Abstract
Objective
Visceral adiposity, defined as a high visceral-to-subcutaneous adipose tissue area ratio (VSR), has been shown to be associated with poor outcomes in several cancers. However, in the surgical field, the significance of visceral adiposity remains controversial. The present study investigated the impact of visceral adiposity as well as sarcopenic factors (low muscularity) on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma (HCC).
Patients and Methods
This retrospective study analyzed data from 606 patients undergoing hepatectomy for HCC at our institution between April 2005 and March 2016. Using preoperative plain computed tomography imaging at the level of the third lumbar vertebra, visceral adiposity, skeletal muscle mass, and muscle quality were evaluated by the VSR, skeletal muscle mass index (SMI), and intramuscular adipose tissue content (IMAC), respectively. The impact of these parameters on outcomes after hepatectomy for HCC was analyzed.
Results
The overall survival rate was significantly lower among patients with a high VSR (p < 0.001) than among patients with a normal VSR. Similarly, the recurrence-free survival rate was significantly lower among patients with a high VSR (p = 0.016). A high VSR, low SMI, and high IMAC contributed to an increased risk of death (p < 0.001) and HCC recurrence (p < 0.001) in an additive manner. Multivariate analysis showed that not only preoperative low muscularity but also visceral adiposity was a significant risk factor for mortality (hazard ratio [HR] = 1.566, p < 0.001) and HCC recurrence (HR = 1.329, p = 0.020) after hepatectomy for HCC.
Conclusions
Preoperative visceral adiposity, as well as low muscularity, was closely related to poor outcomes after hepatectomy for HCC. It is crucial to establish a new strategy including perioperative nutritional interventions with rehabilitation for better outcomes after hepatectomy for HCC.
Key Words: Hepatocellular carcinoma, Intramuscular adipose tissue content, Sarcopenia, Skeletal muscle mass index, Visceral adiposity, Visceral-to-subcutaneous adipose tissue area ratio
Introduction
Hepatocellular carcinoma (HCC) is one of the most common human cancers, and it is the third leading cause of cancer-related death because of its highly malignant potential, characterized by rapid progression, frequent tumor recurrence, and metastasis [1, 2]. Today, surgical treatment, including hepatectomy and liver transplantation, is the only effective treatment for HCC, because there is no successful systemic chemotherapy for patients with advanced HCC [2, 3, 4]. Although advances in preoperative diagnosis, surgical technique and devices, and perioperative management have dramatically improved overall survival (OS) after partial hepatectomy for HCC, the HCC recurrence rate remains high even in patients with curative resection, with a 5-year recurrence rate as high as 70% [5, 6, 7].
Obesity has been well documented as a risk factor for various diseases, including type 2 diabetes mellitus, hypertension, cardiovascular disease, and nonalcoholic fatty liver disease [8, 9]. In addition, prospective studies and meta-analyses have also demonstrated that obese individuals are at increased risk of many types of cancer and have a poor prognosis [10, 11, 12]. On the other hand, several recent studies showed that patients with a higher body mass index (BMI) survive longer than normal-weight patients [13, 14, 15, 16]. These contradictory results, the so-called obesity paradox, might occur because the BMI cannot account for differences in fat distribution because it is an indirect measure of adipose tissue.
In recent studies of body composition assessment, skeletal muscle and adipose tissue were directly evaluated using several imaging techniques such as dual-energy X-ray absorptiometry, computed tomography (CT), magnetic resonance imaging, and bioimpedance analysis. Especially on CT analysis, the areas of skeletal muscle and adipose tissue can be automatically quantified by the CT values peculiar to these tissues, and this has been shown to be a precise and reliable method to evaluate adipose tissue distribution [17, 18, 19]. Using this method, visceral adiposity can be easily calculated as the visceral-to-subcutaneous adipose tissue area ratio (VSR), and a high VSR has been reported to be a useful predictor of poor prognosis in various kinds of disease, including several cancers [20, 21, 22, 23, 24]. Although one study demonstrated the significance of visceral adiposity for patients with HCC, its impact on outcomes in patients undergoing hepatectomy for HCC remains unclear [20].
The present study retrospectively evaluated preoperative visceral adiposity, in addition to muscularity (skeletal muscle mass and muscle quality), and it aimed to investigate the impact of preoperative visceral adiposity and muscularity on outcomes after hepatectomy for HCC.
Patients and Methods
Patients
Between April 2005 and March 2016, a total of 622 patients were admitted to Kyoto University Hospital for hepatectomy for HCC. Sixteen patients who did not undergo preoperative plain CT were excluded from the analysis. This retrospective study therefore analyzed data from 606 patients (484 men, 122 women). The median duration of follow-up for these patients was 42.6 months (interquartile range [IQR] 19.7–71.7). Details about the surgical procedures of hepatectomy, pathological examination, and follow-up after hepatectomy have been described previously [25, 26].
The indications for hepatectomy for HCC at our institution include Child-Pugh grade A or B and a future remnant liver volume ≥25% of the whole liver. Hepatic resection is usually performed using the Cavitron Ultrasonic Surgical Aspirator and a bipolar cautery device equipped with a channel for water dripping, with an intermittent Pringle maneuver, or selective vascular clamping if necessary.
Image Analysis
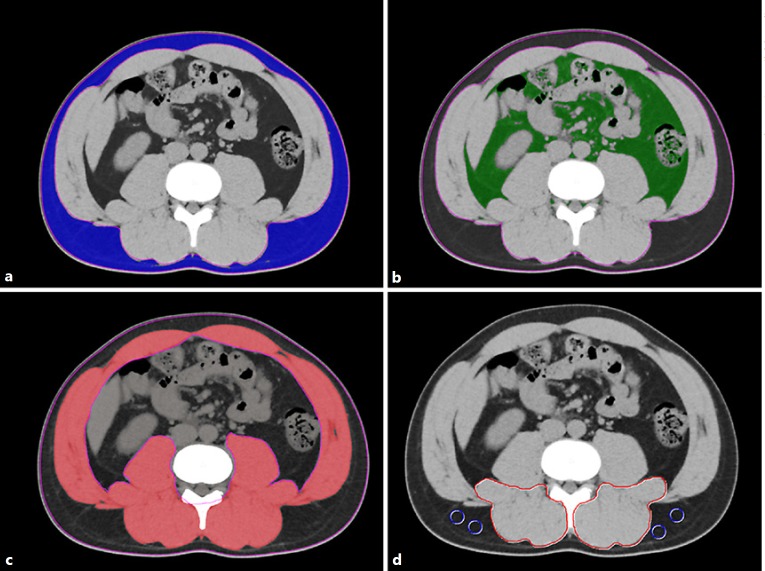
All CT examinations were performed using a multidetector-row CT scanner (Aquilion 64; Toshiba Medical Systems, Tochigi, Japan). The technical parameters for CT were: tube voltage, 120 kV; detector configuration, 0.5 mm × 64 rows; tube current modulation, 0.5 s/rotation (gantry rotation); and reconstruction thickness, 5 mm. Using a cross-sectional CT at the level of the third lumbar vertebra (L3), the adipose tissue area and skeletal muscle were examined using Aquarius iNtuition (TeraRecon, San Mateo, CA, USA). Aquarius iNtuition is an iNtuition client viewer with advanced visualization capabilities. With this software, visceral and subcutaneous adipose tissue and areas of skeletal muscle can be easily and automatically quantified using the CT attenuation values peculiar to these tissues. On the basis of previous reports, subcutaneous and visceral adipose tissue areas were quantified using attenuation values of −190 to −30 Hounsfield units (HU) (Fig. 1a) and −150 to −50 HU (Fig. 1b), respectively [17, 18]. Visceral adiposity was evaluated by the VSR, which was calculated as follows [20]: VSR = visceral adipose tissue area (cm2)/sub cutaneous adipose tissue area (cm2).
Fig. 1.
Cross-sectional CT images at the level of the third lumbar vertebra. a Subcutaneous adipose tissue areas are identified and quantified using CT attenuation values of −190 to −30 HU. b Visceral adipose tissue areas are quantified using attenuation values of −150 to −50 HU. c Skeletal muscle areas are quantified using a CT attenuation value of −29 to 150 HU. d CT attenuation values of subfascial muscular tissue in the multifidus muscle and subcutaneous fat (4 small circles) are examined to calculate the intramuscular adipose tissue content. CT, computed tomography; HU, Hounsfield unit.
