Abstract
Background:
Neuroimaging studies have indicated that prenatal alcohol exposure is associated with alterations in the structure of specific brain regions in children. However, the temporal and regional specificity of such changes and their behavioural consequences are less known. Here we explore the integrity of regional white matter microstructure in infants with in utero exposure to alcohol, shortly after birth.
Methods:
Twenty-eight alcohol-exposed and 28 healthy unexposed infants were imaged using diffusion tensor imaging sequences to evaluate white matter integrity using validated tract-based spatial statistics analysis methods. Second, diffusion values were extracted for group comparisons by regions of interest. Differences in fractional anisotropy (FA), mean diffusivity (MD), axial diffusivity ( AD) and radial diffusivity were compared between groups and associations with measures from the Dubowitz neonatal neurobehavioural assessment were examined.
Results:
Lower AD values (p < 0.05) were observed in alcohol-exposed infants in the right superior longitudinal fasciculus compared with non-exposed infants. Altered FA and MD values in alcohol-exposed neonates in the right inferior cerebellar were associated with abnormal neonatal neurobehaviour.
Conclusion:
These exploratory data suggest that prenatal alcohol exposure is associated with reduced white matter microstructural integrity even early in the neonatal period. The association with clinical measures reinforces the likely clinical significance of this finding. The location of the findings is remarkably consistent with previously reported studies of white matter structural deficits in older children with a diagnosis of foetal alcohol spectrum disorders.
Keywords: DTI, dubowitz, foetal alcohol spectrum disorders, infant, MRI
Introduction
The physical and neurodevelopmental effects of prenatal alcohol exposure have been formally documented in the medical literature since the seminal reports published by Jones et al. (1,2) 40 years ago. The current literature most commonly uses the term foetal alcohol spectrum disorders (FASD) to incorporate a range of conditions relating to maternal alcohol abuse, including a defined range of effects of prenatal alcohol exposure in individuals not meeting full criteria for foetal alcohol syndrome (FAS) (3). Central nervous system (CNS) development and maturation require a carefully patterned sequence of events. These developmental processes are more complex and extend over a longer period than those of any other organ system. Thus, the CNS is particularly vulnerable to prenatal environmental influences (4,5). Animal work and human imaging studies have demonstrated that in utero exposure to alcohol alters brain morphology and neurocircuitry across multiple brain regions (6,7).
White matter microstructure can be measured in vivo with diffusion tensor imaging (DTI) and can estimate the overall directional diffusion of water molecules along fibre pathways (8,9). Analysis of this data allows the degree of microstructural integrity and organisation of areas within the brain tissue to be determined. Traditional scalar metrics derived from DTI data include fractional anisotropy (FA), which quantifies the overall directionality of diffusion, and may represent variations in axon integrity and/or packing density. In contrast, mean diffusivity (MD), which provides a measure of average diffusivity, may reflect myelin degradation, decreased cellular density or increased extra- or intracellular volumes. High FA and low MD values are typically associated with healthier neural microstructure and improved behavioural function, whereas low FA and high MD values may indicate white matter pathology (10,11). Radial diffusivity (RD) specifically reflects perpendicular diffusion towards membranes; RD is higher when myelination is reduced or there is myelin damage. Axial diffusivity (AD) specifically measures diffusion along axons. This is believed to be increased when neurofilaments are damaged (12) or decreased when axonal damage is present (13,14). However, it is also relevant to note that during brain maturation in healthy children and adolescents, axonal pruning and other biological processes may also lead to reduced FA (15,16).
A recent review of DTI studies in older children and young adults exposed to alcohol prenatally reported white matter microstructural abnormalities (lower FA) in the corpus callosum, anterior-posterior fibre bundles and the cerebellum (7,17–22). These abnormalities have also been reported in frontal (23,24) and temporal lobe regions (24,25), and within subcortical structures (globus pallidus, thalamus and putamen) (24,26). Relatively few studies have addressed associations between white matter microstructural abnormalities and specific measures of cognitive and behavioural function (25,27). Although negative findings have been reported for several functional domains, Sowell et al. (25) showed associations between reduced performance on a measure of visuomotor integration and reduced FA in the splenium of the corpus callosum and parietal white matter. Lebel et al. reported significant associations between reduced FA in the left parietal lobe, cerebellum and brainstem with mathematical ability in 5–13-year-old children (27). Correlations between white matter microstructural integrity and eye-blink conditioning have been reported by Fan et al. (28) and oculomotor control by Green et al. (29) in a group of school-age children with prenatal alcohol exposure. These results suggest the clinical significance of the DTI findings in these brain regions, at least in children of school-going age. To date, very little data exists regarding the impact of prenatal alcohol exposure in early infancy before higher-level brain networks have become established or the confounding postnatal environmental influences to which children from these backgrounds are exposed have come into play. Taylor et al. (30) have reported altered AD in 11 alcohol-exposed neonates in six major network areas, including the association networks (superior longitudinal fasciculus, inferior longitudinal fasciculus and uncinate fasciculus). Beyond this recent report, there remains few other data on white matter microstructure at this early period or an association with functional measures such as early neurobehaviour.
