Watch a video presentation of this article
Abbreviations
- CI
confidence interval
- DAA
direct‐acting antiviral
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IFN
interferon
- SVR
sustained virological response
- VA
US Department of Veterans Affairs
Chronic hepatitis C virus (HCV) infection is a leading cause of hepatocellular carcinoma (HCC) worldwide. In the United States, HCV accounts for more than 60% of HCC cases.1, 2 The most important risk factor for HCC among patients with HCV is cirrhosis. Viral eradication is thought to reduce cancer risk by preventing cirrhosis and causing regression of fibrosis. Eliminating HCV also reverses other processes implicated in HCC pathogenesis, such as chronic inflammation and direct carcinogenic effects of the virus.
Direct‐acting antivirals (DAAs) are well‐tolerated agents that cure HCV in more than 95% of treated patients. A growing body of evidence suggests that DAA‐induced sustained virological response (SVR) improves portal hypertension and fibrosis in patients with chronic HCV and may reduce mortality.3 However, the question of whether DAAs prevent HCC has generated substantial controversy. In this review, we examine the impact of DAA therapy on the risk of de novo and recurrent HCC.
The Impact of DAAs on DE NOVO HCC
A primary objective of antiviral therapy is to reduce the risk for HCC. This was indeed the case after interferon (IFN)‐induced SVR. In two meta‐analyses, patients with HCV who achieved SVR with IFN had significantly lower rates of HCC compared with nonresponders (relative risk [RR], 0.26; 95% confidence interval [CI], 0.18‐0.314 versus RR, 0.34; 95% CI, 0.26‐0.465).
In contrast, initial studies in the DAA era produced less promising results. Conti et al.6 described an Italian cohort of 285 patients with HCV‐related cirrhosis who completed a course of DAAs, of whom 9 (3.16%) experienced development of de novo HCC in the first 24 weeks after treatment completion. Additional small studies reported even higher rates of HCC after DAA treatment. These rates compared unfavorably with historic HCC rates in untreated or IFN‐treated patients. It was hypothesized that DAAs cause rapid viral suppression that disrupts cancer immune surveillance, thereby allowing malignant cells to escape immune regulation.
Comparison of crude HCC rates in DAA‐treated patients with that of historic controls, however, fails to account for the higher baseline HCC risk of DAA‐eligible patients because of older age and more advanced liver disease. In a retrospective cohort study of more than 60,000 US Department of Veterans Affairs (VA) patients with chronic HCV, DAA recipients tended to be older than IFN recipients, were more likely to have cirrhosis, and were more likely to have comorbid conditions, such as diabetes and alcohol abuse, that elevate the risk for HCC.7 After adjusting for these confounders in multivariate analysis, the risks for HCC after DAA and IFN treatments were no different (adjusted hazard ratio [aHR], 1.12; 95% CI, 0.95‐1.32). Other studies comparing DAA‐ and IFN‐treated patients have come to similar conclusions.8, 9, 10, 11 (Table 1)
Table 1.
Cohort Studies Evaluating the Association Between DAA Therapy and De Novo HCC
| First Author | Year | Study Design | Data Source | Patients | Start of Follow‐up | Crude HCC Incidence Rate per 100 Person‐Years (Total No. of Patients) | Adjusted HR of HCC* (95% CI) | |
|---|---|---|---|---|---|---|---|---|
| DAA treatment with SVR versus DAA treatment without SVR | SVR | No SVR | ||||||
| Kanwal12 | 2017 | Retrospective cohort | VA | HCV | Antiviral completion | 0.90 (19,518) | 3.45 (2982) | 0.28 (0.22‐0.36) |
| Ioannou7 | 2018 | Retrospective cohort | VA | HCV | 180 days after antiviral initiation | 0.92 (19,909) | 5.19 (2039) | 0.29 (0.23‐0.37) |
| Calvaruso13 | 2018 | Prospective cohort | Italian multicenter cohort | HCV cirrhosis | Antiviral initiation | 2.6% at 1 year (2140) | 8.0% at 1 year (109) | 0.35 (0.19‐0.64)† |
| DAA treatment versus IFN treatment | DAA | IFN | ||||||
| Ioannou7 | 2018 | Retrospective cohort | VA | HCV | 180 days after antiviral initiation | 1.32 (21,948) | 0.81 (35,871) | 1.12 (0.95‐1.32) |
| Singer8 | 2018 | Retrospective cohort | US administrative claims database | HCV | Antiviral initiation | 1.18 (30,183) | 0.98 (12,948) | 0.69 (0.59‐0.81) |
