Abstract
Histopathologic diversity and several distinct histologic subtypes of hepatocellular carcinoma (HCC) are well-recognized. Recent advances in molecular pathology and growing knowledge about the biology associated with distinct histologic features and immuno-profile in HCC allowed pathologists to update classifications. Improving sub-classification will allow for more clinically relevant diagnoses and may allow for stratification into biologically meaningful subgroups. Therefore, immuno-histochemical and molecular testing are not only diagnostically useful, but also are being incorporated as crucial components in predicting prognosis of the patients with HCC. Possibilities of targeted therapy are being explored in HCC, and it will be important for pathologists to provide any data that may be valuable from a theranostic perspective. Herein, we review and provide updates regarding the pathologic sub-classification of HCC. Pathologic diagnostic approach and the role of biomarkers as prognosticators are reviewed. Further, the histopathology of four particular subtypes of HCC: Steatohepatitic, clear cell, fibrolamellar and scirrhous - and their clinical relevance, and the recent consensus on combined HCC-cholangiocarcinoma is summarized. Finally, emerging novel biomarkers and new approaches to HCC stratification are reviewed.
Keywords: Hepatocellular carcinoma, Subtype, Classification, Review, Update
Core tip: We summarize the updated classifications of hepatocellular carcinoma. Immunohistochemistry and molecular pathology are becoming crucial components of prognostication and theranostics. Pathologic and molecular features of clinically relevant subtypes to include steatohepatitic, clear cell, fibrolamellar and scirrhous hepatocellular carcinomas are reviewed. Recent consensus on the combined hepatocellular carcinoma-cholangiocarcinoma, a controversial pathologic entity, is summarized.
INTRODUCTION
Hepatocellular neoplasms constitute a heterogeneous group of disorders that encompasses benign, dysplastic and malignant lesions. These lesions demonstrate a wide morphologic spectrum and include newly identified morphologic subtypes. Knowledge about molecular signatures, provisional classifications, and clinical correlates continues to evolve. A careful synthesis of all clinical, radiologic and pathologic data is essential to establish a proper diagnosis and devise a treatment plan.
This review aims to address the most common questions brought to our (pathologists’) attention by our clinical colleagues. Firstly, the current classification of hepatocellular carcinoma (HCC) will be reviewed. Secondly, the utility of ancillary tests to include immunohistochemistry (IHC) including prognostic biomarkers, and molecular testing will be reviewed. Next, a few subtypes of non-conventional HCC that are deemed clinically relevant will be briefly reviewed. Further, we will summarize the recent consensus on combined HCC-cholangiocarcinoma (cHCC-CCA). Finally, novel diagnostic and/or prognostic biomarkers and comprehensive genomic and epigenomic stratification of HCC are briefly reviewed.
EVOLUTION OF THE HISTOLOGIC (MORPHOLOGIC) CLASSIFICATION OF HEPATOCELLULAR CARCINOMA
The 4th edition of WHO classification of the tumors of the digestive system was published in 2010. In this edition, in addition to the conventional HCC, the WHO recognized 5 morphological subtypes of HCC: Fibrolamellar HCC (FL-HCC), scirrhous HCC (S-HCC), undifferentiated carcinoma, lymphoepithelioma-like carcinoma and sarcomatoid HCC. Several architectural growth patterns and cytological variation of HCC were also described to aid in establishing a diagnosis. These architectural and cytologic variations were not recognized as individual subtypes. The following architectural patterns were described: trabecular (plate-like) pattern, pseudoglandular (acinar) pattern and compact pattern. The cytological variations included pleomorphic cells (bizarre multinucleated, mononuclear giant cells or osteoclast-like giant cells), clear cells, fatty change, bile production, hyaline bodies (Mallory-Denk bodies), pale bodies and ground glass inclusions[1].
Since the publication of the 4th edition WHO classification of the tumors of the digestive system, additional morphologic variations of HCC have been reported in the literature, some with characteristic molecular signature. It was suggested that for a tumor with specific morphologic variation to qualify for a subtype, the following criteria should be met: the tumor should have reproducible microscopic pattern, immuno-histochemical and molecular tests that help in the subcategorization of the tumor should be available, and there should be a particular clinical correlate regarding the proposed subtype.
This approach has led to 12 proposed subtypes and 6 provisional entities, constituting approximately 35% of HCC. In a decreasing order of frequency, these morphologic subtypes are: Steatohepatitic, clear cell, scirrhous, cirrhotomimetic, fibrolamellar carcinoma, combined hepatocellular-cholangiocarcinoma, combined hepatocellular and neuroendocrine, granulocyte colony-stimulating factor producing, sarcomatoid, carcinosarcoma, carcinosarcoma with osteoclast-like giant cells and lymphocyte rich. Out of the 6 provisional entities, chromophobe subtype is the most common. The remaining 5 provisions are of equal frequency: Combined hepatocellular-cholangiocarcinoma with stem cell features, lipid rich, myxoid, syncytial giant cell and transitional cell[2,3]. A subset of the proposed subtypes and provisional entities is likely to be endorsed in the 5th edition WHO classification of the tumors of the digestive system, which is to be released in 2019.
ROLE OF IMMUNOHISTOCHEMISTRY IN HEPATOCELLULAR CARCINOMA DIAGNOSIS
Both well differentiated and poorly differentiated hepatocellular neoplastic lesions pose diagnostic challenge. “Well differentiated” implies that the histomorphology of the tumor closely resembles the native tissue the tumor originates from. Therefore, a well differentiated HCC recapitulates benign liver tissue, mimicking the following benign and pre-neoplastic entities: hepatocellular adenoma, focal nodular hyperplasia (FNH), regenerative nodule, and dysplastic nodule in cirrhotic liver. In order to demonstrate malignancy in a well-differentiated HCC that morphologically resembles benign hepatocytic lesions, IHC such as CD34 (showing diffuse sinusoidal capillarization), and a panel of glutamine synthetase (GS), glypican-3 (GPC-3) and heat shock protein 70 (HSP-70) may be helpful[4,5]. Reticulin special stain remains a very useful test, as the aforementioned benign and pre-neoplastic entities generally retain a reticulin network, whereas HCC does not.
However, these stains are neither 100% specific nor 100% sensitive[6]. Thus, careful review of histomorphology and stringent application of cytomorphologic criteria of malignancy, and clinical and imaging correlation is essential to establish a firm diagnosis of well-differentiated HCC. For example, when immuno-histochemical stains are not helpful in demonstrating malignancy, focal loss of reticulin framework in conjunction with cytomorphologic features of malignancy may be the only adjunctive in arriving at the diagnosis. Yet, differentiating between high grade dysplastic nodule and small, early well-differentiated HCC may be extremely challenging in a biopsy specimen[7].
At the other end of the spectrum, “poor differentiation” implies that a tumor lacks resemblance to the native tissue that the tumor originates from. Consequently, a poorly differentiated HCC would morphologically resemble poorly differentiated carcinomas (malignancy of epithelial origin) from any other site, including metastasis to the liver and poorly differentiated intrahepatic cholangiocarcinoma. While it would be relatively straightforward to document malignancy based on histomorphology, documenting that the tumor demonstrates immuno-histochemical evidence of hepatocellular phenotype, and excluding other lines of differentiation are necessary in arriving at the correct diagnosis of HCC. Several immuno-histochemical markers including hepatocyte in paraffin 1 (HepPar1), Arginase-1, CD10, polyclonal carcinoembryonic antigen, bile salt export pump and GPC-3 have been utilized in this setting with variable sensitivities and specificities depending on the degree of differentiation of HCC[3,4,7,8]. One study suggested that a combination of Arginase 1 and GPC-3 has the highest sensitivity in determining a hepatocellular origin of a poorly differentiated carcinoma[9].
