Abstract
Background:
The use of teledermoscopy in the diagnostic management of pre-cancerous and cancerous skin lesions involves digital dermoscopic images transmitted over telecommunication networks via email or web applications. Teledermoscopy may improve the accuracy in clinical diagnoses of melanoma skin cancer if integrated into electronic medical records and made available to rural communities, potentially leading to decreased morbidity and mortality.
Objective and method:
The purpose of this paper is to present a systematic review of evidence on the use of teledermoscopy to improve the accuracy of skin lesion identification in adult populations. The PRISMA method guided the development of this systematic review. A total of seven scholarly databases were searched for articles published between the years of 2000 and 2015. All studies were critically appraised using the Rosswurm and Larrabee critique worksheet, placed in a matrix for comparison evaluating internal and external validity and inspected for homogeneity of findings.
Results:
Sixteen articles met inclusion criteria for this review. A majority of the studies were cross-sectional and non-experimental. Ten of the 16 focused on interobserver concordance and diagnostic agreement between teledermoscopy and another comparator. Instrumentation in conducting the studies showed inconsistency with reported results.
Discussion:
Higher level evidence is needed to support clinical application of teledermoscopy for accuracy of diagnostic measurement in the treatment of pre-cancerous and cancerous skin lesions in adults. Future research is needed to develop a standardized, reliable and valid measurement tool for implementation in clinical practice.
Keywords: Teledermatology, telehealth, teledermoscopy, melanoma
Background
Melanoma, which is the most common and dangerous of all skin cancers,1 is the most common cancer of young adults under the age of 24.2 Melanoma rates have continued to rise over the past 30 years. It is estimated that approximately 76,380 new melanomas will be diagnosed in the United States by the end of 2016, with 10,130 cases expected to result in mortality.3 The Skin Cancer Foundation reports that one person dies of melanoma every hour.2 Even though melanoma accounts for less than 1% of skin cancers, it remains the leading cause of skin cancer deaths. Melanoma care is expensive; total direct care cost concomitant for malignant melanoma treatment in 2010 was $2.36 billion in the United States and total treatment costs have increased from an annual average of $3.57 billion between 2002 and 2006 to $8.075 billion between 2007 and 2011.4
Prevention is key for melanoma care. Early detection and treatment has the potential to decrease cost, population morbidity and mortality. The Surgeon General created a call to action to prevent skin cancer determining it to be a major public health problem;5 and the United States Preventive Services Task Force (USPSTF) recommends skin cancer screening.6 Access to screening programmes can be limited for rural and underserved populations, creating a need for innovative methods to implement screening programmes.
Progress in the fight against melanoma includes multiple advances in technology for screening and follow-up assessments. Using technology to screen for melanoma in rural areas may be one potential resolution to the screening access issue. Teledermoscopy has been used for screening. Two main types of teledermatology exist for identification of cancerous skin lesions including: store-and-forward (SAF) technology; and video-conferencing (VC) or live interaction (LI). The difference between standard SAF versus teledermoscopy is the addition of the dermoscopic lens – these attachments can be used with standard digital cameras then images sent to a dermatologist (teledermoscopy) or a smartphone (mobile teledermoscopy). Without the dermoscopic lens, a consultation using macro images in considered a SAF teledermatology consultation. VC and LI are skin examinations performed in real-time communication using two-way audio, webcam or virtual conference rooms.7 Mobile teledermoscopy involves the use of high speed, wireless or broadband network connections to mobile devices such as smartphones, digital cameras or computers for follow-up of suspicious lesions to providers.8 This mobile factor can be added to both SAF and VC teleconsultation. Therefore, the following research question has been formulated: What is the evidence related to the use of teledermoscopy to improve the accuracy of skin lesion identification in the adult population?
Methods
The PRISMA method was used to develop this systematic review.9 A review of the literature was conducted between 1 September 2015 and 31 October 2015 using EBCSO host, The Cochrane Library, OVID, PubMed, CINAHL, Academic Search Complete and MEDLINE, with limits set for the years 2000 through 2015. Specific keywords and word combinations included: ‘teledermoscopy’, ‘teledermoscopy and skin lesions’, ‘teledermoscopy and adults’, ‘mobile teledermoscopy’, ‘mobile teledermoscopy and skin lesions’, ‘teledermoscopy and patient-performed’, ‘mobile teledermoscopy and accuracy’, ‘teledermoscopy and clinical screening’ and ‘mobile teledermoscopy and clinical screening’. Both The Cochrane Library and CINAHL database searches yielded no search results.
