Abstract
How a disease is transmitted affects our ability to determine R0, the average number of new cases caused by an infectious host at the onset of an epidemic. R0 becomes progressively more difficult to compute as transmission varies from directly transmitted diseases to diseases that are vector-borne to environmentally transmitted diseases. Pathogens responsible for diseases with environmental transmission are typically maintained in environmental reservoirs that exhibit a complex spatial distribution of local infectious zones (LIZs). Understanding host encounters with LIZs and pathogen persistence within LIZs is required for an accurate R0 and modeling these contacts requires an integrated geospatial and dynamical systems approach. Here we review how interactions between host and pathogen populations and environmental reservoirs are driven by landscape-level variables, and synthesize the quantitative framework needed to formulate outbreak response and disease control.
Keywords: basic reproductive number (R0), indirect disease transmission, disease emergence, disease control, pathogen spillover, animal movement
1. Introduction
Environmental factors play a decisive role in the emergence of infectious diseases, particularly when indirect transmission is involved. Briefly, pathogen transmission can be defined as direct when an infected individual can infect another, such as classical influenza air-borne transmission or HIV sexual transmission. Indirect transmission occurs when the pathogen is acquired from the environment, such as grazing on pathogen-contaminated feed, or a vector, such as an insect, infecting one individual after feeding on another infected individual. Estimating indirect transmission requires that we evaluate the impact of environmental factors on the basic reproductive number, R0, of a pathogen, as has been done in the context of the recent Ebola outbreak in Africa [1], and mosquito-transmitted Zika virus [2]. The number of new cases from a single case, the R0, has long been the basis for assessing outbreak severity and control impacts. Here we provide a brief overview of R0 before providing an overview of challenges to these calculations for pathogens that persist in the environment for long periods.
1.1. A Brief History of R0
1925–1975: R0 is central to our understanding of both population growth and the spread of disease. As reviewed by Heesterbeek [3], Dublin and Lotka [4] were the first to cast R0 as “… the number of female offspring born to one female during her lifetime.” In the context of epidemiology, Dietz [5] defined R0 as “… the number of secondary cases that one case can produce if introduced in a susceptible population.”
1976–1990: The role of R0 in epidemiology received a major boost in 1982 at a conference on Population Biology of Infectious Disease in Dahlem, Germany (R. M. Anderson and R. M. May, organizers). Calculating R0 from data became a major challenge in the 1980s due to inhomogeneities, such as variation in individual susceptibility. This problem was solved by Diekmann et al. [6] in providing a method of calculating a next generation matrix whose dominant eigenvalue is R0. Using R0, however, to determine the actual growth rate of an epidemic requires that the generation time G also be known.
Modern demography: If lx is the proportion of individuals surviving to exact age x and bx is the force of natality (i.e., is the number of female young born to each female in the interval [i,i + 1)), then:
| (1) |
Indirectly transmitted diseases: The next generation matrix approach of Diekmann et al. [6], applicable to directly transmitted diseases, was extended by van Driessche and Watmough [7] to class-structured population processes that accounted for both new within-class infections and the transfer of infection among classes. The structure of this approach could then be applied, for example, to calculating R0 in vector-host-pathogen transmission systems.
Critique: Methods for calculating R0 have been criticized for conflating processes affecting disease transmission. For example, Li et al. [8] pointed to the fact that vertical transmission events may be cancelled out by disease induced mortality events in differential equation models, but not in next-generation matrix methods, leading to ambiguity in the calculation of R0. However, this appears to be a shortcoming of oversimplifying differential equation models of transmission, rather than next-generation matrix methods per se. This conundrum is only resolvable through more careful formulation of the way R0 is computed for ecologically complicated epidemics, such as those that have a determinative environmental component. Long-lived environmental pathogens present exemplar cases for challenging the R0 formulations currently available and require new strategies to estimate transmission.
1.2. Challenges for R0 for Environmentally Maintained Pathogens
For pathogens, such as Bacillus anthracis [9], the causative bacterium of anthrax, and chronic wasting disease (CWD) [10], that persist in spatially limited environmental reservoirs, estimates of R0 to date have not explicitly included their spatial structure, which can be characterized as a distribution of host carcass-generated local infectious zones (LIZs) distributed over the landscape. In such cases, exposure depends on susceptible individuals arriving at the reservoir and contacting the pathogen [11], for example through ingestion [12] or inhalation [13]. These LIZs may remain infectious for some period of time, ranging from hours (Ebola virus [14]) to weeks (Mycoplasma bovis [15]) to months (Brucella abortus [16]) to decades (Bacillus anthracis [17]), with subsequent exposures arising from additional naïve hosts seeking resources within the reservoir. In the case of pathogens that persist across host generations, a traditional susceptible, exposed, infected, recovered (SEIR) model [18] cannot adequately capture exposure at LIZs. Our central tenet is that successfully modeling LIZ exposure dynamics requires an integrated geospatial and mathematical approach, tracking seasonal changes on the landscape and the effects of those changes on host movements (resource selection, site fidelity, and foraging) and pathogen persistence within LIZs (Figure 1).
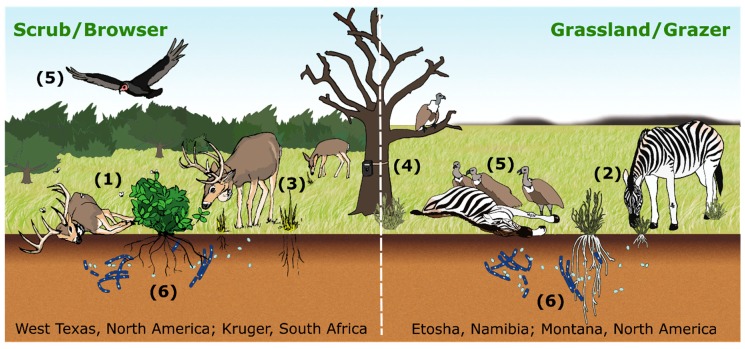
Figure 1.
Conceptual diagram of anthrax transmission from hosts interacting with local infectious zones (LIZs) on two different landscapes. Transmission to browsers, here white-tailed deer in the scrub habitat of west Texas (left panel), can occur through ingestion of contaminated vegetation, which can be amplified by blow flies and biting flies (1). Grazers, here zebras in Etosha National Park, Namibia (right panel), are exposed through ingesting contaminated grasses and soils (2). On both landscapes, host movements are recorded with GPS collars (3), foraging at LIZs is captured with camera traps (4) and mortality is found by following vultures to carcasses (5). B. anthracis persists in soil and may have a soil-borne life cycle in both systems (6). Flies do not play a major role in open grassland grazing systems, particularly when vertebrate scavengers are abundant, but may in the browser systems.
