Abstract
Introduction
The Meta VCI Map consortium performs meta-analyses on strategic lesion locations for vascular cognitive impairment using lesion-symptom mapping. Integration of data from different cohorts will increase sample sizes, to improve brain lesion coverage and support comprehensive lesion-symptom mapping studies.
Methods
Cohorts with available imaging on white matter hyperintensities or infarcts and cognitive testing were invited. We performed a pilot study to test the feasibility of multicenter data processing and analysis and determine the benefits to lesion coverage.
Results
Forty-seven groups have joined Meta VCI Map (stroke n = 7800 patients; memory clinic n = 4900; population-based n = 14,400). The pilot study (six ischemic stroke cohorts, n = 878) demonstrated feasibility of multicenter data integration (computed tomography/magnetic resonance imaging) and achieved marked improvement of lesion coverage.
Discussion
Meta VCI Map will provide new insights into the relevance of vascular lesion location for cognitive dysfunction. After the successful pilot study, further projects are being prepared. Other investigators are welcome to join.
Keywords: Cerebrovascular disease, Vascular cognitive impairment, Stroke, Small vessel disease, Consortium, Data harmonization, Support vector regression, Lesion-symptom mapping, Lesion location
Highlights
-
•
Lesion location is an important determinant of cognitive impact of vascular damage.
-
•
Previous lesion-symptom mapping studies had limited brain lesion coverage.
-
•
Meta VCI Map provides a platform for multicenter lesion-symptom mapping research.
-
•
The pilot study shows that multicenter data integration is feasible and improves coverage.
-
•
Multicenter studies enable comprehensive assessment of strategic lesion locations.
1. Introduction
Cerebrovascular disease is a major cause of cognitive impairment and dementia, either alone or in combination with Alzheimer's disease. This vascular contribution to cognitive decline is collectively referred to as vascular cognitive impairment (VCI) [1]. VCI is a heterogeneous construct, both in terms of cognitive phenotypes and nature and severity of underlying vascular etiologies and comorbid conditions. Despite this heterogeneity, lesion location is likely to be an important determinant of cognitive impact of different manifestations of vascular brain injury. Based on early observations, certain lesion locations are considered to be “strategic,” including the left angular gyrus, thalamus, and basal ganglia. However, a recent statement on VCI from the American Heart Association/American Stroke Association emphasized that current concepts of strategic infarction are largely based on small case series and warrant reexamination in larger imaging studies [1]. For white matter hyperintensities (WMHs), there are also indications that lesion location is related to cognitive impact, but this relationship is less clear [2], [3].
Lesion-symptom mapping (LSM) is an increasingly utilized method in the VCI research field [4], [5]. The concept of LSM is that it examines relationships between the location of brain injury—usually at the level of individual voxels or regions of interest—and behavioral measures such as cognition. This is commonly done through statistical comparisons of patients with and without a lesion for individual voxels or by associating regional lesion volumes with cognition. LSM studies have identified strategic lesion locations for acute infarcts [6] and small vessel disease [3]. A growing number of scientific publications and lesion analysis methods have emerged over the past decades [7]. Yet, most LSM studies are hampered by incomplete lesion coverage of the brain. This is important because the cognitive impact of damage to a particular brain region can only be addressed in the statistical analyses if this region is damaged in a sufficient number of patients. Because vascular lesions are not distributed randomly across the brain, some regions are commonly affected, but others that may be relevant for cognition—such as the orbitofrontal lobes in anterior cerebral artery territory infarction [8], [9]—are rarely affected. As a consequence, previous studies (e.g., [5], [10], [11]) found that even with several hundred subjects, large regions of the brain were not affected by vascular lesions and could therefore not be considered in LSM analyses. Thus, it remains difficult to obtain a comprehensive picture of strategic lesion locations for cognitive dysfunction.
Meta VCI Map is a recently established consortium that aims to perform Meta-analyses on strategic lesion locations for VCI using lesion-symptom Mapping. This consortium provides an international collaborative platform for multicenter LSM projects. By integrating existing data sets with imaging data on vascular lesions and cognitive data, LSM studies can be performed on larger study samples to improve brain lesion coverage.
Meta VCI Map has two key objectives. The first objective is to create vulnerability maps of the brain that show where vascular lesions have the most impact on cognition. Such vulnerability maps would be of diagnostic and prognostic value and could help explain why some patients with vascular lesions develop VCI while others do not. The second objective is to further establish strategic lesion locations that lead to impairment of specific cognitive functions.
In this study, we provide an overview of the design and objectives of this consortium. In addition, we present the methods and results of the pilot study, which aimed to determine the feasibility and potential benefits of multicenter LSM studies.
2. Methods
2.1. Consortium design
2.1.1. Membership and data index
Membership is open to all research groups and consortia willing to share suitable data. Data sets are eligible for inclusion if both brain imaging and data on cognitive performance are available. Study populations typically involve patients with ischemic stroke, memory clinic patients, or population-based subjects. Study design may be cross-sectional or longitudinal. Lesions of interest are infarcts (on computed tomography [CT]/magnetic resonance imaging [MRI]) or WMH (on MRI). Availability of lesion maps is preferred, but not a prerequisite. Meta VCI Map image processing and analysis pipelines allow for inclusion and combination of different types of imaging data (see 2.1.4). Standardized measures of cognitive performance or decline should have been collected, for example, cognitive screening tests (e.g., Montreal Cognitive Assessment [MoCA]), neuropsychological tests (e.g., Trail Making Test), clinical diagnoses (e.g., mild cognitive impairment or dementia), or norm-based scores for individual tests or cognitive domains. Other clinical parameters, such as gait or depressive symptoms, may also be suitable for LSM analyses.
Data sets available from members are listed in the Meta VCI Map data index. This data index is used to select cohorts with comparable study populations and cognitive outcomes that can be used in meta-analyses. These analyses are performed on a project-by-project basis. Membership as such does not require the actual submission of data. Data are only shared in the context of specific projects (see 2.1.2).
Potential members were identified and invited through other collaborative networks in the VCI research field, including STRIVE [12], METACOHORTS [13], and HARNESS (www.harness-neuroimaging.org) and asked to fill in a survey to explore their interest in participating and support creation of the data index. As of November 2018, 47 groups have joined Meta VCI Map, and the data index includes approximately 7800 patients with ischemic stroke, 4900 memory clinic patients, and 14,400 population-based subjects. An overview of cohorts is presented in Table 1. Recruitment of members is an open and ongoing process. Interested parties are invited to sign up through e-mail or the Meta VCI Map website.
Table 1.
Overview of Meta VCI Map member studies
| Study | Country | Sample size | Imaging data | Cognitive screening tests | Cognitive domain-specific assessment | Lesion maps∗ | Key reference(s) |
|---|---|---|---|---|---|---|---|
| Ischemic stroke cohorts | |||||||
| Bundang VCI cohort | Republic of Korea | 2233 | T1, FLAIR, DWI | MMSE, IQCODE | Mem, AEF, Vis, Lan | No | Lim et al., 2014 [14] |
| Center of Excellence in Rehabilitation Medicine, UMC Utrecht—De Hoogstraat Rehabilitation cohort | Netherlands | 150 | CT, T1, FLAIR, DWI | MoCA, MMSE, neglect screening | Mem, AEF, PS, Vis, Lan | Yes | Ten Brink et al., 2016 [15] Ten Brink et al., 2019 [16] |
| Clinical Biological and Pharmacological Factors Influencing Stroke Outcome (BIOSTROKE) | France | 395 | T1, T2, FLAIR, T2-star, DWI | MoCA, MMSE, CDR | N/A | N/A | Ducroquet et al., 2013 [17] |
| Cognition and Affect after Stroke: Prospective Evaluation of Risks (CASPER) | Netherlands | 250 | T1, T2, FLAIR, SWI | MMSE, neglect screening | Mem, AEF, PS, Vis, Lan | Yes | Douven et al., 2016 [18] |
| Cognitive Outcome After Stroke (COAST) | Singapore | 117 | CT, FLAIR, DWI | MoCA, MMSE | Mem, AEF, Vis, Lan | Yes | Dong et al., 2012 [19] |
| Cognitive Deficits in Cerebellar Stroke (CODECS) | Netherlands | 40 | CT, T1, T2, FLAIR, DWI | MoCA, FAB | Mem, AEF, PS, Vis, Lan | Yes | N/A |
| Cognitive function after lacunar stroke MUMC study | Netherlands | 77 | T2, FLAIR, T2-star, DWI | R-CAMCOG | Mem, AEF, PS | No | Huijts et al., 2013 [20] |