Some HCCs aberrantly express non-hepatocellular immuno-histochemical markers of origin and further confound the diagnosis. Shah et al reported that CDX2, an immuno-histochemical marker indicative of intestinal origin, is expressed in 5.2% of HCCs. In their cohort, the aberrant CDX2 expression was common in poorly differentiated HCC[10]. Also, aberrant CK20 expression has been described in 14% of HCCs[11]. Furthermore, a case of poorly differentiated HCC co-expressing CDX2 and CK20 has been recently reported[12]. This would pose a diagnostic pitfall especially when metastatic colorectal carcinoma is a clinical and radiologic differential diagnosis for a liver mass.
Likewise, CK7 expression in HCC is not uncommon. Ward et al compared the immuno-histochemical profile of 22 cases of FL-HCC and 50 non-FL type HCC using tissue microarrays. When “positive” was defined as > 15% of tumor cells staining and “focal positive” as < 15%, 28% and 32% of non-FL type HCC were positive and focal positive for CK7, respectively. All FL-HCCs were positive for CK7[13]. Albumin in situ hybridization (Albumin-ISH) is another promising test. Albumin-ISH is positive in tumors of liver origin such as HCC and intrahepatic cholangiocarcinoma with > 95% and 80%-95% sensitivity, respectively, and is negative in metastatic tumors to the liver[3,8,14].
MOLECULAR BASIS OF HEPATOCELLULAR CARCINOMA AND TARGETED THERAPY
Identification of molecular alterations and signaling pathways that are involved in tumorigenesis is critical for personalized medicine. In HCC, however, it is challenging to identify genetic alterations that are directly related to tumorigenesis, as HCC usually arises in a background of chronic liver disease of many years. The ongoing inflammation and injury lead to the accumulation of a multitude of genetic alterations prior to the development of HCC. The alterations differ between patients with different underlying liver diseases, and between tumor foci within a same patient. This phenomenon is known as “field effect”, and is considered an obstacle in developing a therapy targeting a single mutation[15].
Several studies attempted to classify HCCs according to their molecular signature, but the attempts have not been successfully adopted in the clinical practice[16-19]. However, these attempts are making a significant progress in identifying distinct morphologic subtypes corresponding to specific molecular profiles and clinical correlates. For example, scirrhous subtype, one of the histologic subtypes that were recognized in 2010 WHO, is associated with TSC1/TSC2 mutations. Also, the authors recognized new morphological subtype, “macrotrabecular-massive” subtype, associated with TP53 mutations, FGF19 amplifications and with poor survival and high serum alpha-fetoprotein (AFP) level[20].
The Cancer Genome Atlas Research Network analyzed a total of 559 cases of HCC, and identified 26 genes with significant alterations including some that are promising therapy targets. This study also showed that the promotor region of TERT (regulating cell survival), TP53 (a gene that is frequently involved in carcinogenesis), or CTNNB1 (regulating cell growth and differentiation) is altered in 77% of HCC. However, the authors recognized that it is unlikely to have one therapeutic agent that would effectively target most HCC, given the variety of mutations identified in a given patient (requiring a combination of treatments to target different mutations)[21].
Sorafenib is one of the first generation fms-like tyrosine kinase 3 inhibitors approved for the first-line treatment of advanced HCC. Several protocols and clinical trials reported modest results with survival benefit, especially in patients with hepatitis C[22-24]. Other multikinase inhibitors, regorafenib and cabozantinib, also showed survival benefit as a second-line treatment compared to placebo group in phase 3 clinical trials[23,25,26]. Recent phase 1/2 trial of nivolumab, an immune checkpoint inhibitor, in patients with advanced HCC with or without chronic viral hepatitis reported promising responses[27].
PATHOLOGIC PROGNOSTIC BIOMARKERS
Variable parameters such as serum AFP level, des-gamma-carboxy prothrombin level, tumor size and number, margin status, major vessel invasion, tumor stage, Edmonson-Steiner grade, Child-Pugh score, portal hypertension and cirrhosis, are considered clinical prognosticators of HCC, depending on the treatment modalities and underling liver diseases[28-32]. Pathologically, the overall poor outcome of HCCs expressing stem cell markers such as keratin 19 (K19), epithelial cell adhesion molecule (EpCAM), and CD133 has been reported, potentially via hypoxia-induced epithelial to mesenchymal transition[33-36]. For example, the expression of K19 was associated with high rate of recurrence following radiofrequency ablation, and the HCCs expressing K19, EpCAM or carbonic anhydrase-IX (marker of hypoxia) showed incomplete response to transarterial chemoembolization. The overexpression of CD133 and CD90 in HCCs predicted poor response to sorafenib[37-39]. Histologic grade of HCC is useful in predicting long-term survival. In HCCs with different grades of tumor foci within a same lesion, the worst grade within the tumor appears to dictate its biologic behavior. Therefore, careful approach is warranted when dealing with limited biopsy sample[40].
SELECTED SUBTYPES OF HEPATOCELLULAR CARCINOMA
Steatohepatitic HCC
A possible correlation between non-alcoholic fatty liver disease (NAFLD) and the development of HCC has long been recognized[41]. Moreover, it has been documented that HCC arising in association with non-alcoholic steatohepatitis or metabolic syndrome can develop in non-cirrhotic livers[42,43].
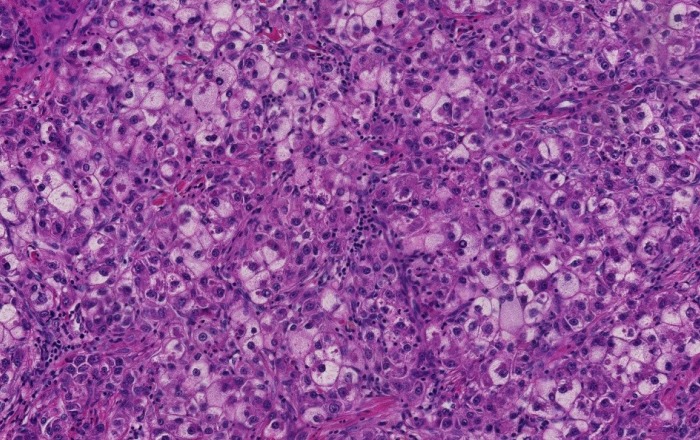
A steatohepatitic variant of HCC was described in 2010. In this study, the authors described multiple histologic features that can be seen in steatohepatitis including macrovesicular steatosis, ballooning, Mallory-Denk Bodies, inflammation and pericellular fibrosis in 35.5% (22 of 62) of HCC arising in HCV induced cirrhosis. 63.6% of patients with steatohepatitic HCC had risk factors of NAFLD (Figure 1). Furthermore, 63.6% of steatohepatitic HCC was associated with background NAFLD, suggesting a link between this variant of HCC and NAFLD[44]. Subsequently the same group established a correlation between the steatohepatitic variant HCC and an underlying steatohepatitis or metabolic risk factors, and suggested a role of steatohepatitis in human hepatocarcinogenesis[45]. A minor subset of steatohepatitic HCC may arise in the absence of background fatty liver disease or metabolic syndrome[46]. This histologic entity and the reports of HCC arising in non-cirrhotic steatohepatitis gained the attention of hepatologists and oncologists. With the increasing incidence of metabolic risk factors and NAFLD especially in the western world, recognition of steatohepatitis without cirrhosis as an additional risk factor for HCC could have significant bearing on HCC screening programs[41,47].
Figure 1.
Steatohepatitic hepatocellular carcinoma. Tumoral cells show features of steatohepatitis including steatosis, ballooning and Mallory-Denk bodies (Hematoxylin and eosin stain, × 200).