In support of this tenet, we present a spatially explicit pathogen-reservoir-host formulation that gives rise to a natural mathematical outbreak description in which both temporal and spatial processes occurring at local and larger scales are linked in meaningful ways and is broadly applicable across several pathogen/host systems (Table 1). We show how such a formulation can help to test the idea that seasonal outbreaks contribute to the accumulation of LIZs to fuel future outbreaks. The spatial and temporal processes involved are driven by vegetation phenology ([19]; or aquatic analogs [20]), climatic variables, and host/LIZ interactions (Figure 1).
Table 1.
Several important diseases caused by pathogens (including bacteria, fungi, prions, and parasites) with environmentally maintained reservoirs.
| Disease | Pathogen | Host | Environmental Reservoir | Local Infectious Zone (LIZ) | Landscape Characteristics | Survival Time in Environment | References |
|---|---|---|---|---|---|---|---|
| Anthrax | Bacillus anthracis | Wildlife and livestock | Host, bones, soil, water, vegetation | Carcass site, water’s edge | Grasslands, scrub/pothole regions | >1 year | [9] |
| Botulism | Clostridium botulinum | Birds & mammals | Host, honey, soil | Carcass site, honeybee colony | Cosmopolitan | >1 year | [21] |
| Bovine mastitis | Mycoplasma bovis | Bovids | Host, soil and/or animal bedding | Bedding within feedlot | Broad conditions | ~1 year (needs futher study) | [22] |
| Brucellosis | Brucella spp. | Wildlife and livestock | Host, soil and/or birthing tissues, aborted fetuses | birthing tissues and aborted fetuses | ~20–80 days (needs further study) | [16] | |
| Cholera | Vibrio cholerae | Humans | Host, feces, zooplankton, saltwater | Estuaries | Periurban, coastal regions | [23,24] | |
| Leptospirosis | Leptospira spp. | Animals, humans | Host, grass, moist soil, water | Grasslands, streams, rivers, ponds, lakes | Periurban, contaminated lakes | [25] | |
| Chronic wasting disease | Prions | Cervids | Host, some soils | Salt/mineral sites, wallows | Host range & soils overlap | [26,27,28] | |
| White-nosed syndrome | Psuedogymnoascus destructans | Hibernating bats | Host, some soils | Bat hibernacula | Cave system or mountain range | [29,30] | |
| Toxoplasmosis | Toxoplasma gondii | Mammals | Host, feces, soil, invertebrates | Soils, streams, bays, estuaries | Periurban areas, coastal regions | [31,32] |
By way of example, in Figure 1 we illustrate anthrax transmission (as an example of environmental transmission) on two landscapes: (1) the scrub habitat of west Texas (left panel), where blow flies [33] and biting flies [34] can increase local case intensity of white-tailed deer (Odocoileus virginianus) predominantly browsing during the outbreak season; and (2) the grasslands of Etosha National Park in Namibia (Etosha; right panel), where grazing near LIZs during the wet season is the primary mode of transmission [11,12]. On each of these landscapes LIZ persistence has been confirmed. Furthermore, movement data and foraging behavior observations (e.g., camera traps [11]) strongly suggest the formulation of a new R0; in these landscapes, it is the host interaction with carcasses (both landscapes) or fly-contaminated browse (Texas scrub; left panel) that drives the exposure and subsequent infection. In Figure 2, we illustrate how such host/LIZ interactions can be compartmentalized in an SEIR framework. The formulation itself suggests that while controlling individual outbreaks may be strategically desirable, reducing the number of local infectious zones may be a better long-term strategy for disease management.
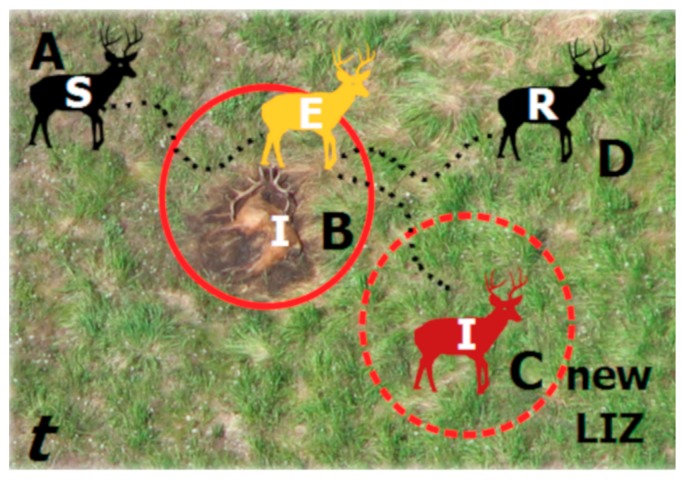
Figure 2.
Components of an SEIR model as applied to environmental transmission during a single outbreak season (t). Susceptible hosts (S) move across the landscape (A) and contact infectious LIZs (I) and become exposed (E; B). As they leave the LIZ, they may succumb to infection and die, becoming a new LIZ, establishing in time t and persisting across future time periods (t + 1,…,n) (C) or recover (R) and survive to a future time period (D).
Under-studied aspects of the role of environment in pathogen transmission are the mechanisms that permit pathogens to be maintained in reservoirs [35,36]. Some pathogens may remain dormant, with no reproductive activity within soil or aquatic environments, while other pathogens can multiply in the environment. For example, non-reproducing Leptospira spirochetes can persist for several months in soils in the absence of a mammalian host [25]. A causative agent of cattle disease, M. bovis persists for long periods and may replicate in sandy soils used as bedding under certain conditions [22]. CWD-causing prions can also survive for long periods in soil [26]. In each case, the role of environmental factors in governing pathogen persistence, such as soil alkalinity, moisture, or specific mineral content, are poorly understood.
Empirical data are needed to assess the time constants of processes involved in indirect pathogen transmission (e.g., replication rates in the environment; half-life decay rates of LIZs), as well as to parameterize transmission models. In the case of soil-borne B. anthracis, recent studies on the environmental conditions that support pathogen persistence include ecological niche modeling [37,38,39]), host seasonal resource selection in risk areas [40], host foraging behavior at LIZs [11,41], seasonal changes in host diet [12], and seasonal fluctuations in host antibody titers to B. anthracis [42]. These studies allow us to parameterize tactical models that estimate the force of infection (within or between species) within an anthrax transmission season on a single landscape. The long-lived nature of LIZs, however, demands the formulation of strategic models able to reliably estimate the number of “offspring” LIZs generated by a “parent” LIZ and to quantify the force of infection (i.e., persistence) across multiple seasons or years. Similarly, in the case of the water-borne bacterium Vibrio cholerae, the causative agent of cholera, sufficient data exist [26,37,40] to construct strategic models that account for transmission enhancement and seasonal affects through V. cholerae associations with zooplankton [23,24,41,42]. Likewise, a growing number of studies find that some bacterial pathogens, including B. anthracis [43], B. thuringiensis [44], Salmonella enterica [45], and Escherichia coli [46], may replicate in the rhizosphere or directly on plants. In addition to providing resources for replication, such environmental reservoirs may promote alternative pathogen transmission routes.