| Li9 | 2018 | Retrospective cohort | VA | HCV cirrhosis | Antiviral initiation excluding HCC diagnosed during first 3 months | 2.52 (1,161) | 3.47 (463) | 1.07 (0.55‐2.08) |
| DAA + SVR | IFN + SVR | |||||||
| Innes10 | 2018 | Retrospective cohort | Scottish HCV database | HCV cirrhosis | Antiviral initiation | 2.53 (272) | 1.26 (585) | 1.15 (0.49‐2.71) |
| Nahon11 | 2018 | Prospective cohort | French multicenter cohort | HCV cirrhosis | Antiviral initiation | 3.5% at 3 years (274) | 3.1% at 3 years (495) | 0.70 (0.28‐1.74) |
| DAA treatment versus no treatment | DAA | Untreated | ||||||
| Singer8 | 2018 | Retrospective cohort | US administrative claims database | HCV | Antiviral initiation, index date in untreated | 1.18 (30,183) | 0.64 (137,502) | 0.84 (0.73‐0.96) |
| Li9 | 2018 | Retrospective cohort | VA | HCV cirrhosis | Antiviral initiation excluding HCC diagnosed during first 3 months, index date in untreated | 2.52 (1161) | 4.53 (1236) | Not calculated |
DAA therapy with or without SVR relative to control group.
Inverse of reported aHR of 2.88.
Large cohort studies also confirm that patients with DAA‐induced SVR have a significantly lower risk for HCC compared with treatment failure or no treatment (Table 1). A study of more than 20,000 VA patients found that DAA recipients who achieved SVR had a more than 70% lower risk for HCC compared with nonresponders (aHR, 0.28; 95% CI, 0.22‐0.36).12 An Italian study of 2249 patients treated with DAAs reported a 2.6% cumulative incidence rate of HCC at 1 year among patients who achieved SVR and 8% among nonresponders, with treatment failure identified as an independent risk factor for HCC.13 Using an administrative claims database, Singer et al.8 demonstrated that DAA recipients have a lower risk for HCC compared with untreated controls (aHR, 0.84; 95% CI, 0.73‐0.96).
Collectively, observational studies support the effectiveness of DAA therapy for primary prevention of HCC. However, achievement of SVR does not obviate the need for continued HCC surveillance in patients with cirrhosis. The residual incidence rate of HCC after SVR ranges from 1.4% to 1.96% per year in the aforementioned studies, which is roughly the incidence rate at which HCC surveillance is considered cost‐effective. As such, we agree with current guideline recommendations that post‐SVR patients with advanced fibrosis receive routine HCC surveillance.14 In contrast, patients with no or minimal fibrosis at the start of antiviral therapy have low residual HCC incidence after SVR and do not warrant surveillance.
Impact of DAAs on HCC Recurrence
Meta‐analyses show that IFN‐based antiviral therapy after curative HCC treatment reduces the likelihood of cancer recurrence and improves survival.15 Whether the same is true of DAAs is an area of ongoing debate due to small, single‐arm studies that suggested that DAAs might precipitate HCC recurrence. The first such study included 58 patients with previously treated HCC, of whom 16 (27.8%) experienced recurrent tumor after a median follow‐up of 6 months from DAA initiation.16 A simultaneous publication reported HCC recurrence in 17/59 patients (28.8%).6 These recurrence rates were alarmingly high compared with historic controls, and some recurrences appeared to be temporally associated with DAA exposure.
Other studies have arrived at different conclusions (Table 2). An analysis of two French cohorts reported lower recurrence rates of 7.7% and 12.7% in patients treated with DAAs followed for a median of ~20 months after antiviral initiation.17 Moreover, there was no difference in recurrence rates between DAA‐treated and untreated patients in multivariate analysis (aHR, 1.09; 95% CI, 0.55‐2.16 and aHR, 0.40; 95% CI, 0.05‐3.03, respectively). A single‐center study of patients awaiting liver transplant compared patients treated with DAA with those who remained untreated and found no difference in HCC recurrence rates after locoregional therapy (aHR, 0.91; 95% CI, 0.58‐1.42).18 Two Japanese cohort studies and a French single‐center study suggested that DAA‐treated patients may in fact have lower rates of HCC recurrence than patients who do not achieve SVR or remain untreated.19, 20, 21
Table 2.
