Abstract
Canine mammary tumors (CMT) represent the most common cancer in noncastrated female dogs. Interestingly, triple-negative tumors are the most common molecular subtype in female dogs. In this study, we proposed to evaluate the expression of vascular endothelial growth factor receptor 2 (VEGFR-2), Platelet-derived growth factor receptor (PDGFR), and microvascular density (MVD) in a group of metastatic and nonmetastatic triple-negative CMT and compare the expression based on clinical parameters. Twenty-six female dogs with triple-negative mammary tumors were divided into three groups: nonmetastatic tumors (NMT) (n = 11), tumors with lymph node metastasis (LNM) (n = 10), and tumors with lung metastasis (LM) (n = 5). We observed increased VEGFR-2 expression in LNM compared with NMT and a positive correlation between tumor grade and VEGFR-2 expression. A positive correlation was noted between VEGFR-2 and PDGFR expression. Regarding microvascular density (MVD), we identified a higher number of vessels in primary tumors with lymph node metastasis and lung metastasis compared with tumors with no metastasis. The primary tumors with lung metastasis exhibited an increased MVD compared with carcinoma with lymph node metastasis. Overall, our results suggest a deregulation of VEGFR-2 and PDGFR and high MVD in metastatic tumors, indicating a role for angiogenesis in tumor progression.
Keywords: angiogenesis, metastasis, mammary neoplasm
1. Introduction
Canine mammary tumors (CMT) are the most common tumor in noncastrated female dogs with a variable clinical behavior [1]. The incidence rates for CMT depend on the geographic origin given that it is a tumor with higher prevalence in countries where castration is not routinely performed [2]. In Brazil, the prevalence of CMT in intact female dogs is approximately 28% to 45% of all tumors in dogs [3,4]. CMTs resemble human breast cancer (BC), and dogs represent an interesting model for comparative studies. The recent Global Cancer Observatory (GLOBOCAN) estimates of cancer presented an expectation of 2,093,876 new cases of BC worldwide and 626,679 deaths related to BC [5].
BC is the most important tumor in women as it is the most diagnosed cancer and the second most common cause of death related to cancer [6]. Human BC is subdivided into molecular subtypes, such as Human Epidermal growth factor Receptor-type 2 (Her-2) enriched, Luminal A, Luminal B, and basal-like [6]. Triple-negative tumors are very important as these tumors represent a therapeutic challenge, and limited therapeutic options are available compared with other subtypes [7]. Recently, a study evaluating a large number of cases subdivided CMT into molecular subtypes and found an increased prevalence of triple-negative tumors in dogs [8]. These results indicate that female dogs serve as a natural model for human BC.
In humans, vascular endothelial growth factor-A (VEGF-A) expression increases based on tumor grade. Thus, a tumor with a higher histological grade presents higher VEGF-A levels. Moreover, increased VEGF-A expression correlates with tumor metastasis, indicating the role of the VEGF pathway in human tumors. Vascular endothelial growth factor receptor 2 (VEGFR-2) is one of the principal mediators of VEGF-A activity [9]. High VEGF-A and VEGFR-2 levels are associated with the worst outcome in patients with BC. The authors of Reference [9] performed a computational analysis of the VEGF-A and VEGFR-2 immunofluorescence and found a cutoff point of 80.5 and 64.3, respectively. Thus, the VEGF-A/VEGFR-2 signaling pathway exhibits prognostic and predictive value in female BC [9]. VEGF expression was previously investigated in CMT. A correlation between VEGF expression and tumor angiogenesis was observed [10], and VEGF overexpression was also found to correlate with lymph node metastasis [11,12].
Platelet-derived growth factor receptor (PDGFR) and c-KIT expression is widely studied in human oncology, and both markers exhibit predictive value in human BC. Imatinib mesylate (Gleevec®) was previously evaluated in advanced/metastatic breast cancer expressing c-KIT or PDGFR [13,14]. However, both studies evaluated a low number of patients due to imatinib toxicity. Thus, it was concluded that imatinib mesylate as a monotherapy does not provide a clinical benefit for BC-affected patients and is associated with important side effects [13,14]. However, PDGFR and c-KIT are overexpressed in human BC and still represent important predictive markers. New studies evaluating other PDGFR/c-KIT inhibitors represent a new therapeutic perspective.
In dogs with mammary tumors, c-KIT exhibits a controversial role in tumorigenesis [15,16,17]. In general, c-KIT is expressed in normal mammary glands. During cancer progression, tumor cells lack c-KIT expression [15,16,17]. Regarding PDGFR, one previous study evaluated gene expression in CMT [17], and no previous study demonstrated PDGFR expression in CMT. Dogs represent, indeed, an important preclinical model for human cancers [18,19,20].
In humans and dogs, the development of metastasis is the major cause of cancer-related deaths [6,15]. The PDGFR and PDGF signaling pathway is responsible for intratumoral lymphogenesis, promoting nodal metastasis [21], and the VEGF/VEGFR pathway induces neovasculogenesis [22]. Microvascular density (MVD) is very important for tumor progression and is induced by the production of proangiogenic factors by tumor cells [23]. MVD in BC is correlated with overall survival and disease-free interval in both humans [23,24] and dogs [25]. Given the importance of dogs as a natural model for human BC, this research aimed to evaluate VEGF and PDGFR expression and assess MVD in metastatic and nonmetastatic CMT.
