Abstract
Background
The purpose of this study was to analyze the effect of breast cancer therapy on fertility concerns and sexuality of young mothers with breast cancer in Germany.
Methods
During a mother-child rehabilitation program, 1,191 young mothers with locoregional primary breast cancer, treated between 2006 and 2014, were recruited. Data included sociodemographic data, TNM stage, tumor biology, therapies, and patient-reported outcomes such as sexuality and fertility concerns.
Results
The mean age at diagnosis was 40 years. Approximately a quarter of the patients stated that family planning had not been completed at the time of diagnosis. Nearly half of all patients had been informed as to how treatment could affect fertility, but counseling at a specialized fertility center was offered to only 13%. Of all patients, 4% took a consultation and 2% underwent fertility preservation procedures.
Conclusion
Our study indicates that only a minority of patients is referred to fertility centers although family planning is incomplete at the time of diagnosis in about 25% of young women with breast cancer. Thus, these patients should not only be informed about the effects of treatment on fertility and sexuality, but should be referred to a fertility center.
Key Words: Breast cancer, Fertility, Sexuality, Family planning
Introduction
With an annual incidence of 72,000 cases in Germany, breast cancer (BC) remains the most frequent malignancy in women. About every 10th woman diagnosed with BC in Germany is younger than 45 years [1] and may not yet have completed her family planning. Younger women are often affected by a more aggressive form of the disease [2], and BC is the most common cause of death in these women [1].
Chemotherapy and anti-hormonal therapy impact fertility either temporarily or permanently [3]. The risk of chemotherapy-induced amenorrhea (CIA) is well known in BC survivors. The destruction of the ovarian reserve can cause premature ovarian failure [4], and the risk for patients younger than the age of 40 years to experience CIA is estimated to range between 10 and 86% [5,6,7].
In view of fertility-threatening effects of BC therapies, the German guideline recommends that all premenopausal women should be informed about the potentially negative side effects of treatment as well as the possibility of ovarian protection [5,8]. In 2006, the so-called FertiPROTEKT network was established in Germany [9]. Within this program, patients are informed about the effects of anticancer treatment on fertility. If necessary and desired, fertility preservation procedures with the use of assistive reproductive technology are performed. In 2015, 76 centers participated in FertiPROTEKT and about 1,000 patients were counseled, among them approximately 425 patients with BC. More than 80% of those patients were childless at the time of diagnosis [10].
Although current guidelines recommend counseling of all BC patients regardless of parenthood, little is known about the rate of counselling and referral to fertility centers if patients are already parents. Therefore, we endeavored to assess the effect of BC diagnosis and therapy on fertility concerns and sexuality, as well as the referral rate for appointments and treatments at fertility centers. Our study focuses on young mothers with BC in Germany.
Patients and Methods
Study Population of Young Mothers with BC and Data Collection
The rehabilitation clinic ‘Klinik Ostseedeich’ in Groemitz, Northern Germany, offers a stationary 3-week mother-child rehabilitation program for women with primary locoregional BC, who have at least 1 child up to the age of 12.
We consecutively recruited 1,191 young mothers with invasive non-metastatic BC diagnosed between 2006 and 2014, who participated in the rehabilitation program between 2010 and 2015. The mean time between diagnosis and rehabilitation/survey was 13 months (standard deviation (SD) 6 months).
Data were obtained from patients' records and questionnaires. Age, number of children, date of diagnosis, TNM stage, tumor biology, and treatment details were retrieved from the records. The questionnaire covered sexual concerns and activities, changes in sex life, and menopausal status. The desire for parenthood (current and at the time of diagnosis) as well as queries regarding sociodemographic factors were also included. The questionnaire contained the standardized, validated BC-specific European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Breast Cancer Module (EORTC QLQ-BR23) [11]. 2 scales of the EORTC QLQ-BR23 (sexual function and sexual enjoyment) were included in our analysis [11]. Only patients who had been sexually active in the last 4 weeks were asked about sexual enjoyment. The final scores of the 2 scales range from 0 to 100, with 100 indicating the highest function and enjoyment. Further topics were professional counseling on fertility aspects and potentially feasible fecundity measures, and whether a pregnancy had occurred after BC.
