Abstract
Aberrant activation of β-catenin signaling is frequently observed in hepatocellular cancer. Although Wnt/β-catenin signaling can be targeted by vitamin D, therapeutic use of vitamin D for this purpose is not currently established. We evaluated the therapeutic use of vitamin D or its analogs using a synthetic transgenic mouse of hepatocarcinogenesis induced by mutant β-catenin, and MET overexpression in which 75% of mice develop well-differentiated HCC within 8 weeks in the absence of fibrosis. Vitamin D receptor expression was similar in both tumoral and nontumoral tissue. There was no significant difference in overall survival, or in tumor progression assessed by imaging, biochemical, or tumor cell burden assessments in mice receiving a vitamin D-supplemented diet containing 12.0 IU VD/g (HVD) compared with a standard diet (SD) containing 2.3 IU VD/g. Furthermore, systemic treatment with calcitriol [vitamin D analog 1α,25(OH)2D3] or EB1089 (synthetic vitamin D analog) by intraperitoneal injection for 4 weeks prolonged median survival but did not increase overall survival compared with controls. Although tumor formation was delayed in males compared with that in females, there was no difference in overall survival between males and females. In conclusion, although 1α,25(OH)2D3 is reported to inhibit β-catenin signaling, as well as proliferation, migration, and differentiation in cancer cells, neither dietary supplementation with vitamin D nor treatment with vitamin D analogs altered the formation or growth of HCC associated with β-catenin activation. These results conclusively demonstrate the lack of utility of targeting vitamin D for therapy of HCC in this setting.
Key words: Liver cancer, Calcitriol, Hepatocyte growth factor, Hepatocellular carcinoma (HCC), Vitamin D
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major health problem and a leading cause of cancer-related death worldwide. Patients with HCC have a poor prognosis, with an overall survival of less than 10%1,2. There are few effective treatment options for HCC that has advanced to a stage when surgical resection or transplantation is not possible. Moreover, these cancers are notorious for their resistance to systemic therapies. The heterogeneity of genetic changes and mutations that are associated with HCC further limit the use of treatment approaches that are based on targeting driver mutations or pathogenic signaling pathways3.
Epidemiological data suggest a protective role of vitamin D against liver cancers4. An association between reduced levels of vitamin D and advanced stage disease, as well as a higher incidence of death from HCC, have been reported5,6. Deficiency of vitamin D is common and is present in 90% of liver cancers in the setting of advanced liver disease7. The most active vitamin D metabolite, 1α,25-dihydroxy vitamin D3 [1α,25(OH)2D3], is a hormone with many documented anticancer effects in vitro. These include inhibitory effects on cancer cell proliferation or migration, and promotion of differentiation8–10. Consistently, several studies have reported antiangiogenic, anti-invasive, or antimetastatic effects of this hormone in vivo, resulting in tumor suppression10. In addition, vitamin D can regulate the expression of many genes and invoke signaling pathways in the liver. Consequently, vitamin D and its analogs have been proposed as treatment options for HCC.
The direct effect of vitamin D on hepatocarcinogenesis is unknown. Most HCC occurs within a background of chronic liver disease and cirrhosis. The association of vitamin D insufficiency with advanced liver disease and cirrhosis, which are also risk factors for HCC, raises an important question with regard to the issue of causality. The Wnt/β-catenin signaling is a target pathway that is antagonized by 1α,25(OH)2D3 11. Mutations in β-catenin are among the most frequent genetic changes observed in HCC. Using a model of HCC induced by β-catenin, a vitamin D target pathway, we sought to directly examine the therapeutic effect of vitamin D on oncogenic tumor growth in the absence of either advanced liver disease and cirrhosis or systemic vitamin D insufficiency.
MATERIALS AND METHODS
Animals
Five-week-old male and female FVB/N mice were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). All animal studies were performed in accordance with a protocol approved by the Mayo Clinic Institutional Animal Care and Use Committee. Mice were maintained on either a standard diet (SD) containing 2.3 IU VD/g or a high vitamin D diet (HVD) identical to SD but supplemented with vitamin D and containing 12.0 IU VD/g (Lab Supply, Highland Village, TX, USA). Equal numbers of male and female mice were used for all experimental groups.
Experimental Model of HCC
HCC was induced using the sleeping beauty transposon/transposase by hydrodynamic transfection (HT) of c-MET, truncated mutation β-catenin, Gaussia luciferase, and sleeping beauty transposase12. Constructs for Gaussia luciferase were provided by Dr. Wei Qui (Loyola University), and all others by Dr. Xin Chen (University of California, San Francisco). Plasmids were amplified using one Shot™ TOP10 chemically competent Escherichia coli cells (Thermo Fisher Scientific, Waltham, MA, USA) and purified using PureYield™ Plasmid Maxiprep System (Promega, Madison, WI, USA). Before injection, endotoxin was removed using MiraCLEAN® Endotoxin Removal Kit (Mirus Bio, Madison, WI,USA), and plasmid DNAs were stored in aliquots of 2 μg/μl. Briefly, 22.5 μg of pT3-EF1a-c-MET (human), 22.5 μg of pT3-EF1a-DN90-β-catenin (human), 5 μg of pT3-Gluc, along with 5 μg of sleeping beauty transposase (SB) were diluted in 2 ml of TransIT-EE Hydrodynamic Delivery Solution (Mirus Bio) and injected hydrodynamically into the lateral tail veins. Details of the experimental procedures have been previously described13–15.