Similarly, skeletal muscle areas that include the psoas, erector spinae, quadratus lumborum, transversus abdominis, external and internal obliques, and rectus abdominis were identified and quantified using attenuation values of −29 to 150 HU (Fig. 1c) [18]. The preoperative skeletal muscle mass was evaluated by the skeletal muscle mass index (SMI), calculated as follows: SMI = cross-sectional areas of skeletal muscle (cm2)/patient's height2 (m2).
In addition, muscle quality was examined by intramuscular adipose tissue content (IMAC) at the L3 level. The IMAC was calculated as follows [24, 25, 26]: IMAC = CT attenuation value of the multifidus muscles (HU)/CT attenuation value of the subcutaneous fat (HU) (Fig. 1d). A higher IMAC indicates a greater amount of adipose tissue within skeletal muscle, and thus a lower quality of skeletal muscle (muscle steatosis).
Cutoff Values for the VSR, SMI, and IMAC
The cutoff values for the VSR, SMI, and IMAC were determined for men and women separately using the data from 657 healthy donors for living donor liver transplantation between 2005 and 2016 [24]. A high VSR was defined as more than 2 standard deviations (SDs) above the mean VSR of younger donors (< 50 years), resulting in cutoff values of 1.325 for males and 0.710 for females. A low SMI was defined as more than 2 SDs below the mean, resulting in cutoff values of 40.31 for males and 30.88 for females. Similarly, a high IMAC was defined as more than 2 SDs above the mean, resulting in cutoff values of −0.358 for males and −0.229 for females.
Analyzed Parameters
First, perioperative parameters were compared between patients with a high VSR and those with a normal VSR. The severity of complications was graded according to the Dindo-Clavien classification [27]. Major complications were defined as any complications of Clavien grade III or higher. Second, the OS and recurrence-free survival (RFS) rates after hepatectomy were investigated in the patients classified according to the VSR, SMI, or IMAC. Moreover, the prognostic factors were analyzed on the basis of the following variables: patient age (≥65 vs. < 65 years), sex (male vs. female), etiology of HCC (HBV [hepatitis B virus] and/or HCV [hepatitis C virus]-related HCC vs. others), the presence of a treatment history for HCC before hepatectomy, the presence of obesity-related comorbidities (type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease), the period of hepatectomy (2005–2009 vs. 2010–2016), platelet count (≥10 × 104 vs. < 10 × 104/mm3), total lymphocyte count (≥1,000 vs. < 1,000/mm3), neutrophil-to-lymphocyte ratio (≥4.0 vs. < 4.0), serum total bilirubin level (≥1.0 vs. < 1.0 mg/dL), serum albumin level (≥3.5 vs. < 3.5 g/dL), indocyanine green retention at 15 min (≥15 vs. < 15%), Child-Pugh class (A vs. B), serum α-fetoprotein (AFP) level (≥20 vs. < 20 ng/dL), des-γ-carboxyprothrombin level (≥40 vs. < 40 mAU/mL), liver histology (normal liver or chronic hepatitis vs. liver fibrosis or liver cirrhosis), tumor size (≥5.0 vs. < 5.0 cm), number of tumors (solitary vs. multiple), microvascular invasion, tumor differentiation (well or moderately differentiated vs. poorly differentiated), pathological stage according to the Liver Cancer Study Group of Japan (I or II vs. III or IV), surgical procedure (lobectomy or more vs. less than segmentectomy), duration of surgery (≥360 vs. < 360 min), estimated operative blood loss (≥500 vs. < 500 mL), BMI (≥25 vs. < 25), preoperative skeletal muscle mass (low SMI vs. normal SMI), skeletal muscle quality (high IMAC vs. normal IMAC), and visceral adiposity (high VSR vs. normal VSR).
Statistical Analysis
Continuous data are presented as medians (IQR); they were nonparametrically analyzed using the Mann-Whitney U test. Categorical variables were compared using the χ2 test or Fisher's exact test, as appropriate. Correlations between continuous variables were assessed using Pearson's correlation coefficient. Cumulative OS and RFS rates were calculated using Kaplan-Meier methods, with differences between curves evaluated using the log-rank test. Among variables identified as significant (p < 0.05) or showing values of p < 0.10 on univariate analysis, candidates for a Cox proportional-hazards model were selected through a stepwise procedure using the minimum AICc method.
The results are shown as hazard ratios (HRs) with 95% CIs. The variation inflation factor and tolerance were calculated to assess multicollinearity before performing multivariate analysis, and multicollinear variables were excluded from the multivariate analysis. Especially, since tumor size, the number of tumors, and vascular invasion are factors constituting a pathological stage according to the Liver Cancer Study Group of Japan, these factors were not included in the multivariate analysis together with the pathological stage.
A p value < 0.05 was considered significant. All statistical data were generated using JMP Pro 12 (SAS Institute, Cary, NC, USA) and Prism 6 (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Patient Characteristics
The baseline characteristics and laboratory data of the 606 patients are shown in Table 1. The median patient age was 68 years (IQR 61–75). Two hundred and twenty-eight patients (38%) had a treatment history for HCC before hepatectomy, such as transcatheter arterial chemoembolization or radiofrequency ablation, while 378 patients (62%) underwent primary hepatectomy as the initial treatment for HCC. The Child-Pugh classes were A and B for 555 (92%) and 51 patients (8%), respectively. The median tumor size was 3.5 cm (IQR 2.2–6.2), and multiple HCCs were found in 189 patients (31%). The pathological stages according to the Liver Cancer Study Group of Japan were I, II, III, and IV for 166 (27%), 195 (32%), 157 (26%), and 88 patients (15%), respectively. Using the cutoff values described above, 252 patients (42%) had a high VSR (visceral adiposity) preoperatively, 84 patients (14%) had a low SMI (low muscle mass) preoperatively, and 258 patients (43%) had a high IMAC (low muscle quality).
Table 1.
