Abstract
Background
The vitamin D system is essential for optimal health in humans. Circulating calcitriol, a key metabolite in maintaining calcium and phosphorus homeostasis, is produced in the kidney. In kidney failure, calcitriol levels progressively decrease, contributing to the development of renal secondary hyperparathyroidism (SHPT).
Summary
For years, SHPT had a central role in the disturbed mineral metabolism of renal patients. As calcitriol deficiency contributes to SHPT development, treatment with calcitriol or other compounds able to activate the vitamin D receptor (VDR) was one of the mainstays of therapy for renal patients in the last 40 years. In this review, we discuss how the treatment with VDR activators (VDRA) evolved during this time in the United States, as well as the main factors responsible for these changes.
Key Messages
Management of SHPT with VDRA in renal patients has undergone a few paradigm shifts over the last 40 years. When treating SHPT, the newly developed therapies as well as VDRA need to be carefully considered and used appropriately. Nephrologists need to use an integrated approach that avoids excessive use of VDRA, ensures replenishment of vitamin D stores, and avoids hypercalcemia and hyperphosphatemia.
Key Words: Vitamin D, Calcitriol, Paricalcitol, Calcifediol, Hemodialysis, Chronic kidney disease, Costs
Introduction
Vitamin D is an important regulator of calcium and phosphate homeostasis and has also numerous extraskeletal effects on the cardiovascular system, central nervous system, endocrine system, immune system, etc., as well as on cell differentiation and cell growth. In chronic kidney disease (CKD), the mineral metabolism is progressively altered. Decreasing 1,25(OH)2D (calcitriol) and rising parathyroid hormone (PTH) levels occur early in the course of renal function decline. Thirteen percent of patients with an estimated glomerular filtration rate (eGFR) > 80 mL/min have calcitriol levels < 22 pg/mL, and 13% patients with an eGFR > 80 mL/min have PTH levels > 65 pg/dL [1]. Further, studies in CKD patients suggested that serum levels of fibroblast growth factor 23 (FGF23), a phosphaturic hormone, increases before PTH in the course of renal function decline [2]. Serum calcium and phosphorus remain normal until eGFR declines below 40 mL/min [1]. The altered mineral metabolism in CKD is thought to contribute to the high mortality noted in this population. While this review focuses on the treatment of secondary hyperparathyroidism (SHPT) with active vitamin D, it is important to remember that concomitant management of associated hyperphosphatemia and hypocalcemia and avoidance of hypercalcemia are imperative, as recommended by multiple sources, more recently by the updated 2017 Kidney Disease: Improving Global Outcomes (KDIGO) CKD-Mineral and Bone Disorder (CKD-MBD) guidelines [3].
Vitamin D Physiology
The vitamin D endocrine system plays an integral role in calcium and phosphorus metabolism. Vitamin D3 is formed by exposure to UV light from 7-dehydrocholesterol in the skin. Plants are the main source of vitamin D2. Vitamins D3 and D2 are collectively known as vitamin D and are biologically inert. Vitamin D from the skin or diet enters the circulation bound to the vitamin D-binding protein and is converted to 25(OH)D (calcidiol) in the liver, primarily by CYP2R1 enzyme. Calcidiol concentration in the circulation is in the ng/mL range, and its half-life is 15–18 days. The serum calcidiol level best reflects vitamin D status because liver hydroxylase is not regulated by negative feedback and, thus, calcidiol production is mainly substrate dependent. Calcidiol undergoes a second hydroxylation, by 25(OH)D-1α-hydroxylase (CYP27B1) enzyme, in the renal proximal tubular cells, forming 1,25(OH)2D (calcitriol). In the kidney, both calcitriol and also calcidiol not used for calcitriol generation undergo further catabolism via the renal 25(OH)-24-hydroxylase (CYP24A1), ultimately resulting in the biologically inactive metabolite calcitroic acid [4]. The renal CYP27B1 is under tight regulatory control, thus maintaining physiologic calcitriol levels in the circulation (upregulated by PTH and downregulated by FGF23, calcitriol itself, calcium, and phosphorus) [5]. Renal calcitriol production accounts largely for the circulating calcitriol levels and its endocrine role in mineral metabolism, also known as classical actions of vitamin D. The calcitriol concentration in the circulation is in the pg/mL range, and its half-life is 4–6 h [6].
Like other steroid hormones, calcitriol exerts its actions via the vitamin D receptor (VDR), which is present in almost all tissues and cells in the human body and is able to activate about 3% of the human genome. After binding to the VDR in cytoplasm, the calcitriol/VDR complex enters the nucleus and binds to the retinoid X receptor (RXR). This complex then interacts with specific DNA sequences (vitamin D response elements) in and around target genes, up- or downregulating their transcription [4, 7]. Calcitriol is the main activator of the VDR; however, calcidiol can also activate it, albeit with ∼100 times less potency than calcitriol. The main endocrine function of calcitriol is to help maintain a tight calcium homeostasis, by increasing intestinal calcium absorption, inducing calcium mobilization from the skeleton, and increasing calcium reabsorption in the distal tubules [5]. Calcitriol has also actions outside of mineral metabolism, known as noncalcemic or pleotropic actions. To this end, multiple tissues and cells, like the gastrointestinal tract, skin, muscles, and vasculature, express CYP27B1 and can produce calcitriol locally in an intracrine/paracrine fashion. In these settings, calcitriol regulates multiple cellular processes, effecting normal and malignant cell growth and differentiation, innate immune function, and cardiovascular function. Unlike the renal enzyme, the extrarenal CYP27B1 expression is regulated by specific local factors, receiving feedback from locally expressed CYP27B1 and CYP24A1 and also available calcidiol [8].