To address gaps in the current literature concerning the presence, timing and regional specificity of altered white matter structural integrity in association with prenatal alcohol exposure, we conducted this study in South Africa, where estimates of prevalence for FAS are extremely high in certain communities. That is, globally quoted prevalence is between 2 and 7/1000 for FAS and between 20 and 50/1000 for FASD (31). In contrast, though no national data are available, in South Africa prevalence has been reported to be as high as 63/1000 for FAS and 155/1000 for FASD in a periurban Western Cape community (32). Using a cohort from this community, the current study thus investigated whether the impact of prenatal alcohol exposure on early white matter microstructural integrity may be discernible in neonates and whether associations with neurobehavioural and physical measures of neonatal neurological health such as head circumference are present. Based on the literature in children and adolescents with FASD, we predicted that disruptions in white matter integrity would occur in association pathways connecting frontal and temporal regions and in the cerebellum (33).
Methods
Study design, population and procedures
The current investigation is a nested sub-study that included infants enroled in a larger population-based birth cohort study, the Drakenstein Child Lung Health Study (DCLS). This larger study was located in the Drakenstein region of the Western Cape, South Africa, in a low- to middle-income community of ~200 000 people in which there is limited migration. This area has a well-established, free primary health care service. Approximately 90% of women in this area seek public sector antenatal care and child health services.
The umbrella study enroled more than 1000 pregnant women, followed them through childbirth until children reached 2 years of age. In this nested sub-study, 116 infants were assessed; 54 infants with significant alcohol exposure and 62 infants with no significant history or biological evidence of substance abuse.
Mothers were recruited at 20–24 weeks gestation, written informed consent obtained, and background data collected for the umbrella study as described by Stein et al. (34). For the group with alcohol exposure, mothers were screened based on a minimum score of 11 (indicating that a participant is at moderate to high risk of experiencing severe problems as a result of their current pattern of use) on the alcohol questions on the ASSIST questionnaire – a widely validated World Health Organization (WHO) scale to assess comorbid substance use (35,36). In addition to this initial screen, mothers were required to give a positive history of alcohol use in any of the three trimesters of pregnancy at levels consistent with WHO moderate–severe alcohol use (either drinking two or more times a week or two or more drinks per occasion; Table 2). After birth, infants from mothers identified through this approach were included for study unless the mothers also had a positive urine screen for other drugs of abuse (any group) (37), the infants were premature (<36 weeks) or had low apgar scores (<7 at 5 min) and/or history of neonatal ICU admission for hypoxic ischaemic encephalopathy or other significant neonatal complication (such as neonatal jaundice requiring phototherapy). Infants were also excluded if they had an identified genetic syndrome or congenital abnormalities.
Table 2.
Alcohol use of mothers by trimester
| Trimester 1 | Trimester 2 | Trimester 3 | |
|---|---|---|---|
| Alcohol usage (n, %) | 24 (86) | 12 (43) | 9 (32) |
| Once per week or less | 18 | 7 | 3 |
| 2 to 3 times per week | 5 | 5 | 6 |
| 4 to 5 times per week | 1 | 0 | 0 |
| Daily | 0 | 0 | 0 |
| Number of drinks per occasion | |||
| <2 | 2 | 1 | 0 |
| 2 to 3 | 6 | 3 | 1 |
| 4 or more | 16 | 8 | 8 |
Two- to four-week-old infants underwent brain imaging, wrapped and fed, in quiet natural (unsedated) sleep. Earplugs and mini-muffs were used for double ear protection; a pulse oximeter was used to monitor pulse and oxygenation, and a qualified neonatal nurse or paediatrician was present with the infant in the scanner room for the duration of the imaging session. At the time of scanning, basic anthropometry was acquired including weight, occipito-frontal head circumference and length. The Dubowitz neurobehavioural scale, a well-validated measure of neonatal neuromotor and neurobehavioural status, was used to study early neurological and behavioural state. This tool includes an optimality score allowing it to be used for quantitative analysis of potential associations with neuroimaging findings. The score is based on the distribution of the scores for each item in a population of low-risk term infants. The total optimality score is the sum of the optimality scores of individual items. However, for this study, specific item clusters were chosen as being of particular interest in this population. As defined by the Dubowitz scale authors, the ‘behaviour’ cluster includes items scoring irritability, cry, consolability, alertness, visual and auditory orientation and eye movements. The ‘abnormal signs’ cluster has focus on posture, tremor and startle items (38,39).
Ethical approval for human subjects research was obtained from the Research Ethics Committee of the Faculty of Health Sciences of University of Cape Town (HREC UCT REF 401/009) for the DCLS. This sub-study protocol was independently reviewed and approved by the same institutional ethics committee (HREC UCT REF 525/2012).