2. Material and Methods
2.1. Study Design
This was a prospective nonrandomized study including 26 female dogs from three institutions: The Veterinary Teaching Hospital of University of Franca (UNIFRAN), the Veterinary Teaching Hospital of São Paulo State University (UNESP), and the Veterinary Teaching Hospital of the Federal University of the Southern Border (UFFS). All procedures were performed in accordance with the national and international guidelines for use of animals in research (protocol number: CEUA 0208/2016). This study was approved by each institutional Ethics Committee for the Use of Animals. The tissue samples were collected between May 2015 and September 2017.
We exclusively included patients with malignant tumors meeting the following criteria: a sufficient amount of tissue in the primary tumor and metastatic foci for immunohistochemical evaluation, received no previous systemic treatment, at least one year of clinical follow-up, only one tumor in the mammary gland, and sentinel lymph node evaluation.
2.2. Patients
We included female dogs with only one mammary gland lesion, independent of the tumor size or location. All dogs underwent a previous cytological examination indicating a mammary gland tumor. The patients underwent sentinel lymph node assessment according to Beserra et al. [26]. Then, unilateral chain mastectomy was performed. The surgical specimens were stored in 10% buffered formalin for 24 h. Then, histological processing was performed. Briefly, 4-μm tissue sections were processed for hematoxylin and eosin staining. The histological classification was performed according to Goldschmidt et al. [27], and tumor grade was evaluated according to Karayannopoulou et al. [28].
2.3. Clinical Evaluation
All patients underwent three-view thoracic radiographic examination, abdominal ultrasound, and complete blood count. Then, we obtained the clinical stage from the staging system established by the World Health Organization for CMT and modified by Sorenmo et al. [2]. Patients were classified as stage I–V [2]. Patients with at least stage III disease and at least tumor grade II received adjuvant chemotherapy with four cycles of 300 mg/m2 carboplatin and 5 mg/kg of firocoxib every 24 h for six months according to the method of Bonolo et al. [29]. Clinical follow-up was performed every three months in the first year with three-view thoracic radiographic examinations and complete blood counts.
2.4. Molecular Phenotype
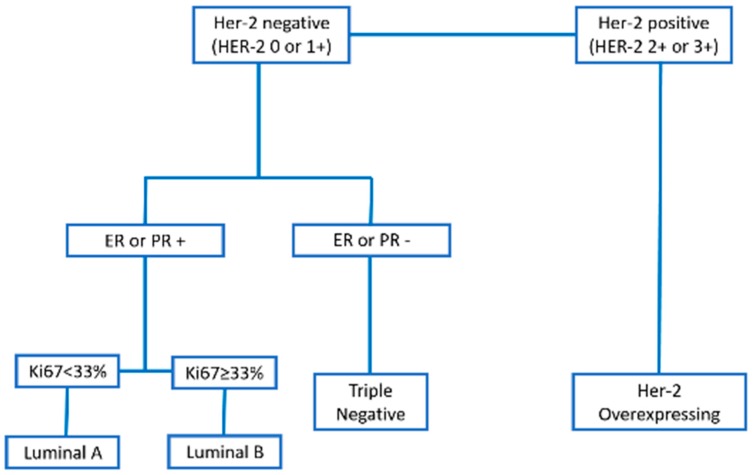
We exclusively included patients with triple-negative mammary tumors. Immunohistochemistry was performed according to Abadie [8]. Then, we used a combination of Estrogen receptor alpha (ERα), progesterone receptor (PR), Her-2, Ki67, cytokeratin 5/6 (CK5/6), and Epidermal growth factor receptor (EGFR) to classify the different molecular phenotypes (Figure 1).
Figure 1.
Classification of the molecular phenotypes of canine mammary carcinomas according to each immunohistochemical marker.
2.5. Tumor Groups
Twenty-six patients met our inclusion criteria, and 42 tissue samples (26 primary tumors and 16 metastasis) were used. Our patients were divided into three groups: patients with malignant mammary tumors with no local or distant metastasis (G1, nonmetastatic tumors), patients with malignant mammary tumors with metastasis to sentinel lymph nodes at diagnosis and no distant metastasis at the diagnosis until the end of this study (G2, tumors with lymph node metastasis), and patients with malignant mammary tumors with negative sentinel lymph nodes at diagnosis and developed late lung metastasis (G3, tumors with distant metastasis). For all groups, at least one year of follow-up of evaluation was considered.