| Cognitive Function After Stroke (CogFAST-UK) | United Kingdom | 100 | CT, T1, FLAIR | MMSE, CDR, CAMCOG IQCODE | N/A | N/A | Allan et al., 2011 [21] |
| Clinical Relevance of Microbleeds in Stroke (CROMIS-2) | United Kingdom | 1000 | T1, T2, FLAIR, T2-star, DWI, SWI | MoCA | Mem, AEF, PS, Vis, Lan | N/A | Charidimou et al., 2015 [22] Wilson et al., 2018 [23] |
| Chinese University of Hong Kong—Stroke Registry Investigating Cognitive Decline (CU-STRIDE) | Hong Kong | 410 | CT, T1, T2, FLAIR, DWI | MoCA, MMSE, CDR, IQCODE | None | Yes | Yang et al., 2015 [24] Mok et al., 2016 [25]; Zhao et al., 2017 [10] |
| Determinants of Dementia After Stroke (DEDEMAS) | Germany | 131 | T1, FLAIR, DTI | MoCA, MMSE | Mem, AEF, PS, Vis, Lan | Yes | Wollenweber et al., 2013 [26] |
| Hallym VCI cohort | Republic of Korea | 994 | T1, FLAIR, DWI | MoCA, MMSE, IQCODE | Mem, AEF, Vis, Lan | No | Yu et al., 2013 [27] |
| Kaohsiung Chang Gung Memorial Hospital stroke cohort | Taiwan | 500 | T1, FLAIR, DWI, DTI | MMSE, CASI | None | No | N/A |
| Linkou Chang Gung Memorial Hospital stroke cohort | Taiwan | 100 | T1, FLAIR, DWI, DTI | MoCA, MMSE | Mem, AEF, Lan | No | N/A |
| Mild Stroke Study II (MSS-II) | United Kingdom | 200 | T1, T2, FLAIR, T2-star, DWI, DTI | MoCA, MMSE, ACE-R | None | Yes | Wardlaw et al., 2017 [28] |
| Neurovascular Underpinnings of Exercise post-Stroke (RISE3) | Canada | 15 | T1, T2, FLAIR | MoCA | Mem, AEF, PS | Yes | Robertson et al., 2015 [29] |
| Prediction of Cognitive Recovery After Stroke (PROCRAS) | Netherlands | 242 | CT, T1, T2, FLAIR, DWI, DTI | MoCA, IQCODE | Mem, AEF, PS, Vis, Lan, emotion recognition | Yes | Aben et al., 2018 [30] |
| Recovery Improved post-Stroke with Exercise (RISE1) | Canada | 13 | T1, T2, FLAIR | MoCA | Mem, AEF, PS | Yes | Robertson et al., 2017 [31] |
| Study of Factors Influencing Post-Stroke Dementia (STROKDEM) | France | 300 | T1, FLAIR, T2-star, DTI | MoCA, MMSE, IQCODE, CDR | N/A | N/A | Bournonville et al., 2018 [32] Delattre et al., 2017 [33] |
| Tel Aviv Brain Acute Stroke Cohort (TABASCO) | Israel | 421 | T1, T2, FLAIR, DWI, DTI | MoCA | Mem, AEF, Vis, Lan | Yes | Ben Assayag et al., 2012 [34] |
| Utrecht Stroke and COGnition (USCOG) | Netherlands | 121 | CT, T1, FLAIR | N/A | Mem, AEF, PS, Vis, Lan | Yes | Biesbroek et al., 2014 [35] |
| Memory clinic cohorts | |||||||
| ADNI-1/2/GO (WMH segmentations by University of California, Davis)† | United States of America | 1231 | T1, T2, FLAIR | MMSE, CDR | Mem, AEF, PS, Vis, Lan | Yes | Jack et al., 2015 [36]http://adni.loni.usc.edu/ |
| ADNI-2/GO (WMH segmentations by University College London)† | United Kingdom | 929 | T1, FLAIR | MoCA, MMSE | Mem, AEF, PS, Vis, Lan | Yes | http://adni.loni.usc.edu/ |
| Alzheimer Center Erasmus MC‡ | Netherlands | 130 | T1, T2, FLAIR | MMSE, FAB | Mem, AEF, PS, Vis, Lan | No | N/A |
| Cognition and Aging Center—Kaohsiung Chang Gung Memorial Hospital (CAC-KCGMH) | Taiwan | 100 | T1, FLAIR | MMSE, FAB, CASI | Mem, AEF, PS, Vis, Lan | No | Huang et al., 2017, 2018 [37], [38] |
| The Dutch Parelsnoer Institute—neurodegenerative diseases‡ | Netherlands | 1200 | T1, T2, FLAIR, DWI | MMSE | Mem, AEF, PS, Vis, Lan | Yes | Aalten et al., 2014 [39] |
| Functional Assessment of Vascular Reactivity (FAVR) and Brain IMPACT | Canada | 150 | T1, T2, FLAIR, DTI | MMSE | Mem, AEF, PS, Vis | Yes | Case et al., 2016 [40] |
| Harmonization | Singapore | 167 | T1, T2, FLAIR | MoCA, MMSE | Mem, AEF, PS, Vis, Lan | Yes | Biesbroek et al., 2016 [41] |
| PRE-MCI | United Kingdom | 91 | FLAIR | MMSE | Mem, AEF, PS, Vis | No | Archer et al., 2006 [42] |
| Prospective Dementia Registry Austria (PRODEM-Austria) | Austria | 819 | T1, T2, FLAIR | MMSE | Mem, AEF, PS, Vis, Lan | Yes | Pusswald et al., 2015 [43] |
| Recovery Improved in Covert Stroke With Exercise (RISE2) | Canada | 47 | T1, T2, FLAIR | MoCA | Mem, AEF, PS | Yes | https://clinicaltrials.gov/ct2/show/NCT02068391 |
| Utrecht-Amsterdam Clinical Features and Prognosis in Vascular Cognitive Impairment (TRACE-VCI)‡ | Netherlands | 860 | T1, T2, FLAIR, SWI | CDR, MMSE, CAMCOG | Mem, AEF, PS, Vis, Lan | Yes | Boomsma et al., 2017 [44] |
| Young Onset Alzheimer's disease (YOAD) cohort | United Kingdom | 69 | T1, T2 | MMSE | Mem, AEF, PS, Vis, Lan | Yes | Slattery et al., 2017 [45] |
| Population-based cohorts | |||||||
| Austrian Stroke Prevention Study (ASPS), Graz Study | Austria | 1188 | T1, T2, FLAIR | MMSE | Mem, AEF, PS, Vis, Lan | Yes | Seiler et al., 2014 [46]; Schmidt et al., 1994, 1999 [47], [48] |
| Calgary Normative Study | Canada | 200 | T1, FLAIR, DTI | MoCA | N/A | Yes | Tsang et al., 2017 [49] |
| Chinese University of Hong Kong – Risk Index for Subclinical brain lesions in Hong Kong (CU-RISK) | Hong Kong | 851 | T1, T2, FLAIR, DTI | MoCA, MMSE | Mem, AEF, PS, Vis, Lan | Yes | Wong et al., 2015 [50] |
| Epidemiology of Dementia in Singapore (EDIS) | Singapore | 1500 | CT, T1, T2, FLAIR, T2-star, DWI | MoCA, MMSE, IQCODE | Mem, AEF, PS, Vis, Lan | Yes | Hilal et al., 2013 [51] |
| Framingham Heart Study | United States | 1820 | T1, T2 | N/A | Mem, AEF, PS, Vis, Lan | Yes | Au et al., 2006 [52] |
| Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) | Netherlands | 550 | T1, T2, FLAIR, T2-star, DWI | MMSE | Mem, AEF, PS | Yes | Shepherd et al., 2014 [53] |
| Rotterdam Study | Netherlands | 5400 | T1, T2, FLAIR, T2-star, DTI | MMSE | Mem, AEF, PS, Vis, Lan | Yes | Ikram et al., 2015, 2017 [54], [55] |
| Southall And Brent Revisited (SABRE) | United Kingdom | 1306 | T1, T2, FLAIR, T2-star, DTI | N/A | Mem, AEF, PS, Lan | Yes | Shibata et al., 2013 [56] Sudre et al., 2018 [57] |
| Sydney Memory and Ageing Study (MAS) | Australia | 540 | T1, FLAIR | MMSE | Mem, AEF, PS, Vis, Lan | Yes | Sachdev et al., 2010 [58] |
| UC Davis ADC Diversity Cohort | United States | 1063 | T1, FLAIR | N/A | Mem, AEF, Vis | Yes | Hinton et al., 2010 [59] |
| Other cohort types | |||||||
| Blood-brain barrier in cerebral small vessel disease cohort | Netherlands | 75 | T1, FLAIR | N/A | Mem, AEF, PS | Yes | Zhang et al., 2017 [60] |
| Discontinuation of Antihypertensive Treatment in Elderly People (DANTE) | Netherlands | 219 | T1, T2, FLAIR, T2-star | MMSE | Mem, AEF, PS | N/A | Moonen et al., 2015 [61] |
| Munich CADASIL cohort | Germany | 125 | T1, FLAIR, T2-star, DTI | MMSE | Mem, PS, Vis | Yes | Duering et al., 2011 [5] |
| Radboud University Nijmegen Diffusion tensor and Magnetic resonance imaging Cohort (RUN DMC) | Netherlands | 503 | T1, T2, FLAIR, T2-star, DWI, DTI | MMSE | Mem, AEF, PS, Vis | Yes | Van Norden et al., 2011 [62] |
Abbreviations: ACE, Addenbrooke's Cognitive Examination; AEF, attention and/or executive functions; CAMCOG, Cambridge Cognitive Examination; CDR, clinical dementia rating; CASI, Cognitive Abilities Screening Instrument; CT, computed tomography; DTI, diffusion tensor imaging; DWI, diffusion-weighted imaging; FAB, frontal assessment battery; FLAIR, fluid-attenuated inversion recovery; IQCODE, Informant Questionnaire for Cognitive Decline in the Elderly; Lan, language; Mem, memory; MMSE, Mini–Mental State Examination; MoCA, Montreal Cognitive Assessment; MRI, magnetic resonance imaging; N/A, (information on data) not available; PS, processing speed; SWI, susceptibility-weighted imaging; Vis, visuospatial functions (e.g., perception, construction); VCI, vascular cognitive impairment; WMH, white matter hyperintensity.
Lesion maps: infarcts for stroke cohorts; white matter hyperintensities for cohorts with memory clinic patients, population-based subjects, and other cohort types.
Lesion segmentations of ADNI data have been provided by different centers (i.e., University of California at Davis and University College London). Therefore, a large degree of overlap in subjects between these data sets must be noted.
The Dutch Parelsnoer Institute—Neurodegenerative diseases cohort is a collaboration between the eight Dutch University Medical Centres (www.parelsnoer.org). This includes subjects from the TRACE-VCI and Alzheimer Center Erasmus MC cohorts; thus, partial overlap between these cohorts must be noted.
The website is active at www.metavcimap.org. It provides a public platform for information on the consortium. This includes the data index of member cohorts and consortia. The website also has a section for technical support (see 2.1.4/2.1.5).
2.1.2. Organization
Meta VCI Map is governed by a Steering Committee, which consists of representatives from study groups who have participated in Meta VCI Map projects. The Steering Committee is responsible for the governance of the consortium and oversees research projects performed within the consortium framework. To support individual projects in various areas of expertise, Working Groups for data analysis, data management, cognitive assessment, and ethics and regulations have been established.
Data will be shared on a project-by-project basis. This enables each consortium member to opt-in or opt-out on sharing data for specific projects, which allows individual investigators to prioritize projects of their interest accordingly. Each project involves individual data sharing agreements between participating centers, defining data use and access for that particular project. This ensures that local requirements for privacy, ownership, and traceability of the data are fulfilled. To accelerate workflow, a data sharing agreement template is provided, which has been optimized for international use (available at https://metavcimap.org/features/downloads).