2. Host Interaction with Local Infectious Zones (LIZs) at the Landscape Level
In the 19th century, Louis Pasteur identified carcass sites as key to anthrax transmission [47], while 45 years ago Van Ness [48] proposed that under certain conditions, B. anthracis maintains high population densities in areas where it multiplies in the environment. Observations of naturally occurring carcass sites in Etosha indicate that spores persist for at least several years after carcass decomposition [11,49]. Environmental reservoirs may be influenced by nutrient availability, weather patterns, and bio-geo-chemical parameters [43,48,50]. Features of the exosporium can affect the ability of B. anthracis spores to bind to different soil types [51,52]. Thus, the rate at which a spore pool decays likely depends on local conditions [9]. CWD prion persistence and infectiousness also varies with soil type [26] and consequences of this variation in environmental persistence was examined with disease modeling [10]. In addition to physical and chemical variables affecting spore persistence, biological interactions between carcass materials and other species occurring in environmental reservoirs may alter the exposure of animal hosts to pathogens [53]. For example, hosts may be attracted to the growth and quality of vegetation that arises from nutrient deposition from carcasses [54,55,56,57]. Using camera traps, host visitation and duration rates at LIZs were quantified in the field for anthrax [11], M. bovis [58] and CWD [59]. These data are readily incorporated into disease models. Landscapes that support LIZ persistence can be characterized using habitat mapping procedures [37,40]: in the case of B. anthracis at continental [37,39,60,61] and local [40,62] scales. Similar approaches were employed for cholera [63] and brucellosis [64]. Kracalik et al. [65,66] used logistic regression approaches to estimate anthrax risk and Osnas et al. [67] employed a hierarchical Bayesian approach to produce spatially explicit estimates of prevalence for a wildlife CWD outbreak.
2.1. Local Infectious Zone (LIZ) Ecology
In the case of anthrax, seasonal peaks in disease incidence are commonly observed [9,12,51]. For example, major anthrax epizootics follow rain events or seasonal changes in green-up trajectories [19], particularly in grass [68] and shrubland [69]. Due to increased nutrient availability, plant growth at LIZs may outpace growth in non-LIZ areas, potentially promoting host foraging. In a field experiment, B. anthracis spores substantially increased the rate of establishment for a native grass, which was also taller when treated with blood [70]. Tall lush growth of plants resulting from a combination of the influence of the bacterium and input of nutrients from decaying carcasses may be attractive to grazing hosts and promote disease transmission [70]. Additionally, host populations may track vegetation responses to environmental triggers and migrate locally, concentrating susceptible hosts in areas where they experience high contact rates with LIZs. Likewise, individuals may change diet preferences seasonally to plants that are greening up. Prevalence rates of Brucella or M. bovis also tend to peak seasonally, with transmission occurring where host populations commingle [71,72]. Unlike B. anthracis, these two pathogens exhibit relatively short-term survival in the environment and thus are likely to have only intra-seasonal LIZ persistence.
2.2. Host Movement Ecology and Transmission
For successful indirect transmission, hosts must contact LIZs. Estimating contact can be done across spatial scales and levels of resource selection as defined by Johnson [73]. As a first estimate of where contact may occur, one can estimate seasonal home ranges for susceptible hosts and compare those to environments that promote LIZ persistence, as was recently done for anthrax in elk (Cervus canadensis) [40] and bison (Bison bison bison) [74]. When modeling the likelihood of host presence in a LIZ region, we are most interested in local utilization distributions (UDs), where space is quantified in some form of density of animal positions over time [75,76]. Several techniques are available for quantifying these local UDs [75] in space and time [77]. New movement analysis tools, coupled with high resolution spatio-temporal data from GPS collars, allow us to directly estimate how individual re-visitation (spatial) and duration of visits [77] (spatio-temporal) to specific areas change seasonally. Such estimates can be used to parameterize movement patterns of hosts and related to LIZ concentrations. Most recently, tools were introduced to evaluate optimal parameter settings for the T-LoCoH (http://tlocoh.r-forge.r-project.org/) package for R, where home ranges and these visitation/fidelity metrics can be calculated, including examples of host/LIZ overlap in Etosha National Park (ENP), Namibia [78,79].
Seasonal and inter-annual variation in observed incidence are driven by factors affecting transmission itself, as well as factors affecting data-gathering. Anthrax incidence in Etosha, for example, is affected by LIZ demography and distribution, animal exposure to LIZs, and the immunological state of individuals [42,80]. Within the context of Johnson [73], third order selection (patch use within a home range), such as individual foraging or interactions at LIZs, can be measured using camera traps set at LIZs, as was done in ENP [11]. Observed anthrax incidence, however, differs from actual incidence because surveillance efforts are typically seasonal, though a modeling approach exists to account for this sampling bias [81]. These difficulties have been documented across disease systems, particularly in wildlife [34,82]. Thus, it is important to keep in mind how ecological and epidemiological processes interact with observation processes. For instance, anthrax outbreaks tend to be observed in Etosha after rainfall with a delay that could be explained by both ecological and observational mechanisms. Rainfall affects animal movement patterns, the splashing of spore-laden soil onto palatable grass leaves, and exposure to interacting microparasites and macroparasites [83,84]. However, there was also a significant correlation with the preponderance of wet season researcher activity [81]. In west Texas, anthrax in deer is also seasonal and has been correlated with seasonal peaks in vegetation greenup [19] and increased biting fly densities [34]. Also, case intensity may be amplified within an outbreak by blow flies [33]; the latter phenomenon can expand the zone of contamination at a LIZ during the first several days after host death.
2.3. Dynamic Thresholds and the Joint Modeling of Reservoir (LIZ) and Host Dynamics
Individual host heterogeneities, along with geographical and environmental discontinuities, might critically amplify, dampen or lag the known effects of pathogen reservoirs. A recent study illustrated host population dynamics and environmental drivers are required to model anthrax outbreak periodicity [85], however the approach was not spatially explicit. Thus, continuity of geographical expansion and homogenous mixing of individuals are unsuitable assumptions for modeling many host-pathogen systems (see [86]). An approach that can account for such inhomogeneities, yet remains relatively simple, is to specify a low-dimensional model of the host, pathogen and reservoir dynamics that explicitly incorporates spatial and temporal lag effects. In reservoir-driven epidemics, considerable effort is currently being invested in unraveling host movement and behavior characteristics that shape the host’s susceptibility, thereby providing data for within-season models of outbreaks that can be coupled to an across-season model of the reservoir or LIZ-population dynamics. Such across-scale stochastic processes are often best characterized by a combination of a fine (fast and small) scale Brownian Motion (BM) process and a coarser scale pure-jump process [87,88], which combination can be often be adequately modeled as a Lévy process [89]. These models have been applied in various biological settings, including epidemiological data [86], with a primer provided here.
