ± 0.01 | 1.31 ± 0.03 | 1.91 ± 0.01 |
| NB12 | 5.05 ± 0.25 | 3.47 ± 0.03 | 0.71 ± 0.01 | 1.43 ± 0.01 |
| NB13 | 5.17 ± 0.02 | 3.83 ± 0.01 | 0.98 ± 0.00 | 1.63 ± 0.01 |
| NB14 | 6.80 ± 0.23 | 4.67 ± 0.02 | 1.07 ± 0.00 | 1.82 ± 0.02 |
| NB15 | 7.81 ± 0.18 | 6.13 ± 0.15 | 1.90 ± 0.04 | 2.10 ± 0.02 |
| NB16 | 5.42 ± 0.11 | 3.72 ± 0.09 | 1.23 ± 0.01 | 1.63 ± 0.01 |
| NB17 | 5.30 ± 0.23 | 3.96 ± 0.02 | 0.61 ± 0.01 | 1.54 ± 0.03 |
| NB18 | 5.93 ± 0.20 | 4.63 ± 0.02 | 1.16 ± 0.06 | 1.80 ± 0.03 |
| NB19 | 6.79 ± 1.04 | 4.83 ± 0.69 | 1.84 ± 0.23 | 1.90 ± 0.12 |
| NS1 | 9.15 ± 0.29 | 7.13 ± 0.02 | 1.35 ± 0.03 | 2.17 ± 0.00 |
| NS2 | 6.29 ± 0.36 | 4.24 ± 0.02 | 1.52 ± 0.03 | 1.79 ± 0.02 |
| NS3 | 7.86 ± 0.08 | 5.87 ± 0.07 | 1.79 ± 0.02 | 2.16 ± 0.01 |
| NS4 | 8.35 ± 0.12 | 6.75 ± 0.02 | 2.01 ± 0.02 | 2.53 ± 0.05 |
| FRA | 8.08 ± 0.04 | 5.81 ± 0.04 | 2.27 ± 0.02 | 2.27 ± 0.02 |
| BRG | 5.67 ± 0.14 | 4.06 ± 0.06 | 0.65 ± 0.02 | 1.63 ± 0.01 |
Values are means ± SD values of at least three replicates.
3.2. Total flavonoid content (TFC)
Flavonoids occur abundantly in Vaccinium species and are important constituents in human diet. They display a range of biological activities in vivo and vitro (Lee and Kim, 2010). TFC results were expressed as mg CE/g fw. The wild clones and cultivars showed wide variation among themselves for TFC (p < 0.05) ranging from 2.78 ± 0.02 to 7.51 ± 0.09 mg for the clones NL51 and NL92, respectively (Table 3). Highest variation was observed among the NL clones (2.70 times) followed by NB (2.20 times), PE (1.77 times) and NS clones (1.68 times). The TFC in NL clones was highest in NL92 followed by NL46 (7.02 ± 0.04 mg). While in PE clones, TFC varied from 3.14 ± 0.03 mg to 5.56 ± 0.01 mg for PE7 and PE11, respectively; these values for NB clones were 3.31 ± 0.04 mg for NB8 to 7.27 ± 0.02 mg for NB2. The TFC of NS wild collections ranged from 4.24 ± 0.02 mg (NS2) to 7.13 ± 0.02 mg (NS1). Franklin possessed more TFC than Bergman 5.81 ± 0.04 and 4.06 ± 0.06 mg, respectively). While three of the four NS clones (NS1, 3 and 4) had significantly more TFC than Franklin, Bergman had lower TFC than all four NS clones. Among the clones from other three provinces, the number of clones with higher TFC than those of both cultivars were 14 in NL (NL1, 11, 19, 21, 25, 33, 46, 47, 62, 69, 81, 92, 93 and 95) and one in NB (NB15). None of the PE clones had higher TFC than both cultivars (Table 3). The TFC values of the present study were more than those reported by de Souza et al. (2014) in red raspberry (0.10 mg CE/g), sweet cherry (0.60 mg CE/g), blueberry (0.48 CE/g), strawberry (0.38 mg CE/g) and blackberry (0.87 mg CE/g. Comparable variation (2.56 times; ranging from 0.78 – 2.0 mg CE/g dry weight) was noticed by Abeywickrama et al. (2016) in five cranberry genotypes. As in TPC, the possible variation in TFC obtained in different studies might be duo to a number of factors including genotype, extraction procedure, growing and storage conditions and maturity stage at harvest (Viskelis et al., 2009; Manganaris et al., 2014).