2.1.3. Ethics
Meta VCI Map supports and encourages sharing of data, while strictly adhering to ethical and legal institutional review board regulations to ensure protection of (data from) human subjects, including the European Union General Data Protection Regulation. Consortium members are responsible for adhering to their local regulations and for proper deidentification of data before sharing. Requirements will differ for each institution and particular research project and therefore must be reviewed on a project-by-project basis.
2.1.4. Image processing
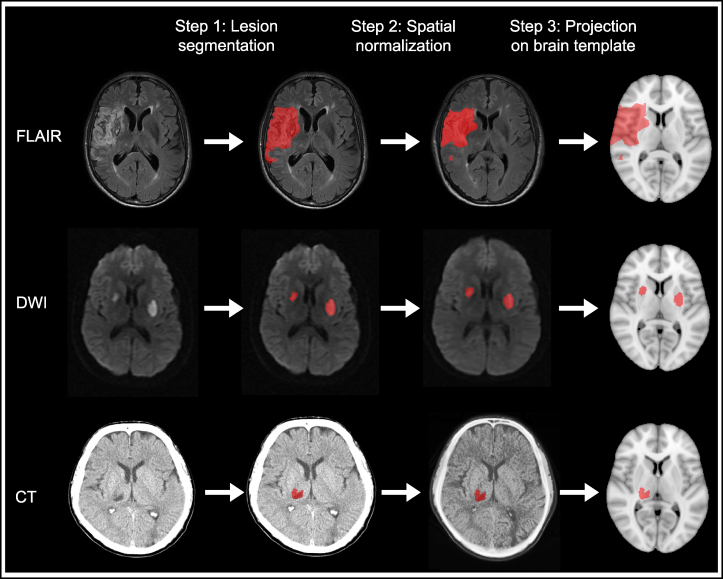
Lesion segmentation and image processing tools are available for determining the location of brain lesions, including infarcts (on CT, fluid-attenuated inversion recovery [FLAIR], or diffusion-weighted imaging [DWI]) or WMH (on FLAIR). An overview of typical image processing steps is shown in Fig. 1. Meta VCI Map provides technical support for each step, thereby allowing interested parties to contribute imaging data at different stages of processing.
Fig. 1.
Typical image and lesion processing pipeline for lesion-symptom mapping studies. Lesion-symptom mapping studies essentially require three image and lesion processing steps to prepare lesion maps. Examples are shown for three common imaging sequences: FLAIR, DWI, and CT. First, the lesion must be delineated on the original scan data (lesion segmentation). This can be done manually, or (semi-)automatically using computer algorithms. Next, the scan and corresponding lesion map are transformed to fit the size and shape of a brain template (spatial normalization). An intermediate registration step to an age-specific template is often used to improve registration accuracy. Finally, the resulting lesion map is projected onto the brain template. This result is compared to the original scan, to determine whether lesion registration was successful. Main criteria are that the key anatomical landmarks of the transformed scan and template should correspond and that the registered lesion map accurately represents the original lesion regarding location, size, and shape. The final lesion map can be used for group comparisons, unrestricted by type and format of the raw imaging data. Abbreviations: CT, computed tomography; DWI, diffusion-weighted imaging; FLAIR, fluid attenuated inversion recovery.
For image and lesion data processing, Meta VCI Map promotes the use of the RegLSM image processing pipeline, which was recently developed in a collaborative LSM project between Utrecht and Hong Kong [10]. RegLSM can transform lesion maps to fit onto a standardized brain template. For the present pilot study, the Montreal Neurological Institute (MNI)-152 template [63] was used, but this can be substituted by other brain templates depending on individual preference [64]. RegLSM provides custom-fit settings for CT [65], MRI T1 and FLAIR [11], and DWI [10] (Fig. 1). It can also process scans from different vendors and field strengths. Furthermore, it can correct for anatomical variation (e.g., ethnicity-based morphometric differences or brain atrophy) by performing intermediate transformation steps using population-adjusted templates. The output is the same for each setting, that is, lesion maps in standardized brain space, which allows lesion data from different imaging modalities (e.g., CT/MRI) and sequences (e.g., T1/FLAIR/DWI) to be combined into one data set.
RegLSM and other downloadable software packages, protocols, and tutorials will be provided on the Meta VCI Map website (see 2.1.1) to facilitate and harmonize data processing. Although we strive to implement standard pipelines across projects, ultimately the selection of particular (or combinations of) pipelines will be decided on a project-by-project basis, but always with rigorous quality controls. Our processing pipelines are in constant development; therefore, we encourage our members to share their tools and knowledge.
2.1.5. Data analysis
Meta-analysis of multicenter data can be challenging due to heterogeneity of data. For imaging data, the ability to detect and localize lesions depends, for example, on the imaging modality and sequence, and timing of assessment. For cognitive data, the wide variety of available neuropsychological test batteries and diagnostic criteria make this challenging to harmonize across cohorts. Meta VCI Map projects will account for heterogeneity by pooling data from cohorts that use internationally validated, commonly used cognitive tests (e.g., MoCA, Trail Making Test), or by recoding data into standardized cognitive outcomes (e.g., dementia yes/no). The data index will allow cohorts to be selected based on the availability of comparable data.
Meta VCI Map will implement state-of-the-art multivariate LSM analysis techniques, which show a higher sensitivity and better spatial accuracy than the original mass-univariate approach [66]. The consortium will facilitate the utilization of multivariate LSM methods by providing software and manuals on its website and offer hands-on support through the Data Analysis Working Group.
2.2. Pilot study
2.2.1. Objectives
As a pilot, we performed a multicenter LSM study in which we integrated data from different cohorts, to test the feasibility of data exchange, image processing, and analysis procedures. A second objective was to determine whether an integrated data set would improve brain lesion coverage.
2.2.2. Cohort and patient selection
The target population for the pilot was based on the responses to the initial survey. We aimed to include at least five different cohorts, to test our processing and analysis pipelines on different types of imaging data from different sources. The initial survey identified patients with ischemic stroke, with symptomatic infarcts as the lesion of interest and MoCA as cognitive outcome, as a suitable target population. This was operationalized in the following inclusion criteria: (1) patients admitted with acute ischemic stroke; (2) availability of brain imaging (CT/MRI) showing acute infarct(s); and (3) MoCA performed within 1 year after stroke. All eligible cohorts (per December 2017) were invited to participate.
2.2.3. Generation of lesion maps
Processing of imaging and lesion data was performed by the project team in Utrecht. Imaging data could be submitted in different formats and at different stages of processing, ranging from raw imaging data to fully prepared lesion maps.
For cohorts that provided raw imaging data, lesion segmentation was performed manually with in-house developed software using MeVisLab (MeVis Medical Solutions AG, Bremen, Germany) [67]. Infarcts were delineated on MRI DWI (<2 weeks after stroke) or FLAIR (≥2 weeks after stroke), or on CT (≥1 day after stroke) if no MRI was available. The acute (symptomatic) lesion was identified based on imaging features, which needed to be compatible with the poststroke interval. In cases of uncertainty based on imaging alone, the reported neurological deficit or the clinician's localization of the infarct was used to help localize the lesion. One trained rater performed lesion segmentation for all scans (E.M.H.), which was reviewed by a second rater (N.A.W.). In case of uncertainty regarding lesion location or classification, a third rater performed a consensus rating (J.M.B.). All raters were blinded to the neuropsychological data. Intraobserver and interobserver agreement were determined using the Dice Similarity Coefficient, which expresses the degree of overlap between the segmented regions on a scale from 0 to 1, on a random subset of 15 scans (5 for CT/FLAIR/DWI each) [68], [69]. Both interobserver (0.81 ± 0.08) and intraobserver (0.83 ± 0.07) agreement were excellent.
Next, segmented lesions were registered to the MNI-152 template using the RegLSM software package (see 2.1.4). Each registered lesion map underwent visual quality control. Lesion maps in MNI-152 space were compared to the original scan, to determine whether key anatomical landmarks corresponded, and whether the registered lesion map accurately represented the infarct regarding location, size, and shape. In case of minor errors, manual adjustments were made using ITK-SNAP v3.6.0 (www.itksnap.org) [70].
2.2.4. Neuropsychological assessment
Cognitive performance was assessed using the MoCA total score as measure of global cognition. In addition, a domain score for language was calculated by combining the scores for the animal picture naming and sentence repetition tasks [71]. This language domain score was included for comparative purposes, to determine whether our LSM analyses would identify different strategic infarct locations for distinct cognitive processes (i.e., global cognition vs. language).
2.2.5. Statistical analyses
Support vector regression (SVR)–based lesion-symptom mapping was performed using a previously published method [10]. Two independent SVR-based analyses were performed: voxel-based LSM (SVR-LSM) and region of interest-based analyses (SVR-ROI). The methodology of SVR-LSM and SVR-ROI is described extensively in the original study [10]. Further details are provided in the Supplementary Material. To allow for optimal comparison with the original study, we applied the same correction factors (i.e., age, sex, years of education, clinical history of stroke and/or TIA, and total infarct volume) and statistical analyses.
3. Results
3.1. Cohort selection and data transfer
In December 2017, nine cohorts of acute ischemic stroke patients with available MoCA scores were invited to participate in the pilot study. Within the time frame of 6 weeks, investigators from six cohorts responded and agreed to participate [10], [19], [26], [28], [30]. Data transfer was straightforward and completed within 2 months, though some regulatory challenges were encountered, mainly due to differences in the required documentation regarding ownership and traceability of data. This has led to further streamlining of data sharing procedures for future projects (see 2.1.2).