Vitamin D in CKD and the Development of SHPT
In CKD, abnormal vitamin D metabolism plays a major role in the development of SHPT. First, low calcidiol levels are highly prevalent in CKD [9] for several reasons: decreased skin conversion of 7-dehydrocholesterol to vitamin D in the setting of uremia, poor nutrition, limited sun exposure, and loss of calcidiol in urine in proteinuric patients. Second, calcitriol levels progressively fall as the GFR decreases, such that > 60% of patients with eGFR < 30 mL/min have levels < 22 pg/mL [1]. This decrease in calcitriol levels is due to several renal factors, such as (a) decreased renal CYP27B1 in the setting of reduced renal mass; (b) loss of renal megalin necessary for uptake of calcidiol by the proximal tubule; and (c) low amounts of calcidiol delivered to the proximal tubular cells for activation to calcitriol [10]. Further, regulatory factors, like elevated FGF23 levels, which occur early in CKD, directly suppress renal CYP27B1 and exacerbate the calcitriol deficiency [11]. As calcitriol levels progressively decrease, the negative feedback it normally exerts on the parathyroid glands becomes ineffective, further promoting the development of SHPT. Phosphate retention, hyperphosphatemia, and the ensuing hypocalcemia are the other major factors contributing to the development of SHPT. In this altered mineral environment and progressively dysfunctional regulators, over time, the parathyroid glands develop diffuse polyclonal hyperplasia [12]. The latter can further undergo monoclonal nodular transformation, an entity associated with reduced expression of calcium-sensing receptors (CaSR) and VDR, rendering the parathyroid glands poorly responsive to treatment with calcimimetics or vitamin D. The consequences of untreated SHPT traditionally are bone disease, vascular calcifications, and abnormal biochemical parameters. For the purpose of this review, we will assume that elevated PTH represents clinically meaningful SHPT and will only review its treatment with active vitamin D.
Treatment of SHPT with Calcitriol
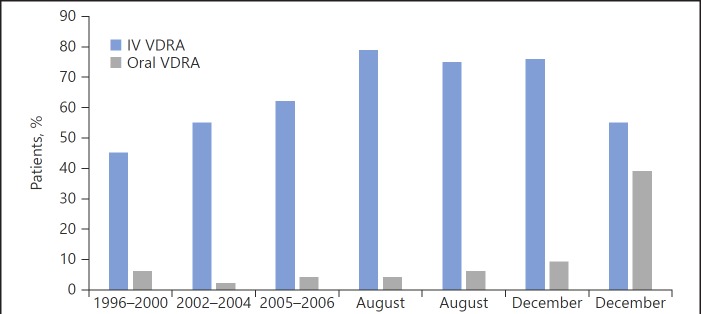
SHPT, as reflected by elevated PTH levels, is not only associated with bone disease but also with poor outcomes in epidemiologic studies of dialysis patients [13]. Even though no randomized controlled trials have demonstrated that suppressing PTH levels improves patient-centered outcomes, treatment of SHPT remains a major focus in the care of renal patients for every practicing nephrologist. In the late 1970s, synthetic calcitriol, the same as the endogenous calcitriol, became available and was approved for oral use in dialysis patients to improve symptomatic renal osteodystrophy (decrease bone pain and increase muscle strength) and biomarkers (alkaline phosphatase and serum PTH) [14]. However, frequent hypercalcemia occurred with doses commonly used. In 1984, Slatopolsky et al. [15] showed that intravenous administration of calcitriol was extremely effective in lowering PTH levels in hemodialysis patients. In 1989, Andress et al. [16] demonstrated that trice weekly intravenous calcitriol administration for 11.5 months to dialysis patients with osteitis fibrosa resulted in a decrease in PTH and alkaline phosphatase levels, with less hypercalcemia than in oral therapies. While over time multiple studies showed a similar effectiveness of the oral and intravenous formulations in reducing PTH and no differences in measurable outcomes, the increased compliance with intravenous calcitriol shifted the use of this medication to primarily the parenteral route in the US. This shift was heavily influenced by the Medicare reimbursement policies in effect at that time, which only covered injectable medications. This shift to intravenous calcitriol was illustrated in the 1996–2001 report of the Dialysis Outcomes and Practice Patterns Study (DOPPS) from the US, Europe, and Japan. In this study, 52% of these patients received some form of vitamin D therapy, with parenteral vitamin D therapy largely restricted to US facilities (44% in the US vs. 4% in Europe and <1% in Japan) [17, 18] (Fig. 1). In 2003, the Kidney Disease Outcomes Quality Initiative (KDOQI) for Bone Metabolism and Disease in CKD expert group recommended intravenous pulse calcitriol as more effective than oral pulse therapy for treatment of SHPT [19], giving this practice a seal of approval.
Fig. 1.
Percentage of patients prescribed vitamin D and route of vitamin D administration in the US during the study phases of DOPPS (DOPPS I [1996–2000], II [2002–2004], and III [2005–2006]; from reference [17]), at the end of August 2010, and any time during the month from August 2014 onward (from references [53] and [18]). IV, intravenous; VDRA, vitamin D receptor activator.
VDR Analogs in the Treatment of SHPT
Further research demonstrated that calcitriol decreases PTH gene transcription and cell proliferation and increases parathyroid gland VDR and CaSR expression, supporting its clinical use for the treatment of SHPT. However, calcitriol dose escalation to effectively suppress PTH in more severe cases of SHPT was limited by its narrow therapeutic window [20]. The dose-dependent development of hypercalcemia and hyperphosphatemia with possible consequent soft tissue and vascular calcifications, as well as concerns for the development of adynamic bone disease, prompted the development of calcitriol analogs for PTH suppression with less calcemic activity [21]. Vitamin D analogs activate the VDR differently, when compared with calcitriol, resulting in a different recruitment of coactivators and corepressors which are cell specific [22]. A brief description of analogs commonly used in nephrology follows.
Prohormones
(a) Alfacalcidol or 1α-hydroxyvitamin D3 is a prohormone which requires 25-hydroxylation in the liver in order to become active 1,25(OH)2D3 and stimulate the VDR. It has been available outside the US in oral and intravenous formulations since 1980. In CKD stages G3 and G4, oral alfacalcidol administration significantly improved the bone histology in patients with abnormal bone turnover prior to therapy [23]. In dialysis patients, both oral and parenteral forms suppress PTH, having an activity similar to calcitriol.
(b) Doxercalciferol or 1α-hydroxyvitamin D2 is also a prohormone that requires 25-hydroxylation in the liver to become active 1,25(OH)2D2. Placebo control studies in dialysis patients demonstrated that both oral and intravenous doxercalciferol suppress PTH with low rates of hypercalcemia and hyperphosphatemia [24]. In the US, the oral form of the drug was approved in 1999 and the parenteral one in 2000.
Direct VDR Activators
(a) Paricalcitol or 19-nor-1α,25-dihydroxyvitamin D2 is an analog of 1,25(OH)2D2, obtained by the elimination of a methylene group from carbon 10. In experimental models of CKD, paricalcitol had one-third the potency of calcitriol in decreasing PTH and one-tenth the potency of calcitriol in increasing serum calcium and/or phosphorus [20]. This makes paricalcitol 3-fold more selective for PTH suppression than calcitriol. In animal models, paricalcitol also improved bone histology. It was approved in the US in the parenteral form in 1998 to treat SHPT. Although less calcemic than calcitriol, hypercalcemia occurred, usually when PTH was suppressed to below target levels. In 2005, oral paricalcitol was approved to treat SHPT in predialysis patients.