3. Discrete, Self-Decomposable (DSD) Parameter Estimation
Discrete, self-decomposable (DSD) stochastic processes [90,91,92] provide a way to combine short-term seasonal outbreaks of a number of pathogen systems (Table 1) modeled by a Lévy process with long-term gradual changes within LIZs modeled by a Brownian (i.e., Gaussian) processes. The first step is to define a stochastic process for the number of LIZs present within the smallest spatial and temporal resolution unit of the data (e.g., the 250 m2 pixel dimensions and 16-day NDVI measurements of the MODIS satellite system). The second step is to formulate a DSD probability generating function that is a composition of two distinct stochastic processes: a stochastic LIZ decay process and an “innovation” (random generation of new LIZs) jump process [91]. The resulting DSD process is then able to model jumps (various formulations can used [88,92]) in the LIZ sample path, as it accounts for abrupt changes in the number of LIZs within a pixel (noting that alternative, as well as accommodating LIZ persistence and LIZ “arrival” process parameters that are dependent on environmental covariates (thereby providing a natural test bed for the relevance of covariates). The arrival of naïve hosts to a pixel establishes a susceptible population, estimated from host movement data. At the population level, resource selection function-based probabilities can be used to define the likelihood a host will choose a pixel [40] (derived from GPS collar data and a use-available framework to model resource selection [93]), while visitation and duration metrics can be used to estimate the length of stay and number of return visits to a given pixel [77] (applying the T-LoCoH metrics to GPS collar data, e.g., [78]). Estimating foraging activity at LIZs within the pixel then effectively provides an informed measure of exposure rate. Camera trap data can be used to estimate foraging activity within a pixel, as was applied in ENP [11].
If LIZ dynamics are modeled as a first-order Markov process, then maximum likelihood estimation allows the calculation of the mean number of LIZs remaining in any pixel after d time steps. In its simplest formulation, the number of LIZs over time follows a Poisson process that implicitly assumes the persistence process is temporally homogeneous, thereby neglecting the effect of the covariates and links to host movement. To make the model spatially explicit, one can model the LIZ decay (epidemiological recovery) and LIZ innovation (epidemiological incidence) processes using environmental covariates in resource selection functions. To account for temporal heterogeneity in the abundance of LIZs, the well-known derivation of the Negative Binomial as a conditional Poisson distribution can be used, where the innovation rate itself is Gamma distributed [94]. The mean of this Gamma process can be made a function of environmental covariates. Then, LIZ persistence can be modeled using a combination of a deterministic trend (e.g., exponential decay) and random fluctuations due to environmental variation. Other elaborations are possible to make this approach more realistic, depending on the quantity and quality of data available to support identification and selection of models with additional complexity [95].
Estimating the probability of a threshold condition for disease emergence, a DSD model can be seeded with a single LIZ within a pixel in an otherwise “clean” landscape. Simple calculations using the model probabilistic structure can then be used to obtain explicit expressions of the average number of newly generated LIZs within any time frame: either after a single iteration of the process, or at the end of a season. Hence, the DSD model is a means to obtaining a “within-year” LIZ reproduction number (R0) as well as assessing the expected number of LIZs that remain on a landscape of a given size after one or more seasons (years). In this way, a DSD model connects the beginning of a given year’s zoonotic season with past dynamics, thereby providing a spatially explicit estimate of R0. With this quantitative framework in place, hypothetical “what-if” games simulating different control measures can lead to informed estimates of their effects. For example, carcass burning or burial (individual LIZ destruction) is a primary means of anthrax control during an epizootic. A DSD model can be used to simulate such a removal process by reducing the increase in LIZs between years and evaluating its impact on R0. A second type of control would be to evaluate the impact of vaccinating a host population, such as a bison herd in Montana [82], on the expected value of R0. These first two kinds of simulations would essentially assess changes to the rate of new LIZ formation due to healthy bison encountering fewer current LIZs (affected by carcass control) or reduced susceptibility (affected by vaccine coverage and efficacy). A third approach to control would be to evaluate the effect of excluding naïve hosts from pixels with LIZs. This would mimic the management strategy of using fences around pastures to keep livestock herds (such as bison in Montana) from areas of known historical outbreaks. Such exclusion would eliminate LIZ contacts by limiting the spatio-temporal jump processes to only non-excluded pixels, thereby lowering the average of the innovation process in the DSD model. The DSD framework provides a way to use empirical data to conduct plausible simulations relating to control strategies that are not possible to directly evaluate on real landscapes, but are likely to inform decisions, with applicability to several pathogens (Table 1).
4. Conclusions
The emergence of diseases caused by environmentally-maintained indirectly transmitted pathogens depends upon local and landscape-level variables, complicating disease modeling efforts. The analytical approaches synthesized here capitalize on advances in our knowledge of pathogen persistence, and high-resolution host movement and foraging behavior to estimate the basic reproductive number, R0 for such pathogens, using LIZs or patches of infection on the landscape. We illustrate that data are required from each the host population, the pathogen population, and the LIZs, as a separate and integral part of the modeling process. Data collection for such an approach should include monitoring and measurement of each population.
This quantitative framework is needed by real world stakeholders of agricultural and wildlife resources for them to manage environmentally transmitted diseases. As an example, anthrax is a globally occurring disease presenting a wide range of control challenges. Eradication of anthrax in Etosha for example, could negatively impact predator/scavenger populations [96], while eradication in commercial bison herds in Montana is highly desirable [82]. Modeling the effects of control in these systems also applies broadly to other landscapes, such as northern Canada, where anthrax threatens the survival of the endangered wood bison [97], and the Republic of Georgia, where livestock and human anthrax is re-emerging [68,98]. However, this modeling strategy is not anthrax specific, and can be extrapolated to other disease systems with environmentally-mediated indirect transmission, such as cholera, chronic wasting disease [27], brucellosis [71], or bovine tuberculosis [99], which are all associated with significant disease risk in multiple hosts, including humans.
Acknowledgments
Figure 1 was drawn and colored by Kelsey Wood.
Author Contributions
Conceptualization, J.K.B., H.H.G., J.M.P., W.M.G.; methodology, J.K.B., H.H.G., J.M.P., S.J.R., W.C.T.; resources, J.K.B., W.M.G., N.C.S.; writing—original draft preparation, J.K.B., H.H.G., J.M.P., S.J.R., W.M.G.; writing—review and editing, all authors; visualization, J.K.B., H.H.G., S.J.R., W.M.G.; supervision, J.K.B., W.M.G.; project administration, J.K.B., W.M.G., N.C.S.; funding acquisition, J.K.B., J.M.P., S.J.R., W.M.G., H.H.G., R.D.H., N.C.S.