3.2. Patient selection and data processing
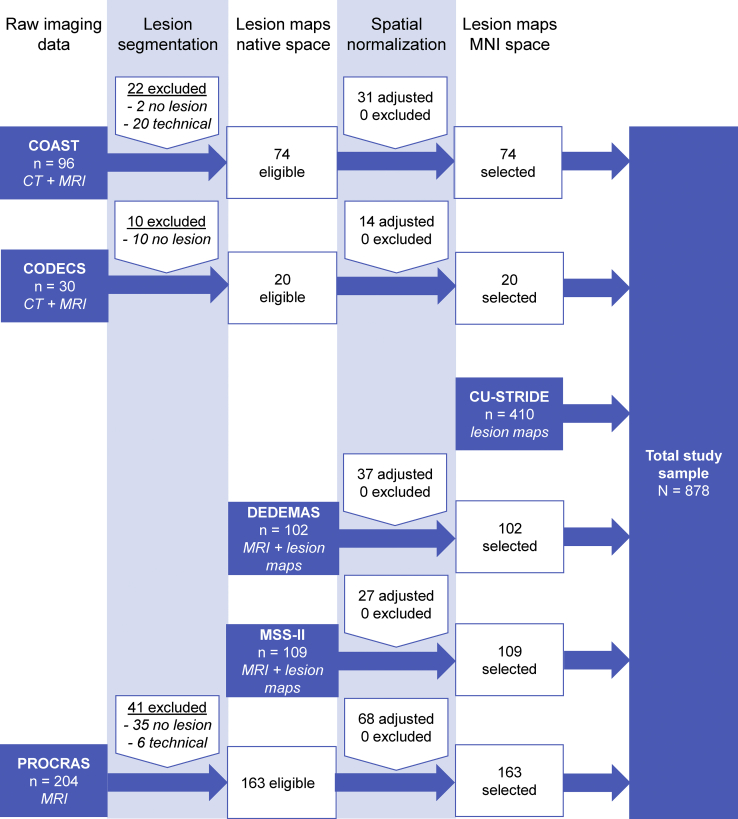
A flowchart of patient selection is shown in Fig. 2. Three cohorts provided raw imaging data (n = 330), two cohorts provided imaging data and lesion maps in native brain space (n = 211), and one cohort provided lesion data in MNI-152 space (n = 410). From the 330 patients with raw imaging data, 73 were excluded because the quality of imaging data was insufficient (n = 26), or if no acute infarct was visible (n = 47).
Fig. 2.
Flowchart of patient selection for the Meta VCI Map pilot study. Patient selection is shown for each cohort separately. Cohorts could join at any given step of image processing. The processing steps—congruent with the pipeline shown in Fig. 1—are shown at the top. Availability of clinical data was a prerequisite to be considered. The blue boxes indicate at what stage the cohort entered the pipeline, and what kind of imaging data was provided. Note that spatial normalization passed visual quality control in all cases, though (minor) manual adjustments were made in 177 (38%) cases.
Lesion segmentation was performed manually for 257 patients (35 DWI; 177 FLAIR; 45 CT). Spatial normalization was performed for 468 patients, which was successful in all cases. Manual adjustments were made in 177 cases, which was comparable for each cohort and type of imaging data. This resulted in a final study sample of 878 subjects. Characteristics of the total and individual study samples are shown in Table 2.
Table 2.
Patient characteristics of pilot study sample
| Characteristics | COAST (n = 74) | CODECS (n = 20) | CU-STRIDE (n = 410) | DEDEMAS (n = 102) | MSS-II (n = 109) | PROCRAS (n = 163) | Total sample (n = 878) |
|---|---|---|---|---|---|---|---|
| Relevant inclusion criteria | Acute ischemic stroke (within 14 days), age ≥21 years | Cerebellar stroke, age ≥18 years | Ischemic stroke, Chinese ethnicity, language: Cantonese | Acute ischemic stroke (within 3 days), age ≥18 years, language: German | Lacunar or mild cortical ischemic stroke (within 4 weeks), age ≥18 years, visible infarct | Acute ischemic stroke, age ≥50 years, language: Dutch | - |
| Relevant exclusion criteria | Significant aphasia or dysarthria, prior dementia, psychiatric comorbidity | Significant aphasia or severe dysarthria, prior cognitive impairment | Significant aphasia, prior dementia, psychiatric comorbidity | Prior dementia | None | Prior dementia, severe stroke requiring long-term care | - |
| Demographic characteristics | |||||||
| Age in years, mean ± SD | 58.4 ± 10.5 | 60.3 ± 16.7 | 68.6 ± 10.4 | 71.0 ± 8.7 | 65.6 ± 11.4 | 69.5 ± 0.76 | 67.6 ± 10.9 |
| Female, n (%) | 21 (28.4) | 10 (50.0) | 163 (39.8) | 35 (34.3) | 41 (37.6) | 53 (32.5) | 323 (36.8) |
| Education in years, mean ± SD | 6.8 ± 3.7 | 13.6 ± 4.6 | 5.9 ± 4.7 | 10.6 ± 2.1 | 12.1 ± 3.2 | 11.4 ± 2.5 | 8.5 ± 4.7 |
| Handedness, left/right/ambidext., n | N/A | 13/4/0 (n = 17)∗ | 396/7/7 | 95/6/1 | 97/12/0 | 153/6/4 | 754/35/12 (n = 801)∗ |
| Clinical history of stroke or TIA | 10 (13.5) | 0 (0.0) | 56 (13.7) | 17 (16.7) | 16 (14.7) | 34 (20.9) | 133 (15.1) |
| Cognitive assessment | |||||||
| MoCA total (max. 30), mean ± SD | 21.0 ± 5.0 | 24.9 ± 3.4 | 19.9 ± 5.9 | 25.0 ± 3.3 | 25.3 ± 3.5 | 22.0 ± 4.3 | 21.8 ± 5.4 |
| MoCA language (max. 5), mean ± SD | 3.9 ± 0.9 | 4.4 ± 0.8 | 4.3 ± 0.9 | 4.7 ± 0.6 | 4.8 ± 0.6 | 4.3 ± 0.9 | 4.4 ± 0.9 |
| Time point of MoCA assessment, n days after stroke onset, median (IQR) | 121 (47) | 3 months† | 154 (47)‡ | 2 (2) | 389 (43) (n = 107)∗ | 3 (3) | 134 (172) (n = 856)∗ |
| Brain imaging | |||||||
| Scan used for lesion segmentation, DWI/FLAIR/CT, n | 30/4/40 | 5/10/5 | 307/0/103 | 102/0/0 | 0/109/0 | 0/163/0 | 444/286/148 |
| Normalized acute infarct volume in milliliters, median (IQR) | 6.82 (30.59) | 16.59 (48.95) | 2.31 (11.99) | 2.52 (11.15) | 2.59 (8.74) | 4.30 (18.88) | 2.88 (13.19) |
| Time point of imaging, n days after stroke onset, median (IQR) | 2 (3) | 5 (24) (n = 18)∗ |
1 (2)‡ | 3 (3) | 4 (6) | 33 (14) | 3 (7) (n = 856)∗ |
Abbreviations: CT, computed tomography; DWI, diffusion-weighted imaging; FLAIR, fluid-attenuated inversion recovery; IQR, interquartile range; MoCA, Montreal Cognitive Assessment; SD, standard deviation; TIA, transient ischemic attack.
Missing data; available sample size (n) is noted behind the respective variable.
3 months is protocol for this study; no exact numbers available, therefore counted as missing data in total sample.
Days after admission to clinical ward (i.e., 0 to 3 days after stroke onset or presentation at emergency department), instead of days after stroke onset.
3.3. Brain lesion coverage
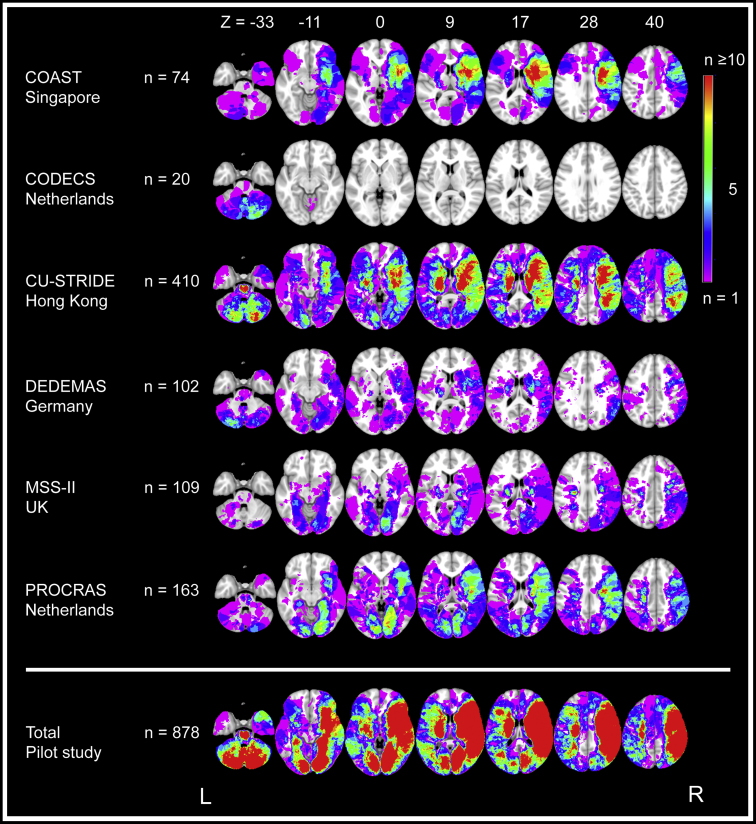
Lesion prevalence maps of individual cohorts and the collective study sample are shown in Fig. 3. Compared with CU-STRIDE, the largest of the six included cohorts (n = 410), lesion coverage in the merged data set (n = 878) showed an absolute increase of 26% (374,100–840,300 voxels; 20%–46% of the MNI-152 template, respectively). This increase was most prominent in the left hemisphere, right occipital lobe, and cerebellum. Meanwhile, coverage of the left hemisphere and orbitofrontal lobes was relatively limited. For the left hemisphere, this was likely due to aphasia being an exclusion criterion in most cohorts, because this would preclude reliable cognitive assessment. This is also reflected in the finding that left hemispheric infarcts were often smaller and did not cover cortical regions in the frontal and temporal lobes (Fig. 4A, B). For the orbitofrontal lobes, coverage was likely limited because anterior cerebral artery infarcts are relatively rare [9].
Fig. 3.