(b) Maxacalcitol or 22-oxa-1α,25-dihydroxyvitamin D3 is similar to calcitriol but has an oxygen between C21 and C23. In clinical studies, maxacalcitol and calcitriol are similarly effective and safe in suppressing PTH [25]. It has been available in Japan, but not in the US, in the parenteral form for the treatment of SHPT since 2000.
(c) Falecalcitriol or 26,27-hexafluoro-1α,25-dihydroxy vitamin D3 is a hexafluoro derivative of 1,25(OH)2D3 also available in Japan. Launched in 2001, the oral form is effective in decreasing PTH in dialysis patients, but it is not available for clinical use in the US [14].
The approval of paricalcitol and doxercalciferol for clinical use in the US represented a new paradigm shift in the treatment of SHPT, where the use of calcitriol, which was limited by the development of hypercalcemia and hyperphosphatemia, is now replaced by new vitamin D analogs with a wider therapeutic window (Fig. 2). This change was fueled by the assumption that, similar to preclinical data, the non-calcitriol VDR activator (VDRA) can be used at higher doses to treat SHPT in humans, with little or no effects on calcium and phosphorus. A double-blind randomized controlled trial in dialysis patients comparing paricalcitol with calcitriol over 32 weeks noted a 50% reduction of PTH more rapidly (87 vs. 108 days median) and with less sustained episodes of hypercalcemia with paricalcitol (18 vs. 33%) [26]. Another small randomized trial of paricalcitol versus calcitriol found that paricalcitol, but not calcitriol, significantly reduced PTH, while serum calcium was only increased by calcitriol, giving further support to the credence that paricalcitol may be a less calcemic analog [27]. A Cochrane Review confirms that VDRA can decrease PTH levels in dialysis (–96 pg/mL, 95% CI −298 to −94) as well as predialysis patients (–49 pg/mL, 95% CI −86 to −13) [28, 29].
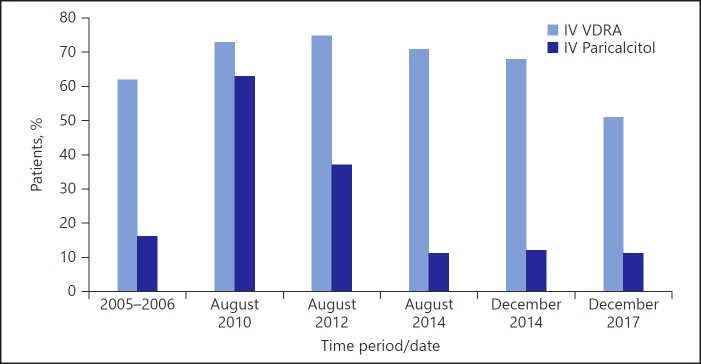
Fig. 2.
Percentage of patients prescribed any IV vitamin D and percentage of patients with IV vitamin D who were prescribed IV paricalcitol in the US during DOPPS III (2005–2006) [17], at the end of August 2010 (DOPPS Practice Monitor [18]), and any time during the month from August 2014 onward (DOPPS Practice Monitor [18]). IV, intravenous; VDRA, vitamin D receptor activator.
Few observational studies in dialysis patients suggested that parenteral use of non-calcitriol VDRA was associated with a decreased risk of hospitalization [30] and, more importantly, with a deceased risk of mortality. In a large historical cohort, parenteral VDRA administration conferred dialysis patients a 26% (HR 0.74, 95% CI 0.71 to 0.79) mortality reduction over those who received none [31]. Another study found a significant survival advantage for patients treated with parenteral paricalcitol over those who received calcitriol [32]. The survival advantage of non-calcitriol VDRA treatment was present even in patients with low PTH, high calcium, and high phosphorus levels. Importantly, no studies to date have reported an association of VDR analogs use with increased mortality [33].
Other contributors to the increased use of VDRA were thought to be:
- treating and maintaining the PTH levels in the fairly narrow PTH target range of 150–300 pg/mL, along with calcium and phosphorus control, recommended by the 2003 KDOQI guidelines;
- several new studies continue to report an association between elevated PTH and relative risk of death [34, 35];
- analysis of the cost-effectiveness ratio of paricalcitol versus calcitriol in CKD patients in the US, using a Markov process model, projected that paricalcitol will offer short- and long-term economic benefits [36].
Using Medicare Part A claims, Beaubrun at al. [37] reported that the parenteral use of vitamin D in dialysis units across the US continued to increase, from 58.6% in 1999 to 84% in 2008. Paricalcitol use increased from 35.6% in 2000 to 66.3% in 2008, doxercalciferol increased from 10% in 2002 to 23.7% in 2008, while calcitriol use decreased from 58.6 to 1.8%.
Another Paradigm Shift in VDRA Use for SHPT
In 2009, the KDIGO CKD-MBD guidelines recommended a wider range for PTH target, of 2–9 the upper limit of normal [38], relaxing the prescription practice of VDRA and conceivably leading to some reduction in the VDRA doses used in clinical practice. Further, KDIGO CKD-MBD could not recommend a preferred route of administration or a dosing frequency for VDRA.
Advantages of paricalcitol over calcitriol from preclinical studies could not be replicated in humans. In a randomized crossover trial, Hansen et al. [39] showed no difference between alfacalcidol and paricalcitol in the reduction of PTH in dialysis patients. Two other small randomized trials outside the US by Večerić-Haler et al. [40] and by Ong et al. [41] showed that oral paricalcitol has similar efficacy and safety to oral calcitriol in dialysis patients. A recent meta-analysis comparing paricalcitol with other VDR analogs in dialysis patients (8 studies, 759 patients) found no significant differences in the percentage of patients with target reduction of PTH from baseline for paricalcitol (RR 1.01, 95% CI 0. 87–1.18; p = 0.85) and, moreover, no differences in the incidence of hypercalcemia (RR 0.95, 95% CI 0.74–1.21; p = 0. 65) and hyperphosphatemia (RR 0.94, 95% CI 0.77–1.16; p = 0.58) [42].
Dose-dependent increases in serum calcium and phosphorus when higher doses of VDR analogs were employed for more severe cases of SHPT brought to the nephrologist a “déjà vu” of calcitriol side effects, such as vascular calcifications, adynamic bone disease, etc. Further, VDRA administration in dialysis patients was shown to increase FGF23 concentrations [43], an established independent predictor of mortality in this population [44].