3.3. Total monomeric anthocyanin content (TAC)
Anthocyanins which belong to the phenolic compounds are the main natural color pigments of berry crops and are, in general, present in coloured fruits. The red colour of cranberries is because of the presence of anthocyanins. Anthocyanins are also well known for their health-promoting properties with strong antioxidant activity. Their concentrations tend to increase when the berries ripen in response to environmental factors including light and temperature (Brouillard et al., 2003; Oszmiański et al., 2018). TAC, expressed as mg C3GE/g fw, for all cranberry genotypes differed significantly (p < 0.05). Diverse genotypes with 17.46 times variation ranging from 0.13 ± 0.01 mg g-1 fw (NL77) to 2.27 ± 0.04 mg (Franklin) TAC were observed in the present study (Table 3). The highest TAC containing genotype Franklin was followed by wild clone NS4 (2.01 ± 0.02 mg). The mean TAC value across all wild collections was highest in the berries of NS clones followed by the clones collected from NB, NL and PE (Table 2). The NL clones had the highest variation (13.23 times) ranging from 0.13 ± 0.01 mg (NL77) to 1.72 ± 0.54 mg (NL93) and it was followed by NB clones (3.62 times) ranging from 0.53 ± 0.15 (NB8) to 1.92 ± 0.01 mg (NB6). Variation among NS clones were the least (1.49 times) with a mean value of 1.67 ± 0.29 mg followed by PE clones (2.48 times) with an average of 0.97 ± 0.21 mg. Among PE clones, PE16 had the highest TAC (1.34 ± 0.17 mg/g fw) while PE13 had lowest TAC (0.54 ± 0.00 mg/g fw). The TAC in NS clones was highest in NS4 (2.01 ± 0.02 mg) and it was followed by NS3 (1.79 ± 0.02 mg), NS2 (1.52 ± 0.03 mg) and NS1 (1.35 ± 0.03 mg). Cultivar Franklin possessed 1.43 times higher TAC than Bergman (Table 3). Current results are more than those reported in blackberry (0.59 mg/g fw), blueberry (0.29 mg/g fw), cherry (0.27 mg/g fw), red raspberry (0.15 mg/g fw), and strawberry (0.16 mg/g fw; de Souza et al., 2014); and in ripe fruits of four cranberry cultivars (0.43–1.18 mg/g fw) (Viskelis et al., 2009). Viskelis et al. (2009) also reported that the total anthocyanin content in three cranberry cultivars was 5–10 times lower when grown in America than when they were grown in Poland. In agreement with the current studies, Abeywickrama et al. (2016) reported 4.11 times variation for TAC in four cranberry wild clones and a cultivar Pilgrim ranging from 0.54 – 2.22 mg/g dry weight. Similar results were also reported by Oszmiański et al. (2017) who observed 2.47 times variation for total anthocyanins in six cultivars. Lu et al. (2017) reported 1.76 times variation of total anthocyanin content in 21 cranberry cultivars grown in New Zealand ranging from 1.63 – 2.87 mg dry weight.
3.4. Antioxidant activity (AA)
The DPPH assay has been widely used for the determination of AA in berry crops (Goyali et al., 2015). The antioxidant activity differed significantly (p < 0.05) among the wild cranberry genotypes and the two cultivars tested. The AA (DPPH radical scavenging activity expressed as mg GAE/g fw) was diverse (p < 0.05) in the present material (Tables 2 and 3) and values varied 2.16 times among the genotypes ranging from 1.17 to 2.53 mg for the clones NL95 and NS4, respectively. Variation was the highest in NL clones (1.85 times) followed by PE (1.68 times), NB (1.47 times) and NS clones (1.41 times). In NL clones, highest AA was observed in the berries of NL92 (2.17 ± 0.02 mg) and it was followed by NL25 (2.14 ± 0.02 mg), NL46 (2.08 ± 0.02 mg), NL11 (2.06 ± 0.03 mg), NL43 (2.06 ± 0.05 mg), NL69 (2.06 ± 0.02 mg), NL47 (2.03 ± 0.02 mg) and NL81 (2.01 ± 0.01 mg). AA in PE clones varied from 1.24 ± 0.02 mg for PE13 to 2.08 ± 0.06 mg for PE10. In the NB wild collections, AA was the highest in NB2 (2.10 ± 0.01 mg) and NB15 (2.10 ± 0.02 GAE/g fw) and the lowest in NB12 (1.43 ± 0.01 mg). The average AA value for four NS clones was the highest among all groups (Table 2) with the highest value of 2.53 ± 0.05 mg for NS4 followed by NS1 (2.17 ± 0.00 mg), NS3 (2.16 ± 0.01 mg) and NS2 (1.79 ± 0.02 mg). The cultivar Franklin (2.27 ± 0.02 mg) had 1.39 times more AA than Bergman (1.63 ± 0.01 mg) under our greenhouse condition (Table 3). These values are, in general, more than those observed by Viskelis et al. (2009) in four cranberry cultivars (0.11–0.15 mg/g). Wild edible fruits were also found to be a good source of bioactive compounds with high antioxidant activities (Morales et al., 2013).