Characteristics of the patients classified according to the VSR
| Characteristic | Total (n = 606) |
High VSR (n = 252) |
Normal VSR (n = 354) |
p value |
|---|---|---|---|---|
| Age, years | 68 (61–75) | 71 (64–80) | 66 (59–77) | <0.001 |
| Sex Male |
484 (80) | 191 (76) | 293 (83) | 0.040 |
| Female | 122 (20) | 62 (24) | 61 (17) | |
| Etiology | ||||
| HBV and/or HCV | 392 (65) | 129 (51) | 263 (74) | <0.001 |
| Others | 214 (35) | 123 (49) | 91 (25) | |
| Previous treatment for HCC Yes |
228 (38) | 99 (39) | 129 (36) | 0.497 |
| No | 378 (62) | 153 (61) | 225 (64) | |
| Diabetes | ||||
| Yes | 180 (30) | 89 (35) | 91 (26) | 0.012 |
| No | 426 (70) | 163 (65) | 263 (74) | |
| Hypertension | ||||
| Yes | 273 (45) | 140 (56) | 133 (38) | <0.001 |
| No | 333 (55) | 112 (44) | 221 (62) | |
| Dyslipidemia | ||||
| Yes | 56 (9) | 32 (13) | 24 (7) | 0.015 |
| No | 550 (91) | 220 (87) | 330 (93) | |
| Cardiovascular disease | ||||
| Yes | 42 (7) | 24 (10) | 18 (5) | 0.036 |
| No | 564 (93) | 228 (90) | 336 (95) | |
| Platelet count, ×104/mm3 | 14.4 (10.5–19.1) | 14.7 (10.6–19.5) | 14.1 (10.4–18.9) | 0.180 |
| Total bilirubin, mg/dL | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | 0.8 (0.6–1.0) | 0.154 |
| Albumin, g/dL | 3.9 (3.6–4.2) | 3.9 (3.6–4.2) | 3.9 (3.6–4.3) | 0.653 |
| Total lymphocyte count, /mm3 | 1,338 (994–1,756) | 1,357 (982–1,721) | 1,328 (1,006–1,778) | 0.454 |
| Neutrophil-to-lymphocyte ratio | 1.9 (1.4–2.7) | 2.1 (1.6–2.8) | 1.8 (1.3–2.6) | 0.046 |
| ICG R15, % | 15 (10–21) | 15 (10–21) | 15 (10–21) | 0.680 |
| Child-Pugh class | ||||
| A | 555 (92) | 229 (91) | 326 (92) | 0.657 |
| B | 51 (8) | 23 (9) | 28 (8) | |
| AFP, ng/mL | 18.3 (5.3–204.1) | 14.9 (5.0–167.5) | 20.2 (5.4–223.7) | 0.454 |
| DCP, mAU/mL | 142 (33–1,568) | 215 (42–3,350) | 97 (31.5–863) | 0.019 |
| Liver histology | ||||
| Normal liver + chronic hepatitis | 305 (50) | 132 (52) | 173 (49) | 0.411 |
| Liver fibrosis + liver cirrhosis | 301 (50) | 120 (48) | 181 (51) | |
| Tumor size, cm | 3.5 (2.2–6.2) | 4.0 (2.5–7.0) | 3.0 (2.0–5.5) | 0.001 |
| Number of tumors | ||||
| Solitary | 417 (69) | 170 (67) | 247 (70) | 0.594 |
| Multiple | 189 (31) | 82 (33) | 107 (30) | |
| MVI | ||||
| Positive | 196 (32) | 87 (35) | 109 (31) | 0.334 |
| Negative | 410 (68) | 165 (65) | 245 (69) | |
| Differentiation of HCC | ||||
| Well | 60 (10) | 28 (11) | 32 (9) | 0.638 |
| Moderate | 364 (60) | 146 (58) | 218 (62) | |
| Poor | 167 (28) | 73 (29) | 94 (27) | |
| Unknown | 15 (2) | 5 (2) | 10 (3) | |
| TNM stage | ||||
| I + II | 361 (60) | 145 (58) | 216 (61) | 0.402 |
| III + IV | 245 (40) | 107 (42) | 138 (39) | |
| Surgical procedure | ||||
| Lobectomy or more | 210 (35) | 95 (38) | 115 (33) | 0.195 |
| Less than segmentectomy | 396 (65) | 157 (62) | 239 (67) | |
| Operative time, min | 367 (277–459) | 367 (283–463) | 366 (270–454) | 0.839 |
| Operative blood loss, mL | 720 (330–1,385) | 800 (357–1,460) | 607 (298–1,307) | 0.651 |
| Postoperative complications (Clavien-Dindo classification) | ||||
| Grade III or higher | 132 (22) | 64 (25) | 68 (19) | 0.073 |
| Below grade III | 474 (78) | 188 (75) | 286 (81) | |
| BMI | 23.2 (21.1–25.1) | 23.2 (21.2–25.6) | 23.2 (21.0–24.9) | 0.852 |
| Preoperative SMI | ||||
| Normal | 522 (86) | 206 (81) | 316 (89) | 0.012 |
| Low | 84 (14) | 46 (18) | 38 (11) | |
| Preoperative IMAC | ||||
| Normal | 348 (57) | 131 (52) | 216 (61) | 0.025 |
| High | 258 (43) | 121 (48) | 137 (39) | |
| Preoperative VSR | ||||
| Males | 1.203 (0.883–1.606) | 1.713 (1.463–2.586) | 0.967 (0.747–1.252) | <0.001 |
| Females | 0.709 (0.491–0.987) | 0.984 (0.807–1.237) | 0.494 (0.377–0.661) | <0.001 |
Values are presented as n (%) or median (IQR). Percentages might not add up to 100% because of rounding. VSR, visceral-to-subcutaneous adipose tissue area ratio; IQR, interquartile range; HBV, hepatitis B virus; HCV, hepatitis C virus; HCC, hepatocellular carcinoma; ICG R15, indocyanine green retention test at 15 min; AFP, α-fetoprotein; DCP, des-γ-carboxyprothrombin; MVI, microvascular invasion; TNM, tumour-node-metastasis (stage defined by the Liver Cancer Study Group of Japan); BMI, body mass index; SMI, skeletal muscle mass index; IMAC, intramuscular adipose tissue content.
Correlations of the Preoperative VSR with Other Parameters
The perioperative parameters of the patients classified according to their VSR are shown in Table 1. The median age was significantly higher among the patients with a high VSR than in the patients with a normal VSR (p < 0.001). The patients with a normal VSR showed more HBV- or HCV-related HCC than those with a high VSR (p < 0.001). In addition, more patients had obesity-related comorbidities, such as type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease, in the high-VSR group than in the normal-VSR group (p = 0.012, p < 0.001, p = 0.015, and p = 0.036, respectively). Although the median tumor size was significantly larger among the patients with a high VSR (p = 0.001), the TNM stage was not significantly different between the two groups (p = 0.402). The operative factors, such as surgical procedure, operative time, and blood loss, were similar between the two groups. Among 252 patients with a high VSR, there were significantly more subjects with a low SMI (p = 0.012) and a high IMAC (p = 0.025). However, there were only weak correlations between VSR and SMI (r = 0.076), and between VSR and IMAC (r = 0.056) (data not shown).
OS and RFS Rates after Hepatectomy for HCC
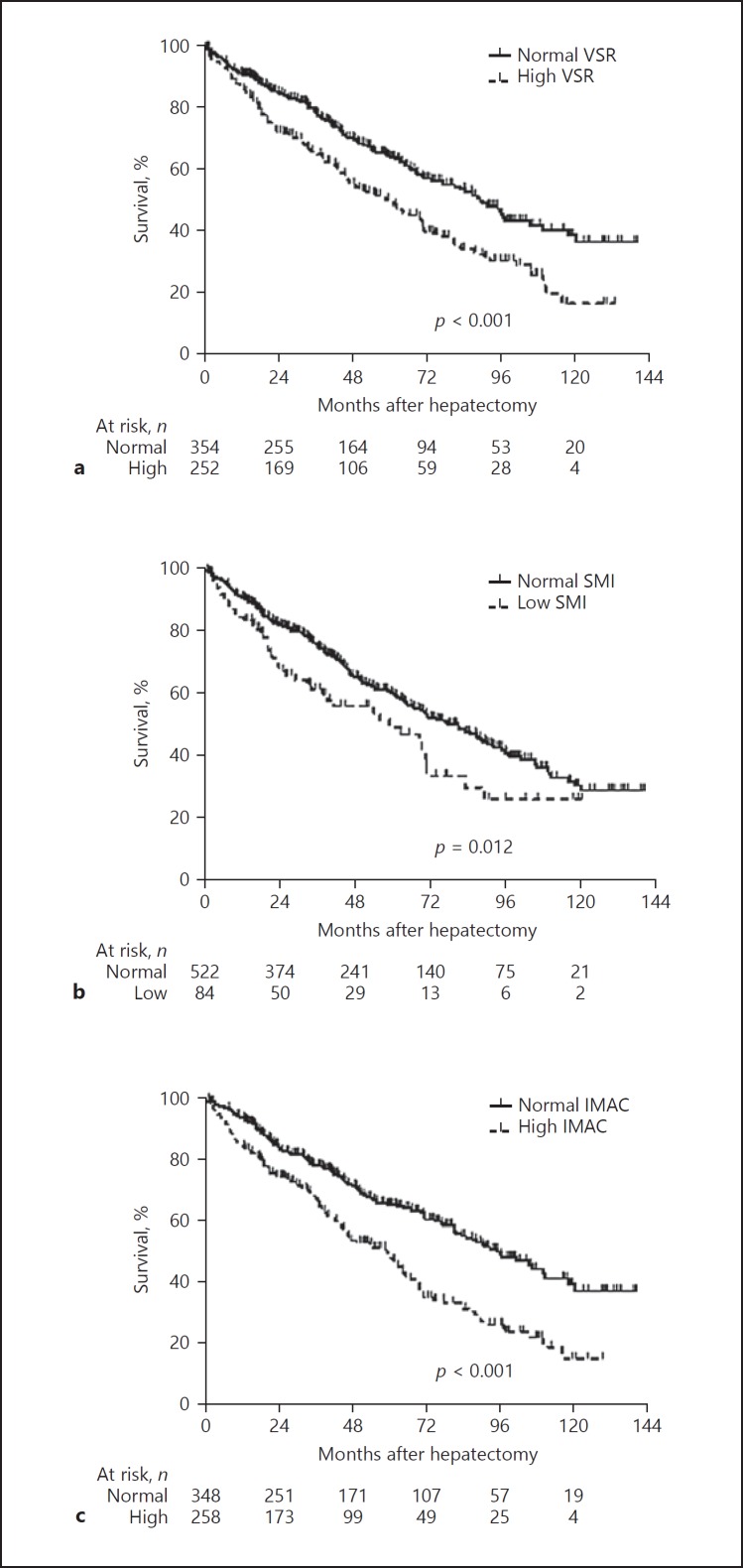
The OS rate after hepatectomy for HCC was significantly lower among the patients with a high VSR (n = 252) than among those with a normal VSR (n = 354; p < 0.001; Fig. 2a). The 1-, 3-, and 5-year OS rates among the patients with a high VSR versus those with a normal VSR were 87.9, 65.3, and 50.5% versus 91.4, 79.4, and 64.9%, respectively. Similarly, the OS rate was significantly lower among the patients with a low SMI (n = 84) than among those with a normal SMI (n = 522; p = 0.012; Fig. 2b). Moreover, the OS rate was significantly lower among the patients with a high IMAC (n = 258) than among those with a normal IMAC (n = 348; p < 0.001; Fig. 2c).