Reference Population of Women with BC and Data Collection
The OVIS study evaluated the oncological health care of 1,927 patients who were diagnosed with primary BC in the federal state of Schleswig-Holstein (2001-2004). Recruitment was performed via the population-based cancer registry of Schleswig-Holstein (response rate 80%). The participants were representative for all BC patients in Schleswig-Holstein [12], and those with primary invasive non-metastatic BC (n = 1,879) were used as an age-heterogeneous comparison group. The mean time between diagnosis and survey was 18 months (SD 7 months).
Statistical Analysis
Absolute and relative frequencies for qualitative data as well as common location and dispersion measures for quantitative measures were used. For subgroup analysis, we stratified our cohort of young mothers with BC by age: <36, 36-39, and ≥40 years.
The EORTC scales were computed according to the manual [13]. The differences between groups were interpreted as clinically relevant if equaling 10 points or more [14].
Data was analyzed using IBM SPSS 22.0 (IBM Corp., Armonk, NY, USA).
Ethics
The study protocol was approved by the local ethics committee (main protocol: reference no. 10-096; Amendment ‘Fertility’). All patients provided informed written consent.
Results
Tumor Characteristics and Treatment
The young mothers with BC had been diagnosed between 2006 and 2014. The mean time between diagnosis and participation in the rehabilitation program was 13 months (SD 5.9). The OVIS patients were diagnosed between 2001 and 2004. Table 1 shows the distribution of TNM status, tumor biology, menopausal status, and civil status. The young patients were more likely to have advanced lymph node involvement and tumors with lower grading compared to the OVIS participants.
Table 1.
Absolute and relative frequencies of characteristics of young mothers and OVIS participants with breast cancer (BC)
| Young mothers with BC |
Women with BC from OVIS study | ||||
|---|---|---|---|---|---|
| total | < 36 years | 36–39 years | ≥ 40 years | ||
| Women, n | 1,191 | 266 | 320 | 605 | 1,879 |
| Time period of diagnosis | 2006–2014 | 2001–2004 | |||
| Age at diagnosis, mean + SD | 39.6 + 5.5 | 32.1 + 2.51 | 37.5 + 1.14 | 44.1 + 3.04 | 58.8 + 11.3 |
| (range), years | (21–54) | (21–35) | (36–39) | (40–54) | (25–85) |
| Age group, n (%) | |||||
| <36 years | 266 (22.3) | n.a. | n.a. | n.a. | 32 (1.7) |
| 36–39 years | 320 (26.9) | 70 (3.7) | |||
| ≥40 years | 605 (50.8) | 1,777 (94.6) | |||
| Menopausal status, n (%) | |||||
| Pre | 180 (16.1) | 51 (20.6) | 51 (16.8) | 78 (13.7) | n.a. |
| Peri | 143 (12.8) | 51 (20.6) | 49 (16.1) | 43 (7.6) | |
| Post | 798 (71.2) | 146 (58.9) | 204 (67.1) | 448 (78.7) | |
| Missing | 70 | 18 | 16 | 36 | |
| Civil status, n (%) | |||||
| Single | 185 (15.6) | 48 (18.2) | 51 (16.1) | 86 (14.3) | 118 (6.3) |
| Married | 832 (70.3) | 200 (75.8) | 223 (70.6) | 409 (67.8) | 1,234 (66.0) |
| Divorced | 150 (12.7) | 16 (6.1) | 41 (13.0) | 93 (15.4) | 191 (10.2) |
| Widowed | 16 (1.4) | - | 1 (0.3) | 15 (2.5) | 328 (17.5) |
| Missing | 8 | 2 | 4 | 2 | 8 |
| Tumor size, n (%) | |||||
| T0 | 85 (7.2) | 32 (12.2) | 26 (8.2) | 27 (4.5) | - |
| T1 | 591 (50.1) | 126 (47.9) | 161 (50.9) | 304 (50.6) | 1,043 (56.2) |
| T2 | 434 (36.8) | 87 (33.1) | 111 (35.1) | 236 (39.3) | 667 (36.0) |
