Atrial fibrillation (AF) is the most commonly encountered sustained arrhythmia and AF management is often impacted by concomitant pathophysiological conditions such as heart failure, liver dysfunction and chronic kidney disease [1]. Another common condition is pulmonary hypertension (PH), which is defined as mean pulmonary artery pressure ≥25 mm Hg at rest or ≥30 mm Hg with exercise. Typically, PH is associated with increased pressure- and volume-overload in the right heart, which may contribute to structural and electrophysiological arrhythmogenic changes [2]. In contrast to patients with left ventricular heart failure, ventricular arrhythmias are rare in patients with PH. However, supraventricular arrhythmias may occur in advanced disease, in particular right-sided arrhythmias such as atrial flutter, but also AF, with a cumulative incidence of 25% in patients after 5 years of PH diagnosis, which is much higher compared to a cumulative 5 year incidence of 3–5% in patients without PH [3]. Recent studies have established that presence of PH is an independent predictor of late recurrence of persistent AF after AF catheter ablation [4]. In addition to AF recurrences, also prognosis seems to be determined by the presence of PH in AF patients. AF diagnosed before or after PH diagnosis was associated with a 3.81-fold increase in the hazard of death in another study, suggesting, that AF occurrence in PH patients might be a marker of progressed underlying disease in some patients [5]. Supraventricular arrhythmias represent an indication for oral anticoagulation, but the long-term risk:benefit ratio of oral anticoagulation may depend on the underlying pathophysiology of PH, mainly because of the difficult scenario of elevated bleeding risk and hemoptysis in some AF patients [6].
In the current issue of this Journal, Bandyopadhyay et al. [7] present the results of a retrospective analysis focusing on the association between PH and mortality (primary outcome), acute kidney injury, length of hospital stay and cost of care (secondary outcomes) in patients undergoing AF ablation using the most recent 2016 National Inpatient Sample (NIS) database. The NIS database is the largest all-payer inpatient publicly available database in the United States, which includes 20% of all hospital discharges of all non-federal hospitals. Among 152.385 patients with a history of AF ablation, 1.855 were found to have PH. Acute kidney injury was demonstrated to be significantly increased after multivariate adjustment in patients having AF ablation with PH compared to the patients undergoing AF ablation without PH. However, neither mortality nor the length of hospital stay or costs differed in patients with vs. without PH.
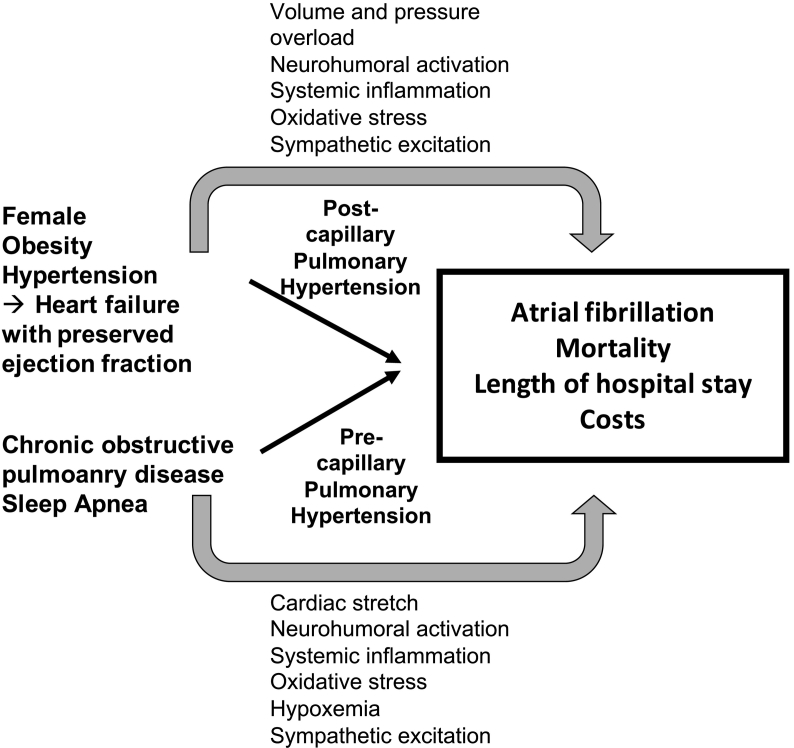