DTI acquisition
Diffusion weighted images were acquired on a Siemens Magnetom 3T Allegra MRI system (Allegra MR2004A, Germany) with an RF transmit/receive head coil using a spin-echo, echo-planar sequence and including 45 non-collinear directions. To overcome limitations with scanning smaller volumes of tissue, voltage was reduced to optimise signal and the head coil was loaded with a wet clay inlay (40×40 cm with a thickness of 2 cm, standard sculpting clay commercially bought – white stoneware clay with grog). Images were obtained in the transverse plane with both anterior–posterior and posterior–anterior phase encoding to control for anatomic distortions and increase signal-to-noise. Parameters were as follows: repetition time (TR) 7900 ms; echo time (TE) 90 ms; slice thickness 1.6 mm; held of view 160 mm; voxel size 1.3 × 1.3 × 1.6 mm3; b-values 0 and 1000 s/mm2. Scan time per phase encoding acquisition was 6:27 min with a total time of 12 : 54 min for both acquisitions.
Data processing
Data were analysed using a whole-brain approach followed by selected regions of interest. First, the FMRIB’s Diffusion Toolbox and Tract-Based Spatial Statistics (TBSS) processing streams (FSL vs. 5.0) (40) were used to investigate diffusion of the whole brain, selecting a representative anatomical template from the sample as is described below. Second, diffusion values were extracted after preprocessing with FSL and data exported to Statistica 12 (StatSoft Inc.) for group comparisons by regions of interest that include white matter tracts that connect temporal and cerebellar regions with cortical areas. Analyses were controlled for gender and age, due to the rapidly evolving white matter maturation that occurs early in infant life (16,41).
Preprocessing using FSL
Acquiring images in such small infants poses technical and logistical challenges. To ensure good quality data, strict criteria (images with at least 12 acquisition volumes without artifact) were deemed as the cut-off for inclusion for data pre-processing and statistical analyses. The Diffusion Toolbox was applied following manual quality control of data from each subject. Each subject’s diffusion weighted image was registered to the corresponding b = 0 image to correct for motion and eddy current distortion. Three b0 images were acquired. The susceptibility-induced off-resonance held was estimated for the pair of images using the FSL top-up tool after which the two images were combined into a single corrected image (42). Images were then brain-extracted with the Brain Extraction Tool and diffusion tensors were calculated at each voxel with a weighted least squares fit of the tensor model to the diffusion data. FA, MD, AD and RD images were subsequently attained for each subject.
Whole-brain TBSS
The standard TBSS pipeline was applied (40). However, the FMRIB adult FA template is not appropriate for neonatal DTI analysis. Standard practice in FSL allows the user to identify an anatomical target after preprocessing that included stringent eddy correction and outlier rejection. Thus, every subject was registered to a representative target that was pre-selected from the control cohort. Each subject was registered to every other subject to find the most representative target, that is, the target with the lowest mean warp coefficient.
Individual FA images were aligned into the target image space and upsampled to 1 × 1 × 1 mm3 voxel size taking into account previous estimated transformations. An average FA map was created and thinned to generate a mean FA skeleton. This skeleton represents the centre of all white matter tracts common to the study group. The skeleton was thresholded at an FA value of 0.2. As this study was explorative, we opted for a more stringent threshold compared with that of some previous infant studies that used a threshold of 0.15. FA and MD data were projected onto this skeleton before statistical analysis.
Variations in DTI metrics were examined voxelwise using FSL’s Randomise tool. Specifically, t-tests and correlational analyses (5000 permutations per test) were used to investigate group differences. Analyses were corrected for multiple comparisons using threshold-free cluster enhancement.
Analysis by regions of interest
Group main effects were investigated using extracted diffusion data by region of interest after FSL preprocessing. Individual brains were registered to the standard FMRIB58_FA template using affine registration. Mean diffusion values were then extracted by subject for regions of interest using the Johns Hopkins University white-matter atlas (43). Regions of interest were major white matter tracts that connect temporal and cerebellar regions to the cortex. These included association fibres (superior longitudinal fasciculus, superior fronto-ocipital fasciculus and uncinate fasciculus), tracts of the brain stem and cerebellum (cerebellar peduncles, corticospinal tract and cerebral peduncle), projection fibres (posterior thalamic radiation, fomix and cingulum) and commissural fibres (corpus callosum). Separate general linear models were used with infant age (in days) and gender as covariates. Results were Bonferroni corrected. Partial correlational analysis was used to investigate associations between diffusion parameters and behaviour, controlled for age and gender. Raw scores on the Dubowitz behaviour and abnormal signs subscales, were converted to optimality scores as described by Dubowitz et al. (39) for comparison. Tests were two-tailed and considered significant at p < 0.05.