In 2004, the calcimimetic drug cinacalcet was introduced on the US market for the treatment of SHPT [45]. Cinacalcet was found to reduce PTH similarly to VDRA in dialysis patients, and also to lower FGF23 levels, presumably via both direct effects on bone cells and indirect effects on mineral metabolism [46]. The favorable biochemical profile of this drug in dialysis patients (lowering PTH, calcium, phosphorus, and FGF23) drew large interest in the nephrology community for its clinical use and stimulated further research in SHPT, aiming to move beyond biochemical parameters and assess patient-centered outcomes. Evaluation of Cinacalcet Hydrochloride Therapy to Lower Cardiovascular Events (EVOLVE) was the largest and most important study to date addressing the impact of this medication on cardiovascular outcomes in dialysis patients [47]. Because EVOLVE did not meet the primary endpoint of reducing the risk of death or clinically important vascular events in dialysis patients, and also due to the high cost of the drug, the KDIGO CKD-MBD 2017 update did not recommend cinacalcet as a first-line therapy for SHPT in this population, rather listing the PTH-lowering therapies in alphabetical order [3]. Recent studies in CKD patients exploring some of the pleiotropic effects of active vitamin D in this population were deceiving. Paricalcitol Capsule Benefits in Renal Failure-Induced Cardiac Morbidity (PRIMO), a randomized controlled trial comparing paricalcitol with placebo over 48 weeks in patients with CKD stages G3a–G4, demonstrated no between-group differences in left ventricular mass index and diastolic function [48]. Another study on the Effect of Paricalcitol on Left Ventricular Mass and Function in CKD – The OPERA Trial, which evaluated patients with CKD stages G3a–G5 with left ventricular (LV) hypertrophy, found no effect on LV mass index and no regression of LV mass after 52 weeks of paricalcitol [49]. Further, hypercalcemia episodes, defined as 2 consecutive serum calcium levels > 10.5 mg/dL in the first study and serum calcium > 10.2 mg/dL in the second one, were more frequent in the paricalcitol groups than in the placebo groups in both studies: 20.9 versus 0.9% and 43.3 versus 3.3%, respectively [50].
Economic considerations continue to influence the management of dialysis patients. Injectable medications were responsible for almost USD 2.8 billion Medicare costs in 2010 of which 18.5% (USD 519 million) were attributable to vitamin D analogues (https://www.usrds.org/2012/pdf/v2_ch11_12.pdf). The Prospective Payment System for end-stage renal disease (ESRD) was launched in January 2011, under which injectable medications became part of the bundled payment system (single payment, per treatment for several components of dialysis care, additional laboratory tests, intravenous medications, etc.) and no longer separately billable by dialysis providers. A Study to Evaluate the Prospective Payment System Impact on Small Dialysis Organizations (STEPPS), exploring trends in dialytic treatment before and after bundle implementation in 51 small dialysis centers, showed a decrease in intravenous vitamin D use from 69.2% in the fourth quarter of 2010 to 60.2 % in the second quarter of 2011, while oral VDRA use increased from 6.1 to 14% during the same period of time (p for trend for all < 0.001) [51]. Analyzing the US hemodialysis patients represented in the US Renal Data System between 2008 and 2013, a 7% reduction in the average dose and starting dose of intravenous vitamin D analogues and a 10% reduction in the rate of vitamin D therapy initiation are reported in a recent study by Spoendlin at al. [52]. This trend is not captured in the DOPPS survey of medical directors from an average of 71 facilities between 2010 and 2014, which indicated stable intravenous vitamin D use (79% in August 2010 to 76% in August 2014) [53].
However, since December 2014, DOPPS data demonstrate that, while the VDRA use remained practically stable, the percentage of patients prescribed intravenous vitamin D has steadily declined from 68% in December 2014 to 51% in December 2017. A concurrent rise in the use of oral vitamin D formulations has also been observed, suggesting substitution of oral calcitriol in place of intravenous therapies. An increase in cinacalcet prescription use from 27 to 30% is reported during the same period of time [18].
Kumar et al. [54] recently described an effective and safe conversion from intravenous paricalcitol to in-center distributed oral calcitriol, with estimated cost savings of USD 564 per person/year. Another small study from Canada examined the conversion from intravenous to oral alfacalcidol and noted that, in addition to effective PTH control, the oral administration was associated with an annual cost saving of CAD 2,246 per patient and an annual nursing time reduction of 25 days [55]. Thus, the last paradigm shift in the treatment of SHPT, the replacement of parenteral VDRA with the less expensive oral forms in ESRD, clearly triggered by our more comprehensive understanding of the mineral metabolism and of the fundamental role of calcimimetics in its management, became apparent only when the bundled payment model in US dialysis entered into effect.
Future Directions
CKD represents the nephrologist's “endocrinopathy,” centered on the calcitriol deficiency that develops as the renal function declines, and its difficult treatment due to the complexity of the associated abnormalities and regulators. Unfortunately, physiologic supplementation of calcitriol in CKD is not possible as calcitriol is only manufactured in pharmacologic doses, 6 orders of magnitude over the physiologic ones (micrograms vs. picograms). At least 2 limitations of the pharmacologic VDRA use deserve mentioning. First, administration of VDRA in pharmacologic doses can increase the catabolism of endogenous vitamin D metabolites (calcitriol and calcidiol), by inducing the CYP24A1 expression [56], thus decreasing their serum concentration. Second, administration of oral VDRA does not correct the vitamin D deficiency that is highly prevalent in CKD patients [57]. Correction of nutritional vitamin D remains a recommendation in the updated KDIGO CKD-MBD 2017, although what constitutes calcidiol sufficiency in ESRD, where calcitriol levels are also low, remains unknown. Few meta-analyses, including a recent one by Zhang et al. [58] in dialysis patients, noted a survival benefit of higher serum calcidiol levels (relative risk of all-cause mortality of 0.78 [95% CI 0.71–0.86] per 10 ng/mL increase in serum calcidiol level).
Both low calcidiol and calcitriol levels have been associated with increased mortality in incident hemodialysis patients [59]. A recent publication of the observational cohort Health, Aging, and Body Composition (Health ABC) Study reports that lower calcitriol levels are independently associated with kidney function decline in this population, where for each 1-standard deviation, lower calcitriol levels were associated with a 30% higher risk for major kidney function decline (95% CI 1.03–1.65; p = 0.03) [60].