Lesion prevalence map for individual cohorts and the collective data set of the Meta VCI Map pilot study. Voxel-based lesion prevalence map of infarcts for individual cohorts and collective data set, shown on the Montreal Neurological Institute 152 T1 template [63]. Every voxel that is damaged in one or more subjects in the cohort is shown in colors ranging from purple (n = 1) to red (n ≥ 10). The right hemisphere is depicted on the right, which is conventional in lesion-symptom mapping studies. To prevent that lesion-symptom mapping analyses are biased by voxels that are only rarely affected, a minimum number of patients with a lesion in a particular voxel is commonly set. Although there is no general rule on where to set this threshold, it is typically set in the range of 5 ≤ n ≤ 10 [66]. In this figure, blue- and purple-colored voxels are damaged in less than five subjects and thus would normally be excluded from lesion-symptom mapping studies. The bottom lesion map was created by merging lesion maps from all the cohorts, which shows a considerably increased number of included voxels after integrating the data from all six cohorts. Note that the left hemisphere is relatively underrepresented; most cohorts used aphasia as an exclusion criterion because it precluded reliable cognitive assessment. Thus, subjects with (large) left hemispheric lesions were often excluded during initial inclusion of stroke patients.
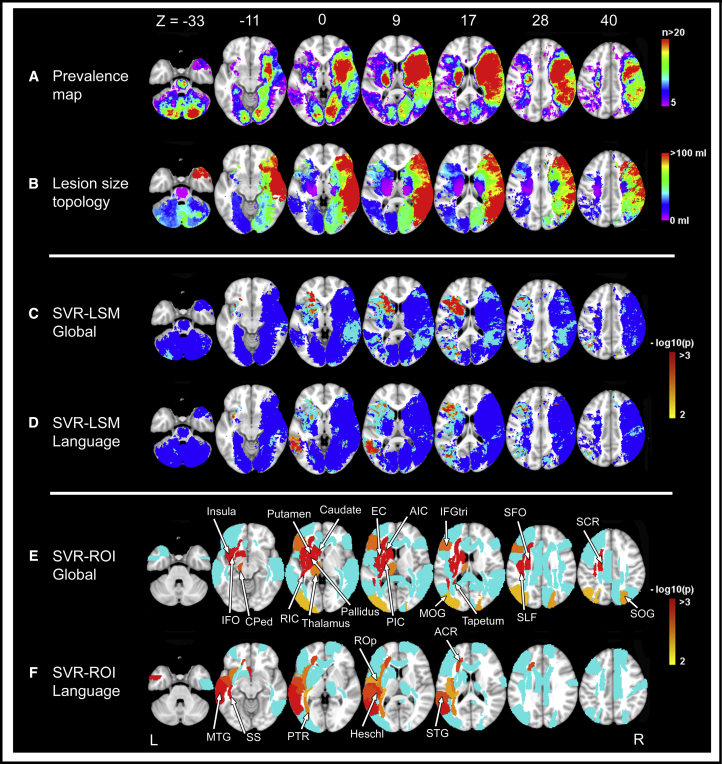
Fig. 4.
Lesion prevalence map and lesion-symptom mapping results. Lesion prevalence map (A), lesion size topographies (B), and SVR-LSM and SVR-ROI results (C–F). The right hemisphere is depicted on the right. (A) Lesion prevalence map showing voxels that are damaged in at least five patients is projected on the 1 mm MNI-152 template [63]. The bar indicates the number of patients with a lesion for each voxel. (B) Lesion size topographies for each voxel lesioned in at least five patients. The bar indicates the median lesion volume (in milliliters) per patient, given that the specific voxel is lesioned. This illustrates whether a particular voxel is more often damaged by relatively large infarcts (red) or small infarcts (purple). In the present study, right hemispheric infarcts were often larger and commonly included cortical areas, while infarcts in the thalamus, brain stem, and internal capsule were often small. (C–D) Results of multivariate lesion-symptom mapping. Voxelwise associations between the presence of a lesion and Montreal Cognitive Assessment (MoCA) total score (C) or language domain score (D) were determined using support vector regression (SVR-LSM). This multivariate approach assesses the intervoxel correlations and identifies which voxels have an independent contribution to the outcome measure. These associations are corrected for age, gender, and education. Significant clusters are shown in colors ranging from yellow (P = .01) to red (P < .001). To visualize the voxels that were included in each step of the analyses, voxels associated with cognition in the univariate analyses (without correction for multiple testing), but not in the multivariate analyses, are shown in light blue. Voxels with no univariate association with cognition are shown in dark blue and were not included in the multivariate analysis. Uncolored voxels were not included in any step of the analyses because these were damaged in less than five individuals. (E–F) Results of multivariate region of interest–based analyses using support vector regression (SVR-ROI). The ROIs where the regional infarct volume was statistically associated with the cognitive functions are colored from yellow (P = .01) to red (P < .001). ROIs that were associated with cognition in the univariate analyses but not in the multivariate analyses are shown in light blue. The names of the significant ROIs are labeled in the figure. Abbreviations: ACR, anterior corona radiata; AIC, anterior limb of internal capsule; CPed, cerebral peduncle; EC, external capsule; IFGtri, inferior frontal gyrus (triangular); IFO, inferior fronto-occipital fasciculus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; PIC, posterior limb of internal capsule; PTR, posterior thalamic radiation; RIC, retrolenticular part of internal capsule; ROp, rolandic operculum; SCR, superior corona radiata; SFO, superior fronto-occipital fasciculus; SLF, superior longitudinal fasciculus; SOG, superior occipital gyrus; SS, sagittal striatum; STG, superior temporal gyrus.
Lesion distributions differed between cohorts, reflective of differences in inclusion criteria and recruitment strategies (Table 2). For example, CODECS only included patients with cerebellar infarcts. Meanwhile, DEDEMAS and MSS-II included subjects with milder stroke symptoms, who generally had smaller infarcts that showed less overlap (Fig. 3).
3.4. Lesion-symptom mapping results
SVR-LSM and SVR-ROI results are shown in Fig. 4 and Supplementary Tables 1 and 2. Both methods identified significant associations between total MoCA scores and infarcts located in the left inferior frontal gyrus, left basal ganglia and their surrounding white matter tracts, left insula, and bilateral occipital lobes. Language domain scores were associated with infarcts located in the left middle and superior temporal gyri, left insula, left posterior thalamic radiation, and left anterior corona radiata.
4. Discussion
The Meta VCI Map consortium will provide new insights into the relevance of vascular lesion location for cognitive dysfunction, by creating a research framework that facilitates collaborative LSM projects. The pilot demonstrates that this novel multicenter LSM approach is feasible and substantially improves brain lesion coverage. Our image processing and LSM analysis pipelines showed that integration of multicenter patient data is feasible, allowing for inclusion of both CT and MRI scans from different data sets.
This pilot study demonstrates the benefits of multicenter LSM. First, we achieved a marked improvement of brain lesion coverage by integrating individual data sets. Compared with the CU-STRIDE study, one of the largest (monocenter) LSM studies to date (n = 410) [8], the increased coverage in our merged data set (n = 878) allowed us to identify strategic infarct locations in previously excluded brain areas. Furthermore, we provided a proof-of-concept that multicenter LSM can identify strategic lesion locations for distinct cognitive processes. For example, infarcts in the basal ganglia and internal capsule were associated with impairment of global cognition, which agrees with the known critical role of these structures [1], whereas infarcts in the left superior temporal lobe and frontal white matter were associated with language impairment, which agrees with well-established neural correlates for language [72], [73].
Several challenges for future multicenter LSM studies were also identified. First, achieving better brain lesion coverage of rarely affected brain regions, such as orbitofrontal regions for infarcts and temporal lobes for WMH, will require even larger study samples. However, although larger multicenter data sets will lead to an increase of statistical power by including more patients with a lesion in each voxel, it must be taken into account that regions with higher lesion frequency will be more likely to be found in the LSM analysis than voxels with a lower lesion frequency [74]. This spatial bias persists in larger data sets because vascular lesion distribution typically follows the vascular tree. Further improvement of LSM methods is therefore an ongoing endeavor of the Data Analysis Working Group. Second, preparation and processing of lesion data is labor intensive, particularly for infarcts because these require manual lesion segmentation and frequent manual adjustments after registration (approximately 25% of cases). In our experience, this issue is less prominent for WMH, where automated segmentation tools are available (e.g., at https://software.harness-neuroimaging.org) and adaptations are generally needed in <5% of subjects (e.g., [41], [75]). Nevertheless, efforts to reduce requirements for manual editing are a key priority of the Data Analysis Working Group. Third, although integration of different types of imaging data at different processing stages is technically feasible, this could introduce variability that must be taken into account, preferably through implementation of standardized protocols for each processing step. Fourth, our pilot study cohort had heterogeneous characteristics in terms of time point of cognitive assessment and inclusion criteria, which might impact our LSM results. Notably, the cognitive impact of acute versus chronic stroke is known to differ due to network reorganization and significantly influences lesion-symptom correlations [6], [7]. Finally, other contributing factors to cognitive decline, such as the presence and burden of other vascular lesions and premorbid cognitive status, should be taken into account in future projects. This should also include markers of neurodegeneration, which often accompany vascular lesions, particularly in older individuals. Of note, LSM techniques are well equipped to deal with such factors, for example, by including biomarkers for amyloid or tau in the analyses.
Future Meta VCI Map projects aim to include thousands of subjects to achieve even better brain lesion coverage. Several Meta VCI Map projects are in preparation. First, for patients with acute ischemic stroke, we aim to create a comprehensive vulnerability map of the brain that shows which infarct locations predict poststroke cognitive impairment, with particular focus on differences between early and late poststroke phases. Second, for infarcts and WMH, we aim to establish strategic lesion locations for domain-specific cognitive functions. Third, multidimensional LSM models will be implemented to study the interplay between MRI manifestations of vascular injury and other contributing factors to cognitive decline, such as risk factors and biomarkers for neurodegeneration.
Current members and other interested parties already show a great willingness to contribute data. Future projects will benefit from a growing Meta VCI Map membership base. Therefore, we invite all investigators who are interested and can contribute data to join Meta VCI Map.
Research in context.
-
1.
Systematic review: We reviewed published literature on lesion-symptom mapping in vascular cognitive impairment (VCI) using PubMed. Potential members for the consortium were identified and invited through existing collaborative networks in the VCI research field.
-
2.
Interpretation: Published lesion-symptom mapping studies proved to have limited lesion coverage. The Meta VCI Map consortium brings international researchers together to perform multicenter lesion-symptom mapping studies. Our initial inventory revealed a great willingness of investigators to share data. The pilot study demonstrates that multicenter data integration is feasible and markedly improves brain lesion coverage. This allows us to study the cognitive impact of vascular lesions in more brain regions.