Fig. 2.
OS rates among patients classified according to body composition variables. a The OS rate after hepatectomy for hepatocellular carcinoma was significantly lower among the patients with a high VSR (n = 252) than among the patients with a normal VSR (n = 354; p < 0.001). b The OS rate was significantly lower among the patients with a low SMI (n = 84) than among the patients with a normal SMI (n = 522; p = 0.012). c The OS rate was significantly lower among the patients with a high IMAC (n = 258) than among the patients with a normal IMAC (n = 348; p < 0.001). OS, overall survival; VSR, visceral-to-subcutaneous adipose tissue area ratio; SMI, skeletal muscle mass index; IMAC, intramuscular adipose tissue content.
The RFS rate after hepatectomy for HCC was significantly lower among the patients with a high VSR than among those with a normal VSR (p = 0.016; Fig. 3a). The 1-, 3-, and 5-year RFS rates among the patients with a high VSR versus those with a normal VSR were 56.5, 33.0, and 20.9% versus 67.6, 41.8, and 32.8%, respectively. The RFS rate was significantly lower among the patients with a low SMI than among those with a normal SMI (p = 0.009; Fig. 3b). Similarly, the RFS rate was significantly lower among the patients with a high IMAC than among those with a normal IMAC (p = 0.047; Fig. 3c).
Fig. 3.
RFS rates among patients classified according to body composition variables. a The RFS rate after hepatectomy for hepatocellular carcinoma was significantly lower among the patients with a high VSR than among the patients with a normal VSR (p = 0.016). b The RFS rate was significantly lower among the patients with a low SMI than among the patients with a normal SMI (p = 0.009). c The RFS rate was significantly lower among the patients with a high IMAC than among the patients with a normal IMAC (p = 0.047). RFS, recurrence-free survival; VSR, visceral-to-subcutaneous adipose tissue area ratio; SMI, skeletal muscle mass index; IMAC, intramuscular adipose tissue content.
In addition, these three factors (high VSR, low SMI, and high IMAC) contributed to an increased risk of death (p < 0.001; Fig. 4a) and HCC recurrence (p < 0.001; Fig. 4b) after hepatectomy for HCC in an additive manner, which suggests that they are complementary predictors of a poor prognosis in patients undergoing hepatectomy for HCC.
Fig. 4.
OS and RFS rates among patients classified by the number of body composition variables. The OS (a) and RFS (b) rates after hepatectomy for hepatocellular carcinoma decreased significantly with an increasing number of prognostic body composition factors (high visceral-to-subcutaneous adipose tissue area ratio, low skeletal muscle mass index, and high intramuscular adipose tissue content) (p < 0.001 and p < 0.001, respectively). OS, overall survival; RFS, recurrence-free survival.
Risk Factors for Poor Outcomes in Patients Undergoing Hepatectomy for HCC
The results of the univariate and multivariate analyses of OS among patients undergoing hepatectomy for HCC are shown in Table 2. The multivariate analysis identified a low serum albumin level (HR = 1.501, p = 0.015), high serum AFP level (HR = 1.590, p < 0.001), advanced TNM stage (HR = 1.969, p < 0.001), preoperative low SMI (HR = 1.333, p = 0.045), high IMAC (HR = 1.774, p < 0.001), and high VSR (HR = 1.566, p = 0.001) as independent risk factors for mortality after hepatectomy (Table 2).
Table 2.
Prognostic factors for overall survival on univariate and multivariate analysis
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | ||||||
| ≥65 years (n = 395) | 1.232 | 0.961–1.592 | 0.101 | |||
| <65 years (n = 211) | 1.000 | (referent) | ||||
| Sex | ||||||
| Male (n = 484) | 1.003 | 0.752–1.364 | 0.982 | |||
| Female (n = 122) Etiology of HCC | 1.000 | (referent) | ||||
| Etiology of HCC | ||||||
| HBV and/or HCV (n = 392) | 0.967 | 0.757–1.242 | 0.788 | |||
| Others (n = 214) | 1.000 | (referent) | ||||
| Previous treatment for HCC | ||||||
| Yes (n = 228) | 1.331 | 1.048–1.688 | 0.019 | |||
| No (n = 378) | 1.000 | (referent) | ||||
| Obesity-related comorbidities | ||||||
| Yes(n = 361) | 1.003 | 0.790–1.278 | 0.978 | |||
| No(n = 245) | 1.000 | (referent) | ||||
| Period of hepatectomy | ||||||
| 2005–2009 (n = 302) | 0.905 | 0.696–1.181 | 0.459 | |||
| 2010–2016 (n = 304) | 1.000 | (referent) | ||||
| Platelet count | ||||||
| <10 × 104/mm3 (n = 128) | 1.410 | 1.071–1.835 | 0.015 | 1.199 | 0.895–1.589 | 0.220 |
| ≥10 × 104/mm3 (n = 478) | 1.000 | (referent) | 1.000 | (referent) | ||
| Total lymphocyte count | ||||||
| <1,000/mm3 (n = 156) | 1.279 | 0.975–1.660 | 0.075 | |||
| ≥1,000/mm3 (n = 450) | 1.000 | (referent) | ||||
| Neutrophil-to-lymphocyte ratio | ||||||
| <4.0 (n = 547) | 0.750 | 0.514–1.143 | 0.174 | |||
| ≥4.0 (n = 59) | 1.000 | (referent) | ||||
| Total bilirubin | ||||||
| ≥1.0 mg/dL (n = 187) | 1.123 | 0.868–1.442 | 0.371 | |||
| <1.0 mg/dL (n = 419) | 1.000 | (referent) | ||||
| Albumin | ||||||
| <3.5 g/dL (n = 100) | 1.799 | 1.329–2.398 | <0.001 | 1.501 | 1.084–2.045 | 0.015 |
| ≥3.5 g/dL (n = 506) | 1.000 | (referent) | 1.000 | (referent) | ||
| ICG R15 | ||||||
| ≥15% (n = 312) | 1.195 | 0.944–1.516 | 0.139 | |||
| <15% (n = 294) | 1.000 | (referent) | ||||
| Child-Pugh class | ||||||
| A (n = 555) | 0.746 | 0.508–1.144 | 0.172 | |||
| B (n = 51) | 1.000 | (referent) | ||||
| AFP | ||||||
| ≥20 ng/dL (n = 294) | 1.792 | 1.412–2.282 | <0.001 | 1.590 | 1.236–2.050 | <0.001 |
| <20 ng/dL (n = 312) | 1.000 | (referent) | 1.000 | (referent) | ||
| DCP | ||||||
| ≥40 mAU/mL (n = 428) | 1.239 | 0.955–1.625 | 0.108 | |||
| <40 mAU/mL (n = 178) | 1.000 | (referent) | ||||
| Liver histology | ||||||
| Normal liver or chronic hepatitis | ||||||
| (n = 305) | 0.899 | 0.709–1.138 | 0.376 | |||
| Liver fibrosis or liver cirrhosis | ||||||
| (n = 301) | 1.000 | (referent) | ||||
| Tumor size | ||||||
| ≥5.0 cm (n = 209) | 1.609 | 1.265–2.041 | <0.001 | |||
| <5.0 cm (n = 397) | 1.000 | (referent) | ||||
| Number of tumors | ||||||
| Multiple (n = 189) | 1.542 | 1.206–1.962 | 0.001 | |||
| Solitary (n = 417) | 1.000 | (referent) | ||||
| MVI | ||||||
| Positive (n = 196) | 1.891 | 1.485–2.400 | <0.001 | |||
| Negative (n = 410) | 1.000 | (referent) | ||||
| Tumor differentiation | ||||||
| Well or moderate (n = 424) | 0.551 | 0.427–0.718 | <0.001 | 0.739 | 0.537–1.040 | 0.081 |
| Poor(n = 167) | 1.000 | (referent) | 1.000 | (referent) | ||
| TNM stage | ||||||
| I or II (n = 361) | 0.454 | 0.357–0.575 | <0.001 | 0.508 | 0.391–0.659 | <0.001 |
| III or IV (n = 245) | 1.000 | (referent) | 1.000 | (referent) | ||
| Surgical procedure | ||||||