2.6. Immunohistochemistry
Immunohistochemical evaluation was performed in 41 paraffin blocks: 11 primary tumors from G1, 10 primary tumors and 10 lymph node metastases from G2, and five primary tumors and five lung metastases from G3. Charged slides with 4-μm tissue sections were cut, deparaffinized, and submitted to antigen retrieval with citrate buffer pH 6.0 in a pressure cooker (Pascal, Dako, Carpinteria, CA, USA). Endogenous peroxidase was blocked with 8% hydrogen peroxide diluted in methanol for 10 min. Then, mouse monoclonal VEGFR (Flk1, Abcam, Cambridge, UK), rabbit monoclonal PDGFR (clone 26E1, Cell signaling, Danvers, MA, USA), and rabbit polyclonal CD31 (ThermoFisher Scientific, Waltham, MA, EUA) antibodies at 1:300, 1:200, and 1:50, respectively, were applied for 18 h. Afterward, incubation with a secondary antibody (Envision, Dako, Carpinteria, CA, USA) for 1 h was performed, and samples were incubated with 3,3′-diaminobenzidine (DAB, Dako, Carpinteria, CA, USA) for 5 min. Counterstaining was performed with Harris hematoxylin for 1 min. The positive controls were selected according to the Protein Atlas recommendations (https://www.proteinatlas.org). For VEGFR, canine liver was used as a positive control. For PGFR-β, normal testis was used as a positive control. For CD31, we used an internal control (blood vessel in each tumor sample). Mouse (Negative Control Mouse, Dako, Carpinteria, CA, USA) and rabbit immunoglobulin (Negative Control Rabbit, Dako, Carpinteria, CA, USA) were used as negative controls. VEGFR, PGFR-β, and CD31 cross-reactivities with canine tissue were provided by the manufacturer.
For VEGFR and PGFR-β, the samples were evaluated by optical microscopy using a semiquantitative score of 0 to 4 [30]. Briefly, 0: absence of labeling, 1: 1% up to 25% of positive cells, 2: 26% up to 50% positive cells, 3: 51% up to 75% positive cells, and 4: >75% positive cells. For CD31, we counted the microvessels in five fields using the 20× objective lens in tissue areas with the highest number of microvessels. The mean of the sum of the five fields was used according to Weidner [23]. For the immunohistochemical analysis, the evaluators were blinded from the patient’s clinical data.
2.7. Statistical Evaluation
The results were previously submitted to Shapiro–Wilk normality tests and analysis of variance (ANOVA). If the variables presented a Gaussian distribution, Tukey’s test or the nonparametric Kruskal–Wallis test was used for microvascular density analysis. Spearman’s test was used to investigate correlations between variables. Regarding the VEGFR and PDGFR immunoexpression, Chi-square or Fisher exact tests were performed. Statistical analyses were performed using the GraphPad Prism® program (version 6.0—GraphPad Software, Inc., San Diego, CA, USA) with a significance level of 0.05.
3. Results
3.1. Clinical and Pathological Evaluation
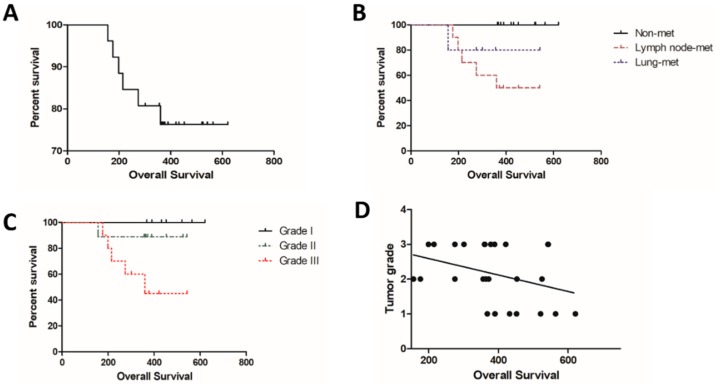
Regarding the pathological parameters, tubulopapillary carcinoma was the most commonly observed diagnosis (8/26), followed by solid carcinoma (7/26), complex carcinoma (5/26), comedocarcinoma (4/26), and mixed carcinoma (2/26). Seven carcinomas were classified as grade I, eight as grade II, and 11 as grade III (Table 1). The clinical parameters are described in Table 1. The mean survival time for all patients independent of the metastatic status was 384.96 days (±123.6) (Figure 2A). Patients with nonmetastatic disease at the diagnosis experienced an increased survival time compared with patients with lymph node metastasis (p = 0.0359) (Figure 2B). Patients with grade III tumors experienced a shorter survival time compared with grades I and II (p = 0.534) (Figure 2C). A negative correlation was observed between tumor grade and overall survival (p = 0.0274; Spearman R = −0.4244). Thus, patients with high tumor grade experienced a reduced survival time (Figure 2D).
Table 1.
Clinical parameters of female dogs affected by mammary gland tumors.
| Clinical Parameters | n | % |
|---|---|---|
| Age (years) | ||
| <10.5 | 16 | 61.5 |
| >10.5 | 10 | 38.5 |
| Neutering Status | ||
| Intact | 26 | 100% |
| Neutered | 0 | 0 |
| Tumor size | ||
| <5 cm | 18 | 69.2% |
| >5 cm | 8 | 30.8% |
| Nodal stage * | ||
| N0 | 16 | 61.5% |
| N1 | 10 | 38.5% |
| Histological grade ** | ||
| Grade I | 7 | 26.9% |
| Grade II | 8 | 30.8% |
| Grade III | 11 | 42.3% |
* Nodal stage is based on the sentinel lymph node at time of diagnosis. ** According to Karayannopoulou et al., 2005 [28].