Funding
Funding for this study was provided by the National Institutes of Health Grant 1R01GM117617-01 to J.K.B., J.M.P., S.J.R., R.D.H., W.M.G. the College of Liberals Arts and Sciences and the Emerging Pathogens Institute at the University of Florida. Additional funding was provided by the Centre for Ecological and Evolutionary Synthesis, University of Oslo and the Sather Foundation. H.H.G. was supported by a grant to JAE from the Alfred P. Sloan Foundation as part of their “Microbiology of the Built Environment” program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dixon M.G., Schafer I.J. Ebola viral disease outbreak—West Africa, 2014. MMWR Morb. Mortal. Wkly. Rep. 2014;63:548–551. [PMC free article] [PubMed] [Google Scholar]
- 2.Bowman C., Gumel A., Van den Driessche P., Wu J., Zhu H. A mathematical model for assessing control strategies against West Nile virus. Bull. Math. Biol. 2005;67:1107–1133. doi: 10.1016/j.bulm.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Heesterbeek J. A brief history of R0 and a recipe for its calculation. Acta Biotheor. 2002;50:189–204. doi: 10.1023/A:1016599411804. [DOI] [PubMed] [Google Scholar]
- 4.Dublin L.I., Lotka A.J. On the true rate of natural increase: As exemplified by the population of the United States, 1920. J. Am. Stat. Assoc. 1925;20:305–339. doi: 10.1080/01621459.1925.10503498. [DOI] [Google Scholar]
- 5.Dietz K. Transmission and Control of Arbovirus Diseases; Proceedings of the SIMS Conference on Epidemiology; Alta, UT, USA. 8–12 July 1974; Philadelphia, PA, USA: Society for Industrial and Applied Mathematics; 1975. pp. 104–121. [Google Scholar]
- 6.Diekmann O., Heesterbeek J., Metz J.A. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990;28:365–382. doi: 10.1007/BF00178324. [DOI] [PubMed] [Google Scholar]
- 7.Van den Driessche P., Watmough J. Reproduction numbers and sub-threshold endemic equilibria for compartmental models of disease transmission. Math. Biosci. 2002;180:29–48. doi: 10.1016/S0025-5564(02)00108-6. [DOI] [PubMed] [Google Scholar]
- 8.Li J., Blakeley D., Smith R.J. The Failure of R0. Comput. Math. Methods Med. 2011;2011:527610. doi: 10.1155/2011/527610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hugh-Jones M., Blackburn J. The ecology of Bacillus anthracis. Mol. Aspects Med. 2009;30:356–367. doi: 10.1016/j.mam.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 10.Almberg E.S., Cross P.C., Johnson C.J., Heisey D.M., Richards B.J. Modeling routes of chronic wasting disease transmission: Environmental prion persistence promotes deer population decline and extinction. PLoS ONE. 2011;6:e19896. doi: 10.1371/journal.pone.0019896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turner W.C., Kausrud K.L., Krishnappa Y.S., Cromsigt J.P.G.M., Ganz H.H., Mapaure I., Cloete C.C., Havarua Z., Küsters M., Getz W.M., et al. Fatal attraction: Vegetation responses to nutrient inputs attract foraging herbivores to infectious anthrax carcass sites. Proc. R. Soc. B Biol. Sci. 2014;281 doi: 10.1098/rspb.2014.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner W.C., Imologhome P., Havarua Z., Kaaya G.P., Mfune J.K., Mpofu I.D., Getz W.M. Soil ingestion, nutrition and the seasonality of anthrax in herbivores of Etosha National Park. Ecosphere. 2013;4:1–19. doi: 10.1890/ES12-00245.1. [DOI] [Google Scholar]
- 13.Palmer M.V., Waters W.R., Whipple D.L. Aerosol exposure of white-tailed deer (Odocoileus virginianus) to Mycobacterium bovis. J. Wildl. Dis. 2003;39:817–823. doi: 10.7589/0090-3558-39.4.817. [DOI] [PubMed] [Google Scholar]
- 14.Piercy T., Smither S., Steward J., Eastaugh L., Lever M. The survival of filoviruses in liquids, on solid substrates and in a dynamic aerosol. J. Appl. Microbiol. 2010;109:1531–1539. doi: 10.1111/j.1365-2672.2010.04778.x. [DOI] [PubMed] [Google Scholar]
- 15.Palmer M.V., Whipple D.L. Survival of Mycobacterium bovis on feedstuffs commonly used as supplemental feed for white-tailed deer (Odocoileus virginianus) J. Wildl. Dis. 2006;42:853–858. doi: 10.7589/0090-3558-42.4.853. [DOI] [PubMed] [Google Scholar]
- 16.Aune K., Rhyan J.C., Russell R., Roffe T.J., Corso B. Environmental persistence of Brucella abortus in the Greater Yellowstone Area. J. Wildl. Manag. 2012;76:253–261. doi: 10.1002/jwmg.274. [DOI] [Google Scholar]
- 17.Mock M., Fouet A. Anthrax. Annu. Rev. Microbiol. 2001;55:647–671. doi: 10.1146/annurev.micro.55.1.647. [DOI] [PubMed] [Google Scholar]
- 18.Li M.Y., Muldowney J.S. Global stability for the SEIR model in epidemiology. Math. Biosci. 1995;125:155–164. doi: 10.1016/0025-5564(95)92756-5. [DOI] [PubMed] [Google Scholar]
- 19.Blackburn J.K., Goodin D.G. Differentiation of Springtime Vegetation Indices Associated with Summer Anthrax Epizootics in West Texas, USA Deer. J. Wildl. Dis. 2013;49:699–703. doi: 10.7589/2012-10-253. [DOI] [PubMed] [Google Scholar]
- 20.Colwell R.R. Infectious disease and environment: Cholera as a paradigm for waterborne disease. Int. Microbiol. 2004;7:285–289. [PubMed] [Google Scholar]
- 21.Nevas M., Lindström M., Hörman A., Keto-Timonen R., Korkeala H. Contamination routes of Clostridium botulinum in the honey production environment. Environ. Microbiol. 2006;8:1085–1094. doi: 10.1111/j.1462-2920.2006.001000.x. [DOI] [PubMed] [Google Scholar]
- 22.Justice-Allen A., Trujillo J., Corbett R., Harding R., Goodell G., Wilson D. Survival and replication of Mycoplasma species in recycled bedding sand and association with mastitis on dairy farms in Utah. J. Dairy Sci. 2010;93:192–202. doi: 10.3168/jds.2009-2474. [DOI] [PubMed] [Google Scholar]
- 23.Colwell R.R., Spira W.M. The ecology of Vibrio cholerae. Cholera. 1992;3:107–127. [Google Scholar]
