Abstract
Background:
Irritable bowel syndrome with diarrhea (IBS-D) is a prevalent gastrointestinal (GI) disorder with a varied presentation, often overlapping with other GI and non-GI disorders. Eluxadoline is a locally active mixed µ- and κ-opioid receptor agonist and δ-opioid receptor antagonist approved for the treatment of IBS-D in adults. As IBS-D is a heterogeneous disease, factors such as patient demographics, symptom severity, and symptom pattern history can potentially inform treatment selection.
Methods:
Here, we report additional prospectively planned analyses of two large double-blind, placebo-controlled studies (IBS-3001 and IBS-3002) enrolling patients meeting Rome III criteria for IBS-D. Patients were randomized 1:1:1 to receive placebo or eluxadoline 75 mg or 100 mg twice daily. Efficacy (abdominal pain, stool consistency, and composite, simultaneous improvement in both) and safety were assessed for prospectively defined patient subgroups stratified by age, sex, race, presence of comorbidities, and baseline disease characteristics.
Results:
Across all age, sex, race, comorbidity, and disease characteristic subgroups, a greater proportion of patients were composite responders with both eluxadoline doses as compared with placebo, including patients with a history of depression or a history of gastroesophageal reflux disease. Among patients aged ⩾65 years, a greater proportion of patients receiving eluxadoline 75 mg were composite, abdominal pain, and stool consistency responders compared with those receiving 100 mg. The proportion of patients with at least one adverse event was slightly higher in patients aged ⩾65 years and also in female patients.
Conclusions:
This analysis suggests that eluxadoline is effective in treating IBS-D across a range of commonly encountered patient types. In contrast to the overall population, patients aged ⩾65 years demonstrated a greater proportion of responders at the lower approved 75 mg eluxadoline dose.
Keywords: abdominal pain, diarrhea, efficacy, eluxadoline, irritable bowel syndrome
Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal (GI) disorder characterized by recurrent abdominal pain and altered bowel movements.1 IBS is diagnosed clinically, based on Rome diagnostic criteria,2 a careful history and examination, and limited diagnostic tests. Symptoms tend to fluctuate over time with variations in both intensity and duration.3 IBS is one of the most commonly diagnosed GI disorders, resulting in 12% of all primary care visits,4 and the most common reason for patient visits to gastroenterologists.5,6 Patients with IBS report that work productivity, time management, and participation in social activities are all significantly affected by the condition.7 Not surprisingly, IBS results in a major demand on health-related resources.8
As IBS is a prevalent yet heterogeneous disorder, patient selection for a given treatment can be challenging and may be influenced by patient demographics, symptom severity, or symptom profile. IBS most commonly presents between the ages of 35 and 50 years; however, IBS can affect people of all ages and patients often have symptoms for years before seeking care.1,7 IBS affects both men and women,1,7 with the overall prevalence being 67% higher in women internationally.9
IBS and other GI disorders, including gastroesophageal reflux disease (GERD) and functional dyspepsia, often occur concurrently. This overlap is associated with increased symptom burden and increased physician consultations.10,11 Approx-imately 40% of patients with IBS have some degree of psychiatric comorbidity,12 including depression, and these comorbidities lead to multiple coinciding symptoms that can make the management of IBS additionally challenging.
IBS with diarrhea (IBS-D) makes up approximately one third of all IBS cases.1 Diarrhea and abdominal pain are the cardinal symptoms of IBS-D, though fecal urgency and bloating are common and the most bothersome symptom varies from patient to patient.2,3,13–15 As IBS-D is a chronic, relapsing disease, the symptom profile can vary greatly over time.3
Eluxadoline is a mixed µ- and κ-opioid receptor agonist and δ-opioid receptor antagonist, approved for the treatment of IBS-D in adults in the United States, European Union, and Canada.16–18 The efficacy of eluxadoline has been demonstrated in two phase III clinical trials, where it simultaneously improved stool consistency and abdominal pain and was associated with greater adequate relief than placebo.19 Further, treatment responses were maintained over 6 months.20 Eluxadoline was generally well tolerated in clinical trials, with the most common adverse events (AEs) being constipation and nausea.19,21,22 Rare events of sphincter of Oddi (SO) spasm and pancreatitis have been reported with eluxadoline, with the risk highest in patients who have undergone a cholecystectomy; as a result, eluxadoline is contraindicated in patients without a gallbladder or patients consuming more than three alcoholic beverages per day.19,21,23
Given the diverse presentation of IBS-D, we sought to determine if eluxadoline was efficacious across a range of relevant subgroups based on demographics, comorbidities, and baseline disease characteristics in the phase III clinical trials. In order to maximize the subgroup population sample sizes, analyses were undertaken only on the pooled phase III studies.
Methods
Study design
IBS-3001 [ClinicalTrials.gov identifier: NCT01553591] and IBS-3002 [ClinicalTrials.gov identifier: NCT01553747] were randomized, double-blind, placebo-controlled studies, the design and results of which have been previously reported.19 Patients with IBS-D were enrolled and randomized 1:1:1 to receive placebo or eluxadoline 75 mg or 100 mg twice daily for a total of 26 (IBS-3002) or 52 (IBS-3001) weeks. The patients completed a 2- to 3-week screening period prior to enrollment, reporting their daily IBS-D symptoms and bowel patterns using an interactive voice response system which also served as an electronic patient diary throughout the trial, where patients reported symptoms of IBS-D as well as their bowel functioning.19
Patients
Patients aged 18–80 years with IBS-D (Rome III) were eligible for enrollment if, during the week before randomization, they reported an average worst abdominal pain score of >3 (on a scale of 0 to 10, with 0 indicating no pain and 10 indicating the worst imaginable pain), an average score for stool consistency of ⩾5.5 on the Bristol Stool Form Scale (BSFS; a 7-point scale where 1 corresponds to hard stool and 7 corresponds to watery diarrhea),24 a score of ⩾5 BSFS for ⩾5 days, and an average IBS-D global symptom score for symptoms of IBS-D of ⩾2 (on a 5-point scale where 0 indicates no symptoms and 4 corresponds to very severe symptoms).19
Assessments
Patients reported both their abdominal pain and stool consistency on a daily basis. Worst abdominal pain in the past 24 h was reported on an 11-point scale. Stool consistency was reported using the BSFS.