| Lobectomy or more (n = 210) | 1.536 | 1.205–1.950 | 0.001 | |||
| Less than segmentectomy (n = 396) | 1.000 | (referent) | ||||
| Operative time | ||||||
| ≥360 min (n = 318) | 1.495 | 1.179–1.899 | 0.001 | |||
| <360 min (n = 288) | 1.000 | (referent) | ||||
| Operative blood loss | ||||||
| ≥500 mL(n = 378) | 1.644 | 1.269–2.151 | <0.001 | 1.012 | 0.758–1.362 | 0.936 |
| <500 mL (n = 228) | 1.000 | (referent) | 1.000 | (referent) | ||
| BMI | ||||||
| ≥25(n = 157) | 0.994 | 0.753–1.296 | 0.964 | |||
| <25 (n = 449) | 1.000 | (referent) | ||||
| Preoperative SMI | ||||||
| Low (n = 84) | 1.506 | 1.077–2.058 | 0.018 | 1.333 | 1.007–1.752 | 0.045 |
| Normal(n = 522) | 1.000 | (referent) | 1.000 | (referent) | ||
| Preoperative IMAC | ||||||
| High (n = 258) | 1.918 | 1.500–2.468 | <0.001 | 1.774 | 1.375–2.302 | <0.001 |
| Normal(n = 348) | 1.000 | (referent) | 1.000 | (referent) | ||
| Preoperative VSR | ||||||
| High(n = 252) | 1.656 | 1.308–2.099 | <0.001 | 1.566 | 1.222–2.008 | <0.001 |
| Normal (n = 354) | 1.000 | (referent) | 1.000 | (referent) | ||
HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; ICG R15, indocyanine green retention test at 15 min; AFP, α-fetoprotein; DCP, des-γ-carboxyprothrombin; MVI, microvascular invasion; TNM, Tumour-Node-Metastasis (stage defined by the Liver Cancer Study Group of Japan); BMI, body mass index; SMI, skeletal muscle mass index; IMAC, intramuscular adipose tissue content; VSR, visceral-to-subcutaneous adipose tissue area ratio.
Table 3 shows the results of the univariate and multivariate analyses of RFS. The multivariate analysis showed that a high serum AFP level (HR = 1.370, p = 0.004), advanced TNM stage (HR = 2.198, p < 0.001), preoperative low SMI (HR = 1.447, p = 0.016), high IMAC (HR = 1.234, p = 0.049), and high VSR (HR = 1.329, p = 0.020) were independent risk factors for HCC recurrence (Table 3).
Table 3.
Prognostic factors for recurrence-free survival on univariate and multivariate analysis
| Univariate analysis |
Multivariate analysis |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | |
| Age | ||||||
| ≥65 years(n = 395) | 0.994 | 0.807–1.229 | 0.955 | |||
| <65 years (n = 211) | 1.000 | (referent) | ||||
| Sex | ||||||
| Male (n = 484) | 1.047 | 0.817–1.359 | 0.721 | |||
| Female (n = 122) | 1.000 | (referent) | ||||
| Etiology of HCC | ||||||
| HBV and/or HCV (n = 392) | 1.111 | 0.900–1.378 | 0.330 | |||
| Others (n = 214) | 1.000 | (referent) | ||||
| Previous treatment for HCC | ||||||
| Yes (n = 228) | 1.377 | 1.122–1.687 | 0.002 | |||
| No (n = 378) | 1.000 | (referent) | ||||
| Obesity-related comorbidities | ||||||
| Yes(n = 361) | 0.867 | 0.709–1.064 | 0.172 | |||
| No(n = 245) | 1.000 | (referent) | ||||
| Period of hepatectomy | ||||||
| 2005–2009 (n = 302) | 1.319 | 1.074–1.626 | 0.008 | |||
| 2010–2016 (n = 304) | 1.000 | (referent) | ||||
| Platelet count | ||||||
| <10 × 104/mm3 (n = 128) | 1.194 | 0.930–1.516 | 0.162 | |||
| ≥10 × 104/mm3 (n = 478) | 1.000 | (referent) | ||||
| Total lymphocyte count | ||||||
| <1,000/mm3 (n = 156) | 1.056 | 0.835–1.330 | 0.633 | |||
| ≥1,000/mm3 (n = 450) | 1.000 | (referent) | ||||
| Neutrophil-to-lymphocyte ratio | ||||||
| <4.0 (n = 547) | 0.942 | 0.675–1.357 | 0.737 | |||
| ≥4.0 (n = 59) | 1.000 | (referent) | ||||
| Total bilirubin | ||||||
| ≥1.0 mg/dL (n = 187) | 1.252 | 1.008–1.548 | 0.042 | |||
| <1.0 mg/dL (n = 419) | 1.000 | (referent) | ||||
| Albumin | ||||||
| <3.5 g/dL (n = 100) | 1.244 | 0.939–1.622 | 0.126 | |||
| ≥3.5 g/dL (n = 506) | 1.000 | (referent) | ||||
| ICG R15 | ||||||
| ≥15% (n = 312) | 1.172 | 0.957–1.435 | 0.124 | |||
| <15% (n = 294) | 1.000 | (referent) | ||||
| Child-Pugh class | ||||||
| A (n = 555) | 0.873 | 0.615–1.287 | 0.479 | |||
| B (n = 51) | 1.000 | (referent) | ||||
| AFP | ||||||
| ≥20 ng/dL (n = 294) | 1.431 | 1.169–1.751 | 0.001 | 1.370 | 1.109–1.695 | 0.004 |
| <20 ng/dL (n = 312) | 1.000 | (referent) | 1.000 | (referent) | ||
| DCP | ||||||
| ≥40 mAU/mL (n = 428) | 1.418 | 1.134–1.787 | 0.002 | |||
| <40 mAU/mL (n = 178) | 1.000 | (referent) | ||||
| Liver histology | ||||||
| Normal liver or chronic hepatitis | ||||||
| (n = 305) | 0.898 | 0.734–1.098 | 0.295 | |||
| Liver fibrosis or liver cirrhosis | ||||||
| (n = 301) | 1.000 | (referent) | ||||
| Tumor size | ||||||
| ≥5.0 cm (n = 209) | 1.549 | 1.254–1.905 | <0.001 | |||
| <5.0 cm (n = 397) | 1.000 | (referent) | ||||
| Number of tumors | ||||||
| Multiple(n = 189) | 1.731 | 1.402–2.130 | <0.001 | |||
| Solitary (n = 417) | 1.000 | (referent) | ||||
| MVI | ||||||
| Positive (n = 196) | 1.934 | 1.566–2.380 | <0.001 | |||
| Negative (n = 410) | 1.000 | (referent) | ||||
| Tumor differentiation | ||||||
| Well or moderate (n = 424) | 0.707 | 0.566–0.889 | 0.003 | 0.851 | 0.673–1.082 | 0.186 |
| Poor(n = 167) TNM stage | 1.000 | (referent) | 1.000 | (referent) | ||
| I or II (n = 361) | 0.461 | 0.377–0.565 | <0.001 | 0.455 | 0.366–0.566 | <0.001 |
| III or IV (n = 245) | 1.000 | (referent) | 1.000 | (referent) | ||
| Surgical procedure | ||||||
| Lobectomy or more (n = 210) | 1.469 | 1.190–1.806 | <0.001 | |||
| Less than segmentectomy (n = 396) | 1.000 | (referent) | ||||
| Operative time | ||||||
| ≥360 min (n = 318) | 1.169 | 0.956–1.431 | 0.128 | |||
| <360 min (n = 288) | 1.000 | (referent) | ||||
| Operative blood loss | ||||||
| ≥500 mL(n = 378) | 1.318 | 1.069–1.631 | 0.010 | 0.962 | 0.767–1.211 | 0.739 |
| <500 mL (n = 228) | 1.000 | (referent) | 1.000 | (referent) | ||
| BMI | ||||||
| ≥25(n = 157) | 1.000 | 0.793–1.250 | 0.999 | |||
| <25 (n = 449) | 1.000 | (referent) | ||||
| Preoperative SMI | ||||||
| Low (n = 84) | 1.456 | 1.087–1.914 | 0.013 | 1.447 | 1.074–1.913 | 0.016 |
| Normal(n = 522) | 1.000 | (referent) | 1.000 | (referent) | ||
| Preoperative IMAC | ||||||
| High (n = 258) | 1.205 | 1.013–1.497 | 0.044 | 1.234 | 1.001–1.493 | 0.049 |
| Normal(n = 348) | 1.000 | (referent) | 1.000 | (referent) | 0.049 | |
| Preoperative VSR | ||||||
| High(n = 252) | 1.283 | 1.047–1.571 | 0.017 | 1.329 | 1.047–1.685 | 0.020 |
| Normal (n = 354) | 1.000 | (referent) | 1.000 | (referent) | ||
HR, hazard ratio; CI, confidence interval; HCC, hepatocellular carcinoma; HBV, hepatitis B virus; HCV, hepatitis C virus; ICG R15, indocyanine green retention test at 15 min; AFP, α-fetoprotein; DCP, des-γ-carboxyprothrombin; MVI, microvascular invasion; TNM, Tumour-Node-Metastasis (stage defined by the Liver Cancer Study Group of Japan); BMI, body mass index; SMI, skeletal muscle mass index; IMAC, intramuscular adipose tissue content; VSR, visceral-to-subcutaneous adipose tissue area ratio.