- 24.Lipp E.K., Huq A., Colwell R.R. Effects of global climate on infectious disease: The cholera model. Clin. Microbiol. Rev. 2002;15:757–770. doi: 10.1128/CMR.15.4.757-770.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bharti A.R., Nally J.E., Ricaldi J.N., Matthias M.A., Diaz M.M., Lovett M.A., Levett P.N., Gilman R.H., Willig M.R., Gotuzzo E. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003;3:757–771. doi: 10.1016/S1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 26.Saunders S.E., Shikiya R.A., Langenfeld K., Bartelt-Hunt S.L., Bartz J.C. Replication efficiency of soil-bound prions varies with soil type. J. Virol. 2011;85:5476–5482. doi: 10.1128/JVI.00282-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saunders S.E., Bartelt-Hunt S.L., Bartz J.C. Occurrence, transmission, and zoonotic potential of chronic wasting disease. Emerg. Infect. Dis. 2012;18:369–376. doi: 10.3201/eid1803.110685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller M.W., Williams E.S., Hobbs N.T., Wolfe L.L. Environmental sources of prion transmission in mule deer. Emerg. Infect. Dis. 2004;10:1003–1006. doi: 10.3201/eid1006.040010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lindner D.L., Gargas A., Lorch J.M., Banik M.T., Glaeser J., Kunz T.H., Blehert D.S. DNA-based detection of the fungal pathogen Geomyces destructans in soils from bat hibernacula. Mycologia. 2011;103:241–246. doi: 10.3852/10-262. [DOI] [PubMed] [Google Scholar]
- 30.Lorch J.M., Lindner D.L., Gargas A., Muller L.K., Minnis A.M., Blehert D.S. A culture-based survey of fungi in soil from bat hibernacula in the eastern United States and its implications for detection of Geomyces destructans, the causal agent of bat white-nose syndrome. Mycologia. 2013;105:237–252. doi: 10.3852/12-207. [DOI] [PubMed] [Google Scholar]
- 31.Miller M., Gardner I., Kreuder C., Paradies D., Worcester K., Jessup D., Dodd E., Harris M., Ames J., Packham A. Coastal freshwater runoff is a risk factor for Toxoplasma gondii infection of southern sea otters (Enhydra lutris nereis) Int. J. Parasitol. 2002;32:997–1006. doi: 10.1016/S0020-7519(02)00069-3. [DOI] [PubMed] [Google Scholar]
- 32.Miller M., Miller W., Conrad P., James E., Melli A., Leutenegger C., Dabritz H., Packham A., Paradies D., Harris M. Type X Toxoplasma gondii in a wild mussel and terrestrial carnivores from coastal California: New linkages between terrestrial mammals, runoff and toxoplasmosis of sea otters. Int. J. Parasitol. 2008;38:1319–1328. doi: 10.1016/j.ijpara.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Blackburn J.K., Mullins J.C., Van Ert M., Hadfield T., O’Shea B., Hugh-Jones M.E. The necrophagous fly anthrax transmission pathway: Empirical and genetic evidence from a wildlife epizootic in West Texas 2010. Vector Borne Zoonotic Dis. 2014;14:576–583. doi: 10.1089/vbz.2013.1538. [DOI] [PubMed] [Google Scholar]
- 34.Blackburn J.K., Hadfield T.L., Curtis A.J., Hugh-Jones M.E. Spatial and temporal patterns of anthrax in white-tailed deer, Odocoileus virginianus, and hematophagous flies in West Texas during the summertime anthrax risk period. Ann. Assoc. Am. Geogr. 2014;104:939–958. doi: 10.1080/00045608.2014.914834. [DOI] [Google Scholar]
- 35.Rohani P., Breban R., Stallknecht D.E., Drake J.M. Environmental transmission of low pathogenicity avian influenza viruses and its implications for pathogen invasion. Proc. Natl. Acad. Sci. USA. 2009;106:10365–10369. doi: 10.1073/pnas.0809026106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown V.L., Drake J.M., Stallknecht D.E., Brown J.D., Pedersen K., Rohani P. Dissecting a wildlife disease hotspot: The impact of multiple host species, environmental transmission and seasonality in migration, breeding and mortality. J. R. Soc. Interface. 2013;10 doi: 10.1098/rsif.2012.0804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blackburn J.K., McNyset K.M., Curtis A., Hugh-Jones M.E. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. Am. J. Trop. Med. Hyg. 2007;77:1103–1110. doi: 10.4269/ajtmh.2007.77.1103. [DOI] [PubMed] [Google Scholar]
- 38.Mullins J., Lukhnova L., Aikimbayev A., Pazilov Y., Van Ert M., Blackburn J.K. Ecological Niche Modelling of the Bacillus anthracis A1.a sub-lineage in Kazakhstan. BMC Ecol. 2011;11:32. doi: 10.1186/1472-6785-11-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mullins J.C., Garofolo G., Van Ert M., Fasanella A., Lukhnova L., Hugh-Jones M.E., Blackburn J.K. Ecological Niche Modeling of Bacillus anthracis on Three Continents: Evidence for Genetic-Ecological Divergence? PLoS ONE. 2013;8:e72451. doi: 10.1371/journal.pone.0072451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris L.R., Proffitt K.M., Asher V., Blackburn J.K. Elk resource selection and implications for anthrax management in Montana. J. Wildl. Manag. 2016;80:235–244. doi: 10.1002/jwmg.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Havarua Z., Turner W.C., Mfune J.K. Seasonal variation in foraging behaviour of plains zebra (Equus quagga) may alter contact with the anthrax bacterium (Bacillus anthracis) Can. J. Zool. 2014;92:331–337. doi: 10.1139/cjz-2013-0186. [DOI] [Google Scholar]
- 42.Cizauskas C.A., Bellan S.E., Turner W.C., Vance R.E., Getz W.M. Frequent and seasonally variable sublethal anthrax infections are accompanied by short-lived immunity in an endemic system. J. Anim. Ecol. 2014 doi: 10.1111/1365-2656.12207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saile E., Koehler T. Bacillus anthracis multiplication, persistence, and genetic exchange in the rhizosphere of grass plants. Appl. Environ. Microbiol. 2006;72:3168–3174. doi: 10.1128/AEM.72.5.3168-3174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hendriksen N.B., Hansen B.M. Long-term survival and germination of Bacillus thuringiensis var. kurstaki in a field trial. Can. J. Microbiol. 2002;48:256–261. doi: 10.1139/w02-009. [DOI] [PubMed] [Google Scholar]
- 45.Gu G., Hu J., Cevallos-Cevallos J.M., Richardson S.M., Bartz J.A., van Bruggen A.H. Internal colonization of Salmonella enterica serovar Typhimurium in tomato plants. PLoS ONE. 2011;6:e27340. doi: 10.1371/journal.pone.0027340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon E.B., Yaron S., Matthews K.R. Transmission of Escherichia coli O157: H7 from contaminated manure and irrigation water to lettuce plant tissue and its subsequent internalization. Appl. Environ. Microbiol. 2002;68:397–400. doi: 10.1128/AEM.68.1.397-400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Debré P. Louis Pasteur. Johns Hopkins University Press; Baltimore, MD, USA: 1998. [Google Scholar]
- 48.Van Ness G.B. Ecology of Anthrax. Science. 1971;172:1303–1307. doi: 10.1126/science.172.3990.1303. [DOI] [PubMed] [Google Scholar]
- 49.Lindeque P.M., Turnbull P.C.B. Ecology and Epidemiology of Anthrax in the Etosha-National-Park, Namibia. Onderstepoort J. Vet. Res. 1994;61:71–83. [PubMed] [Google Scholar]