The molecular profile and the pathway involved in the carcinogenesis of steatohepatitic subtype are different from conventional HCC. For example, CTNNB1 mutation (beta catenin pathway alterations) are less frequent in steatohepatitic HCC compared to conventional HCC[48]. The immune-histochemical profile of steatohepatitic HCC has been compared to the conventional type. While no significant differences in staining pattern with HNF-1α, β-catenin, GS, GPC-3 and HSP-70 were seen between the two, the degree of staining with C-reactive protein and serum amyloid A was higher in steatohepatitic HCC[49].
FNH arising in a background of steatotic liver may demonstrate at least focal, steatohepatitis-like features and mimic steatohepatitic HCC. Careful identification of the typical histologic features of FNH including thick walled blood vessels, ductular reaction and thick fibrous septa, will be helpful in ruling out a steatohepatitic HCC[50]. A study conducted on a Japanese population showed that steatohepatitic HCC has a similar prognosis to conventional type[51].
Clear cell HCC
Cytoplasmic clearing is a defining feature of the broad category of neoplasms so called “clear cell tumors”. It may be a consequence of accumulation of glycogen, cytoplasmic vesicles, lipopolysaccharides or mucopolysaccharides, or simply represent a processing artifact[52]. Clear cell HCC is a well differentiated variant of HCC. Its cytoplasmic clearing is a result of glycogen and less frequently, fat storing in the cytoplasm[53]. The minimum amount of neoplastic cells with clear cytoplasm required for the diagnosis of clear cell HCC varies in the literature; however a cut-off of minimum 50% has been advocated in the recent AFIP fascicle[3].
Rarely, clear cells may be seen in other types of HCC, i.e., clear cell variant of fibrolamellar HCC or lipid-rich HCC secondary to cytoplasmic lipid accumulation. Thus, assigning the right subtype for the tumor in question may be necessary but challenging, particularly when there is a difference in the outcome[54]. Most studies comparing the prognosis of clear cell HCC versus conventional HCC showed a better prognosis of the former[55,56], or at least similar outcomes for both[57,58]. Interestingly, Lee et al[59] reported the presence of IDH1 mutation in 25% of clear cell HCC. These IDH1 mutated clear cell HCCs showed a statistically significant worse prognosis compared to IDH1 wild-type clear cell HCCs. Of note, IDH1 mutations are not uncommon in intrahepatic cholangiocarcinoma, a tumor with a significantly worse prognosis than HCC[60,61].
Cases of clear cell HCC in non-cirrhotic liver or liver without hepatitis are rarely reported in the literature[62,63]. Therefore, when a liver tumor with clear cell features in an otherwise unremarkable background liver is encountered, a metastasis from another primary with clear cell components needs to be excluded. Most common scenario would be a metastatic clear cell renal cell carcinoma or a clear cell carcinoma from the ovaries mimicking clear cell HCC. While a panel of IHC will aid in establishing the diagnosis of a primary vs a metastatic process in the vast majority of cases[64-66], some ovarian clear cell carcinomas may stain with HepPar-1, posing a diagnostic pitfall[65].
Fibrolamellar HCC
FL-HCC is a clinically, histologically and molecularly distinct subtype of HCC when compared to other subtypes of HCC. It frequently occurs in adolescents and young adults, and the mean age at the diagnosis is 25[67-69]. The background liver typically lacks features of chronic hepatitis, inflammation, fibrosis, or preneoplastic lesions. Primarily due to the absence of cirrhosis in the background, the outcome of FL-HCC is usually better than the remaining subtypes. When non FL-HCCs arising in non-cirrhotic liver are compared, both show a similar outcome[70].
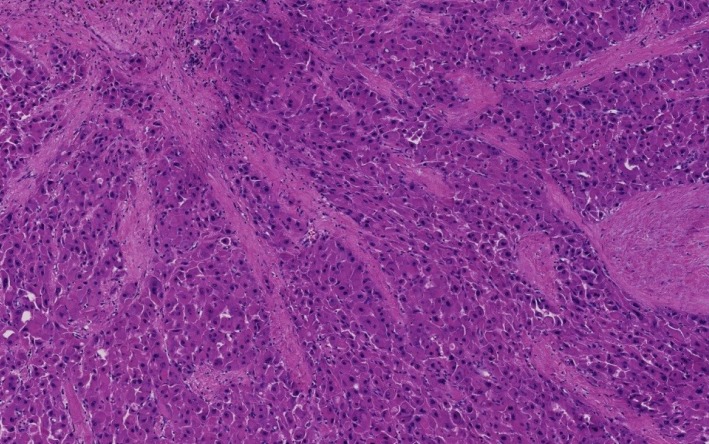
The unique histology of FL-HCC was first described in 1956. FL-HCC consists of large, polygonal eosinophilic cells with abundant cytoplasm resembling hepatocytes. There are prominent nucleoli and pale inclusion bodies and pink bodies (hyaline bodies/globules) within the neoplastic cells, and the extensive intratumoral fibrosis is a characteristic feature. The thick collagenous bands within the tumor are often parallel and “lamellar”, but may be irregular and haphazard[71] (Figure 2). Patchy areas of solid growth with little fibrosis may be noted within FL-HCC[3]. Intratumoral cholestasis is common, and pseudoglandular growth with mucin production may be seen, mimicking combined cholangiocarcinoma component[3].
Figure 2.
Fibrolamellar hepatocellular carcinoma. Multiple fibrous septa composed of parallel collagen fibers separate tumoral trabeculae (Hematoxylin and eosin stain, × 100).
The immuno-profile of FL-HCC is also unique, and shows positivity for the biliary marker CK7[13] and histiocytic marker CD68[72]. Histologic differential diagnosis is S-HCC. Both are characterized by extensive intratumoral fibrosis. Kim et al[73] studied the nature of the fibrous stroma in FL-HCC and S-HCC. The authors showed that the fibrous stroma in FL-HCC is composed of dense lamellated collagen whereas in S-HCC, the fibrous stroma represents aggressive and complex tumoral microenvironment enriched by cancer-associated fibroblasts and tumor-infiltrating macrophages. At the molecular level, FL-HCC harbors a characteristic fusion of DNAJB1 and PRKACA on chromosome 19, secondary to chromosomal deletion in between these two genes[74]. This fusion has been proven to be 100% specific for FL-HCC in the context of hepatic malignancy[75].
Scirrhous HCC
S-HCC is another rare subtype of HCC. The histomorphology of S-HCC is somewhat similar to FL-HCC with striking intratumoral fibrosis and oftentimes non-cirrhotic background liver. While the biologic behavior of S-HCC may be more aggressive than usual HCC with more portal vein invasion in the former, the long term outcome and prognosis of S-HCC is similar to, or better than those of usual HCC[3,76].
