Abstract
Benign metastasizing leiomyomas (BML) represent a rare phenomenon consisting of the extra-uterine spread of smooth muscle cells with similar histological, immunological, and molecular patterns to those of benign uterine leiomyomas. They are considered benign based off their low mitotic activity, lack of anaplasia or necrosis, and limited vascularization. This condition represents an interesting diagnostic and treatment challenge based on their rarity and indolent nature. Our case represents a unique finding of BML in the thoracic spine in a postmenopausal woman many years after hysterectomy and partial oophorectomy. There are currently no standard guidelines for treatment of BML, given the rare nature of this condition, with most patients treated with a combination of surgical resection and radiotherapy, followed by hormonal treatment and radiological surveillance serving as the primary backbone of current management plans. Given that these patients present a unique clinical challenge in terms of diagnosis and management, it is important to delineate and further examine these rare entities.
Key Words: Metastasizing benign leiomyoma, Hormonal therapy
Introduction
Benign metastasizing leiomyomas (BML) represent a rare phenomenon consisting of the extra-uterine spread of smooth muscle cells with similar histological, immunological, and molecular patterns to those of benign uterine leiomyomas. They are considered benign based off their low mitotic activity, lack of anaplasia or necrosis, and limited vascularization [1]. BML were first described in 1939 by Steiner [2]. BML most often occur in the lung, presenting as asymptomatic pulmonary nodules, however they can also be present in the cardiovascular system, lymph nodes, omentum, nervous system and retro-peritoneum and rarely in the spine [3]. The majority of women diagnosed with BML are premenopausal, however a small percentage are found in menopausal or postmenopausal women [4]. The majority of menopausal or postmenopausal cases occur in women who underwent hysterectomy for leiomyomas and were subsequently found to have metastases many years later [5, 6]. Multiple theories exist regarding the mechanism of BML although no clear consensus currently exists. One theory involves coelomic metaplasia and spread of the tumors to locations such as the cardiovascular system, peritoneum, and pleura. Another theory involves lymphovascular spread of the leiomyoma cells and hormonal stimulation of local smooth muscle cells [7, 8]. Histologically, BML lesions are well-differentiated spindle cells without atypia or tumor necrosis, with a low proliferative state which differentiate it from leiomyosarcomas. There are currently no guidelines for the treatment of BML and treatment is currently focused on surgical resection of the lesions in addition to hormonal and radiotherapy as an individualized approach.