Results
From the cohort of infants enroled in the study, 13 scans from the initial alcohol-exposed group (n = 54) and seven scans from the control group (n = 62) were excluded due to movement or other artifacts and DTI imaging data was not acquired (infants awoke during the sequence so it could not be completed) for an additional 13 alcohol-exposed infants and 27 controls. Thus, the final sample for the present analysis included 28 alcohol-exposed infants and 28 healthy infants (Table 1). There were no significant differences in the mean values for gestational age, postnatal age at scanning, weight, length and head circumference for alcohol-exposed infants compared with controls. Although maternal smoking in pregnancy was prevalent in this cohort, there was no difference in this exposure between alcohol-exposed and control infants. There was a significant difference between the scores on the behaviour subscale of the Dubowitz for the infants exposed to alcohol prenatally compared with control infants [t = 2.13, p = 0.04]. Alcohol exposure was clustered to the first trimester, with 86% of mothers reporting alcohol use in this trimester. However, a third of mothers in the exposed group reported continued drinking through the second and third trimesters and those who continued to drink throughout demonstrated a pattern of heavier alcohol use (Table 2).
Table 1.
Demographic, anthropometric and Dubowitz data of infants
| Alcohol-exposed (n = 28) | Controls (n = 28) | Statistics | |
|---|---|---|---|
| Gestation (weeks, SD) | 38.36 (1.81) | 38.50 (1.80) | t = −0.30, p = 0.77 |
| Maternal smoking (yes/no) | 13/15 | 19/9 | −χ2 = 2.63, p = 0.11 |
| Age (days, SD) | 20.71 (5.29) | 19.96 (4.92) | t = 0.55, p = 0.58 |
| Sex (male/female) | 13/15 | 19/9 | χ2 = 2.63, p = 0.11 |
| Weight (kg, SD) | 3.69 (0.56) | 3.99 (0.71) | t = −1.77, p = 0.08 |
| Head circumference (cm, SD) | 35.63 (1.28) | 36.18 (1.75) | t = −1.34, p = 0.18 |
| Length (cm, SD) | 49.81 (3.40) | 50.56 (4.59) | t = −0.69, p = 0.49 |
| Dubowitz optimality scores [mean, (SD)] | |||
| Tone (/10) | 8.09 (1.83) | 8.32 (1.35) | t = 0.54, p = 0.59 |
| Reflex (/6) | 4.83 (0.46) | 4.79 (0.37) | t = 0.42, p = 0.67 |
| Spontaneous movement (/2) | 1.98 (0.09) | 2.00 (0.00) | t = 1.00, p = 0.32 |
| Behaviour (/7) | 4.39 (1.13) | 3.74 (1.14) | t = 2.13, p = 0.04* |
| Abnormal signs (/3) | 2.79 (0.42) | 2.61 (0.92) | t = 0.94, p = 0.35 |
p<0.05.
Whole-brain group comparison of diffusion parameters
There were no significant group differences in any diffusion parameter.
Head circumference was significantly positively correlated with FA, and significantly negatively correlated with MD, AD and RD across groups essentially in all white matter tracts.
Using general linear models by region of interest, there was evidence for a significant difference in diffusion by group in major white matter fibres that interconnect temporal, frontal and parietal regions. There was significantly lower AD in the right superior longitudinal fasciculus of alcohol-exposed infants compared with controls [F(3,52) = 3.46, p = 0.023] after correction for age and gender (Table 3, Fig. 1).
Table 3.
Results of group differences in diffusion parameters by regions of interest
| FA |
MD |
AD |
RD |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hemisphere | F | p | F | p | F | p | F | p | |