The importance of dual supplementation (nutritional and active) vitamin D is evident in cases of more advanced SHPT, where resistance to VDRA has been reported; experimental data in parathyroid glands suggest that resistance to VDRA can be overcome by administration of both nutritional vitamin D and VDRA [61].
Even though calcitriol stimulates FGF23 in physiologic states, reports of elevated FGF23 after vitamin D administration have raised concerns over its use in renal patients. However, a pathogenic effect of FGF23 has been so far only demonstrated in experimental models [62]. Further, emerging data reveal that many stimuli outside of the mineral metabolism are able to increase FGF23 levels (inflammation, iron deficiency [63], high fat diet [64], as well as LV hypertrophy [65]). Lastly, a systematic review and meta-analysis of the prospective studies on the associations between FGF23 and the risk of different cardiovascular diseases suggests that the relationship between FGF23 and cardiovascular risk may be noncausal [66].
The reemergence of calcifediol use in CKD, one of the first vitamin D metabolites to ever achieve commercialization, deserves careful and renewed consideration. In the US, calcidiol was first marketed as oral capsules for patients with CKD and vitamin D-resistant conditions in the 1970s [67] and later discontinued as newer therapies for SHPT emerged. Renewed interest in this metabolite in the recent years resulted in the FDA approval of extended release (ER) calcifediol in the US in 2016. Studies in patients with CKD stages G3–G4 showed that, unlike the immediate release calcifediol, the ER formulation induces a gradual rise in serum 25(OH)D level along with a significant suppression of PTH levels (at least 30% from baseline), in spite of lower mean calcidiol levels [68]. A slight rise in calcitriol levels (from 34.4 to 46.7 pg/mL), proportional to the rise in calcidiol, was also noted, suggesting that the renal and/or extrarenal CYP27B1 were not downregulated by the rising levels of calcitriol. Further, no hypercalcemia or hyperphosphatemia occurred, and FGF23 levels were unchanged. General caution is overall still recommended when using calcifediol due to the possibility of hypercalcemia, through its ability to directly activate VDR at high serum levels in spite of much lower affinity for VDR than calcitriol, as well as its long half-life of 15–18 days [56]. It has been demonstrated long ago that when administering supra-physiological doses of calcidiol to surgically anephric dialysis patients, the extrarenal CYP27B1 can increase the serum calcitriol level (from 5.5 ± 2.4 to 19.6 ± 5.0 pg/mL) [69]. Recently, normalization of both calcidiol and calcitriol levels was demonstrated in dialysis patients by partial body exposure to UVB radiation [70]. While sun exposure might not represent a practical long-term solution for the dialysis population, the availability of ER calcifediol offers a perfect opportunity to further examine the effects of restoring physiologic calcitriol levels in these patients. Enthusiasm to this proposal is perhaps supported by a recent study be Levin at al. [71] in nondialysis patients which showed that calcifediol administration for 6 months decreased pulse wave velocity (PWV) when compared with the calcitriol group (no change in PWV) or placebo (increased PWV). While statistical significance was not reached due to sample size, the combined vitamin D groups had a significantly better PWV than the placebo group (mean change −0.4 m/s, 95% CI −1.2 to 0.4, vs. +1.1 m/s, 95% CI −0.1 to 2.2).
ER calcifediol offers a unique opportunity to treat the dually altered vitamin D metabolism (low calcidiol and calcitriol state) in renal patients and to provide answers to important questions: (1) does calcifediol offer mortality or other patient-centered benefits above 25D repletion alone? (2) Are there any positive or detrimental consequences to a sustained extrarenal production of calcitriol in this setting?
Nephrologists’ fascination with active vitamin D continues, as they explore other pleotropic effects, like reduced infection risks [72], and search for new, noncalcemic analogs [73, 74]. Further research in nephrology should incorporate some of the newer available technologies currently piloted in vitamin D research in the general population, such as the effect of vitamin D therapy on the human transcriptome, in order to better understand and further guide therapies [75].
Conclusion
SHPT is a known complication of calcitriol deficiency in CKD. Calcitriol or other VDRA were the cornerstones of its treatment for years. Progressive research demonstrated that treating SHPT while avoiding hypercalcemia and hyperphosphatemia is of paramount importance. The latter is difficult to secure when using very large doses of active vitamin D. Further, the availability of calcimimetics, which tend to lower serum calcium and phosphorus, led to a combined use of the 2 classes of drugs and, at times, exclusive calcimimetic use to control SHPT. Further, health-care policies and payment models in the US clearly affected nephrologists’ practice. Reiterating statements made by multiple physicians and scientists in our filed, well-designed and -funded randomized controlled studies are desperately needed in the CKD population in order for nephrologists to provide improved care to the CKD population while remaining true to the dictum “primum non nocere.”