Efficacy endpoints
Abdominal pain response was defined as a reduction in pain score of ⩾30% from baseline for ⩾50% of treatment days. Stool consistency response was defined as a BSFS score of <5 or the absence of a bowel movement if accompanied by a reduction of ⩾30% from baseline in the score for the worst abdominal pain, on ⩾50% of treatment days. Composite response was defined as daily reduction of ⩾30% in worst abdominal pain score compared with average baseline pain and, on the same day, a BSFS score of <5 or the absence of a bowel movement if accompanied by a reduction from baseline of ⩾30% in abdominal pain score, with both endpoints being met on ⩾50% of treatment days. In addition to the criteria described above, a minimum of 110 diary-entry days from week 1 to 26 were required to be included as a responder.
Safety
Safety data, including assessment of AEs and serious AEs (SAEs), were collected throughout the study duration and analyzed for the safety analysis set, which includes all patients enrolled who received at least one dose of either eluxadoline or placebo.19
Identification of clinically relevant subgroups
Patient age was calculated in years using the date of informed consent and the date of birth. Information on patient sex and race was collected during the prescreening period (3 weeks prior to randomization). Race categories included White, Black, Asian, American Indian or Alaska Native, Native Hawaiian or other Pacific Islander, or other. Patient body mass index (BMI) was calculated from the height and weight data recorded at prescreening and summarized as the baseline value. Medical history was collected at prescreening and screening, 3 and 2 weeks prior to randomization, respectively. Comorbidities were identified as follows: those with a history of GERD symptoms were identified based on verbatim terms for GERD and its synonyms (acid reflux, dyspepsia, epigastric burning, gastric reflux, and heartburn), and those with a history of depression were identified based on the preferred Medical Dictionary for Regulatory Activities term of depression. Patient IBS symptom history was collected at prescreening and the numbers and percentages of patients with a wax-and-wane versus persistent symptom history were identified based on patient responses to questions asking if IBS symptoms tend to wax/wane or if they tend to be persistent. Patients were also asked to indicate the time period over which symptoms waxed/waned. Baseline abdominal pain was defined as the weekly average of the measurements recorded during the 7 days prior to randomization on an 11-point scale. Patients were categorized by age (<65 or ⩾65 years), sex (male or female), race (Black, White, or other), BMI (<30 or ⩾30 kg/m2), comorbidity history (no GERD symptoms or GERD symptoms; no depression or depression), symptom history (persistent or wax/wane), and baseline average pain score (<5, 5–<8, and ⩾8). Baseline average pain score categories were defined based on clinical definitions such as Rome IV criteria or clinical experience (see Table 1).
Table 1.
Patient subgroup categories.
| Age subgroupsa, n (%) | |
|---|---|
| <65 years | 2182 (90.1) |
| ⩾65 years | 241 (9.9) |
| Sex subgroupsa, n (%) | |
| Female | 1602 (66.1) |
| Male | 821 (33.9) |
| Race subgroupsa, n (%) | |
| Black | 277 (11.4) |
| White | 2084 (86.0) |
| Other | 62 (2.6) |
| BMI subgroupsb, n (%) | |
| <30 kg/m2 | 1276 (52.8) |
| ⩾30 kg/m2 | 1142 (47.2) |
| History of depression subgroupsa, n (%) | |
| No depression | 1809 (74.7) |
| Depression | 614 (25.3) |
| History of GERD symptoms subgroupsa, n (%) | |
| No GERD symptoms | 1681 (69.4) |
| GERD symptoms | 742 (30.6) |
| History of IBS symptoms subgroupsb, n (%) | |
| Persistent | 1913 (79.1) |
| Wax/wane | 505 (20.9) |
| Baseline pain subgroupsa, n (%) | |
| <5 | 555 (22.9) |
| 5–<8 | 1548 (63.9) |
| ⩾8 | 320 (13.2) |
n = 2423; bn = 2418.
BMI, body mass index; GERD, gastroesophageal reflux disease; IBS, irritable bowel syndrome.
Statistical analysis
Data were pooled from IBS-3001 and IBS-3002 and analyses were carried out on the intention-to-treat (ITT) analysis set, defined as all patients randomly assigned to treatment.19 All subgroup analyses of the composite endpoint, abdominal pain endpoint, and stool consistency endpoint over weeks 1–26 in the pooled population were prospectively planned. A summary of previously reported or overlapping analyses is included in Supplementary Table S1. Due to the small sample sizes in some of the subgroups, only descriptive statistics were utilized and no p values are presented.
Results
Baseline demographics and disease characteristics
As previously described, the ITT population comprised 2423 patients from the two studies. The demographics and baseline characteristics were balanced between both studies and across all treatment groups with an average age of approximately 45 years; approximately 65% of patients were female, and approximately 86% were White.19
Efficacy of eluxadoline based on patient demographics
Age
Eluxadoline improved IBS-D symptoms in both age groups (<65 and ⩾65 years) as compared with placebo. The proportion of composite responders was greater with both doses of eluxadoline (75 mg: 24.6%; 100 mg: 30.1%) and as compared with placebo (19.5%) in patients aged <65 years. A slightly higher proportion of responders was observed in patients receiving 100 mg compared with those receiving 75 mg eluxadoline. In contrast, of the patients aged⩾65 years, a higher proportion of patients receiving 75 mg eluxadoline (50.8%) were composite responders as compared with those receiving 100 mg eluxadoline (40.5%) or placebo (19.6%) [see Figure 1(a)]. The proportion of abdominal pain responders was slightly greater than placebo (42.4%) at the 75 mg (44.5%) and the 100 mg (47.7%) eluxadoline doses among patients aged <65 years. Among patients aged ⩾65 years, a higher proportion of patients receiving 75 mg were abdominal pain responders (66.2%) as compared with patients receiving 100 mg eluxadoline (54.1%) or placebo (54.9%) [see Figure 1(b)]. The proportion of stool consistency responders was greater at both eluxadoline doses as compared with placebo in both age groups. However, consistent with the composite and abdominal pain response, the proportion of stool consistency responders increased with increasing dose of eluxadoline (29.2% and 35.9% at 75 mg and 100 mg, respectively) as compared with placebo (23.9%) among patients aged <65 years, whereas a greater proportion of patients aged ⩾65 years were responders with eluxadoline 75 mg (52.3%) than with 100 mg (45.9%) or placebo (23.5%) [see Figure 1(c)].