| T3 | 62 (5.3) | 16 (6.1) | 17 (5.4) | 29 (4.8) | 87 (4.7) |
| T4 | 8 (0.7) | 2 (0.8) | 1 (0.3) | 5 (0.8) | 58 (3.1) |
| Tx | 11 | 3 | 4 | 4 | 22 |
| Axillary lymph node status, n (%) | |||||
| N0 | 682 (57.9) | 155 (59.2) | 187 (59.4) | 340 (56.7) | 1,184 (65.2) |
| N1 | 337 (28.6) | 77 (29.4) | 82 (26.0) | 178 (29.7) | 568 (31.3) |
| N2 | 108 (9.2) | 18 (6.9) | 32 (10.2) | 58 (9.7) | 60 (3.3) |
| N3 | 50 (4.2) | 12 (4.6) | 14 (4.4) | 24 (4.0) | 5 (0.3) |
| Nx | 14 | 4 | 5 | 5 | 62 |
| Grading, n (%) | |||||
| G1 | 71 (6.2) | 7 (2.7) | 17 (5.6) | 47 (8.1) | 208 (11.5) |
| G2 | 529 (46.3) | 91 (35.7) | 127 (41.5) | 311 (53.5) | 1,065 (58.9) |
| G3 | 542 (47.5) | 157 (61.6) | 162 (52.9) | 223 (38.4) | 536 (29.8) |
| Gx | 49 | 11 | 14 | 24 | 70 |
| Receptor status, n (%) | |||||
| ER-positive | 833 (70.4) | 142 (53.8) | 209 (65.5) | 482 (80.2) | n.a. |
| Missing | 7 | 1 | 1 | 4 | |
| PR-positive | 747 (63.1) | 128 (48.3) | 182 (56.9) | 437 (73.0) | |
| Missing | 8 | 1 | 1 | 6 | |
| HER2/neu-positive | 282 (24.1) | 75 (29.0) | 90 (28.5) | 117 (19.7) | |
| Missing | 23 | 7 | 4 | 12 | |
| Triple-negative | 243 (20.6) | 89 (33.8) | 70 (21.9) | 84 (14.0) | |
| Missing | 10 | 3 | 1 | 6 | |
SD = Standard deviation; n.a. = not available; ER = estrogen receptor; PR = progesterone receptor.
Young mothers with BC were treated more aggressively and received mastectomy (either with or without reconstruction) and chemotherapy more often. Nearly all young mothers with hormone receptor-positive tumors received anti-hormonal therapy (table 2).
Table 2.
Absolute and relative frequencies of treatment modalities of young mothers and the OVIS participants with breast cancer (BC)
| Young mothers with BC, n (%) |
Women with BC from OVIS study (n = 1,879) | ||||
|---|---|---|---|---|---|
| total (n = 1,191) | <36 years (n = 266) | 36–39 years (n = 320) | ≥40 years (n = 605) | ||
| Breast Surgery | |||||
| Mastectomy | 212 (17.1) | 39 (14.8) | 67 (21.4) | 106 (17.9) | 532 (29.2) |
| Mastectomy with reconstruction | 246 (21.0) | 76 (28.9) | 66 (21.1) | 104 (17.5) | n.a. |
| BCT | 711 (60.8) | 148 (56.3) | 180 (57.5) | 383 (64.6) | 1,288 (70.8) |
| Missing | 22 | 3 | 7 | 12 | 59 |
| pT1 with BCT | 395 (67.9) | 81 (64.3) | 95 (60.1) | 219 (73.2) | 849 (83.7) |
| Missing | 9 | 1 | 3 | 5 | 29 |
| Axilla Surgery | |||||
| Overall | 1,144 (100) | 263 (100) | 310 (100) | 579 (100) | 1,681 (90.8) |
| SLNB | 627 (56.8) | 127 (49.8) | 165 (53.2) | 335 (57.9) | |
| Axilla dissection | 287 (25.1) | 84 (32.9) | 83 (26.8) | 120 (20.7) | |
| SLNB + axilla dissection | 230 (20.1) | 44 (17.3) | 62 (20.0) | 124 (21.4) | |
| Missing | 47 | 3 | 10 | 26 | 28 |
| Radiation | |||||
| Overall | 1,024 (86.0) | 219 (82.3) | 279 (87.2) | 526 (86.9) | 1,595 (84.9) |
| Missing | 0 | 0 | 0 | 0 | 21 |
| Radiation after BCT | 700 (98.5) | 147 (99.3) | 177 (98.3) | 376 (98.2) | 1,248 (97.6) |
| Missing | 0 | 0 | 0 | 8 | 9 |
| Chemotherapy | |||||
| Overall | 1,061 (89.2) | 260 (97.7) | 297 (92.8) | 504 (83.3) | 1,072 (59.3) |
| Missing | 1 | 0 | 1 | 0 | 71 |
| Anti-endocrine therapy | |||||
| Overall | 865 (72.9) | 158 (59.6) | 223 (69.9) | 484 (80.4) | 1,351 (76.3) |
| SERM (tamoxifen) | 830 (96.0) | 153 (96.8) | 217 (97.3) | 460 (95.0) | |
| AI | 44 (5.1) | 6 (3.8) | 5 (2.2) | 33 (6.8) | |
| GnRH analogues | 267 (30.9) | 104 (65.8) | 108 (48.4) | 55 (11.4) | |
| Missing | 5 | 1 | 1 | 3 | 122 |