Figure 2.
Overall survival of dogs with mammary carcinomas based on clinical parameters. (A) Percent survival of all female dogs independent of metastasis status. (B) Female dogs with nonmetastatic tumors exhibited increased survival time followed by patients with lung metastasis and lymph node metastasis. (C) Overall survival independent of metastasis status according to tumor grade. Patients with grade III experienced a reduced survival time. (D) Negative correlation between tumor grade and overall survival. Patients with low-grade tumors exhibited increased survival time.
3.2. Immunohistochemistry
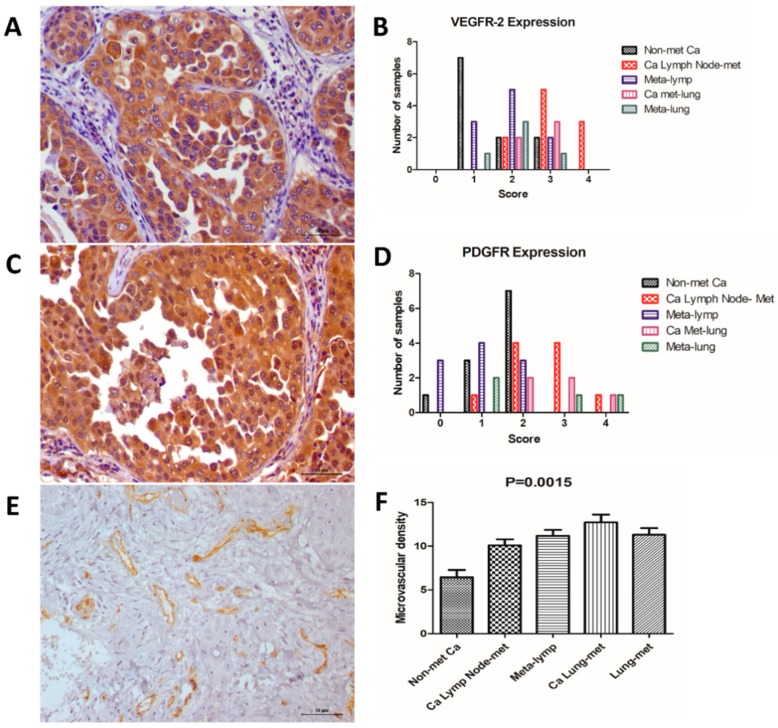
We identified VEGFR-positive expression in all primary and metastatic samples. Patients exhibiting lymph node metastasis at diagnosis exhibited increased VEGFR expression compared with nonmetastatic carcinomas (p = 0.0238). On the other hand, we did not observe a significant difference when we compared primary carcinomas with lung metastasis with nonmetastatic carcinomas (p = 0.1239). We did not observe a significant difference between lymph node metastasis and lung metastasis (p = 0.7243). We also did not observe a significant difference when comparing the primary carcinomas with their respective metastases. We identified a positive correlation between tumor grade and VEGFR expression (p = 0.001; Spearman R = 0.6071). No correlation between VEGFR expression and overall survival was observed (p = 0.125; Spearman R = −0.3087). VEGFR immunoexpression results are presented in Figure 3 and Table 2.
Figure 3.
Immunoexpression of the different markers. (A) Vascular endothelial growth factor receptor 2 (VEGFR-2) (score 4) expression in a mammary carcinoma with lymph node metastasis. (B) Graphic representation of each immunohistochemical score for VEGFR-2 expression in all tumor groups. (C) Platelet-derived growth factor receptor (PDGFR) expression in a mammary carcinoma (score 2) with lymph node metastasis. (D) Graphic representation of each immunohistochemical score for PDGFR expression in all tumor groups. (E) Microvascular density (MVD) in a mammary carcinoma with lymph node metastasis. (F) Graphic representation of MVD, indicating an increased number of vessels in metastatic tumors.
Table 2.
Immunohistochemical evaluation of VEGFR-2, PDGFR, and microvascular density in canine mammary gland tumor samples.
| VEGFR-2 Score | PDGFR Score | Microvascular Density | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 | Mean (SD *) | |
| Nonmetastatic Carcinomas (n = 11) | 0% (n = 0) | 45.5% (n = 5) | 45.5% (n = 5) | 9% (n = 1) | 0% (n = 0) | 9% (n = 1) | 27.3% (n = 3) | 63.7% (n = 7) | 0% (n = 0) | 0% (n = 0) | 6.4 (±2.7) |
| Carcinomas Lymph Node Metastasis (n = 10) | 0% (n = 0) | 0% (n = 0) | 20% (n = 2) | 50% (n = 5) | 30% (n = 3) | 0% (n = 0) | 10% (n = 1) | 40% (n = 4) | 40% (n = 4) | 10% (n = 1) | 10.1 (±2.2) |
| Lymph Node Metastasis (n = 10) | 0% (n = 0) | 30% (n = 3) | 50% (n = 5) | 20% (n = 2) | 0% (n = 0) | 30% (n = 3) | 40% (n = 4) | 30% (n = 3) | 0% (n = 0) | 0% (n = 0) | 11.2 (±2.0) |
| Carcinomas Lung Metastasis (n = 5) | 0% (n = 0) | 0% (n = 0) | 0% (n = 0) | 60% (n = 3) | 40% (n = 2) | 0% (n = 0) | 0% (n = 0) | 40% (n = 2) | 40% (n = 2) | 20% (n = 1) | 12.7 (±2.0) |
| Lung Metastasis (n = 5) | 0% (n = 0) | 20% (n = 1) | 60% (n = 3) | 20% (n = 1) | 0% (n = 0) | 20% (n = 1) | 40% (n = 2) | 0% (n = 0) | 20% (n = 1) | 20% (n = 1) | 11.3 (±1.7) |
* SD: standard deviation.