Figure 1.
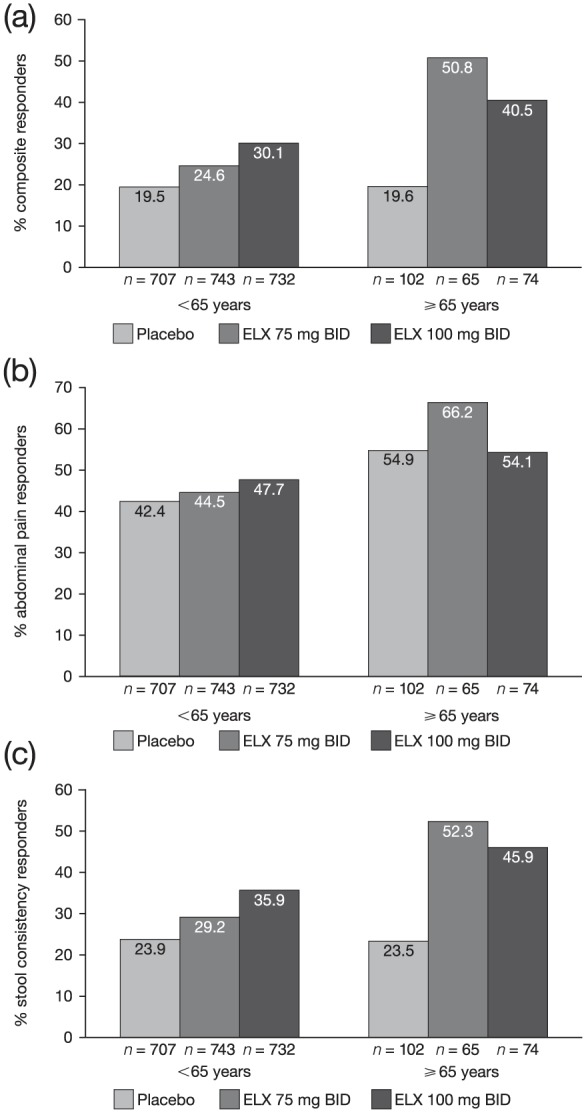
Proportions of responders.
Proportions of composite responders (a)a, abdominal pain responders (b), and stool consistency responders (c) for patients aged <65 and ⩾65 years in the pooled phase III population.
aData for proportions of composite responders for eluxadoline 100 mg versus placebo have been previously published.19
BID, twice daily; ELX, eluxadoline.
Sex
Sex did not appear to have an impact on the efficacy of eluxadoline. A significantly greater proportion of male and female patients who received eluxadoline were composite responders, as compared with placebo.19 The proportions of abdominal pain responders were similar between female and male patients and across all treatment groups [placebo (45.0% of females and 42.2% of males), 75 mg eluxadoline (46.9% of females and 45.0% of males), and 100 mg eluxadoline (48.0% of females and 48.9% of males); see Figure 2(a)]. A greater proportion of female and male patients were stool consistency responders at either dose of eluxadoline (30.5% and 36.4% of females and 32.1% and 37.7% of males at 75 mg and 100 mg, respectively), as compared with placebo (24.1% of females and 23.4% of males) [see Figure 2(b)].
Figure 2.
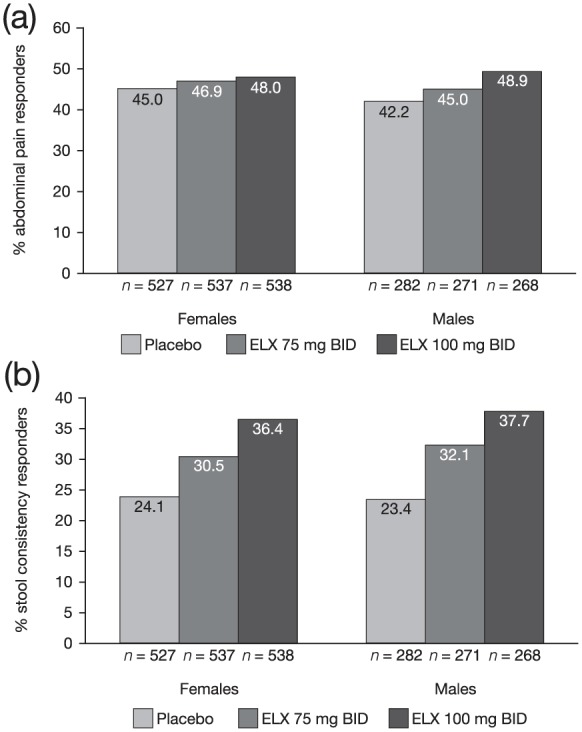
Proportions of abdominal pain responders and stool consistency responders.
Proportions of abdominal pain responders (a) and stool consistency responders (b) for female and male patients in the pooled phase III populationa.
aData for proportions of composite responders for eluxadoline 100 mg versus placebo by sex have been previously published.19
BID, twice daily; ELX, eluxadoline.
Race
Across all reported races, the proportion of composite responders was greater with eluxadoline 75 mg or 100 mg than with placebo; however, it is important to note that White patients comprised 86.0% of the overall patient population (see Supplementary Table S2).
Body mass index
The proportion of composite responders was similar with 75 mg and 100 mg eluxadoline (30.9% and 29.7%, respectively) and higher than placebo (20.8%) among patients with a BMI <30 kg/m2. However, in patients with a BMI ⩾30 kg/m2, the proportion of composite responders was lower with 75 mg than with 100 mg (22.0% and 32.6%, respectively), though the proportion in each group was higher than with placebo (18.2%; see Supplementary Table S2).