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin DPTH, calcium and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007 Jan;71((1)):31–8. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 2.Wolf M. Mineral (Mal)Adaptation to Kidney Disease—Young Investigator Award Address: American Society of Nephrology Kidney Week 2014. Clin J Am Soc Nephrol. 2015 Oct;10((10)):1875–85. doi: 10.2215/CJN.04430415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.KDIGO Clinical Practice Guideline Update for the DiagnosisEvaluation, Prevention and Treatment of Chronic Kidney Disease–Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2017;7((1)):1–59. doi: 10.1016/j.kisu.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christakos S, Dhawan P, Verstuyf A, Verlinden L, Carmeliet G, Vitamin D. Vitamin D: MetabolismMolecular Mechanism of Action and Pleiotropic Effects. Physiol Rev. 2016 Jan;96((1)):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pike JW, Christakos S. Biology and Mechanisms of Action of the Vitamin D Hormone. Endocrinol Metab Clin North Am. 2017 Dec;46((4)):815–43. doi: 10.1016/j.ecl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zittermann A, Ernst JB, Birschmann I, Dittrich M. Effect of Vitamin D or Activated Vitamin D on Circulating 1,25-Dihydroxyvitamin D Concentrations: A Systematic Review and Metaanalysis of Randomized Controlled Trials. Clin Chem. 2015 Dec;61((12)):1484–94. doi: 10.1373/clinchem.2015.244913. [DOI] [PubMed] [Google Scholar]
- 7.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh JC, et al. Molecular mechanisms of vitamin D action. Calcif Tissue Int. 2013 Feb;92((2)):77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 8.Bikle DD. Extraskeletal actions of vitamin D. Ann N Y Acad Sci. 2016 Jul;1376((1)):29–52. doi: 10.1111/nyas.13219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nigwekar SU, Tamez H, Thadhani RI. Vitamin D and chronic kidney disease-mineral bone disease (CKD-MBD) Bonekey Rep. 2014 Feb;3:498. doi: 10.1038/bonekey.2013.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chesney RW. Interactions of vitamin D and the proximal tubule. Pediatr Nephrol. 2016 Jan;31((1)):7–14. doi: 10.1007/s00467-015-3050-5. [DOI] [PubMed] [Google Scholar]
- 11.Kuro-O M. Klotho and endocrine fibroblast growth factors: marker of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant. 2018 May; doi: 10.1093/ndt/gfy126. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 12.Portillo MR, Rodríguez-Ortiz ME. Secondary Hyperparthyroidism: PathogenesisDiagnosis, Preventive and Therapeutic Strategies. Rev Endocr Metab Disord. 2017 Mar;18((1)):79–95. doi: 10.1007/s11154-017-9421-4. [DOI] [PubMed] [Google Scholar]
- 13.Fouque D, Roth H, Pelletier S, London GM, Hannedouche T, Jean G, et al. Control of mineral metabolism and bone disease in haemodialysis patients: which optimal targets? Nephrol Dial Transplant. 2013 Feb;28((2)):360–7. doi: 10.1093/ndt/gfs404. [DOI] [PubMed] [Google Scholar]
- 14.DeLuca HF, Plum LA. Analogs of 1α,25-Dihydroxyvitamin D₃ in Clinical Use. Vitam Horm. 2016;100:151–64. doi: 10.1016/bs.vh.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Slatopolsky E, Weerts C, Thielan J, Horst R, Harter H, Martin KJ. Marked suppression of secondary hyperparathyroidism by intravenous administration of 125-dihydroxy-cholecalciferol uremic patients. J Clin Invest. 1984 Dec;74((6)):2136–2143. doi: 10.1172/JCI111639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andress DL, Norris KC, Coburn JW, Slatopolsky EA, Sherrard DJ. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989 Aug;321((5)):274–9. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- 17.Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, et al. The survival advantage for haemodialysis patients taking vitamin D is questioned: findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant. 2009 Mar;24((3)):963–72. doi: 10.1093/ndt/gfn592. [DOI] [PubMed] [Google Scholar]
- 18.The DOPPS Practice Monitor https://www.dopps.org/dpm/]. Accessed [April 29, 2018. [Google Scholar]
- 19.National Kidney Foundation K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003 Oct;42((4 Suppl 3)):S1–201. [PubMed] [Google Scholar]
- 20.Zand L, Kumar R. The Use of Vitamin D Metabolites and Analogues in the Treatment of Chronic Kidney Disease. Endocrinol Metab Clin North Am. 2017 Dec;46((4)):983–1007. doi: 10.1016/j.ecl.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bover J, Egido J, Fernández-Giráldez E, Praga M, Solozábal-Campos C, Torregrosa JV, et al. Vitamin Dvitamin D receptor and the importance of its activation in patients with chronic kidney disease. Nefrologia. 2015;35((1)):28–41. doi: 10.3265/Nefrologia.pre2014.Sep.11796. [DOI] [PubMed] [Google Scholar]
- 22.Goldsmith DJ, Massy ZA, Brandenburg V. The uses and abuses of Vitamin D compounds in chronic kidney disease-mineral bone disease (CKD-MBD) Semin Nephrol. 2014 Nov;34((6)):660–8. doi: 10.1016/j.semnephrol.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 23.Bover J, Dasilva I, Furlano M, Lloret MJ, Diaz-Encarnacion MM, Ballarin J, et al. Clinical Uses of 1,25-dihydroxy-19-nor-vitamin D(2) (Paricalcitol) Curr Vasc Pharmacol. 2014 Mar;12((2)):313–23. doi: 10.2174/15701611113119990028. [DOI] [PubMed] [Google Scholar]
- 24.Ureña-Torres PA, Cozzolino M, Bover J. [Utilization of alfacalcidol and active vitamin D analogs in chronic kidney disease] Nephrol Ther. 2018 Jun;14((4)):189–200. doi: 10.1016/j.nephro.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 25.Mizobuchi M, Ogata H. Clinical uses of 22-oxacalcitriol. Curr Vasc Pharmacol. 2014 Mar;12((2)):324–8. doi: 10.2174/15701611113119990023. [DOI] [PubMed] [Google Scholar]
- 26.Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003 Apr;63((4)):1483–90. doi: 10.1046/j.1523-1755.2003.00878.x. [DOI] [PubMed] [Google Scholar]
- 27.Abdul Gafor AH, Saidin R, Loo CY, Mohd R, Zainudin S, Shah SA, et al. Intravenous calcitriol versus paricalcitol in haemodialysis patients with severe secondary hyperparathyroidism. Nephrology (Carlton) 2009 Aug;14((5)):488–92. doi: 10.1111/j.1440-1797.2008.01058.x. [DOI] [PubMed] [Google Scholar]
- 28.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev. 2009 Oct;((4)):CD005633. doi: 10.1002/14651858.CD005633.pub2. [DOI] [PubMed] [Google Scholar]