The absence of an association between PH and mortality, length of hospital stay or costs suggests that the outcome in this analysis was not determined by PH alone, but likely results from concomitant pathophysiological conditions and/or cardiovascular risk factors. Importantly, diagnosis of PH and AF in the NIS database is exclusively based on the ICD-10 codes, which does not discriminate between the different WHO groups of PH and does not code for the severity of PH. The latter might be of pre- or post-capillary nature. In patients with cardiovascular diseases, such as AF, increased pulmonary artery pressures are often due to left-heart disease contributing to post-capillary PH. Accordingly, AF patients with PH who underwent AF ablation were more likely female and obese, which are established risk factors for heart failure with preserved ejection fraction. Conceptually, in some patients, AF itself may contribute to increased pulmonary arterial pressures by worsening of diastolic dysfunction, development of tachycardiomyopathy or increased activation of the coagulation system during AF in the lung [8]. However, neither information about AF burden nor AF history were available in this study. Due to a high prevalence of sleep-disordered breathings in AF patients [9,10], PH may also result from long-standing sleep apnea, obesity hypoventilation syndrome or chronic obstructive pulmonary disease which typically result in pre-capillary PH. Grouping different etiologies of PH and the fact that these patients were selected by a cardiologist to undergo AF ablation may have introduced some non-detected variability potentially biasing to the results and the outcome of this analysis.
Interestingly, acute kidney injury after AF ablation was the only component of the secondary outcome in this study, which was associated with PH. The actual mechanisms how acute kidney injury occurs in AF patients with PH undergoing ablation remain unknown, but baseline volume status of the patients and duration of AF ablation procedure may unmask acute kidney injury in patients with preclinical renal damage as a consequence of pre-existing right-ventricular failure in patients with PH. A better baseline assessment of renal function may help to identify AF patients with PH developing acute kidney injury after AF ablation [11].
Taken together, this study suggests that the relative contribution of PH to different outcomes in patients undergoing AF ablation is rather low, and that the main risk of mortality, hospital stay and costs in this study was likely determined by additional concomitant cardiovascular risk factors such as obesity, hypertension, sleep apnea and metabolic syndrome, which are all associated with systemic inflammation, neurohumoral activation, oxidative stress, hypoxemia, sympathetic excitation and atrial cardiomyopathy (Fig. 1) [[12], [13], [14]]. Thus, a more detailed characterization and assessment of the severity of PH in AF patients may help to better identify patients at risk. Whether PH is a modifiable risk factor or rather a marker of underlying disease predisposing to acute kidney injury after AF ablation warrants further prospective study.
Fig. 1.
Contribution of pulmonary hypertension to atrial fibrillation, mortality, length of hospital stay and costs.
Conflict of interest
None (all authors).
Acknowledgements
The authors' work is supported by the National Institutes of Health (R01-HL131517 and R01-HL136389 to D.D.), the German Research Foundation (Do 769/4-1 to D.D.; SFB/TRR219-M02/-S02 to D.L.), the German Society of Cardiology (DGK0914 to D.L.), the German Heart Foundation (F0315 to D.L.), the National Heart Foundation of Australia and The Hospital Research Foundation.