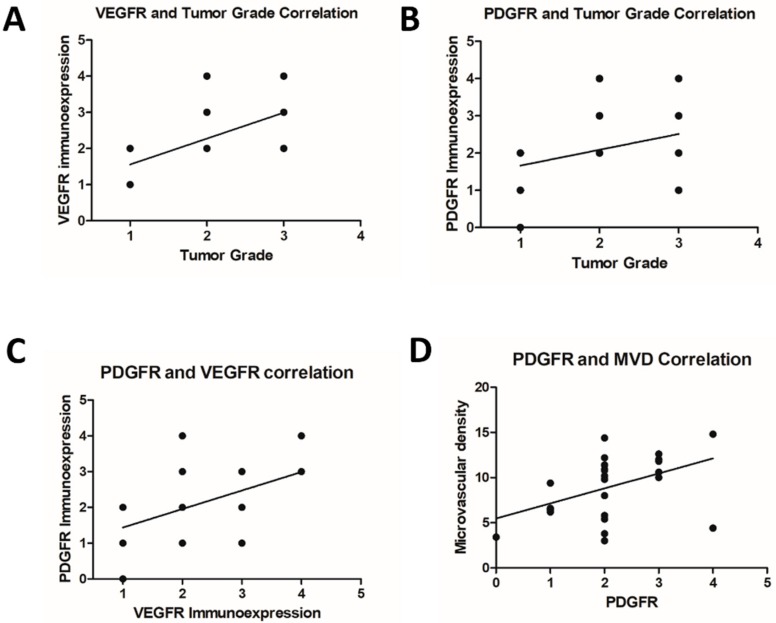
Regarding PDGFR immunoexpression, we observed positive expression in 25 out of 26 samples (Figure 1, Table 2). No significant difference in PDGFR expression was noted among the different groups. No correlation was observed between PDGFR expression and tumor grade (p = 0.0692; Spearman R = 0.3620) or overall survival (p = 0.2581; Spearman R = −0.2301). However, we identified a positive correlation between VEGFR and PDGFR expression. Thus, samples exhibiting the highest scores for VEGFR also presented the highest PDGFR expression (p = 0.01; Spearman R = 0.4959).
Regarding MVD (Figure 3), we identified an increased number of vessels in primary tumors with lymph node metastasis (p = 0.0151) and lung metastasis (p = 0.0046) compared with tumors with no metastasis. Primary tumors with lung metastasis exhibited increased MVD compared with carcinoma with lymph node metastasis (p = 0.0496). Interestingly, we did not find a correlation between MVD and VEGFR expression (p = 0.0827; Spearman R = 0.3467); however, a positive correlation between MVD and PDGFR was observed (p = 0.0102; Spearman R = 0.4946) (Figure 4). Thus, samples with high PDGFR expression also exhibited high MVD (Figure 4).
Figure 4.
Correlation between immunohistochemical markers and different clinical parameters. (A) Positive correlation between VEGFR-2 immunoexpression and tumor grade (p = 0.001; Spearman R = 0.6071). (B) Absence of correlation between PDGFR expression and tumor grade (p = 0.0692; Spearman R = 0.3620). (C) Positive correlation between VEGFR-2 and PDGFR expression (p = 0.01; Spearman R = 0.4959). (D) Positive correlation between PDGFR expression and microvascular density (p = 0.0102; Spearman R = 0.4946).
4. Discussion
Canine mammary gland tumors are one of the most important cancers in intact female dogs and represent a therapeutic challenge. Although surgery and chemotherapy have been used for CMT treatment, there is no standardized chemotherapy or target therapy. This research evaluated VEGFR-2, PDGFR, and MVD in canine mammary tumors, aiming to associate different prognostic factors with these proteins. One interesting aspect of our research is a very restricted criterion used in patient selection. Typically, lymph node metastasis is evaluated after chain mastectomy in inguinal lymph nodes, and the inguinal lymph node is not always draining the tumor. The sentinel lymph node technique allowed us to identify the tumor-draining lymph node and increase the probability of identifying metastasis.
Triple-negative tumors seem to be the most common molecular subtype in dogs [8]. This finding highlights the utility of dogs as a model for human triple-negative BC. We did not include patients with more than one tumor in the mammary chain. This criterion excluded many animals from our study. In the context of multiple mammary tumors, it is not possible to guarantee which nodule the metastasis originated from. Regarding the nonmetastatic group, some patients with multiple tumors exhibited different molecular subtypes (data not shown), making it difficult to establish a prognosis based on the molecular subtype.