3.5. Cluster analysis for antioxidant properties
All 136 wild clones and two cultivars were subjected to cluster analysis using UPGMA multivariate technique and classify them into categories or clusters based on their similarities for their phenolic, flavonoid and monomeric anthocyanin and their antioxidant activity (data not shown). The UPGMA analysis with 136 wild clones and two cultivars identified two main clusters with an outlier, NL95, which had lowest TPC (3.28 ± 0.09 mg) and AA (1.17 ± 0.01 mg) (Table 3). The first cluster (CL I) consisted of 122 clones from all four provinces and had two sub-clusters: CL I-1 and CL I-2. CL I-1 was again divided into two sub-sub clusters: CL I-1a and CL I-1b. CL I-1a had six distinct groups of 81 wild clones while NS2 and NB6 grouped with four NL clones (NL41, 94, 36, 54) in sub-sub-cluster CL I-1b. In CL I-1a, four NL clones (NL1, 19, 21, 47, 62) grouped with NB9 and NL13 grouped with NB1. In this sub-sub class, there were four more groups consisting of 31 (two NB and two PE and 27 NL clones), 20 (15 NL and 5 PE clones), 13 (11 NL and two PE clones) and nine (NL clones) wild clones. Sub-cluster Cl I-2 had two major sub-sub-clusters, the former (CL I-2a) with two groups: one having nine NL and one PE clones and the other with 22 wild clones (12 NL, six NB and five PE clones) and the cultivar Bergman. The sub-sub-cluster CL I-2b had only two clones (NL72 and PE13). Cluster II (CL II) had two sub-clusters; the first with eight NL (NL11, 46, 92, 25, 69, 81, 33, 93), two NS (NS1, 3) and 3 NB clones (NB2, 3, 15) and the second with clone NS4 and the cultivar Franklin. It is interesting that while the cultivar Bergman was grouped with three NL clones (NL55, 58, 63) and NB17 in CL I-2a, Franklin was clustered with NS4 in CL II-1. NS4 (2.53 ± 0.05 mg) had the highest AA and it was followed by Franklin (2.27 ± 0.02 mg).
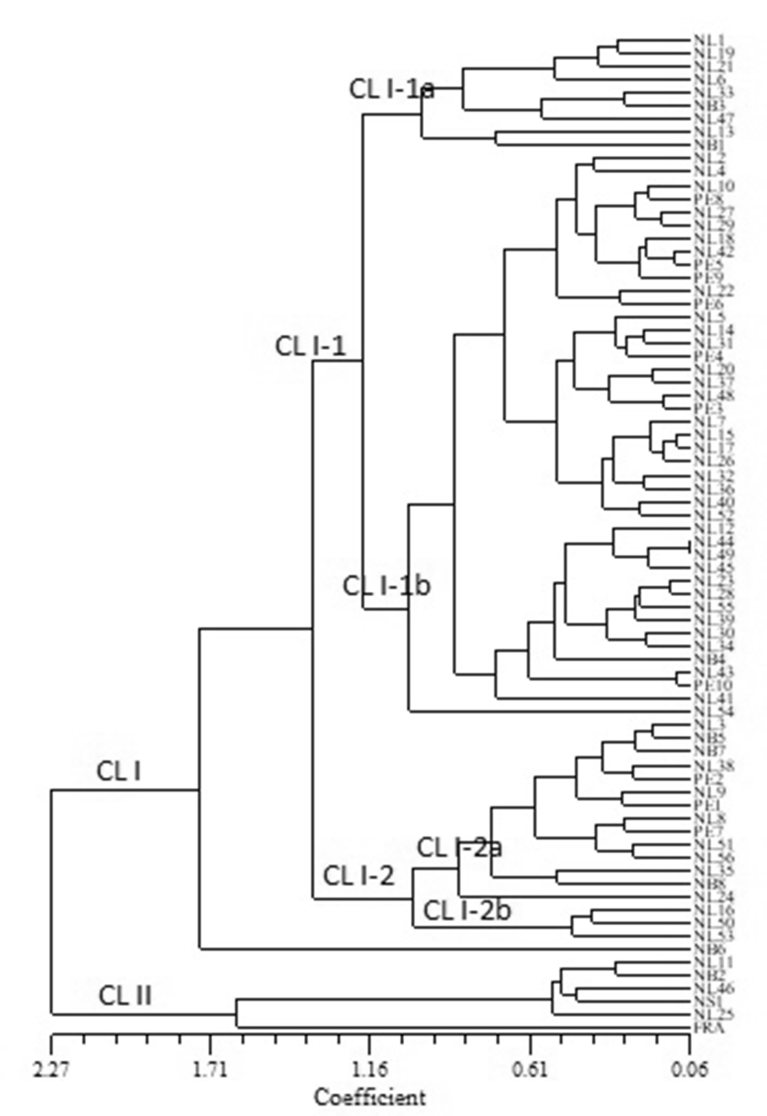
Clustering for antioxidant properties with 75 wild clones and the cultivar Franklin that were used for molecular structure analysis using DNA markers, are presented in Fig. 1. As was with all 138 genotypes, here also 76 genotypes were divided into two main clusters. Cluster I (CL I) was divided into two sub-clusters: CL I-1 and CL 1-2. CL I-1 was again divided into two sub-sub-clusters: CL I-1a and CL I-1b. CL I-1a consisted of two groups: one with NL1, 6, 19, 21, 33, 47 and NB3, and the other with NL13 and NB1. CL I-1b was grouped into two leaving NL54 as an outlier. The first group was again divided into two: one with eight NL (NL2, 4, 10, 18, 22, 27, 29, 42) and four PE clones (PE5, 6, 8, 9); and the second with 14 NL (NL5, 7, 14, 15, 17, 20, 26, 31, 32, 36, 37, 40, 48, 52) and two PE clones (PE3, 4). The second group consisted of 12 NL clones (NL12, 23, 28, 30, 34, 39, 41 43, 44, 45, 49, 55), NB4 and PE10. The sub-cluster CL I-2 had two sub-sub-clusters: CL I-2a and CL I-2b. While the former consisted of eight NL clones (NL3, 8, 9, 24, 35, 38, 51, 56), three NB (NB5, 7, 8) and three PE clones (PE1, 2, 7); the latter had three NL clones (NL16, 50, 53). Cluster II (CL II)was comprised of three NL clones (NL11, 25, 46), NB2, NS1 and the cultivar Franklin. Franklin and NL25 were two outliers while NL11 grouped with NB2 and NL46 with NS1 in CL II (Fig. 1). In the present study, a multivariate analysis was applied to characterize and classify the wild edible fruits according to biochemical and antioxidant characteristics.