| Antibody therapy (trastuzumab) | |||||
| Overall | 264 (23.5) | 74 (29.7) | 85 (27.7) | 105 (18.5) | n.a. |
| Missing | 68 | 17 | 13 | 38 | |
BCT = Breast-conserving therapy; SLNB = sentinel lymph node biopsy; SERM = selective estrogen receptor modulator; AI = aromatase inhibitor; GnRH = gonadotropin-releasing hormone; n.a. = not available.
Amenorrhea
Of 1,121 young mothers who provided information on their menstruation, 54.4% experienced amenorrhea prior to cancer diagnosis and 16.8% post cancer treatment. A further 13% experienced irregular periods. Regular menstrual cycles before and after cancer treatment were reported by only 16% of the young mothers. The likelihood of amenorrhea increased with age (table 1) and with receiving chemotherapy (75 vs. 41%).
Desire to Have Children and Professional Counseling
Approximately a quarter of the young mothers indicated that their family planning had not been completed at the time of diagnosis, with the highest proportion among the youngest patients (table 3). Nearly two-thirds of those women with current family planning activities would have liked to have been counseled, but counseling at a fertility center was offered to only 45% (12% in total). The age at time of diagnosis was a factor influencing the offer of advice. When stratified by time of diagnosis (early years up to 2010 vs. later years), the proportion of women to whom counseling in a fertility center was offered increased from 23.5 to 34%.
Table 3.
Frequencies of young mothers’ agreement with statements on fertility, family planning, counseling, and fertility preservation procedures (overall and by age groups)
| Young mothers with breast cancer, n (%) |
||||
| total
(n = 1,153) |
<36 years
(n = 254) |
36–39 years
(n = 310) |
≥40 years
(n = 589) |
|
|---|---|---|---|---|
| At the time of diagnosis my family plans had not been finally completed | 294/1,153 (25.5) | 139/254 (54.7) | 93/310 (30.0) | 62/589 (10.5) |
| At the time of diagnosis family plans ‘played a role’ | 248/1,129 (22.0) | 115/249 (46.2) | 79/307 (25.7) | 54/573 (9.4) |
| 248/293 (84.6)a | 115/138 (83.3)a | 79/93 (84.9)a | 54/62 (87.1)a | |
| (My family plans had not been completed and) I would have liked to be counseled | 179/288 (62.2) | 88/136 (64.7) | 58/92 (63.0) | 33/60 (55.0) |
| I was informed about how diagnosis and treatment could affect my fertility | 540/1,082 (49.9) | 180/249 (72.3) | 174/302 (57.6) | 186/531 (35.0) |
| Counseling at a specialized (family planning) center was offered to me | 130/1,066 (12.2) | 70/248 (28.2) | 42/298 (14.1) | 18/520 (3.5) |
| I was counseled at a specialized center | 41/1,066 (3.8) | 27/249 (10.8) | 11/296 (3.7) | 3/521 (0.6) |
| I have undergone fertility protecting procedures | 26/1,069 (2.4) | 19/248 (7.7) | 6/299 (2.0) | 1/522 (0.2) |
| Right now, I have family plans | 116/1,122 (10.3) | 63/252 (25.0) | 35/305 (11.5) | 18/565 (3.2) |
| Right now, I am trying to get pregnant | 10/1,112 (0.9) | 2/252 (0.8) | 4/307 (1.3) | 4/553 (0.7) |
| After my breast cancer diagnosis, I was pregnant | 28/1,110 (2.5) | 13/251 (5.1) | 8/306 (2.6) | 7/553 (1.3) |
| After my breast cancer diagnosis, I was pregnant and gave birth to a child | 23/28 (82.1) | 11/13 (84.6) | 6/8 (75.0) | 6/7 (85.7) |
Considering only those women who had not completed family planning at the time of diagnosis.