Hepatic Ultrasound
Ultrasound imaging was performed using the Vevo® 2100 Imaging System (VisualSonics Inc., Toronto, ON, Canada). Mice receiving a standard diet (n = 6) were imaged weekly and sacrificed at selected intervals after hydrodynamic transfection. Ultrasonographic imaging features were correlated with hepatic histology and used to develop an imaging-based scoring system that could be used for noninvasive monitoring of tumor formation and growth.
Serum Collection and Analyses
Blood was collected from the tail vein at pre-determined intervals during the course of the study. Serum was isolated from blood kept at room temperature for 30 min followed by centrifugation at 1,200 × g for 10 min. Serum α-fetoprotein (AFP) and 1,25(OH)2D3 (vitamin D) were analyzed using enzyme immunoassay-based kits (R&D Systems, Minneapolis, MN, USA and Immunodiagnostic Systems, The Boldons, UK). Serum calcium was quantitated using the Calcium Colorimetric Assay kit (Sigma-Aldrich, St. Louis, MO, USA). All assays using kits were done according to the manufacturer’s directions.
Gaussia Luciferase (g-luc) Assay
Serum (5 μl) was plated on a 96-well flat bottom white polystyrene assay plate (costar 3362; Corning). Serum luciferase levels were determined using the BioLux Gaussia Luciferase Assay kit (New England BioLabs, Worcester, MA, USA) according to the manufacturer’s direction. Luminescence measurements were acquired using FLUOstar Omega microplate reader (BMG Labtech, Cary, NC, USA) after controlled injection of 50 μl of substrate mixture into plates containing the serum.
Immunohistochemistry
Formalin-fixed liver tissue samples were embedded in paraffin. Four-micrometer sections were obtained, stained with hematoxylin and eosin (H&E), and immunohistochemistry was performed as follows: slides were deparaffinized, hydrated, and antigen retrieval was performed by soaking slides in EDTA in a 100°C steamer for 25 min. Primary antibody against vitamin D receptor rat monoclonal antibody (dilution 1:25; Thermo Fisher Scientific), anti-rat secondary antibodies (Dako, Carpinteria, CA, USA), and 3,3-diaminobenzidine (DAB; Dako) were used.
PCR Analysis
Total RNA was extracted from mouse livers using TRIzol (Life Technologies), and reverse transcribed to cDNA using iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Real-time quantitative RT-PCR was performed using a LightCycler 96 System (Roche Diagnostics, Mannheim, Germany) to detect VDR mRNA using SYBR green I (SYBR® Advantage® qPCR Premix; Clontech, Mountain View, CA, USA). The primers used were VDR-F, 5′-GAGGTGTCTGAAGCCTGGAG-3′, and VDR-R, 5′-ACCTGCTTTCCTGGGTAGGT-3′. The cycle number at which the reaction crossed a threshold (CT) was determined for each gene, and comparative mRNA expression was assessed using the ΔΔCt method.
Vitamin D Analog Treatment
Stock solutions of calcitriol (Sigma-Aldrich) and EB1089 (Tocris Bioscience, Ellisville, MO, USA) were made in 100% ethanol and stored at −20°C. Appropriate dilutions were made in sterile PBS. The mice in the control group received injections of 1.2% ethanol in PBS (final volume 0.1 ml) intraperitoneally. Mice in treatment groups received calcitriol (2 μg/kg of body weight) and EB1089 (0.5 μg/kg of body weight), injected intraperitoneally three times a week for 4 weeks, with the first administration starting at 3 weeks of hydrodynamic transfection of MET/β-catenin. Study groups for each of the treatment arms comprised 12 animals each, with equal numbers of males and females. Mice were observed for 9 to 12 weeks prior to euthanasia, and a subset was followed until death to assess overall survival.
Statistical Analysis
Data are presented as mean ± standard error, with the number of analyses for each mean indicated. Statistical significance was calculated using the Student t-test. A value of p < 0.05 was considered significant. Correlation between groups was assessed using Pearson’s correlation coefficient. Survival data was analyzed by Kaplan–Meier analysis and compared by log-rank test. Statistical evaluations were carried out using GraphPad Prism 7 software (GraphPad Software, Inc., San Diego, CA, USA).