- 29.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF. Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev. 2009 Oct;((4)):CD008175. doi: 10.1002/14651858.CD008175. [DOI] [PubMed] [Google Scholar]
- 30.Dobrez DG, Mathes A, Amdahl M, Marx SE, Melnick JZ, Sprague SM. Paricalcitol-treated patients experience improved hospitalization outcomes compared with calcitriol-treated patients in real-world clinical settings. Nephrol Dial Transplant. 2004 May;19((5)):1174–81. doi: 10.1093/ndt/gfh123. [DOI] [PubMed] [Google Scholar]
- 31.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005 Apr;16((4)):1115–25. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 32.Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003 Jul;349((5)):446–56. doi: 10.1056/NEJMoa022536. [DOI] [PubMed] [Google Scholar]
- 33.Duranton F, Rodriguez-Ortiz ME, Duny Y, Rodriguez M, Daurès JP, Argilés A. Vitamin D treatment and mortality in chronic kidney disease: a systematic review and meta-analysis. Am J Nephrol. 2013;37((3)):239–48. doi: 10.1159/000346846. [DOI] [PubMed] [Google Scholar]
- 34.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, et al. Changes in serum calciumphosphate, and PTH and the risk of death in incident dialysis patients: a longitudinal study. Kidney Int. 2006 Jul;70((2)):351–7. doi: 10.1038/sj.ki.5001542. [DOI] [PubMed] [Google Scholar]
- 35.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006 Aug;70((4)):771–80. doi: 10.1038/sj.ki.5001514. [DOI] [PubMed] [Google Scholar]
- 36.Nuijten M, Andress DL, Marx SE, Sterz R. Chronic kidney disease Markov model comparing paricalcitol to calcitriol for secondary hyperparathyroidism: a US perspective. Curr Med Res Opin. 2009 May;25((5)):1221–34. doi: 10.1185/03007990902844097. [DOI] [PubMed] [Google Scholar]
- 37.Beaubrun AC, Brookhart MA, Sleath B, Wang L, Kshirsagar AV. Trends and variations in intravenous vitamin D use among hemodialysis patients in the United States. Ren Fail. 2013;35((1)):1–8. doi: 10.3109/0886022X.2012.734260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group KDIGO clinical practice guideline for the diagnosisevaluation, prevention and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD) Kidney Int Suppl. 2009 Aug;((113)):S1–130. doi: 10.1038/ki.2009.188. [DOI] [PubMed] [Google Scholar]
- 39.Hansen D, Rasmussen K, Danielsen H, Meyer-Hofmann H, Bacevicius E, Lauridsen TG, et al. No difference between alfacalcidol and paricalcitol in the treatment of secondary hyperparathyroidism in hemodialysis patients: a randomized crossover trial. Kidney Int. 2011 Oct;80((8)):841–50. doi: 10.1038/ki.2011.226. [DOI] [PubMed] [Google Scholar]
- 40.Večerić-Haler Ž, Romozi K, Antonič M, Benedik M, Ponikvar JB, Ponikvar R, et al. Comparison of the Pharmacological Effects of Paricalcitol Versus Calcitriol on Secondary Hyperparathyroidism in the Dialysis Population. Ther Apher Dial. 2016 Jun;20((3)):261–6. doi: 10.1111/1744-9987.12434. [DOI] [PubMed] [Google Scholar]
- 41.Ong LM, Narayanan P, Goh HK, Manocha AB, Ghazali A, Omar M, et al. Oral Paricalcitol in ESRD Study Group Randomized controlled trial to compare the efficacy and safety of oral paricalcitol with oral calcitriol in dialysis patients with secondary hyperparathyroidism. Nephrology (Carlton) 2013 Mar;18((3)):194–200. doi: 10.1111/nep.12029. [DOI] [PubMed] [Google Scholar]
- 42.Xie Y, Su P, Sun Y, Zhang H, Zhao R, Li L, et al. Comparative efficacy and safety of paricalcitol versus vitamin D receptor activators for dialysis patients with secondary hyperparathyroidism: a meta-analysis of randomized controlled trials. BMC Nephrol. 2017 Aug;18((1)):272. doi: 10.1186/s12882-017-0691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hansen D, Rasmussen K, Pedersen SM, Rasmussen LM, Brandi L. Changes in fibroblast growth factor 23 during treatment of secondary hyperparathyroidism with alfacalcidol or paricalcitol. Nephrol Dial Transplant. 2012 Jun;27((6)):2263–9. doi: 10.1093/ndt/gfr668. [DOI] [PubMed] [Google Scholar]
- 44.Pichler G, Haller MC, Kainz A, Wolf M, Redon J, Oberbauer R. Prognostic value of bone- and vascular-derived molecular biomarkers in hemodialysis and renal transplant patients: a systematic review and meta-analysis. Nephrol Dial Transplant. 2017 Sep;32((9)):1566–78. doi: 10.1093/ndt/gfw387. [DOI] [PubMed] [Google Scholar]
- 45.Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004 Apr;350((15)):1516–25. doi: 10.1056/NEJMoa031633. [DOI] [PubMed] [Google Scholar]
- 46.Sprague SM, Wetmore JB, Gurevich K, Da Roza G, Buerkert J, Reiner M, et al. Effect of Cinacalcet and Vitamin D Analogs on Fibroblast Growth Factor-23 during the Treatment of Secondary Hyperparathyroidism. Clin J Am Soc Nephrol. 2015 Jun;10((6)):1021–30. doi: 10.2215/CJN.03270314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, Goodman WG, et al. EVOLVE Trial Investigators Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012 Dec;367((26)):2482–94. doi: 10.1056/NEJMoa1205624. [DOI] [PubMed] [Google Scholar]
- 48.Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012 Feb;307((7)):674–84. doi: 10.1001/jama.2012.120. [DOI] [PubMed] [Google Scholar]
- 49.Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD—the OPERA trial. J Am Soc Nephrol. 2014 Jan;25((1)):175–86. doi: 10.1681/ASN.2013010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Toussaint ND, Damasiewicz MJ. Do the benefits of using calcitriol and other vitamin D receptor activators in patients with chronic kidney disease outweigh the harms? Nephrology (Carlton) 2017 Mar;22(Suppl 2):51–6. doi: 10.1111/nep.13026. [DOI] [PubMed] [Google Scholar]
- 51.Brunelli SM, Monda KL, Burkart JM, Gitlin M, Neumann PJ, Park GS, et al. Early trends from the Study to Evaluate the Prospective Payment System Impact on Small Dialysis Organizations (STEPPS) Am J Kidney Dis. 2013 Jun;61((6)):947–56. doi: 10.1053/j.ajkd.2012.11.040. [DOI] [PubMed] [Google Scholar]
- 52.Spoendlin J, Schneeweiss S, Tsacogianis T, Paik JM, Fischer MA, Kim SC, et al. Association of Medicare's Bundled Payment Reform With Changes in Use of Vitamin D Among Patients Receiving Maintenance Hemodialysis: An Interrupted Time-Series Analysis. Am J Kidney Dis. 2018 Aug;72((2)):178–87. doi: 10.1053/j.ajkd.2018.03.027. [DOI] [PubMed] [Google Scholar]