- 50.Ganz H.H., Karaoz U., Getz W.M., Versfeld W., Brodie E.L. Diversity and structure of soil bacterial communities associated with vultures in an African savanna. Ecosphere. 2012;3:art47. doi: 10.1890/ES11-00333.1. [DOI] [Google Scholar]
- 51.Manchee R.J., Broster M.G., Melling J., Henstridge R.M., Stagg A.J. Bacillus-Anthracis on Gruinard Island. Nature. 1981;294:254–255. doi: 10.1038/294254a0. [DOI] [PubMed] [Google Scholar]
- 52.Williams G., Linley E., Nicholas R., Baillie L. The role of the exosporium in the environmental distribution of anthrax. J. Appl. Microbiol. 2013;114:396–403. doi: 10.1111/jam.12034. [DOI] [PubMed] [Google Scholar]
- 53.Raymond B., Wyres K.L., Sheppard S.K., Ellis R.J., Bonsall M.B. Environmental factors determining the epidemiology and population genetic structure of the Bacillus cereus group in the field. PLoS Pathog. 2010;6:e1000905. doi: 10.1371/journal.ppat.1000905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Towne E.G. Prairie vegetation and soil nutrient responses to ungulate carcasses. Oecologia. 2000;122:232–239. doi: 10.1007/PL00008851. [DOI] [PubMed] [Google Scholar]
- 55.Danell K., Berteaux D., Bråthen K.A. Effect of muskox carcasses on nitrogen concentration in tundra vegetation. Arctic. 2002:389–392. doi: 10.14430/arctic723. [DOI] [Google Scholar]
- 56.Bump J.K., Peterson R.O., Vucetich J.A. Wolves modulate soil nutrient heterogeneity and foliar nitrogen by configuring the distribution of ungulate carcasses. Ecology. 2009;90:3159–3167. doi: 10.1890/09-0292.1. [DOI] [PubMed] [Google Scholar]
- 57.Melis C., Selva N., Teurlings I., Skarpe C., Linnell J.D.C., Andersen R. Soil and vegetation nutrient response to bison carcasses in Białowieża Primeval Forest, Poland. Ecol. Res. 2007;22:807–813. doi: 10.1007/s11284-006-0321-4. [DOI] [Google Scholar]
- 58.Kukielka E., Barasona J., Cowie C., Drewe J., Gortazar C., Cotarelo I., Vicente J. Spatial and temporal interactions between livestock and wildlife in South Central Spain assessed by camera traps. Prev. Vet. Med. 2013;112:213–221. doi: 10.1016/j.prevetmed.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 59.VerCauteren K.C., Burke P.W., Phillips G.E., Fischer J.W., Seward N.W., Wunder B.A., Lavelle M.J. Elk use of wallows and potential chronic wasting disease transmission. J. Wildl. Dis. 2007;43:784–788. doi: 10.7589/0090-3558-43.4.784. [DOI] [PubMed] [Google Scholar]
- 60.Joyner T., Lukhnova L., Pazilov Y., Temiralyeva G., Hugh-Jones M., Aikimbayev A., Blackburn J. Modeling the potential distribution of Bacillus anthracis under multiple climate change scenarios for Kazakhstan. PLoS ONE. 2010;5:e9596. doi: 10.1371/journal.pone.0009596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Blackburn J. Integrating geographic information systems and ecological niche modeling into disease ecology: A case study of Bacillus anthracis in the United States and Mexico. In: O’Connell K.P., Skowronski E.W., Sulakvelidze A., Bakanidze L., editors. Emerging and Endemic Pathogens: Advances in Surveillance, Detection, and Identification. Springer; Dordrecht, The Netherlands: 2010. pp. 59–88. [Google Scholar]
- 62.Steenkamp P.J. Master’s Thesis. University of Pretoria; Pretoria, South Africa: Jan, 2013. Ecological Suitability Modelling for Anthrax in the Kruger National Park, South Africa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Escobar L.E., Ryan S.J., Stewart-Ibarra A.M., Finkelstein J.L., King C.A., Qiao H., Polhemus M.E. A global map of suitability for coastal Vibrio cholerae under current and future climate conditions. Acta Trop. 2015;149:202–211. doi: 10.1016/j.actatropica.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 64.Jia P., Joyner A. Human brucellosis occurrences in inner mongolia, China: A spatio-temporal distribution and ecological niche modeling approach. BMC Infect. Dis. 2015;15:36. doi: 10.1186/s12879-015-0763-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kracalik I.T., Blackburn J.K., Lukhnova L., Pazilov Y., Hugh-Jones M.E., Aikimbayev A. Analysing the spatial patterns of livestock anthrax in Kazakhstan in relation to environmental factors: A comparison of local (Gi*) and morphology cluster statistics. Geospatial Health. 2012;7:111–126. doi: 10.4081/gh.2012.110. [DOI] [PubMed] [Google Scholar]
- 66.Kracalik I.T., Malania L., Tsertsvadze N., Manvelyan J., Bakanidze L., Imnadze P., Tsanava S., Blackburn J.K. Evidence of Local Persistence of Human Anthrax in the Country of Georgia Associated with Environmental and Anthropogenic Factors. PLoS Negl. Trop. Dis. 2013;7:e2388. doi: 10.1371/journal.pntd.0002388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Osnas E.E., Heisey D.M., Rolley R.E., Samuel M.D. Spatial and temporal patterns of chronic wasting disease: Fine-scale mapping of a wildlife epidemic in Wisconsin. Ecol. Appl. 2009;19:1311–1322. doi: 10.1890/08-0578.1. [DOI] [PubMed] [Google Scholar]
- 68.Parkinson R., Rajic A., Jenson C. Investigation of an anthrax outbreak in Alberta in 1999 using a geographic information system. Can. Vet. J. 2003;44:315–318. [PMC free article] [PubMed] [Google Scholar]
- 69.Turner A., Galvin J., Rubira R., Miller G. Anthrax explodes in an Australian summer. J. Appl. Microbiol. 1999;87:196–199. doi: 10.1046/j.1365-2672.1999.00869.x. [DOI] [PubMed] [Google Scholar]
- 70.Ganz H.H., Turner W.C., Brodie E.L., Kusters M., Shi Y., Sibanda H., Torok T., Getz W.M. Interactions between Bacillus anthracis and Plants May Promote Anthrax Transmission. PLoS Negl. Trop. Dis. 2014;8:e2903. doi: 10.1371/journal.pntd.0002903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Proffitt K.M., Gude J.A., Hamlin K.L., Garrott R.A., Cunningham J.A., Grigg J.L. Elk distribution and spatial overlap with livestock during the brucellosis transmission risk period. J. Appl. Ecol. 2011;48:471–478. doi: 10.1111/j.1365-2664.2010.01928.x. [DOI] [Google Scholar]
- 72.O’Brien D.J., Schmitt S.M., Fitzgerald S.D., Berry D.E., Hickling G.J. Managing the wildlife reservoir of Mycobacterium bovis: The Michigan, USA, experience. Vet. Microbiol. 2006;112:313–323. doi: 10.1016/j.vetmic.2005.11.014. [DOI] [PubMed] [Google Scholar]