Efficacy of eluxadoline based on medical history
A patient history of GERD symptoms (30.6% of all patients) did not seem to affect eluxadoline efficacy; the subgroups receiving either dose of eluxadoline had a greater proportion of composite responders compared with placebo [see Figure 3(a)]. Among patients with a history of GERD symptoms, 31.8% receiving 75 mg eluxadoline and 33.9% receiving 100 mg eluxadoline were composite responders as compared with 19.5% receiving placebo. Of the patients with no history of GERD symptoms, 24.5% receiving 75 mg eluxadoline and 29.7% receiving 100 mg eluxadoline were composite responders as compared with 19.5% receiving placebo.
Figure 3.
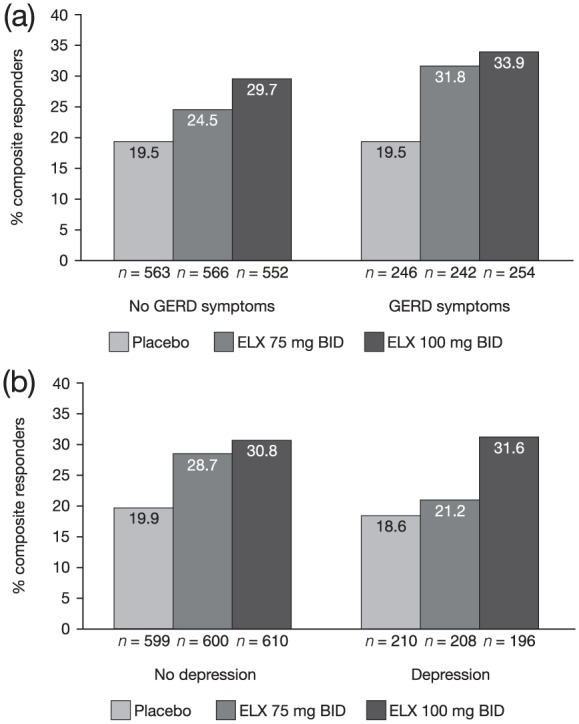
Proportions of composite responders for patients with a history of GERD symptoms and depression.
Proportions of composite responders for patients with a history of GERD symptoms (a) and depression (b) in the pooled phase III population.
BID, twice daily; ELX, eluxadoline; GERD, gastroesophageal reflux disease.
In patients with and without a history of depression, those subgroups receiving eluxadoline doses had a greater proportion of composite responders (100 mg: 31.6% and 30.8%, respectively; 75 mg: 21.2% and 28.7%, respectively) compared with placebo (18.6% and 19.9%, respectively) [see Figure 3(b)].
Efficacy of eluxadoline based on baseline disease characteristics
Symptom profile
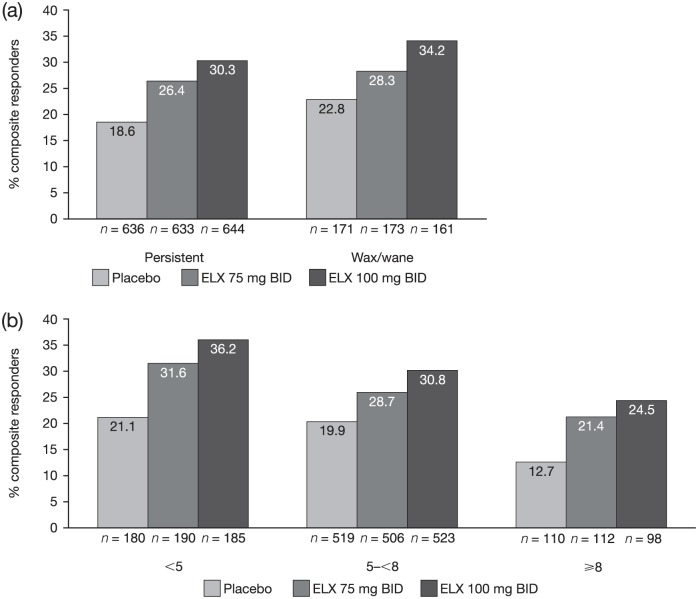
Eluxadoline improved IBS-D symptoms in patients with both persistent and intermittently occurring symptoms. The proportion of composite responders was greater with eluxadoline 100 mg treatment (30.3% of persistent and 34.2% of wax/wane patients) as well as 75 mg eluxadoline treatment (26.4% and 28.3%, respectively) compared with placebo (18.6% and 22.8%, respectively) [see Figure 4(a)].
Figure 4.
Proportions of composite responders for patients.
Proportions of composite responders for patients based on symptom pattern history (a) and baseline pain score (b) in the pooled phase III population.
BID, twice daily; ELX, eluxadoline.
Baseline pain
In general, the proportion of composite responders was highest in the least severe baseline pain subgroup (<5) and lowest in the most severe subgroup (⩾8) across all treatment arms [see Figure 4(b)]. Among those patients reporting a lower baseline pain score (<5), 31.6% receiving 75 mg and 36.2% receiving 100 mg eluxadoline were composite responders, as compared with 21.1% receiving placebo. In those patients with a higher baseline pain score (⩾8), eluxadoline treatment also resulted in a higher proportion of composite responders (21.4% and 24.5% at 75 mg and 100 mg, respectively) compared with placebo (12.7%). However, the difference in the proportion of composite responders versus placebo decreased with increasing severity [see Figure 4(b)].
Safety of eluxadoline based on patient demographics
As reported previously,21 safety analysis for phase II and III clinical trials was completed across subgroups. AEs leading to treatment discontinuation were slightly higher in White patients than in Black patients across all treatment groups (4.3%, 8.7%, and 8.2% in White patients as compared with 3.8%, 5.4%, and 3.2% in Black patients in the placebo, eluxadoline 75 mg, and eluxadoline 100 mg treatment groups, respectively).21 Patients with a BMI ⩾30 kg/m2 had an increased number of AEs compared with patients with a BMI <30 kg/m2. In this analysis, safety data from the combined phase III clinical trials were further examined to identify any potential new safety signals in the age and sex subgroups.