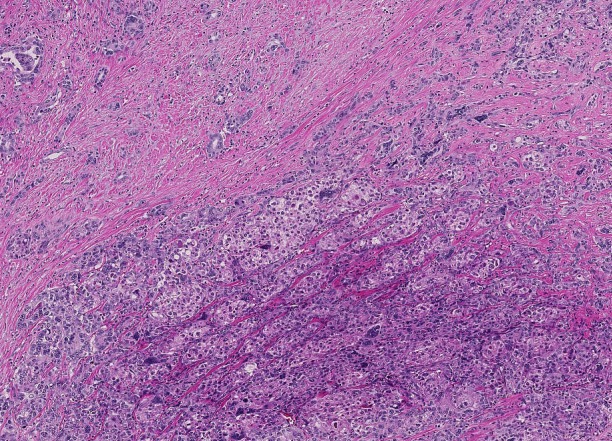
The fibrosis involves at least 50% of the tumor and separates small nests of tumor cells[2] (Figure 3). Due to the abundance of intratumoral fibrous stroma associated with thin trabecular pattern growth, S-HCC may, radiologically and pathologically, closely mimic cholangiocarcinoma[3,76,77]. S-HCC is positive for CK7 similarly to FL-HCC[78]. However, the distinction between these two can be made by co-expression of CD68[72] and the fusion of DNAJB1-PRKACA genes in FL-HCC[74]. On the other hand, TSC1/TSC2 mutations and the overexpression of TGF-beta signaling have been identified in S-HCC[20,79]. While S-HCC is a subtype of HCC, immuno-markers that are associated with adenocarcinoma such as CK7 and EpCAM are commonly expressed whereas HepPar1 expression is less common in S-HCC. A combination of GPC-3 and Arginase 1 was useful in distinguishing S-HCC from cholangiocarcinoma with 100% sensitivity for S-HCC[80].
Figure 3.
Scirrhous hepatocellular carcinoma. Bands of dense fibrosis separate cords and nests of tumoral cells (Hematoxyin and eosin stain, × 200).
COMBINED HEPATOCELLULAR CARCINOMA-CHOLANGIOCARCINOMA
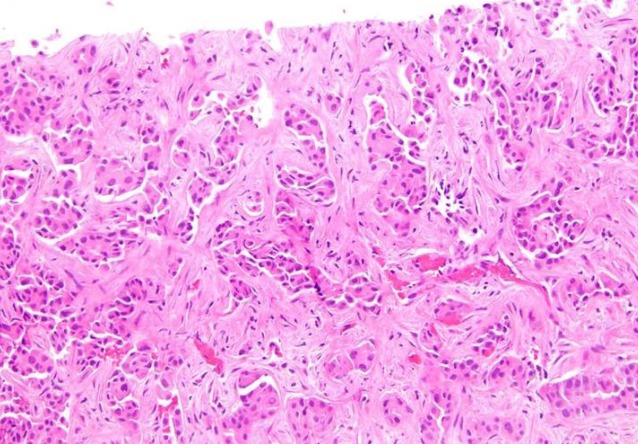
cHCC-CCA is primary liver cancer demonstrating morphologic and immuno-histochemical features of both HCC and CCA (Figure 4). Collision tumor or two separate primaries of HCC and CCA in the same liver do not qualify for cHCC-CCA diagnosis. Variable terminologies have been used in the literature for this entity, including hepatocholangiocarcinoma, biphenotypic HCC-CCA, mixed HCC-CCA, mixed hepatobiliary carcinoma, combined liver cell and bile duct carcinoma, HCC with dual phenotype, HCC with stem/progenitor cell immunophenotype, cholangiolocarcinoma, cholangiolocellular carcinoma (CLC), intermediate HCC and stem cell tumor, making it difficult to collect meaningful data[3,81].
Figure 4.
Combined hepatocellular carcinoma-cholangiocarcinoma. Areas of conventional hepatocellular carcinoma (lower mid to right) are admixed with areas of discrete glands formation (upper left) (Hematoxylin and eosin stain, × 40).
In 2010 WHO, cHCC-CCA was largely divided into two types: Classical type and a subtype with stem-cell features, wherein the latter was further subdivided into typical, intermediate, and cholangiocellular subtypes[1]. However, subsequent studies showed that stem cell features are seen in many other types of HCC and CCA and the WHO criteria for stem cell subtypes are difficult to apply in practice. This observation has led to collaborative efforts and the publication of a consensus paper on working terminology and diagnostic criteria of cHCC-CCA in 2018 by international experts in the field consisting of pathologists, radiologists and clinicians[81].
The consensus paper formally endorsed the terminology cHCC-CCA and recommended that the diagnosis of cHCC-CCA has to be primarily based on morphology on hematoxylin and eosin stain and immuno-histochemical stains are to be used as a supplemental purpose only. In addition, the panel recommended the term "intermediate cell carcinoma" for previous cHCC-CCA with "intermediate cell" stem cell features (primary liver cancer consisting purely of intermediate cells), as a separate entity. "CLC" was recommended as another separate entity with a cut off of 80% required for the diagnosis of pure CLC without mixed components. It was recommended that the morphologic and immuno-histochemical stem/progenitor cell features/phenotypes are to be noted in a comment, but not in the diagnostic line. Further, when more than one morphologic component is present within the same tumor, it was recommended that each component to be listed in the diagnostic line. Therefore, previous cHCC-CCA with "typical" and "cholangiolocellular" stem cell features are termed as cHCC-CCA and cHCC-CCA-CLC, respectively, and the percentage of each component is to be reported[81].
Studies suggest that cHCC-CCA is likely a clonal lesion of stem/progenitor cell origin[33,82,83]. The molecular phenotype of cHCC-CCA is heterogeneous[84]; however, molecular features indicative of stem cell features have been documented[85]. According to the current 8th edition American Joint Committee on Cancer cancer staging manual, cHCC-CCA is staged as intrahepatic CCA[86]. Given the differing treatment options for HCC and CCA, biopsy sample of a large mass lesion needs to be interpreted with great caution and a resection specimen needs to be thoroughly sampled.
EMERGING BIOMARKERS AND COMPREHENSIVE STRATIFICATION OF HEPATOCELLULAR CARCINOMA
Novel diagnostic and/or prognostic biomarkers are emerging as key players in the field of HCC research. For example, annexin A2 is significantly increased in the serum of patients with HCC when compared to patients with chronic liver disease, thus may be diagnostically useful especially when combined with AFP[87,88]. Additional biomarkers, such as osteopontin, Golgi protein-73, squamous cell carcinoma antigen, soluble urokinase plasminogen activator receptor, midkine, AXL, thioredoxins[88], multifucosylated α-1-acid glycoprotein[89], vascular endothelial growth factor, angiopoietin[90], survivin[91] and alpha-1 antitrypsin[92], have shown potential for diagnostic and/or prognostic utility. Some of these are recognized by a major international hepatology society[90].
Likewise, comprehensive genomic and epigenomic approaches are emerging as promising tools to stratify HCCs into clinically relevant subgroups. Bidkhori et al[93] stratified HCCs into three subtypes: Altered kynurenine metabolism, WNT/β-catenin–associated lipid metabolism and PI3K/AKT/mTOR signaling subtype based on the metabolic and signalling pathways and showed differences in survival. Shimada et al[94] stratified HCCs into three major subtypes: Mitogenic and stem cell-like tumors with chromosomal instability (the proliferative subtype), CTNNB1-mutated tumors displaying immune suppression and metabolic disease-associated tumors by multi-platform analysis including transcriptome, exome and methylome profiles and public omics data, and showed a favorable prognosis in the "immuno-genic" subset of the metabolic disease-associated tumors.
CONCLUSION
Our understanding of the pathogenesis of HCC and diagnostic approach is evolving along with rapid advancement of diagnostic tools including molecular pathology. Updated pathologic classification of HCC is a reflection of the accrued knowledge and an effort to facilitate effective communication and collaborative studies. We hope that the effort further advances our understanding of the disease and ultimately improve patient care.
Footnotes
Conflict-of-interest statement: No potential conflicts of interest.
Manuscript source: Invited manuscript
Peer-review started: February 14, 2019
First decision: March 5, 2019
Article in press: March 25, 2019
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jin C, Namisaki T, Sitkin S S-Editor: Yan JP L-Editor: A E-Editor: Song H
Contributor Information
Tony El Jabbour, Department of Pathology and Laboratory Medicine, Albany Medical College, Albany, NY 12208, United States.