-
3.
Future directions: Subsequent Meta VCI Map projects will include thousands of subjects to achieve even better brain lesion coverage, to comprehensively establish strategic lesion locations for cognitive dysfunction. Procedural challenges, also identified through the pilot, are being addressed to allow further upscaling of multicenter study samples.
Acknowledgments
N.A.W. was the study coordinator, contributed to the design of the consortium and the pilot study, analyzed the data, and wrote the manuscript. L.Z. was a coinvestigator, analyzed the data, and contributed to the writing of the manuscript. J.M.B. and H.J.K. were coinvestigators, contributed to the design of the consortium and the pilot study, and contributed to the writing of the manuscript. G.J.B. was the leading investigator and chairman of the consortium, conceived and designed the consortium and the pilot study, and wrote the manuscript. H.J.B., C.P.L.H.C., M.D., R.S.G., O.K.L.H., S.H., P.L.M.K., B.Y.K.L., J.S.L., V.C.T.M., and J.M.W. were members of the Meta VCI Map Steering Committee, who contributed to the design of the consortium and pilot study, and reviewed the content of the draft and final manuscript. Other listed authors contributed to the pilot study and/or were involved in data acquisition and analysis of the cohorts included in the pilot study. Authors included under the banner of “Meta VCI Map consortium” are representatives from the Meta VCI Map member groups, who provided information on their respective cohorts for presentation in the overview table, and reviewed and approved the final version of the article.
Meta VCI Map consortium—collaborating authors: ADNI (Team UC Davis) and UC Davis ADC Diversity Cohort: C. DeCarli, University of California at Davis; E.A. Fletcher, University of California at Davis; P. Maillard, University of California at Davis. ADNI (Team UCL), PRE-MCI, and YOAD: J. Barnes, University College London (UCL) Queen Square Institute of Neurology; C.H. Sudre, School of Biomedical Engineering and Imaging Sciences King's College, London Dementia Research Centre Institute of Neurology–UCL, and Department of Medical Physics and Biomedical Engineering–UCL; J.M. Schott, UCL Queen Square Institute of Neurology. Alzheimer Center Erasmus MC and Rotterdam Study: M.A. Ikram, Erasmus MC—University Medical Center Rotterdam; J.M. Papma, Erasmus MC—University Medical Center Rotterdam; R.M.E. Steketee, Erasmus MC—University Medical Center Rotterdam; M.W. Vernooij, Erasmus MC—University Medical Center Rotterdam. BIOSTROKE and STROKDEM: R. Bordet, University of Lille; R. Lopes, University of Lille. CAC-KCGMH and Kaohsiung Chang Gung Memorial Hospital stroke cohort: C.-W. Huang, Chang Gung Memorial Hospital Taiwan. Calgary Normative Study, FAVR and Brain IMPACT: R. Frayne, University of Calgary; C.R. McCreary, University of Calgary; E.E. Smith, University of Calgary; Calgary Normative Study group (list in Appendix). CASPER, Cognitive function after lacunar stroke MUMC study, and Blood-brain barrier in cerebral small vessel disease cohort: W. Backes, Maastricht UMC+; S. Köhler, Maastricht UMC+; R.J. van Oostenbrugge, Maastricht UMC+; J. Staals, Maastricht UMC+; F. Verhey, Maastricht UMC+. COAST, Harmonization and EDIS: C.Y. Cheng, Singapore National Eye Centre. CogFAST-UK: R.N. Kalaria, Newcastle University Institute of Neuroscience (more in Appendix). CROMIS-2: D. Werring, UCL Institute of Neurology. Linkou Chang Gung Memorial Hospital stroke cohort: J.L. Hsu, Chang-Gung University and Taipei Medical University; K.-L. Huang, Chang-Gung University. DANTE and PROSPER: J. van der Grond, Leiden University Medical Center; J.W. Jukema, Leiden University Medical Center; R.C. van der Mast, Leiden University Medical Center. De Hoogstraat Rehabilitation: T.C.W. Nijboer, Center of Excellence in Rehabilitation Medicine Utrecht, UMC Utrecht & De Hoogstraat Rehabilitation. Framingham Heart Study: Framingham Heart Study group. Hallym VCI cohort: K.-H. Yu, Hallym University. Parelsnoer Neurodegenerative diseases: The Dutch Parelsnoer Institute—neurodegenerative diseases group (list in Appendix). PRODEM-Austria and ASPS: R. Schmidt, Department of Neurology, Division of Neurogeriatrics, Medical University of Graz, Austria; L. Pirpamer, Department of Neurology, Division of Neurogeriatrics, Medical University of Graz, Austria. RISE1, RISE2, and RISE3: B.J. MacIntosh, Sunnybrook Research Institute Toronto, University of Toronto, and Heart and Stroke Foundation Canadian Partnership for Stroke Recovery; A.D. Robertson, Sunnybrook Research Institute Toronto, Heart and Stroke Foundation Canadian Partnership for Stroke Recovery, and University of Waterloo. RUN DMC: F.-E. de Leeuw, Radboud University Medical Center and Donders Institute for Brain, Cognition and Behaviour; A.M. Tuladhar, Radboud University Medical Center and Donders Institute for Brain, Cognition and Behavior. SABRE: N. Chaturvedi, UCL Institute of Cardiovascular Science; T. Tillin, UCL Institute of Cardiovascular Science. Sydney MAS: H. Brodaty, UNSW Sydney; P. Sachdev, UNSW Sydney. TABASCO: TABASCO group (list in Appendix). TRACE-VCI: F. Barkhof, Alzheimer Center Amsterdam, Vrije Universiteit Amsterdam, Amsterdam UMC; W.M. van der Flier, Alzheimer Center Amsterdam, Vrije Universiteit Amsterdam, Amsterdam UMC. USCOG: L.J. Kappelle, University Medical Center Utrecht.
The Meta VCI Map consortium is supported by Vici Grant 918.16.616 from ZonMw, The Netherlands, Organisation for Health Research and Development, to G.J.B.
Additional grant support pilot study cohorts: The CODECS study is supported by a grant from Stichting Coolsingel, the Netherlands. Funding for the PROCRAS study was obtained through ZonMw as part of the ‘TopZorg’ project in 2015 (grant # 842003011). The CU-STRIDE study was funded by the Health and Health Services Research Fund (0708041) of the Food and Health Bureau of the Government of the Hong Kong Special Administrative Region. The CU-RISK study was funded by General Research Fund (grant number GRF CUHK 471911), the National Key Research and Development Program of China (grant number 2016YFC1300600), and the Lui Che Woo Institute of Innovative Medicine, and Therese Pei Fong Chow Research Center for Prevention of Dementia (in memory of Donald H. K. Chow). The Mild Stroke Study 2 was funded by the Wellcome Trust (WT088134/Z/09/A), the Row Fogo Charitable Trust and Chest Heart & Stroke Scotland (long-term follow-up, ref no: Res14/A157). J.M.W. is supported by the Fondation Leducq Transatlantic Network of Excellence for the Study of Perivascular Spaces in Small Vessel Disease (ref no. 16 CVD 05) and the UK Dementia Research Institute at The University of Edinburgh. O.K.L.H. is supported by the College of Medicine and Veterinary Medicine at the University of Edinburgh and the Wellcome Trust.
Footnotes
The authors report no conflicts of interest.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.dadm.2019.02.007.
Contributor Information
Geert Jan Biessels, Email: g.j.biessels@umcutrecht.nl.