Age
As shown in Table 2, the proportion of patients with ⩾1 AE was slightly higher in patients aged ⩾ 65 years at both doses of eluxadoline (66.2% and 65.3% at 75 mg and 100 mg, respectively) compared with patients aged <65 years (59.7% and 57.5%, respectively). The most common AEs in patients aged <65 years receiving eluxadoline 100 mg included constipation and nausea (8.4% and 7.4%, respectively), and in patients aged ⩾65 years the most common AEs were abdominal pain and constipation (12.0% and 10.7%, respectively). The incidence of AEs of special interest, including pancreatitis (six total cases) and elevated levels of aminotransferases (alanine aminotransferase/aspartate aminotransferase) associated with abdominal pain (eight total cases), was low across both age subgroups. Of the six total cases of pancreatitis, only one case occurred in a patient aged ⩾65 years and was later adjudicated as being consistent with SO spasm.
Table 2.
Safety overview and AEs of special interest by age: pooled phase III population.
| Age |
||||||
|---|---|---|---|---|---|---|
| <65 years |
⩾65 years |
|||||
| Placebo (n = 707) | ELX 75 mg (n = 742) | ELX 100 mg (n = 784) | Placebo (n = 101) | ELX 75 mg (n = 65) | ELX 100 mg (n = 75) | |
| Patients with ⩾1 AE, n (%) | 383 (54.2) | 443 (59.7) | 451 (57.5) | 67 (66.3) | 43 (66.2) | 49 (65.3) |
| AEs occurring in ⩾5% of patients, n (%) a | ||||||
| Constipation | 18 (2.5) | 54 (7.3) | 66 (8.4) | 2 (2.0) | 6 (9.2) | 8 (10.7) |
| Nausea | 36 (5.1) | 61 (8.2) | 58 (7.4) | 5 (5.0) | 4 (6.2) | 6 (8.0) |
| Upper respiratory tract infection | 28 (4.0) | 24 (3.2) | 45 (5.7) | 4 (4.0) | 3 (4.6) | 2 (2.7) |
| Abdominal pain | 18 (2.5) | 27 (3.6) | 34 (4.3) | 4 (4.0) | 6 (9.2) | 9 (12.0) |
| Vomiting | 9 (1.3) | 31 (4.2) | 32 (4.1) | 2 (2.0) | 1 (1.5) | 4 (5.3) |
| Dizziness | 12 (1.7) | 20 (2.7) | 26 (3.3) | 5 (5.0) | 1 (1.5) | 2 (2.7) |
| Flatulence | 10 (1.4) | 21 (2.8) | 23 (2.9) | 3 (3.0) | 0 | 4 (5.3) |
| Sinusitis | 24 (3.4) | 25 (3.4) | 20 (2.6) | 2 (2.0) | 2 (3.1) | 4 (5.3) |
| Fatigue | 15 (2.1) | 20 (2.7) | 17 (2.2) | 6 (5.9) | 1 (1.5) | 2 (2.7) |
| Abdominal distension | 11 (1.6) | 18 (2.4) | 16 (2.0) | 2 (2.0) | 3 (4.6) | 6 (8.0) |
| Urinary tract infection | 14 (2.0) | 12 (1.6) | 15 (1.9) | 1 (1.0) | 5 (7.7) | 0 |
| Back pain | 19 (2.7) | 17 (2.3) | 13 (1.7) | 6 (5.9) | 1 (1.5) | 1 (1.3) |
| Dry mouth | 12 (1.7) | 15 (2.0) | 9 (1.1) | 2 (2.0) | 0 | 4 (5.3) |
| AEs of special interest, n (%) | ||||||
| Pancreatitis | 0 | 2 (0.3) | 3 (0.4) | 0 | 1 (1.5) | 0 |
| Adjudicated pancreatitis events | 0 | 2 (0.3) | 3 (0.4) | 0 | 0 | 0 |
| Adjudicated as consistent with SO spasmb | 0 | 0 | 1 (33.3) | 0 | 1 (100) | 0 |
| Associated with alcoholb | 0 | 1 (50.0) | 2 (66.6) | 0 | 0 | 0 |
| Associated with biliary sludgeb | 0 | 1 (50.0) | 0 | 0 | 0 | 0 |
| Prior cholecystectomyb | 0 | 0 | 2 (66.6) | 0 | 1 (100) | 0 |
| Elevated aminotransferases (ALT, AST) associated with abdominal pain | 0 | 1 (0.1) | 7 (0.9) | 0 | 0 | 0 |
| Adjudicated as consistent with SO spasmc | 0 | 1 (100) | 7 (100) | 0 | 0 | 0 |
| Prior cholecystectomyc | 0 | 1 (100) | 7 (100) | 0 | 0 | 0 |
AEs occurring in ⩾5% of patients in any treatment group.
Percentages expressed as a proportion of events reported as pancreatitis; one patient aged ⩾65 years receiving eluxadoline 75 mg had a reported event of pancreatitis adjudicated as SO spasm that did not meet Atlanta criteria for pancreatitis.
Percentages expressed as a proportion of total cases of elevated aminotransferases (ALT, AST) associated with abdominal pain; one of the seven patients in the eluxadoline 100 mg treatment group had congenital agenesis of the gallbladder and is thus included under ‘cholecystectomy’.
Safety analysis set; includes all patients enrolled who received at least one dose of study drug.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELX, eluxadoline; SO, sphincter of Oddi.
Sex
Overall, the proportion of patients with ⩾1 AE was slightly higher in female patients than in male patients (see Table 3). Specifically, 64.6% of female patients receiving eluxadoline 75 mg and 60.3% receiving 100 mg experienced ⩾1 AE, compared with 51.5% and 54.1% of male patients receiving 75 mg and 100 mg, respectively. Female patients had slightly higher incidences of constipation (9.5% versus 6.9%) and vomiting (5.3% versus 2.1%) than male patients receiving the same dose. Four of the six cases of pancreatitis occurred in female patients.