RESULTS
Generation of a Noninvasive Ultrasound-Based Tumor Progression Score
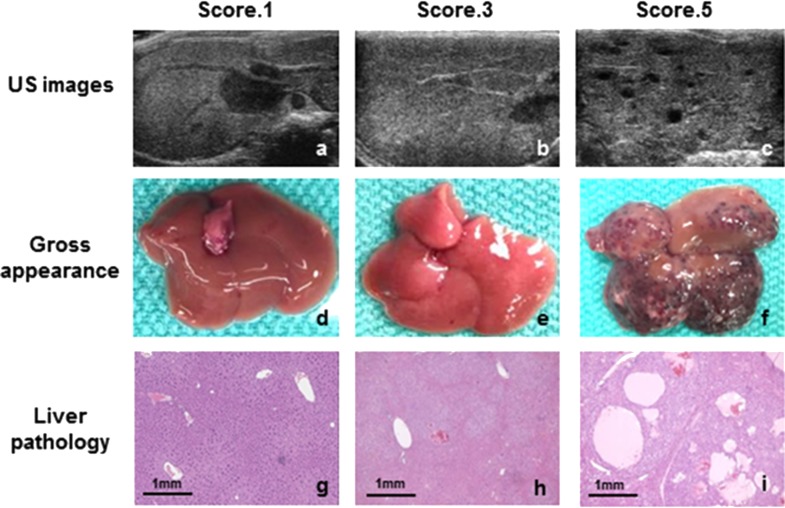
A synthetic transgenic model of HCC induced by MET and β-catenin was used for these studies to recapitulate the subset of human HCC that is characterized by MET expression and β-catenin mutation. The approach uses hydrodynamic transfection (HT) and sleeping beauty transposon/transposase to coexpress MET and mutated β-catenin (Δ-90) within hepatocytes. Most mutations in β-catenin in human HCCs are missense point mutations, and clinically relevant experimental HCC can be generated using full-length β-catenin with point mutations16. For these studies we used a Δ-90-truncated β-catenin mutation to evaluate the impact of altered β-catenin signaling. Successful transfection as determined by incorporation of plasmid DNA was observed in 89.3% of mice. Tumor formation occurred in 75% of animals by 8 weeks after transfection of MET and β-catenin. In order to monitor the rate of tumor formation noninvasively, we began by assessing ultrasonographic features associated with tumor progression. Serial ultrasound imaging was performed at biweekly intervals following hydrodynamic transfection. Mice were euthanized at selected time intervals. Histological examination was then correlated with identifiable features on ultrasonography. The features selected were readily detectable by different observers. Based on these, a five-point ultrasound-based score was then developed (Table 1). Ultrasound images, gross appearance of the liver, and histology are shown in Figure 1.
Table 1.
Ultrasound Scoring System
| US Score | US Findings | Histological Findings |
|---|---|---|
| 1 | Normal: homogeneous parenchyma, clear and smooth vasculature present | Regular hepatic cords are observed without tumor formation |
| 2 | Low intermediate: intermixed homogenous and heterogeneous parenchyma | Small solid tumors are observed in less than about 30% of the liver |
| 3 | Tumor: heterogeneous parenchyma in mosaic pattern, intermingling of high and low echoic area | Solid tumors are observed in about 30–70% of the liver |
| 4 | High intermediate: small hypoechoic lesions with mosaic background | Solid tumors are observed in more than 70% liver with or without small cystic degenerations |
| 5 | Multifocal tumors: multiple hypoechoic lesions | Large solid tumors are observed in entire liver with cystic degeneration |
Figure 1.
Ultrasound imaging–pathology correlation. Ultrasound images were obtained from the right hepatic lobe of MET/β-catenin mice biweekly after tumor induction. At selected intervals, livers were excised for correlative gross and microscopic evaluation. The typical gross appearance and hepatic histopathological changes associated with each of three ultrasound-defined scores is shown. Liver sections were stained with hematoxylin and eosin (H&E). Original magnification 4×.
Gaussia Luciferase (g-luc) Expression as Markers of Tumor Burden
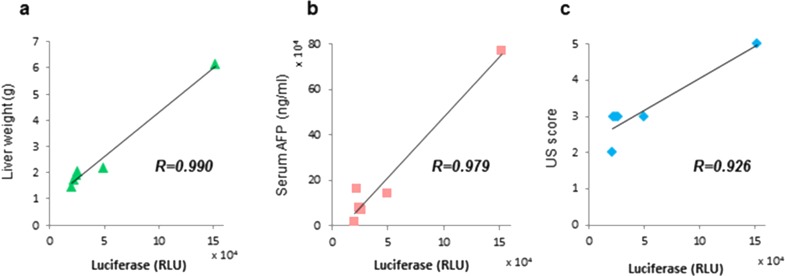
We next evaluated tumor burden using serum luciferase expression. The use of cotransfection with g-luc constructs enables the use of luciferase activity as a measure of tumor cell burden. Six mice underwent hydrodynamic transfection with MET and β-catenin, and were then followed for 6 weeks. Ultrasound examinations were performed 4 and 6 weeks after transfection and scored using our original system (Table 1). Six weeks after hydrodynamic transfection, serum samples were obtained, and AFP and g-luc were assessed. All mice were sacrificed and examined by gross inspection and histopathology. Tumors were observed in the livers of all mice. Liver weight is a conventional approach to measuring the tumor burden because of the multifocal nature of tumorigenesis in this model. The correlation between g-luc and other measures were evaluated, and we observed high correlation coefficients with liver weight (r = 0.990), serum AFP (r = 0.979), and US score (r = 0.926) (Fig. 2). These results validate the use of g-luc as an accurate measure of tumor burden in de novo liver cancer in the MET/β-catenin mice.
Figure 2.
Assessment of tumor burden. Six weeks after hydrodynamic transfection, ultrasound was performed for scoring, after which the mice were sacrificed. Liver weight was measured. Serum was collected and used to assay Gaussia luciferase (g-luc) activity and α-fetoprotein (AFP). g-luc activity showed a high correlation coefficient with (a) liver weight (r = 0.990), (b) serum AFP (r = 0.979), and (c) US score (r = 0.926).