Stephen M Lagana, Department of Pathology and Cell Biology, College of Physicians and Surgeons, Columbia University, New York, NY 10032, United States.
Hwajeong Lee, Department of Pathology and Laboratory Medicine, Albany Medical College, Albany, NY 12208, United States. leeh5@.amc.edu.
References
- 1.Bosman F, Carneiro F, Hruban R, Theise N. Lyon: IARC; 2010. WHO classification of tumours of the digestive system. 4th ed; pp. 205–227. [Google Scholar]
- 2.Torbenson MS. Morphologic Subtypes of Hepatocellular Carcinoma. Gastroenterol Clin North Am. 2017;46:365–391. doi: 10.1016/j.gtc.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Torbenson M, Zen Y, Yeh MM. Washington (DC): American Registry of Pathology; 2018. Tumors of the liver, AFIP Atlas of tumor pathology series 4; pp. 39–112. [Google Scholar]
- 4.Koehne de Gonzalez AK, Salomao MA, Lagana SM. Current concepts in the immunohistochemical evaluation of liver tumors. World J Hepatol. 2015;7:1403–1411. doi: 10.4254/wjh.v7.i10.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Tommaso L, Destro A, Seok JY, Balladore E, Terracciano L, Sangiovanni A, Iavarone M, Colombo M, Jang JJ, Yu E, Jin SY, Morenghi E, Park YN, Roncalli M. The application of markers (HSP70 GPC3 and GS) in liver biopsies is useful for detection of hepatocellular carcinoma. J Hepatol. 2009;50:746–754. doi: 10.1016/j.jhep.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Uthamalingam P, Das A, Behra A, Kalra N, Chawla Y. Diagnostic Value of Glypican3, Heat Shock Protein 70 and Glutamine Synthetase in Hepatocellular Carcinoma Arising in Cirrhotic and Non-Cirrhotic Livers. J Clin Exp Hepatol. 2018;8:173–180. doi: 10.1016/j.jceh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi WT, Ramachandran R, Kakar S. Immunohistochemical approach for the diagnosis of a liver mass on small biopsy specimens. Hum Pathol. 2017;63:1–13. doi: 10.1016/j.humpath.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 8.Koehne de Gonzalez A, Lagana SM. Update on Ancillary Testing in the Evaluation of High-Grade Liver Tumors. Surg Pathol Clin. 2018;11:367–375. doi: 10.1016/j.path.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen T, Phillips D, Jain D, Torbenson M, Wu TT, Yeh MM, Kakar S. Comparison of 5 Immunohistochemical Markers of Hepatocellular Differentiation for the Diagnosis of Hepatocellular Carcinoma. Arch Pathol Lab Med. 2015;139:1028–1034. doi: 10.5858/arpa.2014-0479-OA. [DOI] [PubMed] [Google Scholar]
- 10.Shah SS, Wu TT, Torbenson MS, Chandan VS. Aberrant CDX2 expression in hepatocellular carcinomas: An important diagnostic pitfall. Hum Pathol. 2017;64:13–18. doi: 10.1016/j.humpath.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 11.Mourra N, Azizi L. CK20 positivity in hepatocellular carcinoma: A potential diagnostic pitfall in liver biopsy. Appl Immunohistochem Mol Morphol. 2013;21:94–95. doi: 10.1097/PAI.0b013e31824c4c4a. [DOI] [PubMed] [Google Scholar]
- 12.El Jabbour T, Durie N, Lee H. Coexpression of CDX2 and CK20 in hepatocellular carcinoma, an exceedingly rare co-incidence with potential diagnostic pitfall. Hum Pathol. 2018;81:298. doi: 10.1016/j.humpath.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 13.Ward SC, Huang J, Tickoo SK, Thung SN, Ladanyi M, Klimstra DS. Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Mod Pathol. 2010;23:1180–1190. doi: 10.1038/modpathol.2010.105. [DOI] [PubMed] [Google Scholar]
- 14.Shahid M, Mubeen A, Tse J, Kakar S, Bateman AC, Borger D, Rivera MN, Ting DT, Deshpande V. Branched chain in situ hybridization for albumin as a marker of hepatocellular differentiation: Evaluation of manual and automated in situ hybridization platforms. Am J Surg Pathol. 2015;39:25–34. doi: 10.1097/PAS.0000000000000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez-Gea V, Toffanin S, Friedman SL, Llovet JM. Role of the microenvironment in the pathogenesis and treatment of hepatocellular carcinoma. Gastroenterology. 2013;144:512–527. doi: 10.1053/j.gastro.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JS, Chu IS, Heo J, Calvisi DF, Sun Z, Roskams T, Durnez A, Demetris AJ, Thorgeirsson SS. Classification and prediction of survival in hepatocellular carcinoma by gene expression profiling. Hepatology. 2004;40:667–676. doi: 10.1002/hep.20375. [DOI] [PubMed] [Google Scholar]
- 17.Boyault S, Rickman DS, de Reyniès A, Balabaud C, Rebouissou S, Jeannot E, Hérault A, Saric J, Belghiti J, Franco D, Bioulac-Sage P, Laurent-Puig P, Zucman-Rossi J. Transcriptome classification of HCC is related to gene alterations and to new therapeutic targets. Hepatology. 2007;45:42–52. doi: 10.1002/hep.21467. [DOI] [PubMed] [Google Scholar]
- 18.Hoshida Y, Nijman SM, Kobayashi M, Chan JA, Brunet JP, Chiang DY, Villanueva A, Newell P, Ikeda K, Hashimoto M, Watanabe G, Gabriel S, Friedman SL, Kumada H, Llovet JM, Golub TR. Integrative transcriptome analysis reveals common molecular subclasses of human hepatocellular carcinoma. Cancer Res. 2009;69:7385–7392. doi: 10.1158/0008-5472.CAN-09-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Désert R, Rohart F, Canal F, Sicard M, Desille M, Renaud S, Turlin B, Bellaud P, Perret C, Clément B, Lê Cao KA, Musso O. Human hepatocellular carcinomas with a periportal phenotype have the lowest potential for early recurrence after curative resection. Hepatology. 2017;66:1502–1518. doi: 10.1002/hep.29254. [DOI] [PubMed] [Google Scholar]
- 20.Calderaro J, Couchy G, Imbeaud S, Amaddeo G, Letouzé E, Blanc JF, Laurent C, Hajji Y, Azoulay D, Bioulac-Sage P, Nault JC, Zucman-Rossi J. Histological subtypes of hepatocellular carcinoma are related to gene mutations and molecular tumour classification. J Hepatol. 2017;67:727–738. doi: 10.1016/j.jhep.2017.05.014. [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network. Comprehensive and Integrative Genomic Characterization of Hepatocellular Carcinoma. Cell. 2017;169:1327–1341.e23. doi: 10.1016/j.cell.2017.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan SL, Wong AM, Lee K, Wong N, Chan AK. Personalized therapy for hepatocellular carcinoma: Where are we now? Cancer Treat Rev. 2016;45:77–86. doi: 10.1016/j.ctrv.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 24.Bruix J, Cheng AL, Meinhardt G, Nakajima K, De Sanctis Y, Llovet J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J Hepatol. 2017;67:999–1008. doi: 10.1016/j.jhep.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 25.Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, Yokosuka O, Rosmorduc O, Breder V, Gerolami R, Masi G, Ross PJ, Song T, Bronowicki JP, Ollivier-Hourmand I, Kudo M, Cheng AL, Llovet JM, Finn RS, LeBerre MA, Baumhauer A, Meinhardt G, Han G RESORCE Investigators. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56–66. doi: 10.1016/S0140-6736(16)32453-9. [DOI] [PubMed] [Google Scholar]