Fig. 1.
Unweighted pair-group method with arithmetic averages (UPGMA) dendrogram estimating the genetic distance among 75 cranberry clones and the cultivar Franklin (FRA) designated by codes given in Table 1, based on total phenolic, flavonoid and monomeric anthocyanin as well as their antioxidant activity data.
3.6. Structured diversity
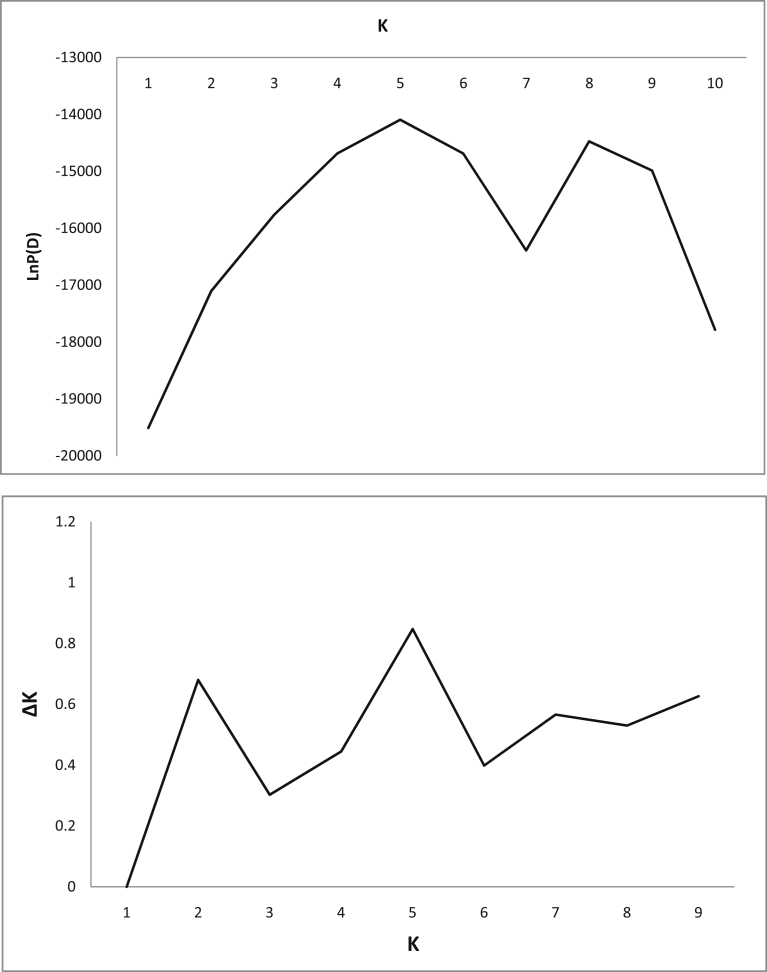
In this study, all ISSR, EST-PCR and EST-SSR primers yielded polymorphic bands in 75 cranberry clones and the cultivar Franklin confirming the results of An et al. (2015). The STRUCTURE program (Pritchard et al., 2000) was used to detect population structure and to assign individuals to subpopulations or groups. This is a model-based Bayesian approach to identify groups of individuals based on a fit to the Hardy–Weinberg equilibrium and linkage equilibrium. Combined data for all three types of marker divided the genotypes into five clusters (K = 5) as was indicated from the plots of probability (Ln) for K and ΔK (Fig. 2) (Pritchard et al., 2000; Evanno et al., 2005). Delta K is an ad hoc statistics and has been recommended for identifying the best-fitting number of groups within a sample (Evanno et al., 2005). However, LnP(D) showed two peaks, one at K = 5 and another at K = 8 (Fig. 2a). Similarly, there were two peaks in ΔK graph, at K = 2 and at K = 5 (Fig. 2b). Both graphs had the highest peak at K = 5. Although ΔK is very useful to identify the correct number of clusters, it can be used together with the other information provided by structure, such as Ln (K) (Evanno et al., 2005).
Fig. 2.
Estimation of the number of clusters (K) best fitting the data based on STRUCTURE runs with K ranging from 1 to 10. (a) Mean natural logarithm probability over 10 replicates compared to K; (b) measure of ΔK for each K value.