Effect of Vitamin D Supplementation on Hypercalcemia
Excessive intake of vitamin D can result in hypercalcemia, which can have unwanted effects. In order to establish an appropriate dietary intake for experimental studies, MET/β-catenin mice received either SD or HVD. Compared with those receiving SD, serum 1α,25(OH)2D3 levels determined after 6 weeks were increased in control mice transfected with SB and g-luc alone receiving HVD (33.1 + 4.4 vs. 66.9 + 12.6 pg/ml, p = 0.035), but were not significantly different in the MET/β-catenin group (59.9 + 12.4 vs. 58.6 + 18.8 pg/ml, p = 0.368). These could reflect a deficiency of 1α,25(OH)2D3. There were no significant differences in either serum calcium levels or body weight between the two groups (Table 2). These results indicate that the HVD was sufficient to increase vitamin D without deleterious effects on serum calcium or on dietary intake.
Table 2.
Systemic Effect of Dietary Vitamin D Supplementation
| Standard Diet | High Vitamin D Diet | p Value | |
|---|---|---|---|
| Calcium (mg/dl) | 10.0 ± 0.32 | 8.98 ± 0.36 | 0.056 |
| Males | 10.4 ± 0.4 | 9.8 ± 0.1 | |
| Females | 9.7 ± 0.5 | 8.4 ± 0.1 | |
| Body weight (g) | |||
| Males | 28.2 ± 1.05 | 29.1 ± 0.2 | 0.281 |
| Females | 22.6 ± 0.3 | 23.3 ± 0.5 | 0.150 |
Data are presented as mean value ± SEM. Statistical significance was calculated with a Student t-test. A value of p < 0.05 was considered significant.
Vitamin D Receptor (VDR) Expression
Vitamin D mediates biological effects and modulates gene expression through its binding to VDR. The classical action of 1α,25(OH)2D3 is mediated by binding of the VDR-9-cis-retinoic acid receptor (RXR) complex to vitamin D response elements17. We examined the expression of VDR by immunohistochemistry in hepatic tissue in the MET/β-catenin and control mice transfected with g-luc plasmid DNA only (Fig. 3). Tumor nodules were present in H&E sections from MET/β-catenin mouse livers (Fig. 3a), whereas normal hepatocytes and regular hepatic cords were observed in control mouse livers (Fig. 3b). VDR expression was observed in the nucleus of liver tumor cells (Fig. 3c) as well as in normal hepatocytes (Fig. 3d). Overall, VDR expression in tumor cells was similar and not higher than that observed in nontumor hepatocytes. Expression of VDR mRNA by RT-PCR was marginally increased in tumors from mice on SD compared with HVD (1.04 ± 0.18 vs. 0.86 ± 0.13, p = 0.47); likewise VDR mRNA was not significantly increased in tumor tissues compared with normal tissue in mice on HVD (1.04 ± 0.18 vs. 1.07 ± 0.05, p = 0.85).
Figure 3.
Vitamin D receptor (VDR) expression. Liver sections were stained with H&E (a, b) or immunohistochemistry performed using anti-VDR antibody (c–e). Normal liver obtained from a mouse transfected with g-luc plasmid DNA only (a, c), liver tumors obtained from MET/β-catenin mouse (b, d), and duodenum (positive control) (e) are shown. Original magnification: 10×.
Effect of Vitamin D Supplementation on HCC Progression
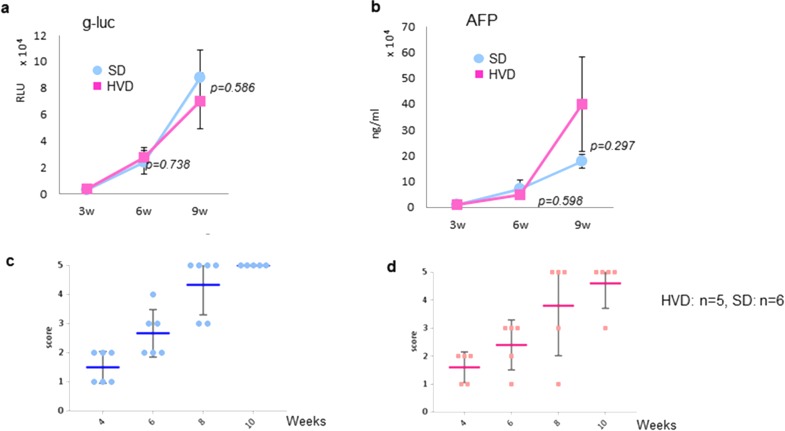
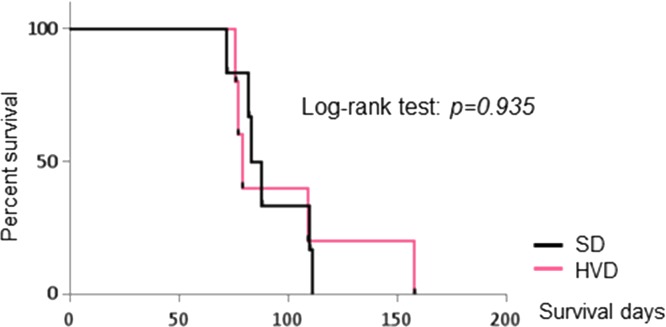
The effects of dietary vitamin D supplementation on tumor progression in MET/β-catenin mice were next assessed. Mice received either SD or HVD from week 5 until the end of the study. Following HT, hepatic ultrasound was performed at 4, 6, 8, and 10 weeks, and serum g-luc expression and AFP were measured at 3, 6, and 9 weeks (Fig. 4). At each time points, there were no significant differences observed in g-luc, AFP, or US score between mice receiving either HVD or SD. The median survival time in HVD group was 79.0 days, whereas that in the SD group was 85.5 days (p = 0.935). Moreover, the survival time of mice receiving HVD was not extended compared with that of SD (Fig. 5). These observations convincingly demonstrate the lack of efficacy of vitamin D supplementation on HCC associated with effects of c-MET and β-catenin expression.