Discussion
The European Working Group on Sarcopenia in Older People in 2010 defined sarcopenia as a syndrome that is characterized by a progressive and generalized loss of skeletal muscle mass and strength [28], and the Asian and Japanese working groups have recently established their own sarcopenia assessment criteria [29, 30]. On the basis of these definitions, low muscularity has been shown to be an independent risk factor for lower overall and disease-free survival with various kinds of diseases [31, 32, 33, 34, 35, 36, 37, 38]. Today, not only skeletal muscle mass but also adipose tissue is recognized as a secretory organ that produces pro- and anti-inflammatory cytokines and adipokines, and the balance between these cytokines plays an important role in various physiological processes such as insulin resistance, lipid metabolism, and obesity-related low-level chronic inflammation [39, 40, 41]. In recent studies, the VSR, which accounts for differences in fat distribution, has been reported to be associated with poor outcomes in several cancers [20, 21, 22, 23, 24]. Therefore, this study newly focused on preoperative adipose tissue distribution, and the impact of visceral adiposity on outcomes after hepatectomy for HCC was investigated. As far as we know, this is the first and largest study to show that preoperative visceral adiposity, as well as low muscularity, is a predictor of poor prognosis after hepatectomy for HCC.
In evaluating obesity or adiposity, the BMI is generally used because it can be easily calculated from body weight and height. However, the BMI cannot directly reflect differences in fat distribution. In the present study, a high BMI (> 25) was not a significant risk factor for postoperative death or HCC recurrence. Nowadays, body composition, including adipose tissue and skeletal muscle, can be directly evaluated using CT imaging, especially in the surgical field. Although recent studies have shown that a higher visceral fat area (VFA) evaluated using CT imaging was significantly associated with poor survival in several cancers [42, 43, 44, 45, 46], the association between VFA and prognosis remains controversial [47, 48, 49]. Our previous study also evaluated the VFA for a definition of visceral obesity, and obesity was considered present when the VFA was ≥100 cm2 in both males and females [50]. Although the OS and RFS rates among the patients with sarcopenic obesity were significantly lower than among the patients with nonsarcopenic nonobesity, there were no significant differences in OS and RFS rates between the sarcopenic nonobese and the sarcopenic obese group, or between the nonsarcopenic nonobese and the nonsarcopenic obese group. These results suggested that the VFA had no impact on postoperative outcomes in HCC patients. Also in the present study, a higher VFA had no impact on OS (p = 0.284; online suppl. Fig. 1a; for all online suppl. material, see www.karger.com/doi/10.1159/000488779) or RFS (p = 0.719; online suppl. Fig. 1b) after hepatectomy for HCC. On the other hand, visceral adiposity as evaluated by the VSR itself was an independent risk factor for death and HCC recurrence after hepatectomy for HCC. In fact, even among the 84 patients with a low SMI, the OS (online suppl. Fig. 2a) and RFS rates (online suppl. Fig. 2b) were significantly lower in the group of patients with a high VSR (n = 46) than in the group with a normal VSR (n = 38; p = 0.048 and p = 0.023, respectively). Similarly, among the 258 patients with a high IMAC, the OS (online suppl. Fig. 3a) and RFS rates (online suppl. Fig. 3b) were significantly lower in the group of patients with a high VSR (n = 121) than in the group with a normal VSR (n = 137; p = 0.027 and p = 0.023, respectively). On the basis of these findings, we consider that the VSR is independent of the SMI and IMAC, and has an added impact on outcomes after hepatectomy for HCC. Previous studies have shown that there are significant differences in the cytokine production profile between visceral and subcutaneous adipose tissue: proinflammatory cytokines such as tumor necrosis factor-α and interleukin-6 are mainly secreted from visceral adipocytes, while adiponectin, one of the anti-inflammatory cytokines, is secreted from subcutaneous adipocytes [51, 52]. These results suggest that the balance between visceral and subcutaneous adipose tissue would be more important than the VFA alone.
The mechanism by which visceral adiposity adversely affects postoperative morbidity and mortality remains unclear. As shown in Table 1, a significantly higher proportion had type 2 diabetes, hypertension, dyslipidemia, and cardiovascular disease in the high-VSR group as compared to the normal-VSR group. However, there were no significant relationships between each comorbidity and poor outcomes after hepatectomy for HCC (Tables 2, 3). In addition, the following six factors were significantly different between the high-VSR and the normal-VSR group: patient age, sex, serum des-γ-carboxyprothrombin level, tumor size, SMI, and IMAC. In order to exclude any effect of these confounding factors on the two groups, we made adjustments for these six variables using a propensity score matching analysis. Even after adjusting for these factors, the OS (online suppl. Fig. 4a) and RFS rates (online suppl. Fig. 4b) were lower among the patients with a high VSR (n = 219) than among the patients with a normal VSR (n = 219; p = 0.001 and p = 0.083, respectively). This result might suggest that any differences in these variables between the two groups could not affect the postoperative outcomes in the HCC patients. A shift of adipose tissue away from subcutaneous to visceral adipose sites with skeletal muscle loss during the aging process leads to an imbalance between proinflammatory adipokines and myokines [53, 54]. This imbalance in the cytokine profile in elderly or sarcopenic populations has been shown to lead to immunosenescence, particularly of the natural killer lymphocytes involved in innate immunity [55]. On the other hand, visceral adiposity paradoxically impairs immune function by altering leukocyte counts, as well as cell-mediated immune responses, although excessive adipose tissue, especially visceral adiposity, activates various kinds of immune cells through an increase in leptin and a decrease in adiponectin [56]. Moreover, several studies have shown an effect of adiponectin and leptin on tumor cells; adiponectin inhibits the oncogenic actions of leptin by blocking Stat 3 and Akt phosphorylation, while leptin promotes the growth and proliferation of tumor cells via activation of various signaling pathways [40, 57]. These results indicate that visceral adiposity with low muscularity would decrease immune function for oncogenesis due to an imbalance in adipokines and other cytokines, which could lead to an increased risk of death and HCC recurrence. Further investigations into the associations between the cytokine profile and visceral adiposity or low muscularity are needed.