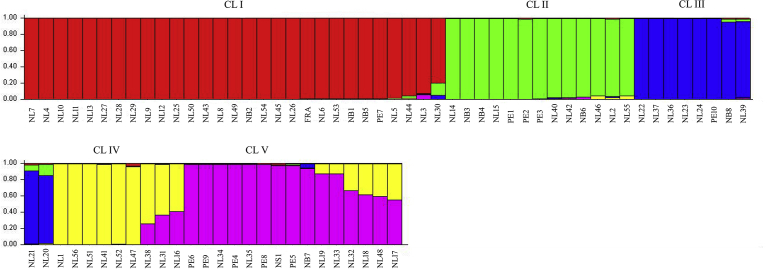
All marker grouped 24 NL, three NB and PE7, and the cultivar Franklin in Cluster I (CL I) (Fig. 3). Around 23% admixtures from Clusters II (CL II) and III (CL III) were observed in NL30 in Cluster I. While CL II had seven NL, three NB and three PE clones; CL III consisted of PE10, NB8 and eight NL clones of which NL20, NL21 were with around 17% and 7%, respectively admixtures from CL II. Nine NL clones were grouped in Cluster IV (CL IV). Three of these clones (NL 16, 31, 38) had around 29-43% admixtures from Cluster V (CL V). CL V possessed eight NL, five PE, NS1 and NB7 clones. In this cluster, NL17 showed the highest admixture of around 43% followed by NL 48 (∼41%), NL18 (∼40%) and NL32 (∼31%). NL 19 and NL 33 had around 11% admixture in this group. All these clones had admixtures from CL IV. In Cluster V, NB7 had around 6% admixture from CL IV (Fig. 3).
Fig. 3.
Distribution of 75 cranberry clones and the cultivar Franklin (FRA) in groups according to STRUCTURE analysis (K = 5), based on 12 ISSR, 10 EST-PCR and 5 EST-SSR primers. The genotypes were represented by vertical bars and each colour is associated to a different group. Genotype identification number is shown on Table 1.
3.7. Comparison between biochemical and genetic clustering
Multiple genetic and environmental factors affect production and accumulation of antioxidant compounds. Clustering based on ISSR, EST-PCR and EST-SSR data for 75 cranberry clones and the cultivar Franklin was different from that based on the total phenolic, flavonoid and anthocyanin contents and antioxidant activity analyses data (Figs. 1 and 3). For example, in biochemical cluster analysis, although seven NL (NL1, 6, 13, 19, 21, 33, 47) and two NB clones (NB1, 3) were grouped in one sub-cluster (Cl I-1; Fig. 1), they were distributed in all five clusters in structure analysis (CL 1: NL6, 13; NB1; CL II: NB3; CL III: NL21; CL IV: NL1, 47; CL V: NL19, 33) (Fig. 3). Similarly, three NL clones (NL11, 25, 46), NB2, NS1 and the cultivar Franklin were grouped in CL II in cluster analysis for antioxidant properties (Fig. 1) but they were found in CL I (NL11, 25; NB2, Franklin), CL II (NL46) and CL V (NS1) in structure analysis (Fig. 3). The poor correspondence between genetic clustering from biochemical data implies that probably these markers differ in their degree of genomic coverage in wild cranberries. Molecular markers are distributed throughout the genome most of which are not expressed at the phenotypic level. The unexpressed noncoding regions of genome that are not accessible to phenotypic expression might be the reason of disagreement between the molecular and chemical diversity (Debnath et al., 2012). Similar results were also reported by Debnath and Sion (2009) in lingonberry and by Debnath and Ricard (2009) in strawberry who observed that clustering based on ISSR data did not coincide with those based on the antioxidant activity and anthocyanin content data. García et al. (2002) did not found correlation between the random amplified polymorphic DNA (RAPD) and morphological data in strawberries.
Although genotype is the major factor in determining berry quality, antioxidant properties in berry crops are also influenced by a number of other factors including pre- and post-harvest environmental conditions, maturity at harvest and storage conditions (De Vittori et al., 2018). DNA markers can directly detect sequence variation among genotypes and have the ability to bypass the factors that affect antioxidant properties. DNA markers can be affected by their types and number, availability across the genome and the scoring precision (Schut et al., 1997). In the present study, three types of markers (ISSR, EST-PCR and EST-SSR) have been used. The use of three types of marker is more effective than one type to estimate relatedness and diversity among genotypes in a diverse wild germplasm (An et al., 2015). It has been suggested that the DNA analysis data combined with data on antioxidant properties could be used effectively for germplasm management and parent selection in berry improvement programs (Debnath and Ricard, 2009; Debnath and Sion, 2009; Debnath et al., 2012).
4. Conclusions
This is the first report of investigation of total phenolic, flavonoid and anthocyanin contents and of antioxidant activity along with molecular diversity analysis using three types of DNA markers: ISSR, EST-PCR and EST-SSR, in a diverse wild cranberry germplasm. Wild cranberries could be a better alternative to the cultivated cultivars as a functional food or dietary supplements, as was suggested by Morales et al. (2013) for wild edible fruits. The study identified 9, 18 and 1 (NS4) wild clones superior to both cultivars for TPC, TFC and AA, respectively that might be used in breeding programs to produce cultivars with high antioxidant properties. Clustering based on combined data on ISSR, EST-PCR and EST-SSR markers was different from that of antioxidant properties. These markers are dispersed throughout the whole genome and many sections of the genome are unexpressed at the phenotypic level. The poor correspondence between genetic distance from biochemical and molecular data indicates that the markers vary in the coverage of the genome. Although no reports are available on using DNA-markers for the analysis of antioxidant properties in cranberries, possibility to use these markers for the same is immense. Extensive reservoir of favourable alleles within wild germplasm is needed for in-depth understanding of the molecular basis for key traits including antioxidant properties of cranberry germplasm. Present study indicates that molecular analysis of genetic diversity and relatedness with DNA based markers along with antioxidant analysis, in a cranberry germplasm collection, can help grouping the wild clones and in identification of subgroups with possible utility for germplasm conservation and utilization in a breeding program. This is invaluable to analyse genetic variability, identify diverse parental combinations to generate wide variability in segregating progenies for further selection and to introduce desirable genes into a cross combination from diverse germplasm. The study should facilitate the use of chemical analysis of fruits and of DNA fingerprints for marker-assisted applications in cranberry improvement.