Figure 4.
The effect of dietary vitamin D supplementation on tumor growth. Animals were randomized to receive either a standard diet (SD) or high vitamin D diet (HVD) after hydrodynamic transfection. g-luc activity (a) and AFP (b) were assessed at 3, 6, and 9 weeks after transfection in both groups. The p values for differences between each group are indicated at 6 and 9 weeks. Ultrasound score was assessed in (c) SD (n = 6) and (d) HVD (n = 5) groups at 2 weekly intervals starting at 4 weeks after transfection. The data represent the mean value ± SEM of the score.
Figure 5.
Effect of dietary vitamin D supplementation on survival. Survival was assessed in mice receiving SD or HVD. Kaplan–Meier analysis was performed, and survival between the groups was compared using log-rank test (p = 0.935).
Effect of Vitamin D Analogs on HCC Progression
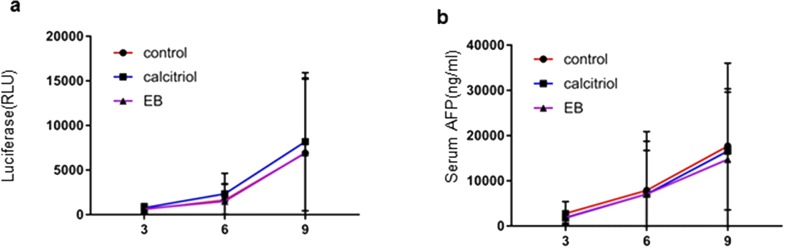
Vitamin D analogs that retain hormonal properties of the natural vitamin D, 1,25-dihydroxyvitamin D3 [1α,25(OH)2D3] are of therapeutic interest. 1α,25(OH)2D3 analogs have been reported to reduce the risk of developing certain malignancies, such as colorectal cancer18 and breast cancer19. We examined in vivo administration of vitamin D analogs, EB1089 (seocalcitol), and calcitriol on HCC formation in MET/β-catenin mice. These analogs were administered in vivo at therapeutically effective concentrations. Calcitriol (1a,25-dihydroxyvitamin D3) is the biologically active form of vitamin D3. EB1089 is a synthetic analog that is 50–200 times potent than calcitriol at inhibiting cell proliferation and differentiation. EB1089 can inhibit the proliferation of the HepG2 HCC cells and was well tolerated with some activity observed in a phase II study in HCC20,21. Serum g-luc expression and AFP were measured at 3, 6, 9, and 12 weeks after HT to determine effects on tumor progression (Fig. 6). There were no significant differences in tumor burden assessed by either luciferase expression or AFP between controls or treatment groups after 9 weeks. Furthermore, median survival was not significantly different between the three groups (Table 3).
Figure 6.
The effect of vitamin D analogs on tumor growth and survival. Animals were randomized to receive an SD alone (control) or SD supplemented with either calcitriol or EB1089 (EB) for 4 weeks, starting at 3 weeks after hydrodynamic transfection. Each group had 12 animals. Tumor growth was monitored using (a) Gaussia luciferase (g-luc) activity or (b) AFP assayed at 3, 6, and 9 weeks after transfection. Data are presented as mean value ± SEM.
Table 3.
Systemic Effects of Vitamin D Analog Administration
| All | Male | Female | ||||||
|---|---|---|---|---|---|---|---|---|
| Calcium (mg/dl) | p Value | Calcium (mg/dl) | p Value | Body Weight (g) | Calcium (mg/dl) | p Value | Body Weight (g) | |
| Control | 9.50 ± 1.01 | – | 9.88 ± 1.00 | – | 30.07 ± 0.91 | 10.96 ± 0.7 | – | 23.3 ± 0.94 |
| Calcitriol | 10.67 ± 0.64 | 0.53 | 9.02 ± 0.46 | 0.69 | 29.03 ± 0.24 | 11.98 ± 1.3 | 0.46 | 22.52 ± 0.7 |
| EB1089 | 9.67 ± 0.71 | 0.99 | 9.19 ± 0.55 | 0.29 | 31.33 ± 0.53 | 10.85 ± 2.06 | 0.99 | 24.02 ± 1.67 |
Data are presented as mean value ± SEM. Statistical significance was calculated with one-way ANOVA. A value of p < 0.05 was considered significant.