- 53.Tentori F, Zepel L, Fuller DS, Wang M, Bieber BA, Robinson BM, et al., The DOPPS practice monitor for US dialysis care PTH levels and management of mineral and bone disorder in US hemodialysis patients. Am J Kidney Dis. 2015 Sep;66((3)):536–539. doi: 10.1053/j.ajkd.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kumar J, Tran NT, Schomberg J, Streja E, Kalantar-Zadeh K, Pahl M. Successful Conversion From Parenteral Paricalcitol to Pulse Oral Calcitriol for the Management of Secondary Hyperparathyroidism in Hemodialysis Patients. J Ren Nutr. 2016 Jul;26((4)):265–9. doi: 10.1053/j.jrn.2016.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lessard M, Ouimet D, Leblanc M, Nadeau-Fredette AC, Bell R, Lafrance JP, et al. Comparison of oral and intravenous alfacalcidol in chronic hemodialysis patients. BMC Nephrol. 2014 Feb;15((27)):27. doi: 10.1186/1471-2369-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dusso AS, Cannata-Andia JB, Vitamin D and Renal Disease . In: Vitamin D. 4th ed. Feldman D, Pike JW, Bouillon R, Giovannucci E, Goltzman D, Hewison M, editors. London: Academic Press; 2018. pp. pp. 445–69. [Google Scholar]
- 57.Goldsmith DJ. Pro: should we correct vitamin D deficiency/insufficiency in chronic kidney disease patients with inactive forms of vitamin D or just treat them with active vitamin D forms? Nephrol Dial Transplant. 2016 May;31((5)):698–705. doi: 10.1093/ndt/gfw082. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y, Darssan D, Pascoe EM, Johnson DW, Pi H, Dong J. Vitamin D status and mortality risk among patients on dialysis: a systematic review and meta-analysis of observational studies. Nephrol Dial Transplant. 2018 Oct;33((10)):1742–51. doi: 10.1093/ndt/gfy016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 59.Parikh C, Gutgarts V, Eisenberg E, Melamed ML. Vitamin D and Clinical Outcomes in Dialysis. Semin Dial. 2015 Nov-Dec;28((6)):604–9. doi: 10.1111/sdi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Selamet U, Katz R, Ginsberg C, Rifkin DE, Fried LF, Kritchevsky SB, et al. Serum Calcitriol Concentrations and Kidney Function DeclineHeart Failure and Mortality in Elderly Community-Living Adults: the Health Aging, and Body Composition Study. Am J Kidney Dis. 2018 Sep;72((3)):419–28. doi: 10.1053/j.ajkd.2018.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Arcidiacono MV, Yang J, Fernandez E, Dusso A. The induction of C/EBPβ contributes to vitamin D inhibition of ADAM17 expression and parathyroid hyperplasia in kidney disease. Nephrol Dial Transplant. 2015 Mar;30((3)):423–33. doi: 10.1093/ndt/gfu311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, et al. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011 Nov;121((11)):4393–408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, et al. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016 Jan;89((1)):135–46. doi: 10.1038/ki.2015.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rios R, Pineda C, Lopez I, Muñoz-Castañeda J, Rodriguez M, Aguilera-Tejero E, et al. Phosphorus restriction does not prevent the increase in fibroblast growth factor 23 elicited by high fat diet. PLoS One. 2018 Jun;13((6)):e0198481. doi: 10.1371/journal.pone.0198481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Matsui I, Oka T, Kusunoki Y, Mori D, Hashimoto N, Matsumoto A, et al. Cardiac hypertrophy elevates serum levels of fibroblast growth factor 23. Kidney Int. 2018 Jul;94((1)):60–71. doi: 10.1016/j.kint.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 66.Marthi A, Donovan K, Haynes R, Wheeler DC, Baigent C, Rooney CM, et al. Fibroblast Growth Factor-23 and Risks of Cardiovascular and Noncardiovascular Diseases: A Meta-Analysis. J Am Soc Nephrol. 2018 Jul;29((7)):2015–27. doi: 10.1681/ASN.2017121334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zella JB, Plum LA, Plowchalk DR, Potochoiba M, Clagett-Dame M, DeLuca HF. Novel, selective vitamin D analog suppresses parathyroid hormone in uremic animals and postmenopausal women. Am J Nephrol. 2014;39((6)):476–83. doi: 10.1159/000362846. [DOI] [PubMed] [Google Scholar]
- 68.Sprague SM, Crawford PW, Melnick JZ, Strugnell SA, Ali S, Mangoo-Karim R, et al. Use of Extended-Release Calcifediol to Treat Secondary Hyperparathyroidism in Stages 3 and 4 Chronic Kidney Disease. Am J Nephrol. 2016;44((4)):316–25. doi: 10.1159/000450766. [DOI] [PubMed] [Google Scholar]
- 69.Dusso A, Lopez-Hilker S, Rapp N, Slatopolsky E. Extra-renal production of calcitriol in chronic renal failure. Kidney Int. 1988 Sep;34((3)):368–75. doi: 10.1038/ki.1988.190. [DOI] [PubMed] [Google Scholar]
- 70.Krause R, Stange R, Roth HJ, Kaase H, Michalsen A, Holick MF. Partial Body UV Exposure in Chronic Kidney Disease and Extrarenal Vitamin D Metabolism. Anticancer Res. 2018 Feb;38((2)):1217–9. doi: 10.21873/anticanres.12342. [DOI] [PubMed] [Google Scholar]
- 71.Levin A, Tang M, Perry T, Zalunardo N, Beaulieu M, Dubland JA, et al. Randomized Controlled Trial for the Effect of Vitamin D Supplementation on Vascular Stiffness in CKD. Clin J Am Soc Nephrol. 2017 Sep;12((9)):1447–60. doi: 10.2215/CJN.10791016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Obi Y, Hamano T, Wada A, Tsubakihara Y, Committee of Renal Data Registry of the Japanese Society for Dialysis Therapy Vitamin D Receptor Activator Use and Cause-specific Death among dialysis Patients: a Nationwide Cohort Study using Coarsened Exact Matching. Sci Rep. 2017 Jan;7((1)):41170. doi: 10.1038/srep41170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khedkar SA, Samad MA, Choudhury S, Lee JY, Zhang D, Thadhani RI, et al. Identification of Novel Non-secosteroidal Vitamin D Receptor Agonists with Potent Cardioprotective Effects and devoid of Hypercalcemia. Sci Rep. 2017 Aug;7((1)):8427. doi: 10.1038/s41598-017-08670-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Pandey R, Zella JB, Zhu JG, Plum LA, Clagett-Dame M, Blaser WJ, et al. Pharmacokinetics of a New Oral Vitamin D Receptor Activator (2-Methylene-19-Nor-(20S)-1α,25-Dihydroxyvitamin D3) in Patients with Chronic Kidney Disease and Secondary Hyperparathyroidism on Hemodialysis. Drugs R D. 2017 Dec;17((4)):597–605. doi: 10.1007/s40268-017-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pasing Y, Fenton CG, Jorde R, Paulssen RH. Changes in the human transcriptome upon vitamin D supplementation. J Steroid Biochem Mol Biol. 2017 Oct;173:93–9. doi: 10.1016/j.jsbmb.2017.03.016. [DOI] [PubMed] [Google Scholar]