| Superior longitudinal fasciculus | R | 1.25 | 0.301 | 1.35 | 0.269 | 3.46 | 0.023† | 0.76 | 0.520 |
| L | 1.49 | 0.228 | 0.56 | 0.643 | 2.25 | 0.093 | 0.15 | 0.932 | |
| Superior fronto-occipital fasciculus | R | 0.60 | 0.615 | 0.66 | 0.581 | 0.95 | 0.424 | 0.41 | 0.748 |
| L | 0.05 | 0.987 | 0.27 | 0.844 | 0.27 | 0.847 | 0.26 | 0.856 | |
| Uncinate fasciculus | R | 2.20 | 0.099 | 0.13 | 0.943 | 0.35 | 0.786 | 0.25 | 0.863 |
| L | 1.61 | 0.197 | 1.26 | 0.296 | 1.35 | 0.268 | 1.21 | 0.317 | |
| Cerebellar peduncle | |||||||||
| Inferior | R | 1.26 | 0.299 | 2.94 | 0.042* | 3.31 | 0.027* | 2.74 | 0.053 |
| L | 1.11 | 0.352 | 0.85 | 0.473 | 0.12 | 0.945 | 1.11 | 0.353 | |
| Middle | 1.02 | 0.390 | 2.40 | 0.078 | 2.89 | 0.044* | 2.12 | 0.109 | |
| Superior | R | 1.28 | 0.292 | 0.38 | 0.770 | 0.90 | 0.449 | 0.41 | 0.746 |
| L | 2.57 | 0.064 | 1.17 | 0.330 | 2.51 | 0.068 | 0.31 | 0.820 | |
| Corticospinal tract | R | 2.43 | 0.076 | 3.05 | 0.037* | 1.98 | 0.129 | 3.56 | 0.020* |
| L | 0.70 | 0.557 | 0.36 | 0.786 | 0.63 | 0.600 | 0.37 | 0.772 | |
| Cerebral peduncle | R | 0.60 | 0.615 | 0.93 | 0.431 | 1.46 | 0.237 | 0.73 | 0.539 |
| L | 0.38 | 0.769 | 0.73 | 0.539 | 1.23 | 0.309 | 0.56 | 0.647 | |
| Posterior thalamic radiation | R | 0.84 | 0.478 | 0.63 | 0.599 | 1.00 | 0.402 | 0.48 | 0.699 |
| L | 1.31 | 0.282 | 2.32 | 0.086 | 2.30 | 0.088 | 2.19 | 0.100 | |
| Fornix | 3.32 | 0.027* | 2.02 | 0.122 | 1.82 | 0.154 | 2.21 | 0.097 | |
| Cingulum | R | 0.49 | 0.689 | 0.88 | 0.458 | 1.23 | 0.310 | 0.69 | 0.562 |
| L | 0.05 | 0.985 | 0.95 | 0.423 | 1.25 | 0.301 | 0.80 | 0.497 | |
| Corpus callosum | |||||||||
| Genu | 1.32 | 0.278 | 1.29 | 0.289 | 1.34 | 0.273 | 1.25 | 0.302 | |
| Body | 0.83 | 0.482 | 2.05 | 0.119 | 2.01 | 0.125 | 2.01 | 0.125 | |
| Splenium | 0.78 | 0.511 | 1.79 | 0.160 | 1.98 | 0.129 | 1.61 | 0.198 | |
AD, axial diffusivity; FA, fractional anisotropy; MD, mean diffusivity; RD, radial diffusivity.
Axial diffusivity was significantly lower in the right superior longitudinal fasciculus of alcohol-exposed infants compared to controls.
Post-hoc investigation of univariate tests of significance, revealed that age contributed significantly to the model.
Significant group difference at p< 0.05 after controlling for age and gender.
Fig. 1.
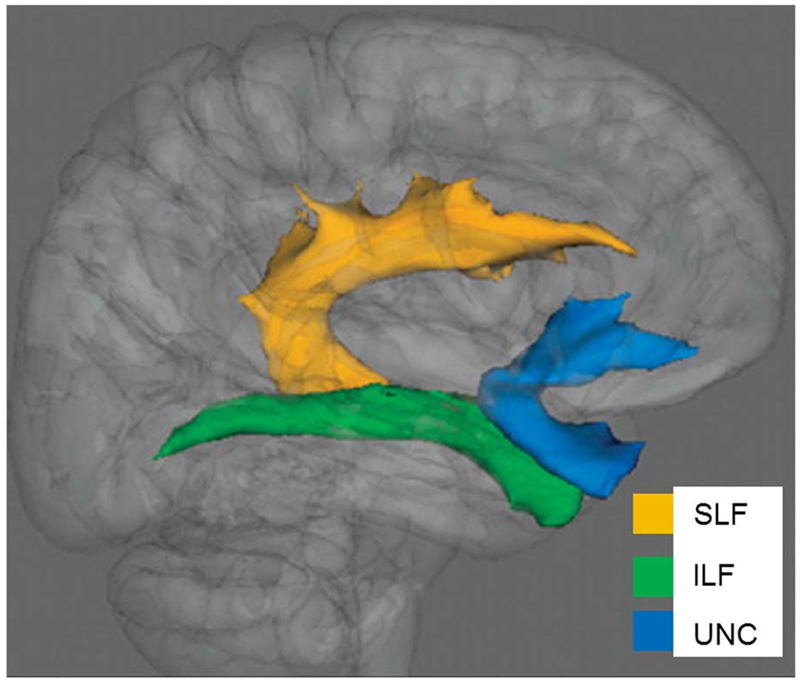
Association tracts superimposed on a 3D brain template. Yellow (SLF): superior longitudinal fasciculus; green (ILF): inferior longitudinal fasciculus; blue (UNC): uncinate fasciculus. Axial diffusivity was significantly lower in the right superior longitudinal fasciculus of alcohol-exposed infants compared with controls.
Behavioural measures scores on the behavioural subscale were positively correlated with FA (r = 0.42, p = 0.033) and negatively correlated with MD (r = −46, p = 0.018) in the right inferior cerebellar peduncle of alcohol-exposed infants.
Discussion
Here, we report the effects of prenatal alcohol exposure on the white matter integrity of exposed infants as compared with a well-matched unexposed control group. Prenatal alcohol-exposed infants were found to have lower AD in the superior longitudinal fasciculus compared with controls. Lower FA and higher MD in the inferior cerebellar peduncle were both correlated significantly with neonatal neurobehavioural scores in infants with prenatal alcohol exposure.