Meta VCI Map consortium:
C. DeCarli, E.A. Fletcher, P. Maillard, J. Barnes, C.H. Sudre, J.M. Schott, M.A. Ikram, J.M. Papma, R.M.E. Steketee, M.W. Vernooij, R. Bordet, R. Lopes, C.-W. Huang, R. Frayne, C.R. McCreary, E.E. Smith, W. Backes, S. Köhler, R.J. van Oostenbrugge, J. Staals, F. Verhey, C.Y. Cheng, R.N. Kalaria, D. Werring, J.L. Hsu, K.-L. Huang, J. van der Grond, J.W. Jukema, R.C. van der Mast, T.C.W. Nijboer, K.-H. Yu, R. Schmidt, L. Pirpamer, B.J. MacIntosh, A.D. Robertson, F.-E. de Leeuw, A.M. Tuladhar, N. Chaturvedi, T. Tillin, H. Brodaty, P. Sachdev, F. Barkhof, W.M. van der Flier, and L.J. Kappelle
Supplementary Data
References
- 1.Gorelick P.B., Scuteri A., Black S.E., Decarli C., Greenberg S.M., Iadecola C. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prins N.D., Scheltens P. White matter hyperintensities, cognitive impairment and dementia: An update. Nat Rev Neurol. 2015;11:157–165. doi: 10.1038/nrneurol.2015.10. [DOI] [PubMed] [Google Scholar]
- 3.Biesbroek J.M., Weaver N.A., Biessels G.J. Lesion location and cognitive impact of cerebral small vessel disease. Clin Sci. 2017;131:715–728. doi: 10.1042/CS20160452. [DOI] [PubMed] [Google Scholar]
- 4.Rorden C., Karnath H.O. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5:812–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- 5.Duering M., Zieren N., Hervé D., Jouvent E., Reyes S., Peters N. Strategic role of frontal white matter tracts in vascular cognitive impairment: A voxel-based lesion-symptom mapping study in CADASIL. Brain. 2011;134:2366–2375. doi: 10.1093/brain/awr169. [DOI] [PubMed] [Google Scholar]
- 6.Karnath H.-O., Rennig J. Investigating structure and function in the healthy human brain: validity of acute versus chronic lesion-symptom mapping. Brain Struct Funct. 2017;222:2059–2070. doi: 10.1007/s00429-016-1325-7. [DOI] [PubMed] [Google Scholar]
- 7.de Haan B., Karnath H.O. A hitchhiker's guide to lesion-behaviour mapping. Neuropsychologia. 2017:1–12. doi: 10.1016/j.neuropsychologia.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Tatemichi T.K., Desmond D.W., Stern Y., Paik M., Sano M., Bagiella E. Cognitive impairment after stroke: frequency, patterns, and relationship to functional abilities. J Neurol Neurosurg Psychiatry. 1994;57:202–207. doi: 10.1136/jnnp.57.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arboix A., García-Eroles L., Sellarés N., Raga A., Oliveres M., Massons J. Infarction in the territory of the anterior cerebral artery: clinical study of 51 patients. BMC Neurol. 2009;9:30. doi: 10.1186/1471-2377-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao L., Biesbroek J.M., Shi L., Liu W., Kuijf H.J., Chu W.W.C. Strategic infarct location for post-stroke cognitive impairment: A multivariate lesion-symptom mapping study. J Cereb Blood Flow Metab. 2018;38:1299–1311. doi: 10.1177/0271678X17728162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biesbroek J.M., Kuijf H.J., van der Graaf Y., Vincken K.L., Postma A., Mali W.P.T.M. Association between subcortical vascular lesion location and cognition: A voxel-based and tract-based lesion-symptom mapping study. The SMART-MR Study. PLoS One. 2013;8 doi: 10.1371/journal.pone.0060541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wardlaw J.M., Smith E.E., Biessels G.J., Cordonnier C., Fazekas F., Frayne R. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dichgans M., Wardlaw J., Smith E., Zietemann V., Seshadri S., Sachdev P. METACOHORTS for the study of vascular disease and its contribution to cognitive decline and neurodegeneration: An initiative of the Joint Programme for Neurodegenerative Disease Research. Alzheimer's Dement. 2016;12:1235–1249. doi: 10.1016/j.jalz.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim J., Kim N., Jang M.U., Han M., Kim S., Baek M.J. Cortical hubs and subcortical cholinergic pathways as neural substrates of poststroke dementia. Stroke. 2014;45:1069–1076. doi: 10.1161/STROKEAHA.113.004156. [DOI] [PubMed] [Google Scholar]
- 15.Ten Brink A.F., Matthijs Biesbroek J., Kuijf H.J., Van der Stigchel S., Oort Q., Visser-Meily J.M.A. The right hemisphere is dominant in organization of visual search-A study in stroke patients. Behav Brain Res. 2016;304:71–79. doi: 10.1016/j.bbr.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Ten Brink A.F., Biesbroek J.M., Oort Q., Visser-Meily J.M.A., Nijboer T.C.W. Peripersonal and extrapersonal visuospatial neglect in different frames of reference: A brain lesion-symptom mapping study. Behav Brain Res. 2019;356:504–515. doi: 10.1016/j.bbr.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 17.Ducroquet A., Leys D., Saabi A.A., Richard F., Cordonnier C., Girot M. Influence of chronic ethanol consumption on the neurological severity in patients with acute cerebral ischemia. Stroke. 2013;44:2324–2326. doi: 10.1161/STROKEAHA.113.001355. [DOI] [PubMed] [Google Scholar]
- 18.Douven E., Schievink S.H.J., Verhey F.R.J., van Oostenbrugge R.J., Aalten P., Staals J. The Cognition and Affect after Stroke - a Prospective Evaluation of Risks (CASPER) study: rationale and design. BMC Neurol. 2016;16:65. doi: 10.1186/s12883-016-0588-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong Y.H., Venketasubramanian N., Chan B.P.L., Sharma V.K., Slavin M.J., Collinson S.L. Brief screening tests during acute admission in patients with mild stroke are predictive of vascular cognitive impairment 3-6 months after stroke. J Neurol Neurosurg Psychiatry. 2012;83:580–585. doi: 10.1136/jnnp-2011-302070. [DOI] [PubMed] [Google Scholar]
- 20.Huijts M., Duits A., van Oostenbrugge R.J., Kroon A.A., de Leeuw P.W., Staals J. Accumulation of MRI markers of cerebral small vessel disease is associated with decreased cognitive function. A study in first-ever Lacunar stroke and hypertensive patients. Front Aging Neurosci. 2013;5:72. doi: 10.3389/fnagi.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allan L.M., Rowan E.N., Firbank M.J., Thomas A.J., Parry S.W., Polvikoski T.M. Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain. 2011;134:3716–3727. doi: 10.1093/brain/awr273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Charidimou A., Wilson D., Shakeshaft C., Ambler G., White M., Cohen H. The clinical relevance of microbleeds in stroke study (CROMIS-2): Rationale, design, and methods. Int J Stroke. 2015;10:155–161. doi: 10.1111/ijs.12569. [DOI] [PubMed] [Google Scholar]
- 23.Wilson D., Ambler G., Shakeshaft C., Brown M.M., Charidimou A., Al-Shahi Salman R. Cerebral microbleeds and intracranial haemorrhage risk in patients anticoagulated for atrial fibrillation after acute ischaemic stroke or transient ischaemic attack (CROMIS-2): A multicentre observational cohort study. Lancet Neurol. 2018;17:539–547. doi: 10.1016/S1474-4422(18)30145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang J., Wong A., Wang Z., Liu W., Au L., Xiong Y. Risk factors for incident dementia after stroke and transient ischemic attack. Alzheimer's Dement. 2015;11:16–23. doi: 10.1016/j.jalz.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Mok V.C.T., Lam B.Y.K., Wang Z., Liu W., Au L., Leung E.Y.L. Delayed-onset dementia after stroke or transient ischemic attack. Alzheimer's Dement. 2016;12:1167–1176. doi: 10.1016/j.jalz.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 26.Wollenweber F.A., Zietemann V., Rominger A., Opherk C., Bayer-Karpinska A., Gschwendtner A. The Determinants of Dementia After Stroke (DEDEMAS) Study: Protocol and pilot data. Int J Stroke. 2014;9:387–392. doi: 10.1111/ijs.12092. [DOI] [PubMed] [Google Scholar]
- 27.Yu K.-H., Cho S.-J., Oh M.S., Jung S., Lee J.-H., Shin J.-H. Cognitive impairment evaluated with vascular cognitive impairment harmonization standards in a multicenter prospective stroke cohort in Korea. Stroke. 2013;44:786–788. doi: 10.1161/STROKEAHA.112.668343. [DOI] [PubMed] [Google Scholar]
- 28.Wardlaw J.M., Makin S.J., Valdés Hernández M.C., Armitage P.A., Heye A.K., Chappell F.M. Blood-brain barrier failure as a core mechanism in cerebral small vessel disease and dementia: evidence from a cohort study. Alzheimer's Dement. 2017;13:634–643. [Google Scholar]
- 29.Robertson A.D., Crane D.E., Rajab A.S., Swardfager W., Marzolini S., Shirzadi Z. Exercise intensity modulates the change in cerebral blood flow following aerobic exercise in chronic stroke. Exp Brain Res. 2015;233:2467–2475. doi: 10.1007/s00221-015-4317-6. [DOI] [PubMed] [Google Scholar]
- 30.Aben H.P., Reijmer Y.D., Visser-Meily J.M., Spikman J.M., de Bresser J., Biessels G.J. A role for new brain magnetic resonance imaging modalities in daily clinical practice: Protocol of the prediction of cognitive recovery after stroke (PROCRAS) study. JMIR Res Protoc. 2018;7:e127. doi: 10.2196/resprot.9431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robertson A.D., Matta G., Basile V.S., Black S.E., Macgowan C.K., Detre J.A. Temporal and spatial variances in arterial spin-labeling are inversely related to large-artery blood velocity. Am J Neuroradiol. 2017;38:1555–1561. doi: 10.3174/ajnr.A5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bournonville C., Hénon H., Dondaine T., Delmaire C., Bombois S., Mendyk A.-M. Identification of a specific functional network altered in poststroke cognitive impairment. Neurology. 2018;90:e1879–e1888. doi: 10.1212/WNL.0000000000005553. [DOI] [PubMed] [Google Scholar]
- 33.Delattre C., Bournonville C., Auger F., Lopes R., Delmaire C., Henon H. Hippocampal deformations and entorhinal cortex ATROPHY as an anatomical signature of long-term cognitive impairment: From the MCAO rat model to the stroke patient. Transl Stroke Res. 2018;9:294–305. doi: 10.1007/s12975-017-0576-9. [DOI] [PubMed] [Google Scholar]
- 34.Ben Assayag E., Korczyn A.D., Giladi N., Goldbourt U., Berliner A.S., Shenhar-Tsarfaty S. Predictors for poststroke outcomes: The Tel Aviv Brain Acute Stroke Cohort (TABASCO) Study Protocol. Int J Stroke. 2012;7:341–347. doi: 10.1111/j.1747-4949.2011.00652.x. [DOI] [PubMed] [Google Scholar]