Effect of Vitamin D Analog on Hypercalcemia
The clinical utility of 1α,25(OH)2D3 is limited by its tendency to cause hypercalcemia. EB1089 has a reduced calcemic activity compared to that of calcitriol22. In order to evaluate these effects, calcium levels were determined after 6 weeks of the start of treatment, and body weight was evaluated every week. The reference range of calcium in serum is 8.8 to 10.4 mg/dl23. Other than in females receiving calcitriol (11.98 mg/dl), the average serum level of calcium was not increased in any group. Moreover, there was no significant difference in body weight change or serum calcium between the three groups (Table 3). These results suggest a lack of systemic toxic effects with vitamin D analog administration.
Gender Differences in Response
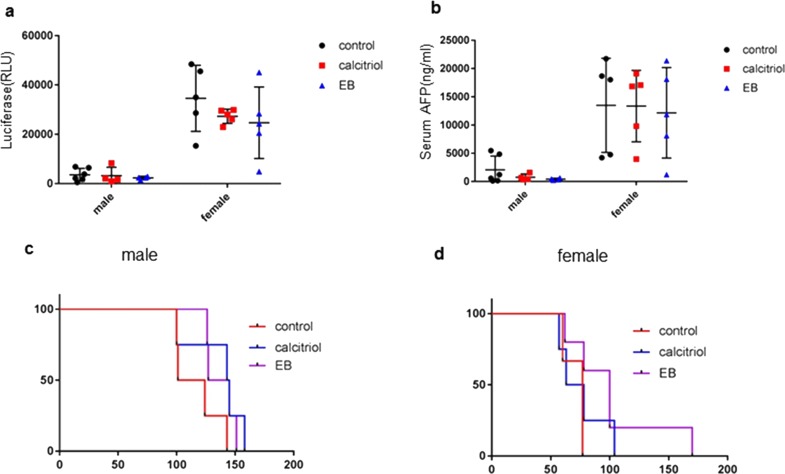
Vitamin D is a circulating sex hormone that can have different effects in males and females. Compared with female mice, male mice with MET/β-catenin-induced HCC have been observed to have a slower rate of tumor growth. Therefore, we examined tumor growth and progression separately in each sex. After 9 weeks of HT, there were no significant differences in g-luc in male mice (p = 0.4328) or in female mice (p = 0.8321) between three groups (EB1089, calcitriol, control) (Fig. 7). Likewise, there were no significant differences of AFP in male mice (p = 0.7432) or in female mice (p = 0.3995) between these groups. We further evaluated the overall survival in each gender separately in three to five males and females in all groups, but did not observe any significant differences in survival times between males and females when assessed using log-rank curve comparisons (Table 4).
Figure 7.
Sex differences in treatment response. The treatment response to vitamin D analogs was assessed separately in male and female mice. Animals were randomized to receive SD alone (control), or SD supplemented with either calcitriol or EB for 4 weeks, starting at 3 weeks after hydrodynamic transfection. Each group had four to six animals. Tumor growth was monitored using (a) Gaussia luciferase (g-luc) activity or (b) AFP assayed at 3, 6, 9, or 12 weeks after transfection Data are presented as mean value ± SEM at 9 weeks. Survival curves were estimated, in three to four animals per group, by Kaplan–Meier analysis and compared by log-rank test (c) in male mice (p = 0.13), and (d) in female mice (p = 0.21).
Table 4.
Effect of Systemic Vitamin D Analog Administration on Survival in Experimental β-Catenin/MET-Induced Hepatocarcinogenesis
| All | Males | Females | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Median Survival (Days) | p Value | n | Median Survival (Days) | p Value | n | Median Survival (Days) | p Value | |
| Control | 7 | 100 | – | 4 | 112.5 | – | 3 | 77 | – |
| Calcitriol | 8 | 100 | 0.61 | 4 | 144 | >0.05 | 4 | 70.5 | >0.05 |
| EB1089 | 9 | 126 | 0.11 | 4 | 136 | 0.08 | 5 | 100 | 0.06 |
Data are presented as mean value ± SEM. Statistical significance was calculated with one-way ANOVA. A value of p < 0.05 was considered significant.
DISCUSSION
Vitamin D has been postulated as a possible treatment for HCC based on studies that have reported an inverse relationship between serum 1,25(OH)2D3 levels and the risk of HCC such as the European Prospective Investigation into Cancer and Nutrition cohort study24. Moreover, overall survival rates from HCC were significantly lower in patients with serum 1α,25(OH)2D3 concentrations ≤10 ng/ml compared with those with concentrations >10 ng/ml25. We directly evaluated the effect of dietary vitamin D supplementation or treatment with 1α,25(OH)2D3 analogs on HCC induced by truncated β-catenin mutations in the absence of chronic liver disease or cirrhosis. The results reported herein conclusively demonstrate the lack of therapeutic efficacy of these interventions in HCC induced by β-catenin in the absence of coexistent liver disease or vitamin D deficiency.
Deficiency of 1α,25(OH)2D3 is associated with advanced stages of HCC and a prognostic marker for poor outcome. Vitamin D deficiency occurs in 90% of liver cancers that occur in the setting of advanced liver disease7. These results of the current study suggest that the observations indicating a relationship between vitamin D and HCC are epiphenomena of concomitant advanced liver disease that could be related to vitamin D insufficiency. Deficiency of vitamin D occurs in many types of chronic liver diseases, and clinical prognosis of chronic liver diseases has been associated with serum vitamin D levels25,26. While supplementation of vitamin D is indicated to correct deficiency, extrapolation of indications for therapeutic use of vitamin D beyond this is not warranted in the absence of preclinical evidence of efficacy.