Table 3.
Safety overview by sex: pooled phase III population.
| Sex | ||||||
|---|---|---|---|---|---|---|
| Female | Male | |||||
| Placebo (n = 525) | ELX 75 mg (n = 537) | ELX 100 mg (n = 569) | Placebo (n = 283) | ELX 75 mg (n = 270) | ELX 100 mg (n = 290) | |
| Patients with ⩾1 AE, n (%) | 315 (60.0) | 347 (64.6) | 343 (60.3) | 135 (47.7) | 139 (51.5) | 157 (54.1) |
| AEs occurring in ⩾5% of patients, n (%) a | ||||||
| Constipation | 14 (2.7) | 43 (8.0) | 54 (9.5) | 6 (2.1) | 17 (6.3) | 20 (6.9) |
| Nausea | 32 (6.1) | 52 (9.7) | 48 (8.4) | 9 (3.2) | 13 (4.8) | 16 (5.5) |
| Abdominal pain | 13 (2.5) | 22 (4.1) | 35 (6.2) | 9 (3.2) | 11 (4.1) | 8 (2.8) |
| Vomiting | 9 (1.7) | 27 (5.0) | 30 (5.3) | 2 (0.7) | 5 (1.9) | 6 (2.1) |
| Upper respiratory tract infection | 20 (3.8) | 18 (3.4) | 30 (5.3) | 12 (4.2) | 9 (3.3) | 17 (5.9) |
| Headache | 28 (5.3) | 21 (3.9) | 25 (4.4) | 10 (3.5) | 11 (4.1) | 14 (4.8) |
| AEs of special interest, n (%) | ||||||
| Pancreatitis | 0 | 3 (0.6) | 1 (0.2) | 0 | 0 | 2 (0.7) |
| Adjudicated pancreatitis events | 0 | 2 (0.4) | 1 (0.2) | 0 | 0 | 2 (0.7) |
| Adjudicated as consistent with SO spasmb | 0 | 0 | 1 (100) | 0 | 0 | 0 |
| Associated with alcoholb | 0 | 1 (50.0) | 0 | 0 | 0 | 2 (100) |
| Associated with biliary sludgeb | 0 | 1 (50.0) | 0 | 0 | 0 | 0 |
| Prior cholecystectomyb | 0 | 0 | 1 (100) | 0 | 0 | 1 (50.0) |
| Elevated aminotransferases (ALT, AST) associated with abdominal pain | 0 | 1 (0.2) | 4 (0.7) | 0 | 0 | 3 (1.0) |
| Adjudicated as consistent with SO spasmc | 0 | 1 (100) | 4 (100) | 0 | 0 | 3 (100) |
| Prior cholecystectomyc | 0 | 1 (100) | 4 (100) | 0 | 0 | 3 (100) |
AEs occurring in ⩾5% of patients in any treatment group.
Percentages expressed as a proportion of events reported as pancreatitis; one female patient receiving eluxadoline 75 mg had a reported event of pancreatitis adjudicated as SO spasm that did not meet Atlanta criteria for pancreatitis.
Percentages expressed as a proportion of total cases of elevated aminotransferases (ALT, AST) associated with abdominal pain; one of the seven patients in the eluxadoline 100 mg treatment group had congenital agenesis of the gallbladder and is thus included under ‘cholecystectomy’.
Safety analysis set; includes all patients enrolled who received at least one dose of study drug.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ELX, eluxadoline; SO, sphincter of Oddi.
Discussion
In this subgroup analysis of the pooled phase III clinical trial data, eluxadoline displayed consistent efficacy across different demographic groups, including sex and race. This is comparable with the responses seen in the overall phase II and III clinical trial program.25,26 However, elderly patients demonstrated apparent increased sensitivity to eluxadoline treatment. Interestingly, among patients aged ⩾65 years, a greater proportion of composite, abdominal pain, and stool consistency responders were observed with eluxadoline 75 mg than with the 100 mg twice-daily dose. The approved starting dose of eluxadoline is 100 mg twice daily. As elderly patients receiving the higher eluxadoline dose also experienced higher rates of GI AEs,21 the Canadian18,27 and European regulatory authorities28 approved a starting dose of 75 mg twice daily in older patients. While speculative, these data suggest that patients may be more sensitive to the effects of eluxadoline with increasing age. As eluxadoline acts locally in the GI tract, any potential increased sensitivity to its effects with age would most likely be related to changes in either opioid receptor expression or signal transduction. This is suggested both by the dose-related incidence of GI AEs and SAEs in the elderly population21 and by reports of increased sensitivity of elderly populations in other opioid prescription settings. An increased sensitivity to centrally acting opioids in the elderly population is well established, though the effects are generally attributable to age-related changes in pharmacokinetics.29 In addition, the predominant beneficial effect of eluxadoline in elderly patients was most pronounced for stool consistency. As colonic transit is reported to slow with age,30 this could partially explain the enhanced efficacy of eluxadoline in these patients. Future studies on the effects of age on opioid receptors could help explain these differences. Moreover, studies examining GI tract metabolism could determine if local eluxadoline exposure was different among age subgroups.
It is likely that elderly IBS-D patients had experienced symptoms for longer periods of time than younger patients, and this longer disease history may have contributed to the heightened treatment response. This is supported by similar proportions of responders between age groups with placebo, but differences in proportions of responders in response to eluxadoline, though future studies correlating response and disease duration would be needed to confirm this.
Treatment adherence or discontinuation due to AEs could also conceivably have contributed to the observed findings. However, given that the proportion of composite responders observed for both doses of eluxadoline was more than double that of the placebo arm in patients aged ⩾65 years, this is unlikely, as patients had to complete a defined proportion of diary-entry days (110 out of 182 days) in order to be considered responders.