A minority of young BC patients received an appointment and counseling at a fertility center and underwent fertility preservation procedures. Again, age was an influential factor. A current desire for children was reported by 10.3% of the women; 2.5% got pregnant after the diagnosis (table 3). With the passage of time post diagnosis, the likelihood of pregnancy and birth increased in our study participants (diagnosis in early years (2006-2010): 10.7% of women gave birth to a child; diagnosis in later years (2013-2014): 2.5%).
Stratification by treatment modalities indicated no clear trend. The observed differences were rather small and not clinically relevant. General information on how diagnosis and treatment might affect fertility and sexuality was given to roughly 50% of all patients. The offer of counseling and the likelihood to be counseled and to undergo fertility-protecting procedures was influenced by having lymph node involvement and the necessity to receive chemotherapy (data not shown). When young mothers with estrogen-positive tumors, either with or without endocrine treatment, were compared, the differences regarding general information and the offer of counseling were only small. Again, only a minority of women with endocrine treatment visited a fertility center (2.8%) and underwent fertility preservation procedures (0.9%).
Sexuality
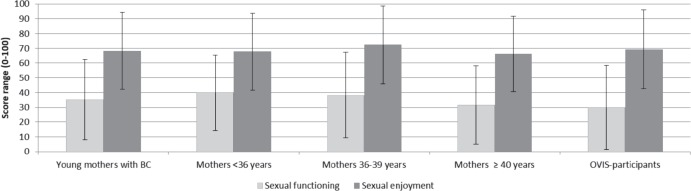
Young mothers with BC scored a mean of 35.2 on the sexual function scale, which was a little higher than the OVIS participants. Overall, women from our study population and the reference population rated their sexual enjoyment as high. No differences according to age were found either in sexual functioning or enjoyment among young mothers (fig. 1).
Fig. 1.
Comparison of sexual functioning and sexual enjoyment (EORTC QLQ-BR23) in young mothers and OVIS participants with breast cancer (BC) (mean values and standard deviation, high values indicate higher functioning and enjoyment).
When stratified by treatment, no clinically relevant differences in terms of sexuality were found for type of surgery, endocrine treatment (yes/no), and chemotherapy (yes/no; data not shown) among young mothers. The scores of sexual function, however, slightly missed the threshold of clinical relevance (mean value in women without chemotherapy: 42.8, with chemotherapy: 33.1).
Discussion
BC is the most prevalent type of cancer in adult women younger than 40 years. Young BC patients are of special interest to the scientific world because of their roles in society as well as the nature of their tumor characteristics. However, the scientific literature on this group is sparse. Topics of sexuality and fertility are becoming more important as life expectancy and quality of life after BC are increasing. These topics require individual enlightenment as well as adapted therapy concepts. Therefore, the treating physician has to weigh the benefits of therapy against the potential harms. This includes being aware of the fact that chemotherapy can damage the ovaries, which may result in a temporary or permanent loss of fertility [3,4].
Desire to Have Children and Professional Counseling
Despite the recommendations that all women who receive chemotherapy prior to the age of 40 and may suffer a loss of ovarian function should be offered a consultation on fertility measures [9], our results show a different reality. A quarter of young mothers indicated that there had been a wish for parenthood at the time of BC diagnosis. The effect of the planned therapy on fertility, however, was discussed with only half of these (chemotherapy-receiving) patients. Consultation in a specialized center was offered to approximately 10%. Among those patients with endocrine treatment, nearly 20% stated that their family planning had not been completed. Approximately 10% of those women were offered counseling, with 3% following through.