Declarations
Author contribution statement
Samir C Debnath: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Dong An: Performed the experiments; Analyzed and interpreted the data.
Funding statement
This work was supported by St. John's Research and Development Centre, Agriculture and Agri-Food Canada, St. John's, Newfoundland and Labrador, Canada.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
St. John's Research and Development Centre, Agriculture and Agri-Food Canada manuscript no. 239. The authors acknowledge the technical help from Juran Goyali, Darryl Martin and Glenn Chubbs.
References
- Abeywickrama G., Debnath S.C., Ambigaipalan P., Shahidi F. Phenolics of selected cranberry genotypes (Vaccinium macrocarpon Ait.) and their antioxidant efficacy. J. Agric. Food Chem. 2016;64:9342–9351. doi: 10.1021/acs.jafc.6b04291. [DOI] [PubMed] [Google Scholar]
- Adams M.D., Kelley J.M., Gocayne J.D., Dubnick M., Polymeropoulos M.H., Hong X., Merril C.R., Wu A., Olde B., Moreno R.F., Kerlavage A.R., McCombie W.R., Venter J.C. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991;252:1651–1656. doi: 10.1126/science.2047873. https://www.ncbi.nlm.nih.gov/pubmed/2047873 [DOI] [PubMed] [Google Scholar]
- An D., Bykova N.V., Debnath S.C. EST-PCR, EST-SSR and ISSR markers to identify a set of wild cranberries and evaluate their relationships. Can. J. Plant Sci. 2015;95:1155–1165. [Google Scholar]
- Blumberg J.B., Camesano T.A., Cassidy A., Kris-Etherton P., Howell A., Manach C., Ostertag L.M., Sies H., Skulas-Ray A., Vita J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013;4:618–632. doi: 10.3945/an.113.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges G., Degeneve A., Mullen W., Crozier A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010;58:3901–3909. doi: 10.1021/jf902263n. [DOI] [PubMed] [Google Scholar]
- Brouillard R., Chassaing S., Fougerousse A. Why are grape/fresh wine anthocyanins so simple and why is it that red wine colour lasts so long. Phytochemistry. 2003;64:1179–1186. doi: 10.1016/s0031-9422(03)00518-1. [DOI] [PubMed] [Google Scholar]
- Debnath S.C. An assessment of the genetic diversity within a collection of wild cranberry (Vacinium macrocarpon Ait.) clones with RAPD-PCR. Genet. Resour. Crop Evol. 2007;54:509–517. [Google Scholar]
- Debnath S.C. Genetic diversity and erosion in berries. In: Ahuja M.R., Jain S.M., editors. vol. 2. Springer Int. Publ.; Switzerland: 2016. pp. 75–129. (Genetic Diversity and Erosion in Plants, Case Histories). [Google Scholar]
- Debnath S.C., Ricard E. ISSR, anthocyanin content and antioxidant activity analyses to characterize strawberry genotypes. J. Appl. Hortic. 2009;11:83–89. https://www.semanticscholar.org/paper/ISSR%2C-anthocyanin-content-and-antioxidant-activity-Debnath-Ricard/ce83b9e5b0c2132fd0f1c47acbdde866f390be48 [Google Scholar]
- Debnath S.C., Sion M. Genetic diversity, antioxidant activities, and anthocyanin contents in lingonberry. Int. J. Fruit Sci. 2009;9:185–199. [Google Scholar]
- Debnath S.C., Siow Y.L., Petkau J., An D., Bykova N.V. Molecular markers and antioxidant activity in berry crops: genetic diversity analysis. Can. J. Plant Sci. 2012;92:1121–1133. [Google Scholar]
- de Souza V.R., Pereira P.A., da Silva T.L., de Oliveira Lima L.C., Pio R., Queiroz F. Determination of the bioactive compounds, antioxidant activity and chemical composition of Brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014;156:362–368. doi: 10.1016/j.foodchem.2014.01.125. [DOI] [PubMed] [Google Scholar]
- Deyhim F., Patil B.S., Villarreal A., Lopez E., Garcia K., Rios R., Garcia C., Gonzales C., Mandadi K. Cranberry juice increases antioxidant status without affecting cholesterol homeostasis in orchidectomized rats. J. Med. Food. 2007;10:49–53. doi: 10.1089/jmf.2006.218. [DOI] [PubMed] [Google Scholar]
- Di Vittori L., Mazzoni L., Battino M., Mezzetti B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. (Canterb.) 2018;233:310–322. [Google Scholar]
- Earl D.A., vonHoldt B.M. Structure harvester: a website and program for visualizing structure output and implementing the Evanno method. Cons. Genet. Resour. 2012;4:359–361. [Google Scholar]
- Evanno G., Regnaut S., Goudet J. Detecting the number of clusters of individuals using the software structure: a simulation study. Mol. Ecol. 2005;14:2611–2620. doi: 10.1111/j.1365-294X.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- FAO . 2018. FAOSTAT: Cranberries. Food and Agriculture Organization (FAO) of the United Nations.http://www.fao.org/faostat/en/#data/QC [Google Scholar]