Another interesting aspect was the inclusion of a group of patients with no metastatic disease detected at diagnosis but with late lung metastasis. In clinical practice, it is relatively common to find female dogs with late lung metastasis after months or even years post surgery. However, given that metastatic disease can appear one or two years late, it was not possible to achieve a high number of patients in this group. We considered overall survival between the diagnosis and the time of current follow-up/death. Thus, the patients had no lung or lymph node metastasis at the diagnosis and the metastasis disease developed late (over five months). In women with BC, patients can present a very late relapse (after 12 years) [31]. In this study, the highest survival time of patients with lung metastasis compared with patients with lymph node metastasis can be related to the lower number of patients in G3.
VEGFR-2 expression is correlated with angiogenesis and modulation of the tumor microenvironment [12]. In human [32] and canine [12] mammary tumors, VEGFR-2 expression is important in tumor growth and development and exhibits prognostic value [12]. Moreover, VEGFR-2 is a tyrosine kinase protein that can be inhibited by different target therapies [33].
Our results strongly suggested that VEGFR-2 is overexpressed in tumors with metastasis, indicating its predictive and prognostic value. Given that the VEGFR-2 inhibitor is not routinely used in human and veterinary oncology, clinical studies in dogs can benefit both species. We demonstrated a correlation between VEGFR-2 expression and tumor grade, indicating that high-grade tumors may require increased angiogenesis to maintain cell proliferation. Although we did not identify a correlation between VEGFR-2 expression and MVD, we identified increased vascular density in metastatic carcinomas.
These results together demonstrate the dependency of high-grade/metastatic tumors on angiogenic factors. Santos et al. [12] investigated VEGFR-2 expression in CMT, and overexpression of this protein was associated with carcinosarcomas (a very aggressive tumor subtype). Although MVD did not correlate with VEGFR-2 expression, we identified a correlation between PDGFR and MVD. PDGFR induces intratumoral lymphogenesis [21], and we identified a correlation between intratumoral vasculogenesis and high levels of PDGFR. In addition, PDGFR and VEGFR-2 exhibited a positive correlation. These results collectively demonstrate the role of angiogenesis in the development and potential aggressiveness of CMT. Interestingly, both primary tumors and their respective metastases were positive for VEGFR and PDGFR immunoexpression. Given that numerous VEGFR/PDGFR inhibitors are available, these results indicate the possible utility of target therapy in patients with CMT. Thus, our results support the idea of future clinical trials investigating the role of VEGFR/PDGFR inhibitors for the treatment of metastatic CMT.
The role of VEGF expression was previously demonstrated in CMT [34,35]. However, these studies did not evaluate the expression of VEGFR-2. Although VEGF expression has been evaluated in CMT, there are few previous studies evaluating VEGFR-2 expression in CMT [12]. Previously, an association between VEGFR-2 and VEGF immunoexpression was described in CMT, indicating an autocrine VEGF/VEGFR2 loop [12]. In this study, we did not evaluate VEGF. Thus, it was not possible to identify any relation between VEGF/VEGFR signaling pathways.
5. Conclusions
Metastatic mammary carcinomas present VEGFR-2 overexpression and high microvascular density, indicating a role of angiogenesis in tumor progression. PDGFR may induce vasculogenesis in metastatic mammary carcinomas. Overall, our results suggest a possible benefit in the use of antiangiogenic and specific target therapies. In future studies, it will be important to demonstrate the presence of the protein via molecular techniques before clinical trials are undertaken.
Author Contributions
Conceptualization, D.S.d.A., A.F.V., C.E.F.-A. and S.G.C.; Methodology, D.S.d.A., A.F.V., P.d.F.L., A.F.L.-F., F.D., F.E., S.G.C., C.E.F.-A.; Writing—Original Draft Preparation, D.S.d.A. and C.E.F.-A.; Writing—Review and Editing, D.S.d.A., A.F.V., P.d.F.L., A.F.L.-F., F.D., F.E., S.G.C., C.E.F.-A.; Supervision, S.G.C. and C.E.F.-A.