Fertility preservation procedures were carried out in 10.5% of those with the desire to have children. This could be due to the fact that such procedures are not covered by health insurance or because cancer treatment was of the highest priority at the time of diagnosis. Additional reasons could include the fact that our BC patients were already mothers, as well as the possible fear of BC progression.
Our findings are in accordance with a Swedish study on fertility-related counseling of a mixed-sex cancer collective: while 48%, aged 18-45 years, received information about the effects of their therapy on fertility, only 14% were informed about fertility preservation procedures [15].
Evidence supports that childbearing after BC is not contraindicated [16]. Still, many young BC survivors reported fears that pregnancy may enhance cancer recurrence, complicate the detection of significant breast changes during pregnancy [17], or result in birth defects because of prior exposure to cytotoxic agents and/or radiation [18].
Fortunately, over the past 10 years, a significant increase in counseling has taken place. Nearly a doubling in counseling rates on fertility and fertility preservation procedures within FertiPROTEKT has occurred, and close to 5% of women has given birth after BC treatment [10]. This increase is also evident in our survey with a higher referral rate for women with more recent diagnoses. The pregnancy rate, however, is lower (2.5%).
Sexuality
Young BC patients reported low to intermediate mean values for sexual function, but higher values for sexual enjoyment. However, no clinically relevant results in sexual functioning and enjoyment were found with regard to surgical procedures, chemotherapy, or endocrine therapy, different age groups, or when compared to older BC patients from our reference population.
Analogous to our findings, a number of other studies also showed no difference in sexual functioning/enjoyment with regard to different surgical procedures (e.g., [19]), while the majority of previous research in women from age-mixed and older collectives showed different results. Patients who underwent breast-conserving therapy reported a greater sense of wellbeing, were often better integrated in their daily lives (both physically and socially), and reported higher sexual activity [20,21]. This could be due to the fact that the female breast is also understood as a sexual symbol of femininity and beauty. A mastectomy may result in a drop in self-perceived attractiveness, sexual function, and satisfaction [22], and increase the sense of security. However, this might be different in younger patients. Some young patients could emotionally benefit from not having lost their breast, while others might feel saver after a mastectomy. With longer time after diagnosis, this personal rating will possibly change, so a longer follow-up survey is desirable.
Concerning sexual function, young mothers with BC and chemotherapy scored a 9.7 lower mean value compared to women without chemotherapy. This corresponds to previous findings that women who had received chemotherapy showed an increased incidence of sexual dysfunction and that women without chemotherapy judged their sexual function and satisfaction higher [23,24].
With regard to sexual satisfaction, we observed that patients without a sustained endocrine therapy experienced higher sexual satisfaction than women with this therapy, but the difference was not clinically relevant. The restricted sexual satisfaction could result from side effects of medication. Tamoxifen, for example, is known to cause vaginal pain, burning, vaginal narrowing, or sexual discomfort [25], while aromatase inhibitors cause night sweats, hot flushes, weight gain, and sexual dysfunction; these side effects were found to negatively impact both quality of life and sexual satisfaction [26].
Strengths and Limitations
The young mothers with BC were recruited in the setting of a rehabilitation program, in which women from all over Germany participated. This program is unique in that accompanying children were also offered psychological counseling. An earlier analysis showed that the young mothers with BC from this rehabilitation clinic can be regarded as a well-mixed collective with a high degree of representativeness for young BC patients in Germany [2]. However, the access criteria introduced a selection bias: At least 1 child up to the age of 12 and non-metastatic BC were mandatory. Thus, no statement can be made about sexuality, fertility, and wishes for children in young childless women with (non-metastatic or metastatic) BC.
Conclusion
Young mothers with BC are a special group with regard to their treatment and prognosis as well as their wishes and fears. When advising young women with BC, it is essential to include topics of prognosis, sexuality, and fertility in the consultation, as many attitudes and views may be based on misinformation about pregnancy-associated risks [27].