- 26.Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, Merle P, Chen Y, Park JW, Blanc JF, Bolondi L, Klümpen HJ, Chan SL, Zagonel V, Pressiani T, Ryu MH, Venook AP, Hessel C, Borgman-Hagey AE, Schwab G, Kelley RK. Cabozantinib in Patients with Advanced and Progressing Hepatocellular Carcinoma. N Engl J Med. 2018;379:54–63. doi: 10.1056/NEJMoa1717002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, Meyer T, Kang YK, Yeo W, Chopra A, Anderson J, Dela Cruz C, Lang L, Neely J, Tang H, Dastani HB, Melero I. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389:2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu J, Zhang X, Cui R, Zhang J, Wang Z, Jia Y, Miao R, Dong Y, Ma X, Fan H, Wang H, Ren L, Li Y, Niu W, Zhang J, Qu K, Liu C. Prognostic predictors for patients with hepatocellular carcinoma receiving adjuvant transcatheter arterial chemoembolization. Eur J Gastroenterol Hepatol. 2019 doi: 10.1097/MEG.0000000000001346. [DOI] [PubMed] [Google Scholar]
- 29.Meguro M, Mizuguchi T, Nishidate T, Okita K, Ishii M, Ota S, Ueki T, Akizuki E, Hirata K. Prognostic roles of preoperative α-fetoprotein and des-γ-carboxy prothrombin in hepatocellular carcinoma patients. World J Gastroenterol. 2015;21:4933–4945. doi: 10.3748/wjg.v21.i16.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Wang Z, Wu L. Combined measurements of tumor number and size helps estimate the outcome of resection of Barcelona clinic liver cancer stage B hepatocellular carcinoma. BMC Surg. 2016;16:22. doi: 10.1186/s12893-016-0135-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goh BK, Chow PK, Teo JY, Wong JS, Chan CY, Cheow PC, Chung AY, Ooi LL. Number of nodules, Child-Pugh status, margin positivity, and microvascular invasion, but not tumor size, are prognostic factors of survival after liver resection for multifocal hepatocellular carcinoma. J Gastrointest Surg. 2014;18:1477–1485. doi: 10.1007/s11605-014-2542-0. [DOI] [PubMed] [Google Scholar]
- 32.Yilmaz C, Karaca CA, Iakobadze Z, Farajov R, Kilic K, Doganay L, Kilic M. Factors Affecting Recurrence and Survival After Liver Transplantation for Hepatocellular Carcinoma. Transplant Proc. 2018;50:3571–3576. doi: 10.1016/j.transproceed.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, Cho JY, Yoo JE, Choi JS, Park YN. Human hepatocellular carcinomas with "Stemness"-related marker expression: Keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707–1717. doi: 10.1002/hep.24559. [DOI] [PubMed] [Google Scholar]
- 34.Guo Z, Li LQ, Jiang JH, Ou C, Zeng LX, Xiang BD. Cancer stem cell markers correlate with early recurrence and survival in hepatocellular carcinoma. World J Gastroenterol. 2014;20:2098–2106. doi: 10.3748/wjg.v20.i8.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan AW, Tong JH, Chan SL, Lai PB, To KF. Expression of stemness markers (CD133 and EpCAM) in prognostication of hepatocellular carcinoma. Histopathology. 2014;64:935–950. doi: 10.1111/his.12342. [DOI] [PubMed] [Google Scholar]
- 36.Nahm JH, Rhee H, Kim H, Yoo JE, San Lee J, Jeon Y, Choi GH, Park YN. Increased expression of stemness markers and altered tumor stroma in hepatocellular carcinoma under TACE-induced hypoxia: A biopsy and resection matched study. Oncotarget. 2017;8:99359–99371. doi: 10.18632/oncotarget.22078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchiya K, Komuta M, Yasui Y, Tamaki N, Hosokawa T, Ueda K, Kuzuya T, Itakura J, Nakanishi H, Takahashi Y, Kurosaki M, Asahina Y, Enomoto N, Sakamoto M, Izumi N. Expression of keratin 19 is related to high recurrence of hepatocellular carcinoma after radiofrequency ablation. Oncology. 2011;80:278–288. doi: 10.1159/000328448. [DOI] [PubMed] [Google Scholar]
- 38.Rhee H, Nahm JH, Kim H, Choi GH, Yoo JE, Lee HS, Koh MJ, Park YN. Poor outcome of hepatocellular carcinoma with stemness marker under hypoxia: Resistance to transarterial chemoembolization. Mod Pathol. 2016;29:1038–1049. doi: 10.1038/modpathol.2016.111. [DOI] [PubMed] [Google Scholar]
- 39.Kim BH, Park JW, Kim JS, Lee SK, Hong EK. Stem Cell Markers Predict the Response to Sorafenib in Patients with Hepatocellular Carcinoma. Gut Liver. 2018 doi: 10.5009/gnl18345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Han DH, Choi GH, Kim KS, Choi JS, Park YN, Kim SU, Park JY, Ahn SH, Han KH. Prognostic significance of the worst grade in hepatocellular carcinoma with heterogeneous histologic grades of differentiation. J Gastroenterol Hepatol. 2013;28:1384–1390. doi: 10.1111/jgh.12200. [DOI] [PubMed] [Google Scholar]
- 41.Starley BQ, Calcagno CJ, Harrison SA. Nonalcoholic fatty liver disease and hepatocellular carcinoma: A weighty connection. Hepatology. 2010;51:1820–1832. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- 42.Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, Kawata S, Uto H, Takami S, Sumida Y, Takamura T, Kawanaka M, Okanoue T Japan NASH Study Group, Ministry of Health, Labour, and Welfare of Japan. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–433; quiz e50. doi: 10.1016/j.cgh.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, Bedossa P, Belghiti J. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: A pathological analysis. Hepatology. 2009;49:851–859. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 44.Salomao M, Yu WM, Brown RS, Jr, Emond JC, Lefkowitch JH. Steatohepatitic hepatocellular carcinoma (SH-HCC): A distinctive histological variant of HCC in hepatitis C virus-related cirrhosis with associated NAFLD/NASH. Am J Surg Pathol. 2010;34:1630–1636. doi: 10.1097/PAS.0b013e3181f31caa. [DOI] [PubMed] [Google Scholar]
- 45.Salomao M, Remotti H, Vaughan R, Siegel AB, Lefkowitch JH, Moreira RK. The steatohepatitic variant of hepatocellular carcinoma and its association with underlying steatohepatitis. Hum Pathol. 2012;43:737–746. doi: 10.1016/j.humpath.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 46.Yeh MM, Liu Y, Torbenson M. Steatohepatitic variant of hepatocellular carcinoma in the absence of metabolic syndrome or background steatosis: A clinical, pathological, and genetic study. Hum Pathol. 2015;46:1769–1775. doi: 10.1016/j.humpath.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Baffy G, Brunt EM, Caldwell SH. Hepatocellular carcinoma in non-alcoholic fatty liver disease: An emerging menace. J Hepatol. 2012;56:1384–1391. doi: 10.1016/j.jhep.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Ando S, Shibahara J, Hayashi A, Fukayama M. β-catenin alteration is rare in hepatocellular carcinoma with steatohepatitic features: Immunohistochemical and mutational study. Virchows Arch. 2015;467:535–542. doi: 10.1007/s00428-015-1836-2. [DOI] [PubMed] [Google Scholar]