Case Presentation
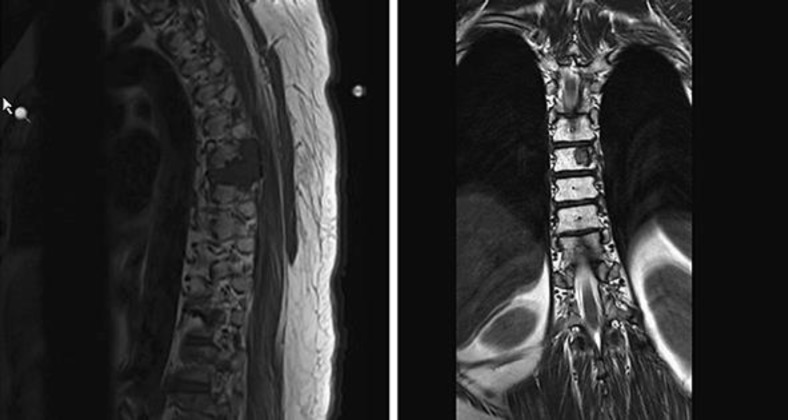
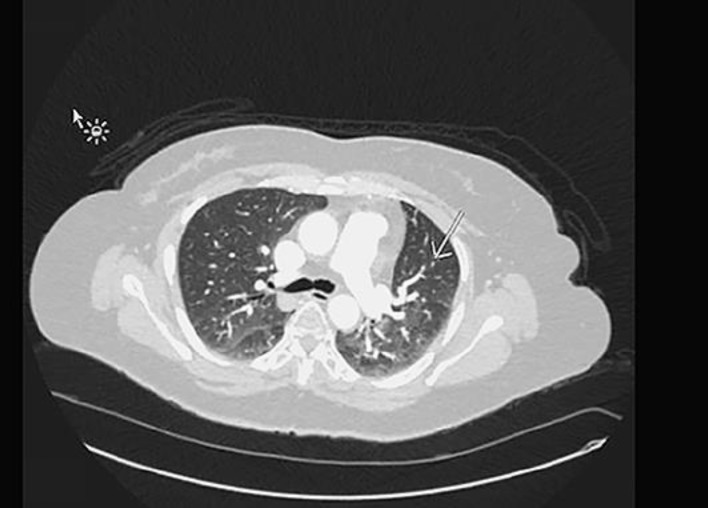
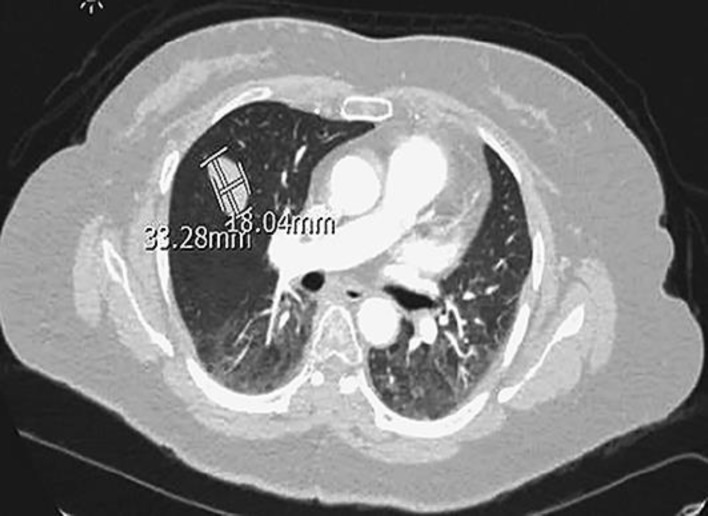
Mrs. Z is a 49-year-old Latina female with a history of HTN, and TAH with left oophorectomy for adenomyosis in 2002, who was diagnosed with metastatic epithelioid leiomyoma involving the spine, lungs, and possibly adrenal gland. She initially presented with 2 months of progressively worsening bilateral lower extremity numbness and weakness in April 2015. MRI of her spine (Fig. 1) showed a T6/T7 mass, measuring 3.8 × 3.0 cm, with epidural compression and bilateral pulmonary nodules, which were further confirmed with CT. The patient then underwent laminectomy and decompression of T5–T8 with pathology consistent with epithelioid leiomyoma composed of spindle cells of smooth muscle origin arranged in fascicles and small clusters. Additionally, there was no obvious cellular atypia or necrosis with a mitotic average of 0–2 per high-power field. Immuno-staining confirmed the cells to be of smooth muscle origin (SMA and Desmin positive) and showed that they were also ER (35%) and PR (95%) positive. CT guided lung biopsy confirmed metastatic leiomyoma. The patient's estradiol level was checked and was found to be elevated at a premenopausal level of 50 which was abnormal given her previous history of total abdominal hysterectomy and left oophorectomy. The patient was started on Depo Lupron and Arimidex given her elevated estradiol level and ER positive and PR positive pathology. Additionally, she received radiotherapy to the spine in June 2015. Follow up PET CT in July, showed low FDG avid with similar target disease in the lung and T7 spine and possibly in the adrenal gland. The patient was continued on Arimidex. Follow up MRI and CT's initially showed decreasing disease however eventually showed new spinal metastases in June 2016. The patient then underwent right oophorectomy a month later, to minimize endogenous hormonal exposure. Pathology of her right ovary was benign. Follow up CT in May 2017 showed the metastatic leiomyoma with stable metastatic disease involving the lung parenchyma (Fig. 2, 3) and the thoracic spine with no new suspicious nodules.