Studies Evaluating the Association Between DAA Therapy and Recurrent HCC
| First Author | Year | Data Source | HCC Treatments | Median Time From HCC Treatment to DAA Initiation (Months) | Start of Follow‐up | Median Duration of Follow‐up in Patients Treated with DAA (Months) | Number of Patients Treated With DAA | Number of Controls | HCC Recurrence (Proportion) | Comparison of HCC Recurrence in Patients Treated With DAA Versus Controls |
|---|---|---|---|---|---|---|---|---|---|---|
| Reig16 | 2016 | Spanish multicenter cohort | Resection | 11.2 | Antiviral initiation | 5.7 | 58 | — | 27.6% | — |
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Conti6 | 2016 | Italian regional database | Resection | 12.4 | Antiviral completion | 6 | 59 | — | 28.8% | — |
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Percutaneous ethanol | ||||||||||
| Bielen23 | 2017 | Belgian multicenter cohort | Liver transplant | 12 | Antiviral completion | — | 41 | — | 15% | — |
| Resection | ||||||||||
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Cabibbo24 | 2017 | Italian regional database | Resection | 11 | Antiviral initiation | 8.7 | 143 | — | 20.3% | — |
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Ogawa25 | 2018 | Japanese multicenter cohort | Resection | 14.4 | Antiviral initiation | 17 | 152 | — | 17.1% | — |
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Radioembolization | ||||||||||
| ANRS17 | 2016 | French multicenter cohort | — | 21.6* | Antiviral initiation | 20.2 | 189 | 78 (untreated) | 12.7% | aHR, 1.09 (95% CI, 0.55‐2.16) |
| ANRS17 | 2016 | French multicenter cohort | Resection | __ | Antiviral initiation | 21.3 | 13 | 66 (untreated) | 7.7% | HR, 0.40 (95% CI, 0.05‐3.03) |
| Radiofrequency ablation | ||||||||||
| Ikeda26 | 2017 | Japanese single center | Resection | 10.7 | Antiviral initiation | 21.5 | 89 | 89 (untreated) | 19.3% | aHR, 0.35 (95% CI, 0.19‐0.65) |
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Particle radiation therapy | ||||||||||
| Nagata19 | 2017 | Japanese multicenter cohort | Resection | — | HCC curative treatment | 27.6 | 83 | 60 (IFN treated) | 22.9% DAA + SVR | P = 0.022 |
| Radiofrequency ablation | ||||||||||
| 40.0% DAA − SVR | ||||||||||
| (3‐year incidence) | ||||||||||
| 45.1% DAA | P = 0.54 | |||||||||
| 54.2% IFN | ||||||||||
| (5‐year incidence) | ||||||||||
| Virlogeux21 | 2017 | French single center | Resection | 7.2 | Antiviral initiation | — | 23 | 45 (untreated) | 47.8% | HR, 0.24 (95% CI, 0.10‐0.55) |
| Radiofrequency ablation | ||||||||||
| Chemoembolization | ||||||||||
| Huang18 | 2018 | US single center | Resection | 11.9† | Complete response | — | 61 | 51 (untreated) | 47% DAA | aHR, 0.91 (95% CI, 0.58‐1.42) |
| Radiofrequency ablation | 49.8% no DAA | |||||||||
| (1‐year incidence) | ||||||||||
| Cryotherapy | ||||||||||
| Percutaneous ethanol | ||||||||||
| Mashiba20 | 2018 | Japanese multicenter cohort | — | 10.9 | Antiviral completion | 7.7 | 368 | 148 (IFN treated) | — | HR, 0.95 (95% CI, 0.61‐1.45)‡ |
From HCC diagnosis to inclusion in cohort.
From HCC diagnosis to DAA initiation.
IFN versus DAA therapy, restricted to SVR patients.
Various methodological limitations hamper the interpretation of existing studies and prevent firm conclusions from being drawn. These limitations are detailed in a recent meta‐analysis.22 (Fig. 1) First, sample sizes of studies are typically small, and many do not include control arms. Second, studies are inconsistent in excluding HCC or suspicious nodules prior to DAA treatment. Consequently, some “recurrences” may actually represent prevalent tumors that were present at the start of antiviral therapy. Third, most studies do not specify a standard surveillance protocol after curative treatment, leading to heterogeneity in ascertainment of recurrence. Fourth, studies variably include patients with non–early‐stage HCC, patients with past recurrences, and patients who received noncurative locoregional therapies, all of which would be expected to increase recurrence rates. Lastly, determining when to start the clock in terms of follow‐up varies. Follow‐up may be calculated from the time of HCC treatment, antiviral therapy initiation, or antiviral completion, each of which produces different estimates of recurrence rates. The delay between HCC treatment and antiviral initiation also varies widely. In a meta‐regression analysis, the most common factor associated with recurrence is a short interval between HCC cure and DAA initiation, suggesting that many cases of “recurrence” are in fact patients who have not achieved a durable complete response.