Funding
We would like to thank the São Paulo Research Foundation (FAPESP) for their financial support (grant number: 2015/02798-5).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Misdorp W. Tumors of the mammary gland. In: Meuten D.J., editor. Tumors in Domestic Animals. 4th ed. Iowa State Press; Ames, IA, USA: 2002. pp. 575–606. [Google Scholar]
- 2.Sorenmo K.U., Worley D.R., Goldschmidt M.H. Tumors of the Mammary Gland. In: Withrow S.J., Vail D.M., Page R.L., editors. Withrow & MacEwen’s Small Animal Clinical Oncology. 5th ed. Elsevier; St Louis, MO, USA: 2013. pp. 538–556. [Google Scholar]
- 3.De Nardi A.B., Rodaski S., Sousa R.S., Costa T.A., Macedo T.R., Rodigheri S.M., Rios A., Piekarz C.H. Prevalência de neoplasias e modalidades de tratamentos em cães, atendidos no hospital veterinário da Universidade Federal do Paraná. Arch. Vet. Sci. 2002;7:15–26. doi: 10.5380/avs.v7i2.3977. [DOI] [Google Scholar]
- 4.Santos I.F.C., Cardoso J.M.M., Oliveira K.C., Laisse C.J.M., Bessa S.A.T. Prevalência de neoplasias diagnosticadas em cães no Hospital Veterinário da Universidade Eduardo Mondlane, Moçambique. Arq. Bras. Med. Vet. Zootec. 2013;65:773–782. doi: 10.1590/S0102-09352013000300025. [DOI] [Google Scholar]
- 5.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Cancer Genome Atlas Network Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCart Reed A.E., Kalita-de Croft P., Kutasovic J., Saunus J.M., Lakhani S.R. Recent advances in breast cancer research impacting clinical diagnostic practice. J. Pathol. 2018 doi: 10.1002/path.5199. accepted. [DOI] [PubMed] [Google Scholar]
- 8.Abadie J., Nguyen F., Loussouarn D., Peña L., Gama A., Rieder N., Belousov A., Bemelmans I., Jaillardon L., Ibisch C., et al. Canine invasive mammary carcinomas as models of human breast cancer. Part 2: Immunophenotypes and prognostic significance. Breast Cancer Res. Treat. 2018;167:459–468. doi: 10.1007/s10549-017-4542-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghosh S., Sullivan C.A., Zerkowski M.P., Molinaro A.M., Rimm D.L., Camp R.L., Chung G.G. High levels of vascular endothelial growth factor and its receptors (VEGFR-1, VEGFR-2, neuropilin-1) are associated with worse outcome in breast cancer. Hum. Pathol. 2008;39:1835–1843. doi: 10.1016/j.humpath.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Queiroga F.L., Pires I., Parente M., Gregório H., Lopes C.S. COX-2 over-expression correlates with VEGF and tumour angiogenesis in canine mammary cancer. Vet. J. 2011;189:77–82. doi: 10.1016/j.tvjl.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C.W., Lin D.G., Wang J.Q., Li C.Y., Deng G.Z. Expression and significance of PTEN and VEGF in canine mammary gland tumours. Vet. Res. Commun. 2008;32:463–472. doi: 10.1007/s11259-008-9049-7. [DOI] [PubMed] [Google Scholar]
- 12.Santos A., Lopes C., Gärtner F., Matos A.J. VEGFR-2 expression in malignant tumours of the canine mammary gland: A prospective survival study. Vet. Comp. Oncol. 2016;14:83–92. doi: 10.1111/vco.12107. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M., Morandi P., Krishnamurthy S., Reuben J.M., Lee B.N., Francis D., Booser D.J., Green M.C., Arun B.K., Pusztai L., et al. Imatinib mesylate (Gleevec) in advanced breast cancer-expressing C-Kit or PDGFR-beta: Clinical activity and biological correlations. Ann. Oncol. 2008;19:1713–1719. doi: 10.1093/annonc/mdn352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modi S., Seidman A.D., Dickler M., Moasser M., D’Andrea G., Moynahan M.E., Menell J., Panageas K.S., Tan L.K., Norton L., et al. A phase II trial of imatinib mesylate monotherapy in patients with metastatic breast cancer. Breast Cancer Res. Treat. 2005;90:157–163. doi: 10.1007/s10549-004-3974-0. [DOI] [PubMed] [Google Scholar]
- 15.Salvador R.C.L., Raposo T.M.M., Fonseca-Alves C.E., Terra E.M., Varallo G.R., Laufer-Amorim R. BMC Proceedings. Volume 7. BioMed Central; London, UK: 2013. Evaluation of c-KIT protein expression in canine mammary tumors; p. 63. [Google Scholar]
- 16.Brunetti B., Beha G., Benazzi C., Bondin V., De Tolla L., Sarli G. CD117 expression influences proliferation but not survival in canine mammary tumours. J. Comp. Pathol. 2014;151:202–206. doi: 10.1016/j.jcpa.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Koltai Z., Szabó B., Jakus J., Vajdovich P. Tyrosine kinase expression analyses in canine mammary gland tumours—A pilot study. Acta Vet. Hung. 2018;66:294–308. doi: 10.1556/004.2018.027. [DOI] [PubMed] [Google Scholar]
- 18.London C.A., Hannah A.L., Zadovoskaya R., Chien M.B., Kollias-Baker C., Rosenberg M., Downing S., Post G., Boucher J., Shenoy N., et al. Phase I dose-escalating study of SU11654, a small molecule receptor tyrosine kinase inhibitor, in dogs with spontaneous malignancies. Clin. Cancer Res. 2003;9:2755–2768. [PubMed] [Google Scholar]