Fig. 1.
T-spine imaging with spine metastases.
Fig. 2.
Stable scattered less than 4 mm micronodules, left upper lobe.
Fig. 3.
Stable perifissural mass in the right middle lobe.
Discussion
Benign metastasizing leiomyomas are rare tumors composed of benign smooth muscle cells derived from uterine leiomyomas presenting in an extrauterine site. They are most often encountered in premenopausal women with a known history of leiomyomas [2, 8]. Many women diagnosed with BML have a history of uterine leiomyoma resection or hysterectomy making the diagnoses challenging in patients post hysterectomy [9]. Our patient presents a unique case in that her records indicated that her previous hysterectomy was due to adenomyosis and not leiomyoma, although it is possible however that she had leiomyomas at the time of hysterectomy and was misdiagnosed at the time.
The mean age of women at the time of primary surgery is 38.5 years, and at the time of BML diagnosis is 47.3 years. Most common site of involvement are the lungs, lymph nodes, abdomen/pelvis, nervous system and bone. Spine metastases are rare and have only been reported in a handful of case reports over the years.
BML have characteristic immune-histochemical markers for muscle (SMA and Desmin) in addition to being estrogen and progesterone positive which is atypical for extrauterine smooth muscle tumors [2, 10]. Immuno-histochemical analysis is required for accurate diagnosis. Additionally, BML exhibit the same clonal pattern and genomic markers of uterine leiomyomas [11]. These tumors most often metastasize to the lungs and have been only very rarely reported in the spine [3, 12]. We present a unique case involving a postmenopausal women with BML metastatic to the thoracic spine and lungs, and literature search reveals there are only limited case reports involving BML to the spine [3, 12, 13]. These patients present a unique clinical challenge as spine BML requires a high level of suspicion and can be challenging to diagnose. Symptoms may vary from pain to weakness and radiculopathy as seen in our patient. Spine BML have typical MRI findings of a well circumscribed mass without any bony destruction or invasion and low T1 and T2 signal intensity with strong homogenous enhancement [3]. Understanding these characteristic findings can enable faster and more accurate diagnosis.
There are currently no guidelines for treatment of BML, given how rare the condition is, and most treatment is based on location and disease burden [2, 5]. BML are indolent in nature and treatment can involve targeted hormonal therapies and surgery [2]. Spine BML present even more of a treatment challenge as they are so rare and current reports offer no consensus for treatment. Surgical resection and radiotherapy are often the best option. Hormonal treatment for BML using hormonal suppression has been found to result in disease regression or control in up to 79% of patients, however postmenopausal patients tend to have poorer outcomes [2]. Even with hormonal treatment and surgical castration postmenopausal patients have been known to have disease progression [6]. This is demonstrated in our patient who after treatment with hormonal therapy eventually showed disease progression. At this point it was decided to proceed with right oophorectomy to decrease hormonal exposure. As BML are an indolent disease long term radiological surveillance is required with either CT or MRI to monitor for disease progression [5].
Conclusions
In conclusion, BML is a condition that presents an interesting diagnostic and treatment challenge based on their rarity and indolent nature. Our case represents a unique finding of BML in the thoracic spine in a postmenopausal woman many years after hysterectomy and partial oophorectomy. There are currently no standard guidelines for treatment of BML, given the rare nature of this condition, with most patients treated with a combination of surgical resection and radiotherapy, followed by hormonal treatment and radiological surveillance serving as the primary backbone of current management plans. Given that these patients present a unique clinical challenge in terms of diagnosis and management, it is important to delineate and further examine these rare entities.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
The authors have no conflicts of interest to disclose.