These findings contrast with the results of experimental studies that report inhibitory effects of vitamin D analogs on HCC cell lines27,28. The systemic administration of vitamin D could have immunomodulatory or other effects that can mask true antitumor responses in vivo. The use of appropriate in vivo models that recapitulate the systemic and tumor microenvironment are necessary in order to evaluate the efficacy of treatments for HCC.
Vitamin D deficiency has been reported to promote HCC growth in smad3+/− mice through regulation of TLR7 expression and β-catenin activation29. Vitamin D deprivation induced a high tumor burden in Smad3 mutant mice through aberrant Toll-like receptor (TLR) expression as well as Wnt signaling, whereas vitamin D treatment restored levels of TGF-β pathway members and suppressed β-catenin29. Antagonism of the Wnt/β-catenin pathway by 1α,25(OH)2D3 has been reported in human colon carcinoma cells, with inhibition of expression of several β-catenin/TCF target genes such as c-MYC, TCF1, LEF1, and AXIN230,31. Various mechanisms have been proposed, all of which are dependent on VDR expression11,32,33. However, divergent results have been observed with vitamin D treatment in this setting. Administration of 1α,25(OH)2D3 analogs decreased tumor growth in APCmin/+ mice, a model characterized by upregulation of Wnt/β-catenin pathway34. However, in another study using the same model, 1α,25(OH)2D3 analogs did not alter the growth rate of colonic tumors35.
These divergent effects could have arisen from differences in treatment duration, effects of preexisting vitamin D deficiency on other signaling pathways and genes, or variations in VDR expression. Genetic variations in the expression or activity of VDR and vitamin D-metabolizing enzymes in HCC, as well as systemic effects related to the underlying liver disease and associated comorbidities could impact on therapeutic responses to vitamin D17. We used a model of HCC with a defined oncogenetic driver for which direct modulation by vitamin D was expected. However, these findings do not exclude an antitumor effect of vitamin D in HCC induced by other gene mutations. The use of synthetic transgenic mice models provides the opportunity for targeted assessment of therapeutic efficacy in HCC with other defined genetic drivers. Taken together, our observations emphasize the need for careful preclinical assessment of therapeutic strategies for HCC based on the use of vitamin D or its analogs.
Footnotes
The authors declare no conflicts of interests.
REFERENCES
- 1. Mittal S, El-Serag HB. Epidemiology of hepatocellular carcinoma: Consider the population. J Clin Gastroenterol. 2013;47(Suppl):S2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wallace MC, Preen D, Jeffrey GP, Adams LA. The evolving epidemiology of hepatocellular carcinoma: A global perspective. Expert Rev Gastroenterol Hepatol. 2015;9(6):765–79. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–90. [DOI] [PubMed] [Google Scholar]
- 4. Giovannucci E. Epidemiology of vitamin D and colorectal cancer. Anticancer Agents Med Chem. 2013;13(1):11–9. [PubMed] [Google Scholar]
- 5. Krishnan AV, Feldman D. Mechanisms of the anti-cancer and anti-inflammatory actions of vitamin D. Annu Rev Pharmacol Toxicol. 2011;51:311–36. [DOI] [PubMed] [Google Scholar]
- 6. Giovannucci E, Liu Y, Rimm EB, Hollis BW, Fuchs CS, Stampfer MJ, Willett WC. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J Natl Cancer Inst. 2006;98(7):451–9. [DOI] [PubMed] [Google Scholar]
- 7. Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, Donato MF, Piva A, Di Carlo V, Dioguardi N. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325(10):675–80. [DOI] [PubMed] [Google Scholar]
- 8. Fleet JC, DeSmet M, Johnson R, Li Y. Vitamin D and cancer: A review of molecular mechanisms. Biochem J. 2012;441(1):61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pereira F, Larriba MJ, Munoz A. Vitamin D and colon cancer. Endocr Relat Cancer 2012;19(3):R51–71. [DOI] [PubMed] [Google Scholar]
- 10. Leyssens C, Verlinden L, Verstuyf A. Antineoplastic effects of 1,25(OH)2D3 and its analogs in breast, prostate and colorectal cancer. Endocr Relat Cancer 2013;20(2):R31–47. [DOI] [PubMed] [Google Scholar]