- 35.Biesbroek J.M., van Zandvoort M.J.E., Kuijf H.J., Weaver N.A., Kappelle L.J., Vos P.C. The anatomy of visuospatial construction revealed by lesion-symptom mapping. Neuropsychologia. 2014;62:68–76. doi: 10.1016/j.neuropsychologia.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 36.Jack C.R., Barnes J., Bernstein M.A., Borowski B.J., Brewer J., Clegg S. Magnetic resonance imaging in Alzheimer's Disease Neuroimaging Initiative 2. Alzheimers Dement. 2015;11:740–756. doi: 10.1016/j.jalz.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C.-W., Hsu S.-W., Tsai S.-J., Chen N.-C., Liu M.-E., Lee C.-C. Genetic effect of interleukin-1 beta (C-511T) polymorphism on the structural covariance network and white matter integrity in Alzheimer's disease. J Neuroinflammation. 2017;14:12. doi: 10.1186/s12974-017-0791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang C.-W., Hsu S.-W., Chang Y.-T., Huang S.-H., Huang Y.-C., Lee C.-C. Cerebral perfusion insufficiency and relationships with cognitive deficits in Alzheimer's disease: A multiparametric neuroimaging study. Sci Rep. 2018;8:1541. doi: 10.1038/s41598-018-19387-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aalten P., Ramakers I.H., Biessels G.J., de Deyn P.P., Koek H.L., OldeRikkert M.G. The Dutch Parelsnoer Institute - Neurodegenerative diseases; methods, design and baseline results. BMC Neurol. 2014;14:254. doi: 10.1186/s12883-014-0254-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Case N.F., Charlton A., Zwiers A., Batool S., McCreary C.R., Hogan D.B. Cerebral amyloid angiopathy is associated with executive dysfunction and mild cognitive impairment. Stroke. 2016;47:2010–2016. doi: 10.1161/STROKEAHA.116.012999. [DOI] [PubMed] [Google Scholar]
- 41.Biesbroek J.M., Weaver N.A., Hilal S., Kuijf H.J., Ikram M.K., Xu X. Impact of strategically located white matter hyperintensities on cognition in memory clinic patients with small vessel disease. PLoS One. 2016;11:1–17. doi: 10.1371/journal.pone.0166261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Archer H.A., MacFarlane F., Price S., Moore E.K., Pepple T., Cutler D. Do symptoms of memory impairment correspond to cognitive impairment: a cross sectional study of a clinical cohort. Int J Geriatr Psychiatry. 2006;21:1206–1212. doi: 10.1002/gps.1644. [DOI] [PubMed] [Google Scholar]
- 43.Pusswald G., Lehrner J., Hagmann M., Dal-Bianco P., Benke T., Marksteiner J. Gender-Specific Differences in Cognitive Profiles of Patients with Alzheimer's Disease: Results of the Prospective Dementia Registry Austria (PRODEM-Austria) J Alzheimer's Dis. 2015;46:631–637. doi: 10.3233/JAD-150188. [DOI] [PubMed] [Google Scholar]
- 44.Boomsma J.M.F., Exalto L.G., Barkhof F., van den Berg E., de Bresser J., Heinen R. Vascular cognitive impairment in a Memory Clinic Population: Rationale and design of the “Utrecht-Amsterdam Clinical Features and Prognosis in Vascular Cognitive Impairment” (TRACE-VCI) Study. JMIR Res Protoc. 2017;6:e60. doi: 10.2196/resprot.6864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slattery C.F., Zhang J., Paterson R.W., Foulkes A.J.M., Carton A., Macpherson K. ApoE influences regional white-matter axonal density loss in Alzheimer's disease. Neurobiol Aging. 2017;57:8–17. doi: 10.1016/j.neurobiolaging.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seiler S., Pirpamer L., Hofer E., Duering M., Jouvent E., Fazekas F. Magnetization transfer ratio relates to cognitive impairment in normal elderly. Front Aging Neurosci. 2014;6:263. doi: 10.3389/fnagi.2014.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt R., Lechner H., Fazekas F., Niederkorn K., Reinhart B., Grieshofer P. Assessment of cerebrovascular risk profiles in healthy persons: definition of research goals and the Austrian Stroke Prevention Study (ASPS) Neuroepidemiology. 1994;13:308–313. doi: 10.1159/000110396. [DOI] [PubMed] [Google Scholar]
- 48.Schmidt R., Fazekas F., Kapeller P., Schmidt H., Hartung H.P. MRI white matter hyperintensities: Three-year follow-up of the Austrian Stroke Prevention Study. Neurology. 1999;53:132–139. doi: 10.1212/wnl.53.1.132. [DOI] [PubMed] [Google Scholar]
- 49.Tsang A., Lebel C.A., Bray S.L., Goodyear B.G., Hafeez M., Sotero R.C. White matter structural connectivity is not correlated to cortical resting-state functional connectivity over the healthy adult lifespan. Front Aging Neurosci. 2017;9:144. doi: 10.3389/fnagi.2017.00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wong A., Law L.S.N., Liu W., Wang Z., Lo E.S.K., Lau A. Montreal cognitive assessment: One cutoff never fits all. Stroke. 2015;46:3547–3550. doi: 10.1161/STROKEAHA.115.011226. [DOI] [PubMed] [Google Scholar]
- 51.Hilal S., Ikram M.K., Saini M., Tan C.S., Catindig J.A., Dong Y.H. Prevalence of cognitive impairment in Chinese: Epidemiology of dementia in Singapore study. J Neurol Neurosurg Psychiatry. 2013;84:686–692. doi: 10.1136/jnnp-2012-304080. [DOI] [PubMed] [Google Scholar]
- 52.Au R., Massaro J.M., Wolf P.A., Young M.E., Beiser A., Seshadri S. Association of White Matter Hyperintensity Volume With Decreased Cognitive Functioning. Arch Neurol. 2006;63:246. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 53.Shepherd J., Blauw G.J., Murphy M.B., Cobbe S.M., Bollen E.L., Buckley B.M. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. Am J Cardiol. 1999;84:1192–1197. doi: 10.1016/s0002-9149(99)00533-0. [DOI] [PubMed] [Google Scholar]
- 54.Ikram M.A., van der Lugt A., Niessen W.J., Koudstaal P.J., Krestin G.P., Hofman A. The Rotterdam Scan Study: Design update 2016 and main findings. Eur J Epidemiol. 2015;30:1299–1315. doi: 10.1007/s10654-015-0105-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ikram M.A., Brusselle G.G.O., Murad S.D., van Duijn C.M., Franco O.H., Goedegebure A. The Rotterdam Study: 2018 update on objectives, design and main results. Eur J Epidemiol. 2017;32:807–850. doi: 10.1007/s10654-017-0321-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shibata D., Tillin T., Beauchamp N., Heasman J., Hughes A.D., Park C. African Caribbeans have greater subclinical cerebrovascular disease than Europeans: this is associated with both their elevated resting and ambulatory blood pressure and their hyperglycaemia. J Hypertens. 2013;31:2391–2399. doi: 10.1097/HJH.0b013e328364f5bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudre C.H., Smith L., Atkinson D., Chaturvedi N., Ourselin S., Barkhof F. Cardiovascular risk factors and white matter hyperintensities: Difference in susceptibility in South Asians compared with europeans. J Am Heart Assoc. 2018;7:e010533. doi: 10.1161/JAHA.118.010533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sachdev P.S., Brodaty H., Reppermund S., Kochan N.A., Trollor J.N., Draper B. The Sydney Memory and Ageing Study (MAS): Methodology and baseline medical and neuropsychiatric characteristics of an elderly epidemiological non-demented cohort of Australians aged 70-90 years. Int Psychogeriatrics. 2010;22:1248–1264. doi: 10.1017/S1041610210001067. [DOI] [PubMed] [Google Scholar]
- 59.Hinton L., Carter K., Reed B.R., Beckett L., Lara E., DeCarli C. Recruitment of a community-based cohort for research on diversity and risk of dementia. Alzheimer Dis Assoc Disord. 2010;24:234–241. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang C.E., Wong S.M., van de Haar H.J., Staals J., Jansen J.F.A., Jeukens C.R.L.P.N. Blood–brain barrier leakage is more widespread in patients with cerebral small vessel disease. Neurology. 2017;88:426–432. doi: 10.1212/WNL.0000000000003556. [DOI] [PubMed] [Google Scholar]
- 61.Moonen J.E.F., Foster-Dingley J.C., de Ruijter W., van der Grond J., Bertens A.S., van Buchem M.A. Effect of discontinuation of antihypertensive treatment in elderly people on cognitive functioning—the DANTE Study Leiden. JAMA Intern Med. 2015;175:1622. doi: 10.1001/jamainternmed.2015.4103. [DOI] [PubMed] [Google Scholar]
- 62.van Norden A.G., de Laat K.F., Gons R.A., van Uden I.W., van Dijk E.J., van Oudheusden L.J. Causes and consequences of cerebral small vessel disease. The RUN DMC study: A prospective cohort study. Study rationale and protocol. BMC Neurol. 2011;11:29. doi: 10.1186/1471-2377-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fonov V., Evans A.C., Botteron K., Almli C.R., McKinstry R.C., Collins D.L. Unbiased average age-appropriate atlases for pediatric studies. Neuroimage. 2011;54:313–327. doi: 10.1016/j.neuroimage.2010.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.de Haan B., Karnath H.O. ‘Whose atlas I use, his song I sing?’ – The impact of anatomical atlases on fiber tract contributions to cognitive deficits after stroke. Neuroimage. 2017;163:301–309. doi: 10.1016/j.neuroimage.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 65.Kuijf H.J., Biesbroek J.M., Viergever M.A., Biessels G.J., Vincken K.L. Springer; Cham: 2013. Registration of Brain CT Images to an MRI Template for the Purpose of Lesion-Symptom Mapping; pp. 119–128. [Google Scholar]
- 66.Sperber C., Karnath H.O. On the validity of lesion-behaviour mapping methods. Neuropsychologia. 2017;115:17–24. doi: 10.1016/j.neuropsychologia.2017.07.035. [DOI] [PubMed] [Google Scholar]
- 67.Ritter F., Boskamp T., Homeyer A., Laue H., Schwier M., Link F. Medical image analysis. IEEE Pulse. 2011;2:60–70. doi: 10.1109/MPUL.2011.942929. [DOI] [PubMed] [Google Scholar]
- 68.Dice L.R. Measures of the amount of ecologic association between species. Ecology. 1945;26:297–302. [Google Scholar]
- 69.Crum W.R., Camara O., Hill D.L.G. Generalized Overlap Measures for Evaluation and Validation in Medical Image Analysis. IEEE Trans Med Imaging. 2006;25:1451–1461. doi: 10.1109/TMI.2006.880587. [DOI] [PubMed] [Google Scholar]
- 70.Yushkevich P.A., Piven J., Hazlett H.C., Smith R.G., Ho S., Gee J.C. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 71.Lam B., Middleton L.E., Masellis M., Stuss D.T., Harry R.D., Kiss A. Criterion and convergent validity of the montreal cognitive assessment with screening and standardized neuropsychological testing. J Am Geriatr Soc. 2013;61:2181–2185. doi: 10.1111/jgs.12541. [DOI] [PubMed] [Google Scholar]
- 72.Mirman D., Chen Q., Zhang Y., Wang Z., Faseyitan O.K., Coslett H.B. Neural organization of spoken language revealed by lesion-symptom mapping. Nat Commun. 2015;6:6762. doi: 10.1038/ncomms7762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dronkers N.F., Wilkins D.P., Van Valin R.D., Redfern B.B., Jaeger J.J. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 74.Kimberg D.Y., Coslett H.B., Schwartz M.F. Power in Voxel-based Lesion – Symptom Mapping. J Cogn Neurosci. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- 75.Leeuwis A.E., Weaver N.A., Biesbroek J.M., Exalto L.G., Kuijf H.J., Hooghiemstra A.M. Impact of white matter hyperintensity location on depressive symptoms in memory clinic patients: a lesion-symptom mapping study. J Psychiatry Neurosci. 2019 doi: 10.1503/jpn.180136. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.