- 49.Taniai M, Hashimoto E, Tobari M, Kodama K, Tokushige K, Yamamoto M, Takayama T, Sugitani M, Sano K, Kondo F, Fukusato T. Clinicopathological investigation of steatohepatitic hepatocellular carcinoma: A multicenter study using immunohistochemical analysis of adenoma-related markers. Hepatol Res. 2018;48:947–955. doi: 10.1111/hepr.13203. [DOI] [PubMed] [Google Scholar]
- 50.Deniz K, Moreira RK, Yeh MM, Ferrell LD. Steatohepatitis-like Changes in Focal Nodular Hyperplasia, A Finding to Distinguish From Steatohepatitic Variant of Hepatocellular Carcinoma. Am J Surg Pathol. 2017;41:277–281. doi: 10.1097/PAS.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 51.Shibahara J, Ando S, Sakamoto Y, Kokudo N, Fukayama M. Hepatocellular carcinoma with steatohepatitic features: A clinicopathological study of Japanese patients. Histopathology. 2014;64:951–962. doi: 10.1111/his.12343. [DOI] [PubMed] [Google Scholar]
- 52.Kwon TJ, Ro JY, Mackay B. Clear-cell carcinoma: An ultrastructural study of 57 tumors from various sites. Ultrastruct Pathol. 1996;20:519–527. doi: 10.3109/01913129609016356. [DOI] [PubMed] [Google Scholar]
- 53.Bannasch P, Ribback S, Su Q, Mayer D. Clear cell hepatocellular carcinoma: Origin, metabolic traits and fate of glycogenotic clear and ground glass cells. Hepatobiliary Pancreat Dis Int. 2017;16:570–594. doi: 10.1016/S1499-3872(17)60071-7. [DOI] [PubMed] [Google Scholar]
- 54.Cheuk W, Chan JK. Clear cell variant of fibrolamellar carcinoma of the liver. Arch Pathol Lab Med. 2001;125:1235–1238. doi: 10.5858/2001-125-1235-CCVOFC. [DOI] [PubMed] [Google Scholar]
- 55.Liu Z, Ma W, Li H, Li Q. Clinicopathological and prognostic features of primary clear cell carcinoma of the liver. Hepatol Res. 2008;38:291–299. doi: 10.1111/j.1872-034X.2007.00264.x. [DOI] [PubMed] [Google Scholar]
- 56.Ji SP, Li Q, Dong H. Therapy and prognostic features of primary clear cell carcinoma of the liver. World J Gastroenterol. 2010;16:764–769. doi: 10.3748/wjg.v16.i6.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang SH, Watanabe J, Nakashima O, Kojiro M. Clinicopathologic study on clear cell hepatocellular carcinoma. Pathol Int. 1996;46:503–509. doi: 10.1111/j.1440-1827.1996.tb03645.x. [DOI] [PubMed] [Google Scholar]
- 58.Emile JF, Lemoine A, Azoulay D, Debuire B, Bismuth H, Reynès M. Histological, genomic and clinical heterogeneity of clear cell hepatocellular carcinoma. Histopathology. 2001;38:225–231. doi: 10.1046/j.1365-2559.2001.01096.x. [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Shin DH, Park WY, Shin N, Kim A, Lee HJ, Kim YK, Choi KU, Kim JY, Yang YI, Lee CH, Sol MY. IDH1 R132C mutation is detected in clear cell hepatocellular carcinoma by pyrosequencing. World J Surg Oncol. 2017;15:82. doi: 10.1186/s12957-017-1144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee H, Ross JS. The potential role of comprehensive genomic profiling to guide targeted therapy for patients with biliary cancer. Therap Adv Gastroenterol. 2017;10:507–520. doi: 10.1177/1756283X17698090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lee WS, Lee KW, Heo JS, Kim SJ, Choi SH, Kim YI, Joh JW. Comparison of combined hepatocellular and cholangiocarcinoma with hepatocellular carcinoma and intrahepatic cholangiocarcinoma. Surg Today. 2006;36:892–897. doi: 10.1007/s00595-006-3276-8. [DOI] [PubMed] [Google Scholar]
- 62.Clayton EF, Furth EE, Ziober A, Xu T, Yao Y, Hwang PG, Bing Z. A case of primary clear cell hepatocellular carcinoma in a non-cirrhotic liver: An immunohistochemical and ultrastructural study. Rare Tumors. 2012;4:e29. doi: 10.4081/rt.2012.e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Takahashi A, Saito H, Kanno Y, Abe K, Yokokawa J, Irisawa A, Kenjo A, Saito T, Gotoh M, Ohira H. Case of clear-cell hepatocellular carcinoma that developed in the normal liver of a middle-aged woman. World J Gastroenterol. 2008;14:129–131. doi: 10.3748/wjg.14.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Murakata LA, Ishak KG, Nzeako UC. Clear cell carcinoma of the liver: A comparative immunohistochemical study with renal clear cell carcinoma. Mod Pathol. 2000;13:874–881. doi: 10.1038/modpathol.3880156. [DOI] [PubMed] [Google Scholar]
- 65.Fan Z, van de Rijn M, Montgomery K, Rouse RV. Hep par 1 antibody stain for the differential diagnosis of hepatocellular carcinoma: 676 tumors tested using tissue microarrays and conventional tissue sections. Mod Pathol. 2003;16:137–144. doi: 10.1097/01.MP.0000052103.13730.20. [DOI] [PubMed] [Google Scholar]
- 66.Hou TC, Wu CC, Yang CR, Wang J. Synchronous renal cell carcinoma and clear cell hepatocellular carcinoma mimicking metastatic disease. Pathol Res Pract. 2010;206:342–345. doi: 10.1016/j.prp.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 67.Craig JR, Peters RL, Edmondson HA, Omata M. Fibrolamellar carcinoma of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer. 1980;46:372–379. doi: 10.1002/1097-0142(19800715)46:2<372::aid-cncr2820460227>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 68.Torbenson M. Fibrolamellar carcinoma: 2012 update. Scientifica (Cairo) 2012;2012:743790. doi: 10.6064/2012/743790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eggert T, McGlynn KA, Duffy A, Manns MP, Greten TF, Altekruse SF. Fibrolamellar hepatocellular carcinoma in the USA, 2000-2010: A detailed report on frequency, treatment and outcome based on the Surveillance, Epidemiology, and End Results database. United European Gastroenterol J. 2013;1:351–357. doi: 10.1177/2050640613501507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kakar S, Burgart LJ, Batts KP, Garcia J, Jain D, Ferrell LD. Clinicopathologic features and survival in fibrolamellar carcinoma: Comparison with conventional hepatocellular carcinoma with and without cirrhosis. Mod Pathol. 2005;18:1417–1423. doi: 10.1038/modpathol.3800449. [DOI] [PubMed] [Google Scholar]
- 71.Edmondson HA. Differential diagnosis of tumors and tumor-like lesions of liver in infancy and childhood. AMA J Dis Child. 1956;91:168–186. doi: 10.1001/archpedi.1956.02060020170015. [DOI] [PubMed] [Google Scholar]