References
- 1.Khan AT, Shehmar M, Gupta JK. Uterine fibroids: current perspectives. Int J Womens Health. 2014 Jan;6((1)):95–114. doi: 10.2147/IJWH.S51083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lopes ML, Carvalho L, Costa A, Lebenthal A, Godleski J, McNamee C. [Benign metastasizing leiomyomas] Acta Med Port. 2003 Nov-Dec;16((6)):455–8. [PubMed] [Google Scholar]
- 3.Hur JW, Lee S, Lee JB, Cho TH, Park JY. What are MRI findings of Spine Benign Metastasizing Leiomyoma? Case report with literature review. Eur Spine J. 2015 May;24((S4 Suppl 4)):S600–5. doi: 10.1007/s00586-015-3774-8. [DOI] [PubMed] [Google Scholar]
- 4.Moon H, Park SJ, Lee HB, Kim SR, Choe YH, Chung MJ, et al. Pulmonary benign metastasizing leiomyoma in a postmenopausal woman. Am J Med Sci. 2009 Jul;338((1)):72–4. doi: 10.1097/MAJ.0b013e31819c7160. [DOI] [PubMed] [Google Scholar]
- 5.Efared B, Atsame-Ebang G, Sani R, Tahiri L, Sidibe IS, Erregad F, et al. Unexpected pulmonary tumor: metastasis from a benign uterine leiomyoma in a post-menopausal woman: a case report. BMC Res Notes. 2017 Dec;10((1)):662. doi: 10.1186/s13104-017-2998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silva I, Tomé V, Oliveira J. Benign metastasising leiomyoma: a progressive disease despite chemical and surgical castration. BMJ Case Rep. 2012 Mar;2012(mar26 1):bcr0120125505. doi: 10.1136/bcr.01.2012.5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Awonuga AO, Shavell VI, Imudia AN, Rotas M, Diamond MP, Puscheck EE. Pathogenesis of benign metastasizing leiomyoma: a review. Obstet Gynecol Surv. 2010 Mar;65((3)):189–95. doi: 10.1097/OGX.0b013e3181d60f93. [DOI] [PubMed] [Google Scholar]
- 8.Lee HJ, Choi J, Kim KR. Pulmonary benign metastasizing leiomyoma associated with intravenous leiomyomatosis of the uterus: clinical behavior and genomic changes supporting a transportation theory. Int J Gynecol Pathol. 2008 Jul;27((3)):340–5. doi: 10.1097/PGP.0b013e3181656dab. [DOI] [PubMed] [Google Scholar]
- 9.Kayser K, Zink S, Schneider T, Dienemann H, André S, Kaltner H, et al. Benign metastasizing leiomyoma of the uterus: documentation of clinicalimmunohistochemical and lectin-histochemical data of ten cases. Virchows Arch. 2000 Sep;437((3)):284–92. doi: 10.1007/s004280000207. [DOI] [PubMed] [Google Scholar]
- 10.Lee SR, Choi YI, Lee SJ, Shim SS, Lee JH, Kim YK, et al. Multiple cavitating pulmonary nodules: rare manifestation of benign metastatic leiomyoma. J Thorac Dis. 2017 Jan;9((1)):E1–5. doi: 10.21037/jtd.2016.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowen JM, Cates JM, Kash S, Itani D, Gonzalez A, Huang D, et al. Genomic imbalances in benign metastasizing leiomyoma: characterization by conventional karyotypicfluorescence in situ hybridization and whole genome SNP array analysis. Cancer Genet. 2012 May;205((5)):249–54. doi: 10.1016/j.cancergen.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berti AF, Santillan A, Velasquez LA. Benign metastasizing leiomyoma of the cervical spine 31 years after uterine leiomyoma resection. J Clin Neurosci. 2015 Sep;22((9)):1491–2. doi: 10.1016/j.jocn.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 13.Joseph V, Chacko G, Raghuram L, Rajshekhar V. Benign metastasizing leiomyoma causing spinal cord compression. Surg Neurol. 2003 Dec;60((6)):575–7. doi: 10.1016/s0090-3019(03)00268-4. [DOI] [PubMed] [Google Scholar]