There are several limitations to this study. First, it is retrospective and was conducted at a single institution. Larger, multicenter studies are needed to confirm the results of the present study. Second, it is necessary to consider whether the use of the cutoff values for body composition parameters that we had previously investigated was adequate in this study. In our previous studies [23, 25, 26, 37], the cutoff values for body composition parameters had been determined based on receiver operating characteristic curves. Although the use of receiver operating characteristic curves provides a more accurate and objective method than the use of SDs for designing cutoff levels, these values differ markedly between study populations. Therefore, in order to avoid this potential problem, we have established optimal cutoffs that could be universally applied to other research into body composition [24]. A previous study defined sex-specific cutoff levels for low skeletal muscle mass as > 2 SDs below the means of young adults [58]. In addition, the Asian Working Group for Sarcopenia has also recommended using 2 SDs below the mean muscle mass of a young reference group or the lower quintile when determining cutoffs [29]. We therefore decided to define donors < 50 years old as the reference group, and to use these values to determine cutoff levels. Finally, although the preoperative body composition was shown to be significant, it remains unclear whether pre- and postoperative interventions such as nutritional therapy and rehabilitation could change adiposity or muscularity and improve postoperative outcomes. Our previous study had shown that perioperative nutritional therapy, including an immunomodulating diet enriched with hydrolyzed whey peptide, branched-chain amino acid nutrients, and synbiotics, significantly reduced posttransplant mortality among sarcopenic recipients of a liver transplantation [35]. We consider that it would take a long time to improve visceral adiposity and the sarcopenic status by preoperative nutritional intervention and rehabilitation. However, there is the dilemma that the duration of preoperative interventions for cancer patients should be limited because of the progression of the disease during these interventions. Therefore, we now start the preoperative intervention as early as possible after patients have consulted us at our institution, without extension of the period to operation. Further prospective investigations are needed to reveal whether this preoperative “short-term” nutritional intervention and rehabilitation improves body composition and results in better outcomes after hepatectomy for HCC. In addition, because the risk of postoperative death and HCC recurrence was significantly higher among the patients showing all three factors (high VSR, low SMI, and high IMAC), new selection criteria for hepatectomy that take into account preoperative body composition need to be established.
In conclusion, preoperative visceral adiposity, as well as low muscularity, was closely related to postoperative death and HCC recurrence after hepatectomy for HCC. It would be crucial to establish a new strategy for hepatectomy that takes both preoperative body composition and tumor factors into account. Such a strategy could lead to better outcomes after hepatectomy for HCC.
Statement of Ethics
All study protocols were approved by the Ethics Committee of Kyoto University (approval No. R0061) and were conducted in accordance with the 1996 revision of the Declaration of Helsinki.
Disclosure Statement
The authors declare no conflict of interest.
Funding Sources
No financial support was received in relation to this study.
Supplementary Material
Supplementary data
Supplementary data
Supplementary data
Supplementary data
References
- 1.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sourcesmethods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Kaido T. Selection criteria and current issues in liver transplantation for hepatocellular carcinoma. Liver Cancer. 2016;5:121–127. doi: 10.1159/000367749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akamatsu N, Cillo U, Cucchetti A, Donadon M, Pinna AD, Torzilli G, Kokudo N. Surgery and hepatocellular carcinoma. Liver Cancer. 2016;6:44–50. doi: 10.1159/000449344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 6.Kudo M. Breakthrough imaging in hepatocellular carcinoma. Liver Cancer. 2016;5:47–54. doi: 10.1159/000367761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makino Y, Imai Y, Igura T, Kogita S, Sawai Y, Fukuda K, Iwamoto T, Okabe J, Takamura M, Fujita N, Hori M, Takehara T, Kudo M, Murakami T. Feasibility of extracted-overlay fusion imaging for intraoperative treatment evaluation of radiofrequency ablation for hepatocellular carcinoma. Liver Cancer. 2016;5:269–279. doi: 10.1159/000449338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heindel JJ, Newbold R, Schug TT. Endocrine disruptors and obesity. Nat Rev Endocrinol. 2015;11:653–661. doi: 10.1038/nrendo.2015.163. [DOI] [PubMed] [Google Scholar]
- 10.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesityand mortality from cancer in a prospectively studied cohort of US adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 12.Arnold M, Pandeya N, Byrnes G, Renehan AG, Stevens GA, Ezzati M, Ferlay J, Miranda JJ, Romieu I, Dikshit R, Forman D, Soerjomataram I. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16:36–46. doi: 10.1016/S1470-2045(14)71123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, Mikklineni N, Fiegoli B, Kim PH, Voss MH, Shen H, Laird PW, Sander C, Reuter VE, Motzer RJ, Hsieh JJ, Russo P. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105:1862–1870. doi: 10.1093/jnci/djt310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin L, Birdsell L, Macdonald N, Reiman T, Clandinin MT, McCargar LJ, Murphy R, Ghosh S, Sawyer MB, Baracos VE. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factorindependent of body mass index. J Clin Oncol. 2013;31:1539–1547. doi: 10.1200/JCO.2012.45.2722. [DOI] [PubMed] [Google Scholar]
- 15.Schlesinger S, Siegert S, Koch M, Walter J, Heits N, Hinz S, Jacobs G, Hampe J, Schafmayer C, Nöthlings U. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014;25:1407–1418. doi: 10.1007/s10552-014-0435-x. [DOI] [PubMed] [Google Scholar]
- 16.Amptoulach S, Gross G, Kalaitzakis E. Differential impact of obesity and diabetes mellitus on survival after liver resection for colorectal cancer metastases. J Surg Res. 2015;199:378–385. doi: 10.1016/j.jss.2015.05.059. [DOI] [PubMed] [Google Scholar]
- 17.Vehmas T, Kairemo KJ, Taavitsainen MJ. Measuring visceral adipose tissue content from contrast enhanced computed tomography. Int J Obes Relat Metab Disord. 1996;20:570–573. [PubMed] [Google Scholar]
- 18.Mitsiopoulos N, Baumgartner RN, Heymsfield SB, Lyons W, Gallagher D, Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol (1985) 1998;85:115–112. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 19.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O'Donnell CJ, Hoffmann U. Comparison of anthropometricarea- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31:500–506. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 20.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, Nakagomi R, Kondo M, Nakatsuka T, Minami T, Sato M, Uchino K, Enooku K, Kondo Y, Asaoka Y, Tanaka Y, Ohtomo K, Shiina S, Koike K. Sarcopenia, intramuscular fat depositionand visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63:131–140. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 21.Grignol VP, Smith AD, Shlapak D, Zhang X, Del Campo SM, Carson WE. Increased visceral to subcutaneous fat ratio is associated with decreased overall survival in patients with metastatic melanoma receiving anti-angiogenic therapy. Surg Oncol. 2015;24:353–358. doi: 10.1016/j.suronc.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okamura A, Watanabe M, Mine S, Nishida K, Imamura Y, Kurogochi T, Kitagawa Y, Sano T. Clinical impact of abdominal fat distribution on prognosis after esophagectomy for esophageal squamous cell carcinoma. Ann Surg Oncol. 2016;23:1387–1394. doi: 10.1245/s10434-015-5018-x. [DOI] [PubMed] [Google Scholar]
- 23.Okumura S, Kaido T, Hamaguchi Y, Kobayashi A, Shirai H, Fujimoto Y, Iida T, Yagi S, Taura K, Hatano E, Okajima H, Uemoto S. Impact of skeletal muscle massmuscle quality and visceral adiposity on outcomes following resection of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2017;24:1037–1045. doi: 10.1245/s10434-016-5668-3. [DOI] [PubMed] [Google Scholar]