- 72.Ross HM, Daniel HD, Vivekanandan P, Kannangai R, Yeh MM, Wu TT, Makhlouf HR, Torbenson M. Fibrolamellar carcinomas are positive for CD68. Mod Pathol. 2011;24:390–395. doi: 10.1038/modpathol.2010.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim YJ, Rhee H, Yoo JE, Alves VAF, Kim GJ, Kim HM, Herman P, Chagas A, Kim H, Park YN. Tumour epithelial and stromal characteristics of hepatocellular carcinomas with abundant fibrous stroma: Fibrolamellar versus scirrhous hepatocellular carcinoma. Histopathology. 2017;71:217–226. doi: 10.1111/his.13219. [DOI] [PubMed] [Google Scholar]
- 74.Honeyman JN, Simon EP, Robine N, Chiaroni-Clarke R, Darcy DG, Lim II, Gleason CE, Murphy JM, Rosenberg BR, Teegan L, Takacs CN, Botero S, Belote R, Germer S, Emde AK, Vacic V, Bhanot U, LaQuaglia MP, Simon SM. Detection of a recurrent DNAJB1-PRKACA chimeric transcript in fibrolamellar hepatocellular carcinoma. Science. 2014;343:1010–1014. doi: 10.1126/science.1249484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Graham RP, Jin L, Knutson DL, Kloft-Nelson SM, Greipp PT, Waldburger N, Roessler S, Longerich T, Roberts LR, Oliveira AM, Halling KC, Schirmacher P, Torbenson MS. DNAJB1-PRKACA is specific for fibrolamellar carcinoma. Mod Pathol. 2015;28:822–829. doi: 10.1038/modpathol.2015.4. [DOI] [PubMed] [Google Scholar]
- 76.Lee JH, Choi MS, Gwak GY, Lee JH, Koh KC, Paik SW, Yoo BC, Choi D, Park CK. Clinicopathologic characteristics and long-term prognosis of scirrhous hepatocellular carcinoma. Dig Dis Sci. 2012;57:1698–1707. doi: 10.1007/s10620-012-2075-x. [DOI] [PubMed] [Google Scholar]
- 77.Kurogi M, Nakashima O, Miyaaki H, Fujimoto M, Kojiro M. Clinicopathological study of scirrhous hepatocellular carcinoma. J Gastroenterol Hepatol. 2006;21:1470–1477. doi: 10.1111/j.1440-1746.2006.04372.x. [DOI] [PubMed] [Google Scholar]
- 78.Matsuura S, Aishima S, Taguchi K, Asayama Y, Terashi T, Honda H, Tsuneyoshi M. 'Scirrhous' type hepatocellular carcinomas: A special reference to expression of cytokeratin 7 and hepatocyte paraffin 1. Histopathology. 2005;47:382–390. doi: 10.1111/j.1365-2559.2005.02230.x. [DOI] [PubMed] [Google Scholar]
- 79.Seok JY, Na DC, Woo HG, Roncalli M, Kwon SM, Yoo JE, Ahn EY, Kim GI, Choi JS, Kim YB, Park YN. A fibrous stromal component in hepatocellular carcinoma reveals a cholangiocarcinoma-like gene expression trait and epithelial-mesenchymal transition. Hepatology. 2012;55:1776–1786. doi: 10.1002/hep.25570. [DOI] [PubMed] [Google Scholar]
- 80.Krings G, Ramachandran R, Jain D, Wu TT, Yeh MM, Torbenson M, Kakar S. Immunohistochemical pitfalls and the importance of glypican 3 and arginase in the diagnosis of scirrhous hepatocellular carcinoma. Mod Pathol. 2013;26:782–791. doi: 10.1038/modpathol.2012.243. [DOI] [PubMed] [Google Scholar]
- 81.Brunt E, Aishima S, Clavien PA, Fowler K, Goodman Z, Gores G, Gouw A, Kagen A, Klimstra D, Komuta M, Kondo F, Miksad R, Nakano M, Nakanuma Y, Ng I, Paradis V, Nyun Park Y, Quaglia A, Roncalli M, Roskams T, Sakamoto M, Saxena R, Sempoux C, Sirlin C, Stueck A, Thung S, Tsui WMS, Wang XW, Wee A, Yano H, Yeh M, Zen Y, Zucman-Rossi J, Theise N. cHCC-CCA: Consensus terminology for primary liver carcinomas with both hepatocytic and cholangiocytic differentation. Hepatology. 2018;68:113–126. doi: 10.1002/hep.29789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Durnez A, Verslype C, Nevens F, Fevery J, Aerts R, Pirenne J, Lesaffre E, Libbrecht L, Desmet V, Roskams T. The clinicopathological and prognostic relevance of cytokeratin 7 and 19 expression in hepatocellular carcinoma. A possible progenitor cell origin. Histopathology. 2006;49:138–151. doi: 10.1111/j.1365-2559.2006.02468.x. [DOI] [PubMed] [Google Scholar]
- 83.Theise ND, Yao JL, Harada K, Hytiroglou P, Portmann B, Thung SN, Tsui W, Ohta H, Nakanuma Y. Hepatic 'stem cell' malignancies in adults: four cases. Histopathology. 2003;43:263–271. doi: 10.1046/j.1365-2559.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- 84.Moeini A, Sia D, Zhang Z, Camprecios G, Stueck A, Dong H, Montal R, Torrens L, Martinez-Quetglas I, Fiel MI, Hao K, Villanueva A, Thung SN, Schwartz ME, Llovet JM. Mixed hepatocellular cholangiocarcinoma tumors: Cholangiolocellular carcinoma is a distinct molecular entity. J Hepatol. 2017;66:952–961. doi: 10.1016/j.jhep.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 85.Coulouarn C, Cavard C, Rubbia-Brandt L, Audebourg A, Dumont F, Jacques S, Just PA, Clément B, Gilgenkrantz H, Perret C, Terris B. Combined hepatocellular-cholangiocarcinomas exhibit progenitor features and activation of Wnt and TGFβ signaling pathways. Carcinogenesis. 2012;33:1791–1796. doi: 10.1093/carcin/bgs208. [DOI] [PubMed] [Google Scholar]
- 86.Amin MB, Edge S, Greene F, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. Chicago: Springer International Publishing: American Joint Commission on Cancer; 2017. AJCC Cancer Staging Manual. 8th ed; pp. 295–302. [Google Scholar]
- 87.El-Abd N, Fawzy A, Elbaz T, Hamdy S. Evaluation of annexin A2 and as potential biomarkers for hepatocellular carcinoma. Tumour Biol. 2016;37:211–216. doi: 10.1007/s13277-015-3524-x. [DOI] [PubMed] [Google Scholar]
- 88.Tsuchiya N, Sawada Y, Endo I, Saito K, Uemura Y, Nakatsura T. Biomarkers for the early diagnosis of hepatocellular carcinoma. World J Gastroenterol. 2015;21:10573–10583. doi: 10.3748/wjg.v21.i37.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tanabe K, Kitagawa K, Kojima N, Iijima S. Multifucosylated Alpha-1-acid Glycoprotein as a Novel Marker for Hepatocellular Carcinoma. J Proteome Res. 2016;15:2935–2944. doi: 10.1021/acs.jproteome.5b01145. [DOI] [PubMed] [Google Scholar]
- 90.European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 91.Su C. Survivin in survival of hepatocellular carcinoma. Cancer Lett. 2016;379:184–190. doi: 10.1016/j.canlet.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 92.Abdel-Wahab R, Hassan M, Wolff RA, Lacin S, Al-Shamsi HO, Raghav KPS, Shalaby AS, Yao JC, Kaseb AO. Association of elevated alpha-1 antitrypsin with advanced clinicopathologic features of hepatocellular carcinoma. J Clin Oncol. 2017;35:289–289. [Google Scholar]
- 93.Bidkhori G, Benfeitas R, Klevstig M, Zhang C, Nielsen J, Uhlen M, Boren J, Mardinoglu A. Metabolic network-based stratification of hepatocellular carcinoma reveals three distinct tumor subtypes. Proc Natl Acad Sci U S A. 2018;115:E11874–E11883. doi: 10.1073/pnas.1807305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Shimada S, Mogushi K, Akiyama Y, Furuyama T, Watanabe S, Ogura T, Ogawa K, Ono H, Mitsunori Y, Ban D, Kudo A, Arii S, Tanabe M, Wands JR, Tanaka S. Comprehensive molecular and immunological characterization of hepatocellular carcinoma. EBioMedicine. 2019;40:457–470. doi: 10.1016/j.ebiom.2018.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]