- 19.Nakano Y., Kobayashi T., Oshima F., Fukazawa E., Yamagami T., Shiraishi Y., Takanosu M. Imatinib responsiveness in canine mast cell tumors carrying novel mutations of c-KIT exon 11. J. Vet. Med. Sci. 2014;76:545–548. doi: 10.1292/jvms.13-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foskett A., Manley C., Naramore R., Gordon I.K., Stewart B.M., Khanna C. Tolerability of oral sorafenib in pet dogs with a diagnosis of cancer. Vet. Med. 2017;8:97–102. doi: 10.2147/VMRR.S149678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao R., Björndahl M.A., Religa P., Clasper S., Garvin S., Galter D., Meister B., Ikomi F., Tritsaris K., Dissing S., et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 22.Shibuya M. Vascular Endothelial Growth Factor (VEGF) and Its Receptor (VEGFR) Signaling in Angiogenesis: A Crucial Target for Anti- and Pro-Angiogenic Therapies. Genes Cancer. 2011;2:1097–1105. doi: 10.1177/1947601911423031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weidner N., Folkman J., Pozza F., Bevilacqua P., Allred E.N., Moore D.H., Meli S., Gasparini G. Tumor angiogenesis: A new significant and independent prognostic indicator in early-stage breast carcinoma. J. Nat. Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 24.Bosari S., Lee A.K.C., DeLellis R.A., Wiley B.D., Heatley G.J., Silverman M.L. Microvessel quantification and prognosis in invasive breast carcinoma. Hum. Pathol. 1992;23:755–761. doi: 10.1016/0046-8177(92)90344-3. [DOI] [PubMed] [Google Scholar]
- 25.Graham J.C., Myers R.K. The prognostic significance of angiogenesis in canine mammary tumors. J. Vet. Intern. Med. 1999;13:416–418. doi: 10.1111/j.1939-1676.1999.tb01456.x. [DOI] [PubMed] [Google Scholar]
- 26.Beserra H.E.O., Cavalcante R.V., de Pinho Pessoa A.W., Pinheiro L.G.P. Technical of sentinel lymph detection in canine mammary gland using Patent Blue V and technetium [sup. 99m] Tc/Tecnica de deteccao do linfonodo sentinela da glandula mamaria de cadelas utilizando Azul Patente V e tecnecio [sup. 99m] Tc/Tecnica para la deteccion del ganglio linfatico centinela en glandulas mamarias de perras con Azul Patente y tecnecio [sup. 99m] Tc. Vet. Zootec. 2011;18:57–60. [Google Scholar]
- 27.Goldschimidt M., Peña L., Rasotto R., Zappuli V. Classification and grading of canine mammary tumors. Vet. Pathol. 2011;48:117–131. doi: 10.1177/0300985810393258. [DOI] [PubMed] [Google Scholar]
- 28.Karayannopoulou M., Kaldrymidou E., Constantinidis T.C., Dessiris A. Histological grading and prognosis in dogs with mammary carcinomas: Application of a humangrading method. J. Comp. Pathol. 2005;133:246–252. doi: 10.1016/j.jcpa.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Bonolo De Campos C., Lavalle G.E., Monteiro L.N., Pêgas G.R.A., Fialho S.L., Balabram D., Cassali G.D. Adjuvant Thalidomide and Metronomic Chemotherapy for the Treatment of Canine Malignant Mammary Gland Neoplasms. In Vivo. 2018;32:1659–1666. doi: 10.21873/invivo.11429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonseca-Alves C.E., Kobayashi P.E., Rivera Calderón L.G., Felisbino S.L., Rinaldi J.C., Drigo S.A., Rogatto S.R., Laufer-Amorim R. Immunohistochemical panel to characterize canine prostate carcinomas according to aberrant p63 expression. PLoS ONE. 2018;13:1–16. doi: 10.1371/journal.pone.0199173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Omidvari S., Hamedi S.H., Mohammadianpanah M., Nasrolahi H., Mosalaei A., Talei A., Ahmadloo N., Ansari M. Very late relapse in breast cancer survivors: A report of 6 cases. Iran. J. Cancer Prev. 2013;6:113–117. [PMC free article] [PubMed] [Google Scholar]
- 32.Yan J.D., Liu Y., Zhang Z.Y., Liu G.Y., Xu J.H., Liu L.Y., Hu Y.M. Expression and prognostic significance of VEGFR-2 in breast cancer. Pathol. Res. Pract. 2015;211:539–543. doi: 10.1016/j.prp.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Sloan B., Scheinfeld N.S. Pazopanib, a VEGF receptor tyrosine kinase inhibitor for cancer therapy. Curr. Opin. Investing. Drug. 2008;9:1324–1335. [PubMed] [Google Scholar]
- 34.Raposo T.P., Pires I., Carvalho M.I., Prada J., Argyle D.J., Queiroga F.L. Tumour-associated macrophages are associated with vascular endothelial growth factor expression in canine mammary tumours. Vet. Comp. Oncol. 2015;13:464–474. doi: 10.1111/vco.12067. [DOI] [PubMed] [Google Scholar]
- 35.Camacho L., Peña L., Gil A.G., Martín-Ruiz A., Dunner S., Illera J.C. Immunohistochemical vascular factor expression in canine inflammatory mammary carcinoma. Vet. Pathol. 2014;51:737–748. doi: 10.1177/0300985813503568. [DOI] [PubMed] [Google Scholar]