- 24.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Shirai H, Yagi S, Kamo N, Okajima H, Uemoto S. Impact of skeletal muscle mass indexintramuscular adipose tissue content and visceral to subcutaneous adipose tissue area ratio on early mortality of living donor liver transplantation. Transplantation. 2017;101:565–574. doi: 10.1097/TP.0000000000001587. [DOI] [PubMed] [Google Scholar]
- 25.Hamaguchi Y, Kaido T, Okumura S, Ito T, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E, Uemoto S. Preoperative intramuscular adipose tissue content is a novel prognostic predictor after hepatectomy for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2015;22:475–485. doi: 10.1002/jhbp.236. [DOI] [PubMed] [Google Scholar]
- 26.Hamaguchi Y, Kaido T, Okumura S, Kobayashi A, Fujimoto Y, Ogawa K, Mori A, Hammad A, Hatano E, Uemoto S. Muscle steatosis is an independent predictor of postoperative complications in patients with hepatocellular carcinoma. World J Surg. 2016;40:1959–1968. doi: 10.1007/s00268-016-3504-3. [DOI] [PubMed] [Google Scholar]
- 27.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6,336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15:95–101. doi: 10.1016/j.jamda.2013.11.025. [DOI] [PubMed] [Google Scholar]
- 30.Nishikawa H, Shiraki M, Hiramatsu A, Moriya K, Hino K, Nishiguchi S. Japan Society of Hepatology guidelines for sarcopenia in liver disease (1st edition): recommendation from the working group for creation of sarcopenia assessment criteria. Hepatol Res. 2016;46:951–963. doi: 10.1111/hepr.12774. [DOI] [PubMed] [Google Scholar]
- 31.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 32.Englesbe MJ, Patel SP, He K, Lynch RJ, Schaubel DE, Harbaugh C, Holcombe SA, Wang SC, Segev DL, Sonnenday CJ. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211:271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, IJzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 34.Meza-Junco J, Montano-Loza AJ, Baracos VE, Prado CM, Bain VG, Beaumont C, Esfandiari N, Lieffers JR, Sawyer MB. Sarcopenia as a prognostic index of nutritional status in concurrent cirrhosis and hepatocellular carcinoma. J Clin Gastroenterol. 2013;47:861–870. doi: 10.1097/MCG.0b013e318293a825. [DOI] [PubMed] [Google Scholar]
- 35.Kaido T, Ogawa K, Fujimoto Y, Ogura Y, Hata K, Ito T, Tomiyama K, Yagi S, Mori A, Uemoto S. Impact of sarcopenia on survival in patients undergoing living donor liver transplantation. Am J Transplant. 2013;13:1549–1556. doi: 10.1111/ajt.12221. [DOI] [PubMed] [Google Scholar]
- 36.Durand F, Buyse S, Francoz C, Laouénan C, Bruno O, Belghiti J, Moreau R, Vilgrain V, Valla D. Prognostic value of muscle atrophy in cirrhosis using psoas muscle thickness on computed tomography. J Hepatol. 2014;60:1151–1157. doi: 10.1016/j.jhep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Hamaguchi Y, Kaido T, Okumura S, Fujimoto Y, Ogawa K, Mori A, Hammad A, Tamai Y, Inagaki N, Uemoto S. Impact of quality as well as quantity of skeletal muscle on outcomes after liver transplantation. Liver Transpl. 2014;20:1413–1419. doi: 10.1002/lt.23970. [DOI] [PubMed] [Google Scholar]
- 38.Voron T, Tselikas L, Pietrasz D, Pigneur F, Laurent A, Compagnon P, Salloum C, Luciani A, Azoulay D. Sarcopenia impacts on short- and long-term results of hepatectomy for hepatocellular carcinoma. Ann Surg. 2015;261:1173–1183. doi: 10.1097/SLA.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 39.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 40.Dalamaga M. Interplay of adipokines and myokines in cancer pathophysiology: emerging therapeutic implications. World J Exp Med. 2013;3:26–33. doi: 10.5493/wjem.v3.i3.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer – mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10:455–465. doi: 10.1038/nrendo.2014.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guiu B, Petit JM, Bonnetain F, Ladoire S, Guiu S, Cercueil JP, Krausé D, Hillon P, Borg C, Chauffert B, Ghiringhelli F. Visceral fat area is an independent predictive biomarker of outcome after first-line bevacizumab-based treatment in metastatic colorectal cancer. Gut. 2010;59:341–347. doi: 10.1136/gut.2009.188946. [DOI] [PubMed] [Google Scholar]
- 43.Ladoire S, Bonnetain F, Gauthier M, Zanetta S, Petit JM, Guiu S, Kermarrec I, Mourey E, Michel F, Krausé D, Hillon P, Cormier L, Ghiringhelli F, Guiu B. Visceral fat area as a new independent predictive factor of survival in patients with metastatic renal cell carcinoma treated with antiangiogenic agents. Oncologist. 2011;16:71–81. doi: 10.1634/theoncologist.2010-0227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee CS, Murphy DJ, McMahon C, Nolan B, Cullen G, Mulcahy H, Sheahan K, Barnes E, Fennelly D, Ryan EJ, Doherty GA. Visceral adiposity is a risk factor for poor prognosis in colorectal cancer patients receiving adjuvant chemotherapy. J Gastrointest Cancer. 2015;46:243–250. doi: 10.1007/s12029-015-9709-0. [DOI] [PubMed] [Google Scholar]
- 45.Nault JC, Pigneur F, Nelson AC, Costentin C, Tselikas L, Katsahian S, Diao G, Laurent A, Mallat A, Duvoux C, Luciani A, Decaens T. Visceral fat area predicts survival in patients with advanced hepatocellular carcinoma treated with tyrosine kinase inhibitors. Dig Liver Dis. 2015;47:869–876. doi: 10.1016/j.dld.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 46.Pecorelli N, Carrara G, De Cobelli F, Cristel G, Damascelli A, Balzano G, Beretta L, Braga M. Effect of sarcopenia and visceral obesity on mortality and pancreatic fistula following pancreatic cancer surgery. Br J Surg. 2016;103:434–442. doi: 10.1002/bjs.10063. [DOI] [PubMed] [Google Scholar]
- 47.Gaujoux S, Torres J, Olson S, Winston C, Gonen M, Brennan MF, Klimstra DS, D'Angelica M, DeMatteo R, Fong Y, House M, Jarnagin W, Kurtz RC, Allen PJ. Impact of obesity and body fat distribution on survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2908–2916. doi: 10.1245/s10434-012-2301-y. [DOI] [PubMed] [Google Scholar]
- 48.Kaneko G, Miyajima A, Yuge K, Yazawa S, Mizuno R, Kikuchi E, Jinzaki M, Oya M. Visceral obesity is associated with better recurrence-free survival after curative surgery for Japanese patients with localized clear cell renal cell carcinoma. Jpn J Clin Oncol. 2015;45:210–216. doi: 10.1093/jjco/hyu193. [DOI] [PubMed] [Google Scholar]
- 49.Lee HW, Jeong BC, Seo SI, Jeon SS, Lee HM, Choi HY, Jeon HG. Prognostic significance of visceral obesity in patients with advanced renal cell carcinoma undergoing nephrectomy. Int J Urol. 2015;22:455–461. doi: 10.1111/iju.12716. [DOI] [PubMed] [Google Scholar]
- 50.Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, Kamo N, Yagi S, Taura K, Okajima H, Uemoto S. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002555. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Marra F, Bertolani C. Adipokines in liver diseases. Hepatology. 2009;50:957–969. doi: 10.1002/hep.23046. [DOI] [PubMed] [Google Scholar]
- 52.Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R, Arsenescu V. Adipose tissue immune response: novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014;37:1337–1353. doi: 10.1007/s10753-014-9914-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pararasa C, Bailey CJ, Griffiths HR. Ageing, adipose tissuefatty acids and inflammation. Biogerontology. 2015;16:235–248. doi: 10.1007/s10522-014-9536-x. [DOI] [PubMed] [Google Scholar]
- 54.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissueinflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 55.Lutz CT, Quinn LS. Sarcopenia, obesityand natural killer cell immune senescence in aging: altered cytokine levels as a common mechanism. Aging (Albany NY) 2012;4:535–546. doi: 10.18632/aging.100482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Milner JJ, Beck MA. The impact of obesity on the immune response to infection. Proc Nutr Soc. 2012;71:298–306. doi: 10.1017/S0029665112000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma D, Wang J, Fu PP, Sharma S, Nagalingam A, Mells J, Handy J, Page AJ, Cohen C, Anania FA, Saxena NK. Adiponectin antagonizes the oncogenic actions of leptin in hepatocellular carcinogenesis. Hepatology. 2010;52:1713–1722. doi: 10.1002/hep.23892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, Lindeman RD. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147:755–763. doi: 10.1093/oxfordjournals.aje.a009520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data
Supplementary data
Supplementary data
Supplementary data