White matter microstructural abnormalities have been described in the anterior–posterior association pathways in prenatal alcohol-exposed children (33), specifically in the superior longitudinal fasciculus (22) and more recently even in the neonatal age group in these areas (30). Our findings in a larger, independent group of infants exposed to alcohol during prenatal life, replicating the report of reduced AD in the superior longitudinal fasciculus, reinforces the significance of these findings. The superior longitudinal fasciculus is one of the major fibre bundles linking frontal, temporal and parietal association cortices. Functions of these networks are believed to include associative tasks, higher level motor tasks, visual perception and attention. While these are not functions that can be teased out in the neonatal age group, they are functional areas, which have been identified as being affected in children with prenatal alcohol exposure (44,45). Following up these infants with developmental and behavioural assessments as they mature may help further define the functional significance of the abnormal white matter microstructure in these tracts.
Cerebellar structure has been found to be affected by prenatal alcohol exposure in children even taking into account differences in overall brain volumes (7,46). In addition, O’Hare et al. (47) demonstrated impaired verbal learning as a cognitive correlate of abnormal cerebellar vermis morphology. Further, Fan et al., (28) have reported lower FA and higher MD associated with eye-blink conditioning and Green et al., (29) a correlation of FA with oculomotor control in a group of school-age children with prenatal alcohol exposure. In our alcohol-exposed infants, the presence of an association between infant neurobehaviour scores with bilateral reduced FA and higher MD in the cerebellar peduncles, which link the cerebellum to the important cerebral motor and language networks, reinforces the role of these pathways in the primary effects of alcohol exposure on early brain development.
There was a significant association between reduced FA and smaller head size in these infants across both groups. This was a robust and widespread finding across multiple white matter areas and suggests that infants who have poorer overall brain growth in the antenatal and immediate postnatal period demonstrate poorer white matter organisation. Although there was a reduced mean occipito-frontal circumference in the alcohol-exposed group compared with the unexposed controls, this finding did not reach statistical significance. This is not entirely surprising as the infants in the alcohol-exposed group have not been categorised by anything other than their exposure status and many of them may represent milder forms of the FASD spectrum. Follow-up of this group of prenatal alcohol-exposed children will be critical. Clinical outcomes may well vary in later childhood where other factors such as social, nutritional and other environmental factors may have impact.
The rate of brain development and in particular the rapidly maturing white matter tracts in the 1st weeks and months of life, as well as the paucity of imaging literature in this age group mean that the dynamics of exactly how DTI metrics relate to tissue microstructural integrity and organisation is still incompletely understood. What is known is that the rate of white matter maturation is most rapid in the first 3 months of life (as compared with any other period) and that deep white matter structures (such as those we have reported here), already achieve approximately one half of the mean adult FA at this age. However, peripheral white matter remains barely discernible at the thresholds of detection (8,48). In this context, one has to assume that abnormal results especially in the large midline tracts should be considered as potentially relevant.
Limitations of the current study relate to the reduction of sample size due to movement artefact, this also led to an unbalanced ratio of female to male infant data used in the current analyses. In addition, although information was collected on the timing and severity of alcohol exposure, the number of infants for whom we acquired usable DTI data was insufficient to make meaningful comparisons between these exposure categories in order to help define particular windows of vulnerability to the effects of alcohol during the prenatal period. It is important to note that analyses were conducted in infants before any formal diagnosis could be made and thus effect sizes are expected to be smaller. Further, given the strong influence of age on DTI parameters (reflecting the rapid changes in myelination and maturation), although this was controlled for in the analysis, may have masked group differences resulting from prenatal alcohol exposure. The paucity of data in this age cohort is an important knowledge gap and it remains highly relevant to provide observations of differences in white matter integrity to inform future studies and aid with the interpretation of the available data in older children. These data, representing congruent trends in an age-group, which has not been previously described, is a meaningful addition to the literature in this important area.
In conclusion, the results reported here indicate that the neurobiological effects of prenatal alcohol exposure are observable in newborns, with reduced white matter integrity in major midline white matter tracts. The location of the findings is consistent with previously reported studies of white matter tracts in older children with FASD. Future work with larger cohorts may be able to identify specific windows of vulnerability to the effects of alcohol on the developing brain. In addition, the presence of findings such as these in such a young cohort presents the possibility of exploring the effectiveness of early therapeutic approaches using non-invasive neuroimaging techniques.
Significant outcomes.
White matter microstructural integrity altered in newborns exposed to alcohol during antenatal period.
Main effects were seen in central fibre tracts, particularly the superior longitudinal fasciculus.
These data, represent congruent trends in an age-group about which very little is currently known.
Limitations.
The size of the sample limited the ability to make meaningful comparisons between exposure severity and timing categories in order to help define particular windows of vulnerability to the effects of alcohol during the prenatal period.
Acknowledgements
The authors thank the study staff and the staff at Paarl Hospital, Mbekweni and TC Newman clinics for their support of the study. They thank the families and children who participated in this study. Authors’ Contributions: K.D. designed and ran the study, directed analysis, interpreted the results and wrote the manuscript. A.R. and J.F. were involved in the analysis and provided critical review of the manuscript. K.L., H.Z., D.S., N.K., F.H., R.W. all participated in study design and in critical review of the manuscript.