- 73.Johnson D.H. The comparison of usage and availability measurements for evaluating resource preference. Ecology. 1980;61:65–71. doi: 10.2307/1937156. [DOI] [Google Scholar]
- 74.Nekorchuk D.M., Morris L.R., Asher V., Hunter D.L., Ryan S.J., Blackburn J.K. Potential Bacillus anthracis Risk Zones for Male Plains Bison (Bison bison bison) in Southwestern Montana, USA. J. Wildl. Dis. 2019;55:136–141. doi: 10.7589/2017-09-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Getz W.M., Wilmers C.C. a local nearest-neighbor convex-hull construction of home ranges and ultization distributions. Ecography. 2004;27:489–505. doi: 10.1111/j.0906-7590.2004.03835.x. [DOI] [Google Scholar]
- 76.Jennrich R., Turner F. Measurement of non-circular home range. J. Theor. Biol. 1969;22:227–237. doi: 10.1016/0022-5193(69)90002-2. [DOI] [PubMed] [Google Scholar]
- 77.Lyons A.J., Turner W.C., Getz W.M. Home Range Plus: A Space-Time Characterization of Movement over Real Landscapes. Mov. Ecol. 2013;1:2. doi: 10.1186/2051-3933-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dougherty E.R., Carlson C.J., Blackburn J.K., Getz W.M. A cross-validation-based approach for delimiting reliable home range estimates. Mov. Ecol. 2017;5:19. doi: 10.1186/s40462-017-0110-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dougherty E.R., de Valpine P., Carlson C.J., Blackburn J.K., Getz W.M. Commentary to: A cross-validation-based approach for delimiting reliable home range estimates. Mov. Ecol. 2018;6:10. doi: 10.1186/s40462-018-0128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bagamian K.H., Alexander K.A., Hadfield T.L., Blackburn J.K. Ante- and postmortem diagnostic techniques for anthrax: Rethinking pathogen exposure and the geographic extent of the disease in wildlife. J. Wildl. Dis. 2013;49:786–801. doi: 10.7589/2013-05-126. [DOI] [PubMed] [Google Scholar]
- 81.Bellan S.E., Gimenez O., Choquet R., Getz W.M. A Hierarchical Distance Sampling Approach to Estimating Mortality Rates from Opportunistic Carcass Surveillance Data. Methods Ecol. Evol. 2013 doi: 10.1111/2041-210x.12021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blackburn J.K., Asher V., Stokke S., Hunter D.L., Alexander K.A. Dances with Anthrax: Wolves (Canis lupus) Kill Anthrax Bacteremic Plains Bison (Bison bison bison) in Southwestern Montana. J. Wildl. Dis. 2014;50:393–396. doi: 10.7589/2013-08-204. [DOI] [PubMed] [Google Scholar]
- 83.Turner W.C., Getz W.M. Seasonal and demographic factors influencing gastrointestinal parasitism in ungulates of Etosha National Park. J. Wildl. Dis. 2010;46:1108–1119. doi: 10.7589/0090-3558-46.4.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cizauskas C.A., Turner W.C., Wagner B., Küsters M., Vance R.E., Getz W.M. Gastrointestinal helminths may affect host susceptibility to anthrax through seasonal immune trade-offs. BMC Ecol. 2014;14:27. doi: 10.1186/s12898-014-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez J.P., Nekorchuk D.M., Mao L., Ryan S.J., Ponciano J.M., Blackburn J.K. Decoupling environmental effects and host population dynamics for anthrax, a classic reservoir-driven disease. PLoS ONE. 2018;13:e0208621. doi: 10.1371/journal.pone.0208621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bhadra A., Ionides E.L., Laneri K., Pascual M., Bouma M., Dhiman R.C. Malaria in Northwest India: Data analysis via partially observed stochastic differential equation models driven by Lévy noise. J. Am. Stat. Assoc. 2011;106:440–451. doi: 10.1198/jasa.2011.ap10323. [DOI] [Google Scholar]
- 87.Böttcher B. Feller processes: The next generation in modeling. Brownian motion, Lévy processes and beyond. PLoS ONE. 2010;5:e15102. doi: 10.1371/journal.pone.0015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kallenberg O. Foundations of Modern Probability. Springer; New York, NY, USA: 2002. [Google Scholar]
- 89.Bertoin J. On the first exit time of a completely asymmetric stable process from a finite interval. Bull. Lond. Math. Soc. 1996;28:514–520. doi: 10.1112/blms/28.5.514. [DOI] [Google Scholar]
- 90.Steutel F., Van Harn K. Discrete analogues of self-decomposability and stability. Ann. Probab. 1979:893–899. doi: 10.1214/aop/1176994950. [DOI] [Google Scholar]
- 91.McKenzie E. Some ARMA models for dependent sequences of Poisson counts. Adv. Appl. Probab. 1988:822–835. doi: 10.2307/1427362. [DOI] [Google Scholar]
- 92.Leonenko N., Savani V., Zhigljavsky A. Autoregressive Negative Binomial Processes. [(accessed on 14 March 2019)];2007 Volume 51:25–47. Annales de l'Institut de Statistique de l'Universite de Paris. Available online: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.332.4844&rep=rep1&type=pdf. [Google Scholar]
- 93.Manly B., McDonald L., Thomas D., McDonald T., Erickson W. Resource Selection by Animals: Statistical Analysis and Design for Field Studies. Kluwer; New York, NY, USA: 2002. [Google Scholar]
- 94.Fisher R.A., Corbet A.S., Williams C.B. The relation between the number of species and the number of individuals in a random sample of an animal population. J. Anim. Ecol. 1943;12:42–58. doi: 10.2307/1411. [DOI] [Google Scholar]
- 95.Ponciano J.M., Burleigh J.G., Braun E.L., Taper M.L. Assessing parameter identifiability in phylogenetic models using Data Cloning. Syst. Biol. 2012;61:955–972. doi: 10.1093/sysbio/sys055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Getz W.M. Disease and the dynamics of food webs. PLoS Biol. 2009;7:e1000209. doi: 10.1371/journal.pbio.1000209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dragon D., Elkin B., Nishi J., Ellsworth T. A review of anthrax in Canada and implications for research on the disease in northern bison. J. Appl. Microbiol. 1999;87:208–213. doi: 10.1046/j.1365-2672.1999.00872.x. [DOI] [PubMed] [Google Scholar]
- 98.Kracalik I., Malania L., Tsertsvadze N., Manvelyan J., Bakanidze L., Imnadze P., Tsanava S., Blackburn J.K. Human Cutaneous Anthrax, Georgia 2010–2012. Emerg. Infect. Dis. 2014;20:261–264. doi: 10.3201/eid2002.130522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wint G., Robinson T., Bourn D., Durr P., Hay S., Randolph S., Rogers D. Mapping bovine tuberculosis in Great Britain using environmental data. Trends Microbiol. 2002;10:441–444. doi: 10.1016/S0966-842X(02)02444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]