References
- 1.Dan G.A., Dobrev D. Antiarrhythmic drugs for atrial fibrillation: imminent impulses are emerging. Int. J. Cardiol. Heart. Vasc. 2018;21:1–15. doi: 10.1016/j.ijcha.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau D.H., Linz D., Schotten U., Mahajan R., Sanders P., Kalman J.M. Pathophysiology of paroxysmal and persistent atrial fibrillation: rotors, foci and fibrosis. Heart Lung Circ. 2017;26:887–893. doi: 10.1016/j.hlc.2017.05.119. [DOI] [PubMed] [Google Scholar]
- 3.Olsson K.M., Nickel N.P., Tongers J., Hoeper M.M. Atrial flutter and fibrillation in patients with pulmonary hypertension. Int. J. Cardiol. 2013;167:2300–2305. doi: 10.1016/j.ijcard.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y.Q., Zhang F.L., Wang W.W., Chen X.H., Chen J.H., Chen L.L. The correlation of pulmonary arterial hypertension with late recurrence of paroxysmal atrial fibrillation after catheter ablation. J. Thorac. Dis. 2018;10:2789–2794. doi: 10.21037/jtd.2018.04.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith B., Genuardi M.V., Koczo A., Zou R.H., Thoma F.W., Handen A., Craig E., Hogan C.M., Girard T., Althouse A.D., Chan S.Y. Atrial arrhythmias are associated with increased mortality in pulmonary arterial hypertension. Pulm. Circ. 2018 doi: 10.1177/2045894018790316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang T.Y., Liao J.N., Chao T.F., Vicera J.J., Lin C.Y., Tuan T.C., Lin Y.J., Chang S.L., Lo L.W., Hu Y.F., Chung F.P., Chen S.A. Oral anticoagulant use for stroke prevention in atrial fibrillation patients with difficult scenarios. Int. J. Cardiol. Heart Vasc. 2018;20:56–62. doi: 10.1016/j.ijcha.2018.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bandyopadhyay D., Devanabanda A.R., Hajra A., Tummala R., Ghosh R., Chakraborty S., Banerjee U., Herzog E. Impact of pulmonary hypertension in patients undergoing atrial fibrillation ablation: a nationwide study. Int. J. Cardiol. Heart Vasc. 2019;23 doi: 10.1016/j.ijcha.2019.100348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chibana H., Tahara N., Itaya N., Ishimatsu T., Sasaki M., Sasaki M., Nakayoshi T., Ohtsuka M., Yokoyama S., Sasaki K.I., Ueno T., Fukumoto Y. Pulmonary artery dysfunction in chronic thromboembolic pulmonary hypertension. Int. J. Cardiol. Heart Vasc. 2017;17:30–32. doi: 10.1016/j.ijcha.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Linz D., Baumert M., Catcheside P., Floras J., Sanders P., Levy P., Cowie M.R., McEvoy D.R. Assessment and interpretation of sleep disordered breathing severity in cardiology: clinical implications and perspectives. Int. J. Cardiol. 2018;271:281–288. doi: 10.1016/j.ijcard.2018.04.076. [DOI] [PubMed] [Google Scholar]
- 10.Linz D., McEvoy R.D., Cowie M.R., Somers V.K., Nattel S., Lévy P., Kalman J.M., Sanders P. Associations of obstructive sleep apnea with atrial fibrillation and continuous positive airway pressure treatment: a review. JAMA Cardiol. 2018;3:532–540. doi: 10.1001/jamacardio.2018.0095. [DOI] [PubMed] [Google Scholar]
- 11.Linz D., Hohl M., Dobrev D. Can cystatin C-based estimated glomerular filtration rate help to guide individualized risk factor modification programs? Int. J. Cardiol. Heart Vasc. 2018;19:100–101. doi: 10.1016/j.ijcha.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baumert M., Immanuel S.A., Stone K.L., Harrison S.L., Redline S., Mariani S., Sanders P., McEvoy R.D., Linz D. Composition of nocturnal hypoxemic burden and its prognostic value for cardiovascular mortality in older community-dwelling men. Eur. Heart J. 2018 doi: 10.1093/eurheartj/ehy838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Linz D., Elliott A.D., Hohl M., Malik V., Schotten U., Dobrev D., Nattel S., Böhm M., Floras J., Lau D.H., Sanders P. Role of autonomic nervous system in atrial fibrillation. Int. J. Cardiol. 2018 doi: 10.1016/j.ijcard.2018.11.091. [DOI] [PubMed] [Google Scholar]
- 14.Goette A., Kalman J.M., Aguinaga L., Akar J., Cabrera J.A., Chen S.A., Chugh S.S., Corradi D., D'Avila A., Dobrev D., Fenelon G., Gonzalez M., Hatem S.N., Helm R., Hindricks G., Ho S.Y., Hoit B., Jalife J., Kim Y.H., Lip G.Y.H., Ma C.S., Marcus G.M., Murray K., Nogami A., Sanders P., Uribe W., Van Wagoner D., Nattel S. Atrial cardiomyopathies: definition, characterization and clinical implication. Heart Rhythm. 2017;14:e3–e40. doi: 10.1016/j.hrthm.2016.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]