Financial Support
The study was funded by the Bill and Melinda Gates Foundation [OPP 1017641] and an ABMRF start-up grant. Support was also received for this research from the South African MRC, NRF, SAMA and Harry Crossley Foundation.
Footnotes
Conflicts of Interest
None.
References
- 1.Jones KL, Smith DW, Ulleland CN, Streissguth AP. Pattern of malformation in offspring of chronic alcoholic mothers. Lancet 1973;1:1267–1271. [DOI] [PubMed] [Google Scholar]
- 2.Jones KL, Smith DW, Hansen JW. The fetal alcohol syndrome: clinical delineation. Ann N Y Acad Sci 1976;273:130–139. [DOI] [PubMed] [Google Scholar]
- 3.Riley EP, Alejandra Infante M, Warren K Fetal alcohol spectrum disorders: an overview. Neuropsychol Rev 2011;21:73–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodier PM. Vulnerable periods and processes during central nervous system development. Environ Health Perspective 1994;102(Suppl. 2):121–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodier PM. Environmental causes of central nervous system maldevelopment. Pediatrics 2004;113:1076–1083. [PubMed] [Google Scholar]
- 6.O’Leary-Moore S, Parnell S, Lipinski R Magnetic resonance-based imaging in animal models of fetal alcohol spectrum disorder. Neuropsychol Rev 2011;21:167–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lebel C, Roussotte F, Sowell ER . Imaging the impact of prenatal alcohol exposure on the structure of the developing human brain. Neuropsychol Rev 2011;21:102–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pannek K, Guzzetta A, Colditz P, Rose S. Diffusion MRI of the neonate brain: acquisition, processing and analysis techniques. Pediatr Radiol 2012;42:1169–1182. [DOI] [PubMed] [Google Scholar]
- 9.Beaulieu C The basis of anisotropic water diffusion in the nervous system – a technical review. NMR Biomed 2002;15:435–455. [DOI] [PubMed] [Google Scholar]
- 10.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics 2007;4:316–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci 2008;34:51–61. [DOI] [PubMed] [Google Scholar]
- 12.Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 2002;17:1429–1436. [DOI] [PubMed] [Google Scholar]
- 13.Kinoshita Y, Ohnishi A, Kohshi K, Yokota A. Apparent diffusion coefficient on rat brain and nerves intoxicated with methylmercury. Environ Res 1999;80:348–354. [DOI] [PubMed] [Google Scholar]
- 14.Harsan LA, Poulet P, Guignard B et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res 2006;83:392–402. [DOI] [PubMed] [Google Scholar]
- 15.Paus T, Pesaresi M, French L. White matter as a transport system. Neuroscience 2014;276:117–125. [DOI] [PubMed] [Google Scholar]
- 16.Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L . The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 2014;276:48–71. [DOI] [PubMed] [Google Scholar]
- 17.Wozniak JR, Muetzel RL. What does diffusion tensor imaging reveal about the brain and cognition in fetal alcohol spectrum disorders. Neuropsychol Rev 2011;21:133–147. [DOI] [PubMed] [Google Scholar]
- 18.Wozniak JR, Mueller BA, Chang PN, Muetzel RL, Caros L, Lim KO. Diffusion tensor imaging in children with fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2006;30:1799–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wozniak JR, Muetzel RL, Mueller BA et al. Microstructural corpus callosum anomalies in children with prenatal alcohol exposure: an extension of previous diffusion tensor imaging findings. Alcohol Clin Exp Res 2009;33:1825–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma X, Coles CD, Lynch ME et al. Evaluation of corpus callosum anisotropy in young adults with fetal alcohol syndrome according to diffusion tensor imaging. Alcohol Clin Exp Res 2005;29:1214–1222. [DOI] [PubMed] [Google Scholar]
- 21.Li L, Coles CD, Lynch ME, Hu X. Voxelwise and skeleton-based region of interest analysis of fetal alcohol syndrome and fetal alcohol spectrum disorders in young adults. Hum Brain Mapp 2009;30:3265–3274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Treit S, Lebel C, Baugh L, Rasmussen C, Andrew G, Beaulieu C . Longitudinal MRI reveals altered trajectory of brain development during childhood and adolescence in fetal alcohol spectrum disorders. J Neurosci 2013;33:10098–10109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fryer SL, Schweinsburg BC, Bjorkquist OA, Frank LR, Mattson SN, Spadoni AD, Riley EP. Characterisation of white matter microstructure in fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2009;33:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lebel C, Rasmussen C, Wyper K, Walker L, Andrew G, Yager J . Brain diffusion abnormalities in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 2008;32:1732–1740. [DOI] [PubMed] [Google Scholar]