- Galletta G.J., Ballington J.R. Blueberries, cranberries and lingonberries. In: Janick J., Moore J.N., editors. Fruit Breeding, Vol. II, Vine and Small Fruit Crops. Prentice Hall; New York, NY: 1996. pp. 1–107. [Google Scholar]
- García M.G., Ontivero M., Diaz Ricci J.C., Castagnaro A. Morphological traits and high resolution RAPD markers for the identification of the main strawberry varieties cultivated in Argentina. Plant Breed. 2002;121:76–80. [Google Scholar]
- Goyali J.C., Igamberdiev A.U., Debnath S.C. Propagation methods affect fruit morphology and antioxidant properties but maintain clonal fidelity in lowbush blueberry. Hortscience. 2015;50:888–896. [Google Scholar]
- Hatano T., Kagawa H., Yasuhara T., Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effects. Chem. Pharmac. Bull. 1988;36:1090–2097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Howell A.B. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol. Nutr. Food Res. 2007;51:732–737. doi: 10.1002/mnfr.200700038. [DOI] [PubMed] [Google Scholar]
- Kalgaonkar S., Gross H.B., Yokoyama W., Keen C.L. Effects of a flavonol-rich diet on select cardiovascular parameters in a golden Syrian hamster model. J. Med. Food. 2010;13:108–115. doi: 10.1089/jmf.2008.0295. [DOI] [PubMed] [Google Scholar]
- Kraujalytė V., Venskutonis P.R., Pukalskas A., Česoniene L., Daubaras R. Antioxidant properties, phenolic composition and potentiometric sensor array evaluation of commercial and new blueberry (Vaccinium corymbosum) and bog blueberry (Vaccinium uliginosum) genotypes. Food Chem. 2015;188:583–590. doi: 10.1016/j.foodchem.2015.05.031. [DOI] [PubMed] [Google Scholar]
- Lee J.H., Kim G.H. Evaluation of antioxidant and inhibitory activities for different subclasses flavonoids on enzymes for rheumatoid arthritis. J. Food Sci. 2010;75:H212−H217. doi: 10.1111/j.1750-3841.2010.01755.x. [DOI] [PubMed] [Google Scholar]
- Lee J., Durst R., Wrolstad R. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J. AOAC Int. 2005;88:1269–1278. https://www.ncbi.nlm.nih.gov/pubmed/16385975 [PubMed] [Google Scholar]
- Leitao D.P.S., Polozello A.C.M., Ito I.Y., Spadaro A.C. Antibacterial screening of anthocyanic and proanthocyanic fractions from cranberry juice. J. Med. Food. 2005;8:36–40. doi: 10.1089/jmf.2005.8.36. [DOI] [PubMed] [Google Scholar]
- Lu Y., Pekerti B.N., Toh Z.S., Broom F., Savage G., Liu S.Q., Huang D. Physico-chemical parameters and proanthocyanidin profiles of cranberries cultivated in New Zealand. J. Food Compos. Anal. 2017;63:1–7. [Google Scholar]
- Manganaris G.A., Goulas V., Vicente A.R., Terry L.A. Berry antioxidants: small fruits providing large benefits. J. Sci. Food Agric. 2014;94:825–833. doi: 10.1002/jsfa.6432. [DOI] [PubMed] [Google Scholar]
- McKay D.L., Blumberg J.B. Cranberries (Vaccinium macrocarpon) and cardiovascular disease risk factors. Nutr. Rev. 2007;65:490–502. doi: 10.1111/j.1753-4887.2007.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Mishra K., Ojha H., Chaudhury N.K. Estimation of antiradical properties of antioxidants using DPPH assay: a critical review and results. Food Chem. 2012;130:1036–1043. [Google Scholar]
- Morales P., Ferreira I.C., Carvalho A.M., Fernández-Ruiz V., Sánchez-Mata M.C., Cámara M., Wild edible fruits as a potential source of phytochemicals with capacity to inhibit lipid peroxidation. Eur. J. Lipid Sci. Technol. 2013;115:176–185. [Google Scholar]
- Neto C.C., Amoroso J.W., Liberty A.M. Anticancer activities of cranberry phytochemicals: an update. Mol. Nutr. Food Res. 2008;52(suppl 1):S18–S27. doi: 10.1002/mnfr.200700433. [DOI] [PubMed] [Google Scholar]
- Oszmiański J., Kolniak-Ostek J., Lachowicz S., Gorzelany J., Matłok N. Phytochemical compounds and antioxidant activity in different cultivars of cranberry (Vaccinium macrocarpon L) J. Food Sci. 2017;82:2569–2575. doi: 10.1111/1750-3841.13924. [DOI] [PubMed] [Google Scholar]
- Oszmiański J., Lachowicz S., Józef Gorzelany J., Matłok N. The effect of different maturity stages on phytochemical composition and antioxidant capacity of cranberry cultivars. Eur. Food Res. Technol. 2018;244:705–719. [Google Scholar]
- Pritchard J.K., Stephens M., Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–956. doi: 10.1093/genetics/155.2.945. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1461096/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P.M., Sarla N., Siddiq E.A. Inter simple sequence repeat (ISSR) polymorphism and its application in plant breeding. Euphytica. 2002;128:9–17. [Google Scholar]