We have also reported that a slightly higher proportion of patients aged ⩾65 years had a baseline pain score of <5, which could explain their increased response to the lower approved dose.31 Overall, these differences should be interpreted with caution as only 9.9% of patients included in this study were aged ⩾65 years, and further studies are warranted. Additional analyses to identify whether demographic characteristics such as age cluster with particular clinical characteristics could be of value to provide further context. Ideally, this would be performed in large, prospective studies with endpoints defined a priori.
IBS affects both men and women, with only a slight increase in prevalence among women.1 As reported previously, the proportion of composite responders was the same among both male and female patients.19 In this analysis, eluxadoline demonstrated efficacy in men and women, with respect to both abdominal pain and stool consistency, which is consistent with the previous findings for composite response.
GI disorders such as IBS have been reported as part of symptom complexes with other GI and non-GI disorders, including GERD symptoms and depression, respectively. The presence of multiple disorders can often create challenges when treating and assessing patients; therefore, patients with and without patient-reported symptoms of GERD and depression were analyzed for response to eluxadoline treatment. Notably, eluxadoline treatment was as effective in patients with these conditions as for the overall population. However, formal validated questionnaires regarding depression or GERD symptoms would be necessary to more accurately define patient populations with these specific comorbidities. Whether other comorbidities (e.g. functional dyspepsia) or overall comorbidity burden have an impact on eluxadoline efficacy is still unknown and may warrant further investigation.
Another challenge in treating IBS-D is the dynamic symptom profiles of patients, who experience a variety of symptoms that can fluctuate over time.1 IBS-D is a chronic condition that requires a long-term management plan, whether patients have persistent symptoms or flares of symptoms that occur intermittently. In this analysis, eluxadoline was effective in patients who reported persistent or waxing/waning symptoms at baseline. As IBS-D is a persistent disorder for the majority of patients, this supports the effectiveness of eluxadoline in treating the IBS-D patient population. In addition, the proportion of responders was greater than placebo with both doses of eluxadoline across all baseline pain subgroups.
Overall, there were no new safety signals in any of the analyzed subgroups, with a similar safety profile across age and sex subgroups when comparing eluxadoline and placebo treatment groups. However, it was noted that the proportions of patients with ⩾1 AE among female patients and patients aged ⩾65 years were higher than among male patients and patients aged <65 years, respectively. The incidences of the most common AEs, constipation and nausea, are consistent with previously published work.19,21 As has already been discussed, previously reported safety data support the recommendation for lower (75 mg) dosing in the ⩾65 years population, with higher incidences of GI AEs and SAEs at the 100 mg dose in the elderly population.21
While this analysis provides meaningful data about the efficacy of eluxadoline across patient subtypes, there is also an important limitation. For many of the subgroups, the population sizes are relatively small, and whether the data are translatable to the population at large is uncertain.
Altogether, this analysis provides evidence that eluxadoline is an effective IBS-D therapy irrespective of age, sex, race, presence of comorbidities, or baseline disease characteristics.
Supplemental Material
Supplemental material, Supplementary_Material for Impact of patient and disease characteristics on the efficacy and safety of eluxadoline for IBS-D: a subgroup analysis of phase III trials by Brian E. Lacy, Lucinda A. Harris, Lin Chang, Susan Lucak, Catherine Gutman, Leonard S. Dove, Paul S. Covington and Anthony Lembo in Therapeutic Advances in Gastroenterology
Acknowledgments
The authors would like to thank the study participants for their contribution to knowledge in this area. Editorial assistance in the writing and revision of the draft manuscript on the basis of detailed discussion and feedback from all authors was provided by Katie L. Beski, PhD, of Complete HealthVizion, Inc., Chicago, IL, USA, funded by Allergan plc.
Leonard S. Dove, PhD, was involved in the design and execution of the eluxadoline clinical trials, as well as the data analysis and interpretation. Brian E. Lacy, MD, PhD, Paul S. Covington, MD, Lin Chang, MD, Susan Lucak, MD, Catherine Gutman, PhD, Lucinda A. Harris, MD, and Anthony Lembo, MD were involved in the analysis and interpretation of the data.
Footnotes
Funding: This study was funded by Allergan plc, Dublin, Ireland.
Conflict of interest statement: Financial arrangements of the authors with companies whose products may be related to the present report are listed below, as declared by the authors. Brian E. Lacy has participated on advisory boards for Actavis, Inc., an affiliate of Allergan plc, Forest Laboratories, Inc., an affiliate of Allergan plc, Ironwood Pharmaceuticals, and Salix Pharmaceuticals. Lucinda A. Harris has participated on advisory boards for Actavis, Inc., an affiliate of Allergan plc, Ironwood Pharmaceuticals, Napo Pharmaceuticals, Shire, Synergy, and QOL Medical; received grant support from Alvine Pharmaceuticals and Rhythm Pharmaceuticals; received fees for Continuing Medical Education lectures from Forest Laboratories, Inc., an affiliate of Allergan plc, and Ironwood Pharmaceuticals; and served as a consultant for Salix Pharmaceuticals. Lin Chang has participated on advisory boards for Cairn Diagnostics, IM HealthScience, Napo Pharmaceuticals, Salix Pharmaceuticals, and Synergy; has received grant support from Ardelyx; and has been a consultant and speaker for Allergan plc. Susan Lucak has served as an advisor or consultant for Actavis, Inc., an affiliate of Allergan plc, Ironwood Pharmaceuticals, Prometheus Laboratories, Salix Pharmaceuticals, and Takeda; and has served as a speaker for or received lecture fees from Actavis, Inc., an affiliate of Allergan plc, Ironwood Pharmaceuticals, Salix Pharmaceuticals, and Takeda. Catherine Gutman is an employee of Allergan plc. Leonard S. Dove serves as a consultant for Allergan plc. Paul S. Covington has served as a consultant for Allergan plc. Anthony Lembo serves as a consultant for Allergan plc, Ironwood Pharmaceuticals, Napo Pharmaceuticals, Prometheus Laboratories, Salix Pharmaceuticals, and Shire.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Brian E. Lacy, Division of Gastroenterology and Hepatology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA.