Figure 1.
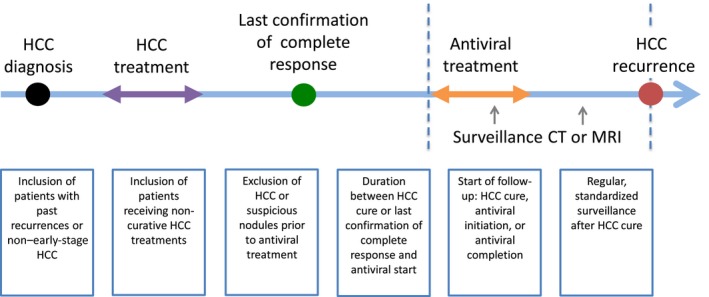
Sources of heterogeneity in studies examining the association between DAAs and HCC recurrence.
Given the inconclusiveness of existing evidence, some clinicians are hesitant to initiate DAA therapy in patients with previously treated HCC. Yet withholding treatment risks further liver decompensation, and the possibility remains that treatment may actually lower the risk for recurrence. Hence we propose DAA therapy be considered in patients with prior HCC provided a durable complete response has been demonstrated. It seems reasonable to wait at least 6 months after the first demonstration of complete response and to obtain two multiphase computed tomography or magnetic resonance imaging studies to document absence of HCC before initiating antivirals.
Conclusion
DAAs dramatically enhanced our ability to cure chronic HCV and prevent its downstream complications. Several large cohort studies demonstrate that DAA‐induced SVR reduces the risk for de novo HCC. However, the residual risk for HCC after SVR means that surveillance is still required for patients with advanced fibrosis and cirrhosis. Additional well‐designed studies are needed to determine the consequences of DAA therapy in patients with previously treated HCC, although the initial concern that DAAs increase recurrence risk has generally not been borne out in subsequent studies.
The contents do not represent the views of the US Department of Veterans Affairs or the US government.
Potential conflict of interest: Nothing to report.
References
- 1. Goldberg D, Ditah IC, Saeian K, et al. Changes in the prevalence of hepatitis C virus infection, nonalcoholic steatohepatitis, and alcoholic liver disease among patients with cirrhosis or liver failure on the waitlist for liver transplantation. Gastroenterology 2017;152:1090‐1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beste LA, Leipertz SL, Green PK, et al. Ioannou GN. Trends in burden of cirrhosis and hepatocellular carcinoma by underlying liver disease in US veterans, 2001‐2013. Gastroenterology 2015;149:1471‐1482. [DOI] [PubMed] [Google Scholar]
- 3. Backus LI, Belperio PS, Shahoumian TA, et al. Impact of sustained virologic response with direct‐acting antiviral treatment on mortality in patients with advanced liver disease. Hepatology. 10.1002/hep.29408 [DOI] [PubMed] [Google Scholar]
- 4. Morgan RL, Baack B, Smith BD, et al. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta‐analysis of observational studies. Ann Intern Med 2013;158:329‐337. [DOI] [PubMed] [Google Scholar]
- 5. Singal AK, Singh A, Jaganmohan S, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus‐related cirrhosis. Clin Gastroenterol Hepatol 2010;8:192‐199. [DOI] [PubMed] [Google Scholar]
- 6. Conti F, Buonfiglioli F, Scuteri A, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV‐related cirrhosis treated with direct‐acting antivirals. J Hepatol 2016;65:727‐733. [DOI] [PubMed] [Google Scholar]
- 7. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct‐acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol 2018;68:25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Singer AW, Reddy KR, Telep LE, et al. Direct‐acting antiviral treatment for hepatitis C virus infection and risk of incident liver cancer: A retrospective cohort study. Aliment Pharmacol Ther 2018;47:1278‐1287. [DOI] [PubMed] [Google Scholar]
- 9. Li DK, Ren Y, Fierer DS, et al. The short‐term incidence of hepatocellular carcinoma is not increased after hepatitis C treatment with direct‐acting antivirals: An ERCHIVES study. Hepatology 2018;67:2244‐2253. [DOI] [PubMed] [Google Scholar]
- 10. Innes H, Barclay ST, Hayes PC, et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: Role of the treatment regimen. J Hepatol 2018;68:646‐654. [DOI] [PubMed] [Google Scholar]