- Rohlf F.J. Exeter Software; Setauket, New York: 1998. NTSYS-pc. Numerical Taxonomy and Multivariate Analysis System.http://www.exetersoftware.com/cat/update_NTSYSpc.shtml [Google Scholar]
- Rosenberg N.A., Burke T., Elo K., Feldmann M.W., Freidlin P.J., Groenen M.A.M., Hillel J., Maki-Tanila A., Tixier-Boichard M., Vignal A., Wimmers K., Weigend S. Empirical evaluation of genetic clustering methods using multilocus genotypes from 20 chicken breeds. Genetics. 2001;159:699–713. doi: 10.1093/genetics/159.2.699. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1461842/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Khanna S., Alessio H.M., Vider J. Antiangiogenic property of edible berries. Free Radic. Res. 2002;36:1023–1031. doi: 10.1080/1071576021000006662. [DOI] [PubMed] [Google Scholar]
- Schut J.W., Qi X., Stam P. Association between relationship measures based on AFLP markers, pedigree data and morphological traits in barley. Theor. Appl. Genet. 1997;95:1161–1168. [Google Scholar]
- Seeram N.P., Adams L.S., Hardy M.L., Heber D. Total cranberry extract versus its phytochemical constituents: antiproliferative and synergistic effects against human tumor cell lines. J. Agric. Food Chem. 2004;52:2512–2517. doi: 10.1021/jf0352778. [DOI] [PubMed] [Google Scholar]
- Singleton V.L., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965;16:144–158. http://www.ajevonline.org/content/16/3/144 [Google Scholar]
- Sokal R.R., Michener C.D. A statistical method for evaluating systematic relationships. Univ. Kansas Sci. Bull. 1958;28:1409–1438. https://archive.org/details/cbarchive_133648_astatisticalmethodforevaluatin1902/page/n1 [Google Scholar]
- Sun J., Hai Liu R.H. Cranberry phytochemical extracts induce cell cycle arrest and apoptosis in human MCF-7 breast cancer cells. Cancer Lett. 2006;241:124–134. doi: 10.1016/j.canlet.2005.10.027. [DOI] [PubMed] [Google Scholar]
- Vander Kloet S.P. Agr. Can. Publ.; Ottawa, Ontario, Canada: 1988. The Genus Vaccinium in North America.http://publications.gc.ca/site/eng/463154/publication.html 1828. [Google Scholar]
- Varshney R.K.1., Graner A., Sorrells M.E. Genic microsatellite markers in plants: features and applications. Trends Biotechnol. 2005;23:48–55. doi: 10.1016/j.tibtech.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Vattem D.A., Jang H.D., Levin R., Shetty K. Synergism of cranberry phenolics with ellagic acid and rosmarinic acid for antimutagenic and DNA protection functions. J. Food Biochem. 2006;30:98–116. [Google Scholar]
- Viskelis P., Rubinskiene M., Jasutiene I., Sarkinas A., Daubaras R., Cesoniene L. Anthocyanins, antioxidative, and antimicrobial properties of american cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J. Food Sci. 2009;74:C157−C161. doi: 10.1111/j.1750-3841.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- Weising K., Atkinson R.G., Gardner R.C. Genomic fingerprinting by microsatellite-primed PCR: a critical evaluation. PCR Methods Appl. 1995;4:249–255. doi: 10.1101/gr.4.5.249. [DOI] [PubMed] [Google Scholar]
- Weiss E.L., Lev-Dor R., Sharon N., Ofek I. Inhibitory effect of a high-molecular-weight constituent of cranberry on adhesion of oral bacteria. Crit. Rev. Food Sci. Nutr. 2002;42:285–292. doi: 10.1080/10408390209351917. [DOI] [PubMed] [Google Scholar]
- Weiss E.I., Kozlovsky A., Steinberg D., Lev-Dor R., Bar Ness Greenstein R., Feldman M., Sharon N., Ofek I. A high molecular mass cranberry constituent reduces mutans streptococci level in saliva and inhibits in vitro adhesion to hydroxyapatite. FEMS Microbiol. Lett. 2004;232:89–92. doi: 10.1016/S0378-1097(04)00035-7. [DOI] [PubMed] [Google Scholar]
- Weiss E.I., Houri-Haddad Y., Greenbaum E., Hochman N., Ofek I., Zakay-Rones Z. Cranberry juice constituents affect influenza virus adhesion and infectivity. Antivir. Res. 2005;66:9–12. doi: 10.1016/j.antiviral.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Zhishen J., Mengcheng T., Jianming W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–559. [Google Scholar]
- Zielinski H., Kozlowska H. Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J. Agric. Food Chem. 2000;48:2008–2016. doi: 10.1021/jf990619o. [DOI] [PubMed] [Google Scholar]
- Zuo Y., Wang C., Zhan J. Separation, characterization, and quantitation of benzoic and phenolic antioxidants in American cranberry fruit by GC-MS. J. Agric. Food Chem. 2002;50:3789–3794. doi: 10.1021/jf020055f. [DOI] [PubMed] [Google Scholar]