- 25.Sowell ER, Johnson A, Kan E et al. Mapping white matter integrity and neurobehavioral correlates in children with fetal alcohol spectrum disorders. J Neurosci 2008;28: 1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lebel C, Mattson SN, Riley EP et al. A longitudinal study of the long-term consequences of drinking during pregnancy: heavy in utero alcohol exposure disrupts the normal processes of brain development. J Neurosci 2012;32: 15243–15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebel C, Rasmussen C, Wyper K, Andrew G, Beaulieu C . Brain microstructure is related to math ability in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 2010;34:354–363. [DOI] [PubMed] [Google Scholar]
- 28.Fan J, Meintjes EM, Molteno CD et al. White matter integrity of the cerebellar peduncles as a mediator of effects of prenatal alcohol exposure on eyeblink conditioning. Hum Brain Mapp 2015; doi: 10.1002/hbm.22785 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green CR, Lebel C, Rasmussen C, Beaulieu C, Reynolds JN . Diffusion tensor imaging correlates of saccadic reaction time in children with fetal alcohol spectrum disorder. Alcohol Clin Exp Res 2013;37:1499–1507. [DOI] [PubMed] [Google Scholar]
- 30.Taylor PA, Jacobson SW, van der Kouwe A et al. A DTI-based tractography study of effects on brain structure associated with prenatal alcohol exposure in newborns. Hum Brain Mapp 2015;36:170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.May PA, Gossage JP, Kalberg K, Robinson L, Buckley D, Manning M. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev Disabil Res Rev 2009;15: 176–192. [DOI] [PubMed] [Google Scholar]
- 32.May PA, Blankenship J, Marais A et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a south african population-based study. Alcohol Clin Exp Res 2013;37:818–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Donald KA, Eastman E, Howells FM et al. Neuroimaging effects of prenatal alcohol-exposure on the developing human brain: a magnetic resonance imaging review. Acta Neuropsychiatrica 2015;1–19 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 34.Stein DJ, Koen N, Donald KA et al. Investigating the psychosocial determinants of child health in Africa: The Drakenstein Child Health Study. J Neurosci Methods 2015; pii: S0165-0270(15)00099-0. doi: 10.1016/j.jneumeth.2015.03.016 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Humeniuk R, Ali R, Babor TF et al. Validation of the alcohol, smoking and substance involvement screening test (ASSIST). Addiction 2008;103:1039–1047. [DOI] [PubMed] [Google Scholar]
- 36.Jackson PB, Williams DR, Stein DJ, Herman A, Williams SL, Redmond DL. Race and psychological distress: The South African Stress and Health Study. J Health Soc Behav 2010;51:458–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lozarno J, Garcia-Algar O, Vall O, De LA Torre R, Scaravelli G, Pichini S. Biological matrices for the evaluation of in utero exposure to drugs of abuse. Ther Drug Monit 2007;29:711–734. [DOI] [PubMed] [Google Scholar]
- 38.Noble Y, Boyd R. Neonatal assessments for the preterm infant up to 4 months corrected age: a systematic review. Dev Med Child Neurol 2012;54:129–139. [DOI] [PubMed] [Google Scholar]
- 39.Dubowitz L, Mercurio E, Dubowitz V. An optimality score for the neurological examination of the term newborn. J Pediatr 1998;133:406–416. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM, Jenkinson M, Johansen-Berg H et al. Tract-based spatial statistics: voxel-wise analysis of multi-subject diffusion data. Neuroimage 2006;31:1487–1505. [DOI] [PubMed] [Google Scholar]
- 41.Dubois J, Hertz-Pannier L, Dehaene-Lambertz G, Cointepas Y, Le Bihan D. Assessment of the early organization and maturation of infants’ cerebral white matter fiber bundles: a feasibility study using quantitative diffusion tensor imaging and tractography. Neuroimage 2006;30:1121–1132. [DOI] [PubMed] [Google Scholar]
- 42.Andersson JLR, Jenkinson M, Smith S. Non-linear registration, aka spatial normalisation FMRIB technical report TR07JA2. www.fmrib.ox.ac.uk/analysis/techrep 2007, accessed 20 December 2014.
- 43.Mori S, Wakana S, van Zijl PCM, Nagae-Poetscher LM. MRI atlas of human white matter. Amsterdam: Elsevier, 2005. [Google Scholar]
- 44.Mattson S, Crocker N, Nguyen T . Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychol Rev 2011;21:81–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mattson SN, Roesch SC, Fagerlund Å et al. Toward a neurobehavioral profile of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 2010;34:1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mattson SN, Riley EP, Jernigan TL et al. A decrease in the size of the basal ganglia following prenatal alcohol exposure: a preliminary report. Neurotoxicol Teratol 1994;16:283–289. [DOI] [PubMed] [Google Scholar]
- 47.O’Hare ED, Kan E, Yoshii J et al. Mapping cerebellar vermal morphology and cognitive correlates in prenatal alcohol exposure. Neuroreport 2005;16:1285–1290. [DOI] [PubMed] [Google Scholar]
- 48.Provenzale JM, Liang L, DeLong D, White LE. Diffusion tensor imaging assessment of brain white matter maturation in the first postnatal year. Am J Radiol 2007;189:476–486. [DOI] [PubMed] [Google Scholar]