Individualized information and coordination of both oncological and reproductive medical treatments are crucial. Therefore, all BC patients of childbearing age should be routinely and actively informed with regard to possible fertility issues resulting from the required therapy. Timely referral to a fertility center at the time of diagnosis, rather than after the treatment, should become standard.
As a result of our study, all BC patients up to the age of 40 are actively approached at their first attendance at our Breast Center to determine their desire to have children, and are offered counseling in the local fertility center.
Further studies regarding the causality as to why no consultations and fertility preservation procedures were carried out are needed to improve the care of this special group of women.
Disclosure Statement
We declare that we have no conflict of interest.
Acknowledgment
We thank ‘Klinik Ostseedeich’ in Groemitz for allowing the scientific monitoring of their patients. We are especially grateful to the young mothers with BC for their cooperation in this study.
References
- 1.Robert Koch-Institut und GEKID (Hrsg) Krebs in Deutschland 2009/2010. KID. 2013 www.krebsdaten.de/Krebs/DE/Content/Publikationen/Krebs_in_Deutschland/kid_2013/krebs_in_deutschland_2013.html. [Google Scholar]
- 2.Banz-Jansen C, Heinrichs A, Hedderich M, Waldmann A, Wedel B, Mebes I, Diedrich K, Rody A, Fischer D. Are there changes in characteristics and therapy of young patients with early-onset breast cancer in Germany over the last decade? Arch Gynecol Obstet. 2013;288:379–383. doi: 10.1007/s00404-013-2738-7. [DOI] [PubMed] [Google Scholar]
- 3.Tschudin S, Bitzer J. Psychological aspects of fertility preservation in men and women affected by cancer and other life-threatening diseases. Hum Reprod Update. 2009;15:587–597. doi: 10.1093/humupd/dmp015. [DOI] [PubMed] [Google Scholar]
- 4.Maltaris T, Boehm D, Dittrich R, Seufert R, Koelbl H. Reproduction beyond cancer: a message of hope for young women. Gynecol Oncol. 2006;103:1109–1121. doi: 10.1016/j.ygyno.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 5.S3-Leitlinie Mammakarzinom www.awmf.org/leitlinien/detail/ll/032-045OL.html.
- 6.Vriens IJ, De Bie AJ, Aarts MJ, de Boer M, van Hellemond IE, Roijen JH, van Golde RJ, Voogd AC, Tjan-Heijnen VC. The correlation of age with chemotherapy-induced ovarian function failure in breast cancer patients. Oncotarget. 2017;8:11372–11379. doi: 10.18632/oncotarget.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ademuyiwa FO, Cyr A, Ivanovich J, Thomas MA. Managing breast cancer in younger women: challenges and solutions. Breast Cancer (Dove Med Press) 2015;8:1–12. doi: 10.2147/BCTT.S68848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. www.ago-online.de/de/infothek-fuer-aerzte/leitlinienempfehlungen/mamma/
- 9.Fertiprotekt www.fertiprotekt.de.