Lucinda A. Harris, Mayo Clinic, Scottsdale, AZ, USA
Lin Chang, University of California, Los Angeles, CA, USA.
Susan Lucak, Weill Cornell Medical Center, New York, NY, USA.
Catherine Gutman, Allergan plc, Madison, NJ, USA.
Leonard S. Dove, Former employees of Furiex Pharmaceuticals, Inc., an affiliate of Allergan plc, Madison, NJ, USA
Paul S. Covington, Former employees of Furiex Pharmaceuticals, Inc., an affiliate of Allergan plc, Madison, NJ, USA
Anthony Lembo, Beth Israel Deaconess Medical Center, Boston, MA, USA.
References
- 1. Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 2014; 6: 71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 3. Chey WD, Kurlander J, Eswaran S. Irritable bowel syndrome: a clinical review. J Am Med Assoc 2015; 313: 949–958. [DOI] [PubMed] [Google Scholar]
- 4. Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002; 123: 2108–2131. [DOI] [PubMed] [Google Scholar]
- 5. Soares RLS. Irritable bowel syndrome: a clinical review. World J Gastroenterol 2014; 20: 12144–12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm 2004; 10: 299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hungin APS, Chang L, Locke GR, et al. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther 2005; 21: 1365–1375. [DOI] [PubMed] [Google Scholar]
- 8. Buono JL, Carson RT, Flores NM. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes 2017; 15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Houghton LA, Heitkemper M, Crowell MD, et al. Age, gender, and women’s health and the patient. Gastroenterology 2016; 150: 1332–1343.e4. [DOI] [PubMed] [Google Scholar]
- 10. Jarbøl DE, Rasmussen S, Balasubramaniam K, et al. Self-rated health and functional capacity in individuals reporting overlapping symptoms of gastroesophageal reflux disease, functional dyspepsia and irritable bowel syndrome – a population based study. BMC Gastroenterol 2017; 17: 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vakil N, Stelwagon M, Shea EP, et al. Symptom burden and consulting behavior in patients with overlapping functional disorders in the US population. United European Gastroenterol J 2016; 4: 413–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fadgyas-Stanculete M, Buga AM, Popa-Wagner A, et al. The relationship between irritable bowel syndrome and psychiatric disorders: from molecular changes to clinical manifestations. J Mol Psychiatry 2014; 2: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atarodi S, Rafieian S, Whorwell PJ. Faecal incontinence—the hidden scourge of irritable bowel syndrome: a cross-sectional study. BMJ Open Gastroenterol 2014; 1: e000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lucak S, Chang L, Halpert A, et al. Current and emergent pharmacologic treatments for irritable bowel syndrome with diarrhea: evidence-based treatment in practice. Therap Adv Gastroenterol 2017; 10: 253–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Serag HB, Pilgrim P, Schoenfeld P. Natural history of irritable bowel syndrome. Aliment Pharmacol Ther 2004; 19: 861–870. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Food and Drug Administration. Viberzi (eluxadoline) highlights of prescribing information, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/206940s002lbl.pdf (2017, accessed 14 February 2018).
- 17. European Medicines Agency. Truberzi (eluxadoline) EPAR summary for the public, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Summary_for_the_public/human/004098/WC500213371.pdf (2016, accessed 14 February 2018).
- 18. Health Canada. Summary basis of decision – Viberzi, https://hpr-rps.hres.ca/reg-content/summary-basis-decision-detailTwo.php?lang=en&linkID=SBD00346 (2017, accessed 14 February 2018).
- 19. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016; 374: 242–253. [DOI] [PubMed] [Google Scholar]
- 20. Chey WD, Dove LS, Andrae DA, et al. Early response predicts a sustained response to eluxadoline in patients with irritable bowel syndrome with diarrhoea in two phase 3 studies. Aliment Pharmacol Ther 2017; 45: 1319–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cash BD, Lacy BE, Schoenfeld PS, et al. Safety of eluxadoline in patients with irritable bowel syndrome with diarrhea. Am J Gastroenterol 2017; 112: 365–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Food and Drug Administration. Viberzi (eluxadoline) highlights of prescribing information, http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206940s000lbl.pdf (2015, accessed 14 February 2018).
- 23. Cash BD, Schoenfeld PS, Lacy BE, et al. Adverse event profile of eluxadoline over time in patients with irritable bowel syndrome with diarrhea. Am J Gastroenterol 2015; 110(Suppl. 1): S748–S749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol 1997; 32: 920–924. [DOI] [PubMed] [Google Scholar]
- 25. Fragkos KC. Spotlight on eluxadoline for the treatment of patients with irritable bowel syndrome with diarrhea. Clin Exp Gastroenterol 2017; 10: 229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018; 113: 1–18. [DOI] [PubMed] [Google Scholar]
- 27. Allergan plc. Viberzi™ product monograph – Canada, https://allergan-web-cdn-prod.azureedge.net/allergancanadaspecialty/allergancanadaspecialty/media/actavis-canada-specialty/en/products/pms/2017–01–26-viberzi-pm-english.pdf (2017, accessed 14 February 2018).
- 28. European Medicines Agency. Truberzi assessment report, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/004098/WC500213370.pdf (2016, accessed 14 February 2018).
- 29. Cavalieri TA. Management of pain in older adults. J Am Osteopath Assoc 2005; 105: 12S–17S. [PubMed] [Google Scholar]
- 30. De Giorgio R, Ruggeri E, Stanghellini V, et al. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol 2015; 15: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lacy B, Harris LA, Chang L, et al. Efficacy and safety of eluxadoline in elderly patients with irritable bowel syndrome with diarrhea. J Can Assoc Gastroenterol 2018; 1(Suppl. 2): 252–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Material for Impact of patient and disease characteristics on the efficacy and safety of eluxadoline for IBS-D: a subgroup analysis of phase III trials by Brian E. Lacy, Lucinda A. Harris, Lin Chang, Susan Lucak, Catherine Gutman, Leonard S. Dove, Paul S. Covington and Anthony Lembo in Therapeutic Advances in Gastroenterology