- 11. Nahon P, Layese R, Bourcier V, et al. Incidence of hepatocellular carcinoma after direct antiviral therapy for HCV in patients with cirrhosis included in surveillance programs. Gastroenterology 2018;155:1436‐1450. [DOI] [PubMed] [Google Scholar]
- 12. Kanwal F, Kramer J, Asch SM, et al. Risk of hepatocellular cancer in HCV patients treated with direct‐acting antiviral agents. Gastroenterology 2017;153:996‐1005. [DOI] [PubMed] [Google Scholar]
- 13. Calvaruso V, Cabibbo G, Cacciola I, et al. Incidence of hepatocellular carcinoma in patients with HCV‐associated cirrhosis treated with direct‐acting antiviral agents. Gastroenterology 2018;155:411‐421. [DOI] [PubMed] [Google Scholar]
- 14. American Association for the Study of Liver Diseases and Infectious Diseases Society of America . Recommendations for testing, managing, and treating hepatitis C. Available at: http://www.hcvguidelines.org. Accessed October 1, 2018. [DOI] [PMC free article] [PubMed]
- 15. Singal AK, Freeman DH, Anand BS. Meta‐analysis: interferon improves outcomes following ablation or resection of hepatocellular carcinoma. Aliment Pharmacol Ther 2010;32:851‐858. [DOI] [PubMed] [Google Scholar]
- 16. Reig M, Mariño Z, Perelló C, et al. Unexpected high rate of early tumor recurrence in patients with HCV‐related HCC undergoing interferon‐free therapy. J Hepatol 2016;65:719‐726. [DOI] [PubMed] [Google Scholar]
- 17. ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts) . Lack of evidence of an effect of direct‐acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol 2016;65:734‐740. [DOI] [PubMed] [Google Scholar]
- 18. Huang AC, Mehta N, Dodge JL, et al. Direct‐acting antivirals do not increase the risk of hepatocellular carcinoma recurrence after local‐regional therapy or liver transplant waitlist dropout. Hepatology 2018;68:449‐461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nagata H, Nakagawa M, Asahina Y, et al. Effect of interferon‐based and ‐free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol 2017;67:933‐939. [DOI] [PubMed] [Google Scholar]
- 20. Mashiba T, Joko K, Kurosaki M, et al. Does interferon‐free direct‐acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS One 2018;13:e0194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Virlogeux V, Pradat P, Hartig‐Lavie K, et al. Direct‐acting antiviral therapy decreases hepatocellular carcinoma recurrence rate in cirrhotic patients with chronic hepatitis C. Liver Int 2017;37:1122‐1127. [DOI] [PubMed] [Google Scholar]
- 22. Saraiya N, Yopp AC, Rich NE, et al. Systematic review with meta‐analysis: recurrence of hepatocellular carcinoma following direct‐acting antiviral therapy. Aliment Pharmacol Ther 2018;48:127‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bielen R, Moreno C, Van Vlierberghe H, et al. The risk of early occurrence and recurrence of hepatocellular carcinoma in hepatitis C‐infected patients treated with direct‐acting antivirals with and without pegylated interferon: A Belgian experience. J Viral Hepat 2017;24:976‐981. [DOI] [PubMed] [Google Scholar]
- 24. Cabibbo G, Petta S, Calvaruso V, et al. Is early recurrence of hepatocellular carcinoma in HCV cirrhotic patients affected by treatment with direct‐acting antivirals? A prospective multicentre study. Aliment Pharmacol Ther 2017;46:688‐695. [DOI] [PubMed] [Google Scholar]
- 25. Ogawa E, Furusyo N, Nomura H, et al. Short‐term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct‐acting anti‐viral treatment. Aliment Pharmacol Ther 2018;47:104‐113. [DOI] [PubMed] [Google Scholar]
- 26. Ikeda K, Kawamura Y, Kobayashi M, et al. Direct‐acting antivirals decreased tumor recurrence after initial treatment of hepatitis c virus‐related hepatocellular carcinoma. Dig Dis Sci 2017;62:2932‐2942. [DOI] [PubMed] [Google Scholar]