- 10.Germeyer A. Registerdaten 2015. 12. Arbeitstreffen des Netzwerkes FertiPROTEKT in Heidelberg19. Februar 2016. static1.squarespace.com/static/560a328fe4b0 e8c4f373857e/t/57206c853c44d81ea19e790b/ 1461742728020/registerdaten_fertiprotekt_2015.pdf. [Google Scholar]
- 11.Sprangers MA, Groenvold M, Arraras JI, Franklin J, te Velde A, Muller M, Franzini L, Williams A, de Haes HC, Hopwood P, Cull A, Aaronson NK. The European Organization for Research and Treatment of Cancer breast cancer-specific quality-of-life questionnaire module: first results from a three-country field study. J Clin Oncol. 1996;14:2756–2768. doi: 10.1200/JCO.1996.14.10.2756. [DOI] [PubMed] [Google Scholar]
- 12.Pritzkuleit R, Waldmann A, Raspe H, Katalinic A. The population-based oncological health care study OVIS - recruitment of the patients and analysis of the non-participants. BMC Cancer. 2008;8:311. doi: 10.1186/1471-2407-8-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer. 2001;37:1331–1334. doi: 10.1016/s0959-8049(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 14.Osoba D, Rodrigues G, Myles J, Zee B, Pater J. Interpreting the significance of changes in health-related quality-of-life scores. J Clin Oncol. 1998;16:139–144. doi: 10.1200/JCO.1998.16.1.139. [DOI] [PubMed] [Google Scholar]
- 15.Armuand GM, Rodriguez-Wallberg KA, Wettergren L, Ahlgren J, Enblad G, Höglund M. Sex differences in fertility-related information received by young adult cancer survivors. J Clin Oncol. 2012;30:2147–2153. doi: 10.1200/JCO.2011.40.6470. [DOI] [PubMed] [Google Scholar]
- 16.De Bree E, Makrigiannakis A, Askoxylakis J, Melissas J, Tsiftsis DD. Pregnancy after breast cancer. A comprehensive review. J Surg Oncol. 2010;101:534–542. doi: 10.1002/jso.21514. [DOI] [PubMed] [Google Scholar]
- 17.Lee RJ, Wakefield A, Foy S, Howell SJ, Wardley AM, Armstrong AC. Facilitating reproductive choices: the impact of health services on the experiences of young women with breast cancer. Psychooncology. 2011;20:1044–1052. doi: 10.1002/pon.1826. [DOI] [PubMed] [Google Scholar]
- 18.Siegel K, Gorey E, Gluhosk V. Pregnancy decision making among women previously treated for breast cancer. J Psychosoc Oncol. 1997;15:27–42. [Google Scholar]
- 19.Kedde H, Van de Wiel H, Schultz WW, Wijsen C. Sexual dysfunction in young women with breast cancer. Support Care Cancer. 2013;21:271–280. doi: 10.1007/s00520-012-1521-9. [DOI] [PubMed] [Google Scholar]
- 20.Arndt V, Stegmaier C, Ziegler H, Brenner H. Quality of life over 5 years in women with breast cancer after breast-conserving therapy versus mastectomy: a population-based study. J Cancer Res Clin Oncol. 2008;134:1311–1318. doi: 10.1007/s00432-008-0418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engel J, Kerr J, Schlesinger‐Raab A, Sauer H, Hölzel D. Quality of life following breast‐conserving therapy or mastectomy: results of a 5‐year prospective study. Breast J. 2004;10:223–231. doi: 10.1111/j.1075-122X.2004.21323.x. [DOI] [PubMed] [Google Scholar]
- 22.Karabulut N, Erci B. Sexual desire and satisfaction in sexual life affecting factors in breast cancer survivors after mastectomy. J Psychosoc Oncol. 2009;27:332–343. doi: 10.1080/07347330902979101. [DOI] [PubMed] [Google Scholar]
- 23.Broeckel JA, Thors CL, Jacobsen PB, Small M, Cox CE. Sexual functioning in long-term breast cancer survivors treated with adjuvant chemotherapy. Breast Cancer Res Treat. 2002;75:241–248. doi: 10.1023/a:1019953027596. [DOI] [PubMed] [Google Scholar]
- 24.Ganz PA, Rowland JH, Desmond K, Meyerowitz BE, Wyatt GE. Life after breast cancer: understanding women's health-related quality of life and sexual functioning. J Clin Oncol. 1998;16:501–514. doi: 10.1200/JCO.1998.16.2.501. [DOI] [PubMed] [Google Scholar]
- 25.Baumgart J, Nilsson K, Evers AS, Kallak TK, Poromaa IS. Sexual dysfunction in women on adjuvant endocrine therapy after breast cancer. Menopause. 2013;20:162–168. doi: 10.1097/gme.0b013e31826560da. [DOI] [PubMed] [Google Scholar]
- 26.Christinat A, Di Lascio S, Pagani O. Hormonal therapies in young breast cancer patients: whenwhat and for how long? J Thorac Dis. 2013;5:S36–S46. doi: 10.3978/j.issn.2072-1439.2013.05.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonçalves V, Sehovic I, Quinn G. Childbearing attitudes and decisions of young breast cancer survivors: a systematic review. Hum Reprod Update. 2014;20:279–292. doi: 10.1093/humupd/dmt039. [DOI] [PMC free article] [PubMed] [Google Scholar]