- 11. Larriba MJ, Gonzalez-Sancho JM, Barbachano A, Niell N, Ferrer-Mayorga G, Munoz A. Vitamin D is a multilevel repressor of Wnt/b-catenin signaling in cancer cells. Cancers (Basel) 2013;5(4):1242–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yant SR, Park J, Huang Y, Mikkelsen JG, Kay MA. Mutational analysis of the N-terminal DNA-binding domain of sleeping beauty transposase: Critical residues for DNA binding and hyperactivity in mammalian cells. Mol Cell Biol. 2004;24(20):9239–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bell JB, Podetz-Pedersen KM, Aronovich EL, Belur LR, McIvor RS, Hackett PB. Preferential delivery of the Sleeping Beauty transposon system to livers of mice by hydrodynamic injection. Nat Protoc. 2007;2(12):3153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tward AD, Jones KD, Yant S, Cheung ST, Fan ST, Chen X, Kay MA, Wang R, Bishop JM. Distinct pathways of genomic progression to benign and malignant tumors of the liver. Proc Natl Acad Sci USA 2007;104(37):14771–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shang N, Arteaga M, Zaidi A, Stauffer J, Cotler SJ, Zeleznik-Le NJ, Zhang J, Qiu W. FAK is required for c-Met/beta-catenin-driven hepatocarcinogenesis. Hepatology 2015;61(1):214–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tao J, Xu E, Zhao Y, Singh S, Li X, Couchy G, Chen X, Zucman-Rossi J, Chikina M, Monga SP. Modeling a human hepatocellular carcinoma subset in mice through coexpression of met and point-mutant beta-catenin. Hepatology 2016;64(5):1587–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: Potential for anticancer therapeutics. Nat Rev Cancer 2007;7(9):684–700. [DOI] [PubMed] [Google Scholar]
- 18. van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ, Schiffelers RM. Microvesicles and exosomes: Opportunities for cell-derived membrane vesicles in drug delivery. J Control Release 2012;161(2):635–44. [DOI] [PubMed] [Google Scholar]
- 19. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: Incidence and risk factors. Gastroenterology 2004;127(5 Suppl 1):S35–50. [DOI] [PubMed] [Google Scholar]
- 20. Dai S, Wei D, Wu Z, Zhou X, Wei X, Huang H, Li G. Phase I clinical trial of autologous ascites-derived exosomes combined with GM-CSF for colorectal cancer. Mol Ther. 2008;16(4):782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Escudier B, Dorval T, Chaput N, Andre F, Caby MP, Novault S, Flament C, Leboulaire C, Borg C, Amigorena S, et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of the first phase I clinical trial. J Transl Med. 2005;3(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Spina C, Tangpricha V, Yao M, Zhou W, Wolfe MM, Maehr H, Uskokovic M, Adorini L, Holick MF. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J Steroid Biochem Mol Biol. 2005;97(1–2):111–20. [DOI] [PubMed] [Google Scholar]
- 24. Fedirko V, Duarte-Salles T, Bamia C, Trichopoulou A, Aleksandrova K, Trichopoulos D, Trepo E, Tjonneland A, Olsen A, Overvad K, et al. Prediagnostic circulating vitamin D levels and risk of hepatocellular carcinoma in European populations: A nested case-control study. Hepatology 2014;60(4):1222–30. [DOI] [PubMed] [Google Scholar]
- 25. Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55(9):2624–8. [DOI] [PubMed] [Google Scholar]
- 26. Chen EQ, Shi Y, Tang H. New insight of vitamin D in chronic liver diseases. Hepatobiliary Pancreat Dis Int. 2014;13(6):580–5. [DOI] [PubMed] [Google Scholar]
- 27. Ghous Z, Akhter J, Pourgholami MH, Morris DL. Inhibition of hepatocellular cancer by EB1089: In vitro and in vive study. Anticancer Res. 2008;28(6a):3757–61. [PubMed] [Google Scholar]
- 28. Chiang KC, Yeh CN, Chen MF, Chen TC. Hepatocellular carcinoma and vitamin D: A review. J Gastroenterol Hepatol. 2011;26(11):1597–603. [DOI] [PubMed] [Google Scholar]
- 29. Chen J, Katz LH, Munoz NM, Gu S, Shin JH, Jogunoori WS, Lee MH, Belkin MD, Kim SB, White JC, et al. Vitamin D deficiency promotes liver tumor growth in transforming growth factor-beta/Smad3-deficient mice through Wnt and Toll-like receptor 7 pathway modulation. Sci Rep. 2016;6:30217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. MJ L. The inhibition of Wnt/beta-catenin signalling by 1alpha,25-dihydroxyvitamin D3 is abrogated by Snail1 in human colon cancer cells. Endocr Relat Cancer 2007;14(1):141–51. [DOI] [PubMed] [Google Scholar]
- 31. Larriba MJ, Ordonez-Moran P, Chicote I, Martin-Fernandez G, Puig I, Munoz A, Palmer HG. Vitamin D receptor deficiency enhances Wnt/beta-catenin signaling and tumor burden in colon cancer. PLoS One 2011;6(8):e23524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, et al. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154(2):369–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aguilera O, Pena C, Garcia JM, Larriba MJ, Ordonez-Moran P, Navarro D, Barbachano A, Lopez de Silanes I, Ballestar E, Fraga MF, et al. The Wnt antagonist DICKKOPF-1 gene is induced by 1alpha,25-dihydroxyvitamin D3 associated to the differentiation of human colon cancer cells. Carcinogenesis 2007;28(9):1877–84. [DOI] [PubMed] [Google Scholar]
- 34. Xu H, Posner GH, Stevenson M, Campbell FC. Apc(MIN) modulation of vitamin D secosteroid growth control. Carcinogenesis 2010;31(8):1434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Irving AA, Halberg RB, Albrecht DM, Plum LA, Krentz KJ, Clipson L, Drinkwater N, Amos-Landgraf JM, Dove WF, DeLuca HF. Supplementation by vitamin D compounds does not affect colonic tumor development in vitamin D sufficient murine models. Arch Biochem Biophys. 2011;515(1–2):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]