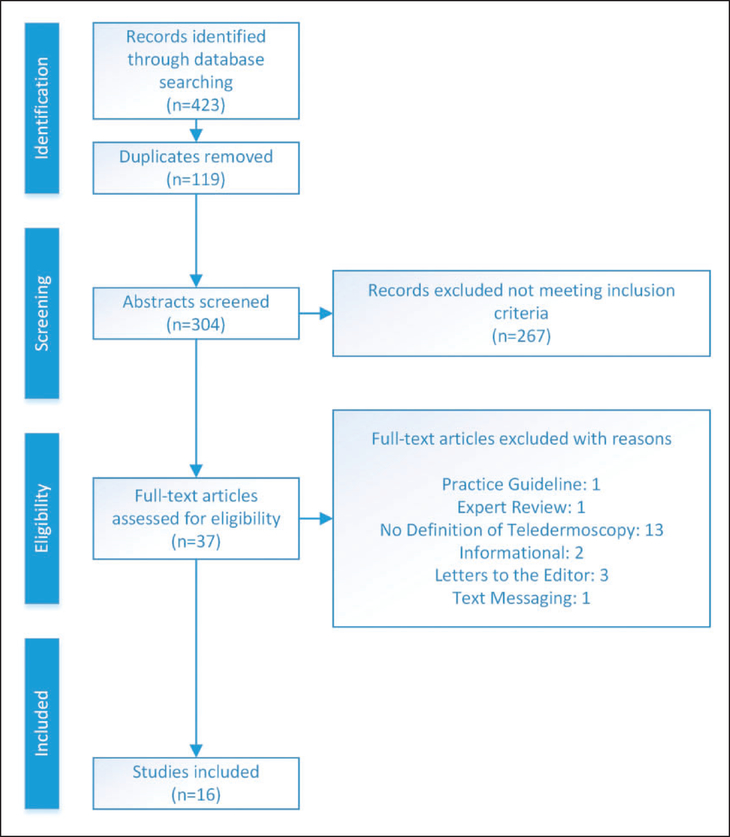
The completed search yielded 423 articles with 119 duplicates eliminated. Thus, 304 abstracts were reviewed for inclusion with the following inclusion criteria: (1) adults 18 years of age or older, (2) adults with skin lesions, (3) mobile teledermoscopy and (4) patient-performed or clinical screening teledermoscopy. One author independently extracted and reviewed the initial literature for inclusion, with accuracy confirmed by two additional authors for selection bias purposes. Thirty-seven abstracts met the inclusion criteria and the full-text articles were obtained. The Rosswurm and Larrabee critique worksheet was utilized to screen each article to reduce bias, determine validity, interpret results and evaluate applicability in clinic practice and future research.10 After critiquing the full-text articles, 21 articles were removed from this review due to failure in meeting inclusion criteria. Articles eliminated included the following: 13 articles focused specifically on teledermatology with no definition of teledermoscopy in the actual studies;11–23 three letters to the editor;24–26 two purely informational articles without defined study groups or methodologies;27,28 one practice guideline from the American Telemedicine Association on the Practice Guidelines for Teledermatology;29 one expert review which focused on distinguishing malignant tumours from benign utilizing dermoscopy;30 and one article involving only text-messaging as a form of early detection.31 Figure 1 summarizes this literature screening process.
Figure 1.
The literature screening process.
Results
A total of 16 articles remained for inclusion. Table 1 includes a description of each article (in alphabetical order by first author last name), year of publication, country, type of teledermoscopy used, type of measurement data and strengths and weaknesses of the study. Similarities and differences were noted in overall approach for the included studies. Ten of the 16 studies focused on some level of interobserver agreement or diagnostic concordance when evaluating the accuracy of the lesion diagnosis between teledermatologists, clinical dermatologists and general practitioners.32–41 Nine studies examined the comparison between teledermoscopy and biopsy and histopathology.32,34,35,37–40,42,43 Two of these stated that face-to-face evaluation was used to confirm lesion diagnosis,33,44 and one study specifically stated that both lesion histopathology and face-to-face comparison were used to confirm final diagnoses.38 Eight studies focused on the use of a mobile device to perform teledermoscopy,32,36,38,41,42,44–46 yet only one included use of an actual mobile phone application or web-application.32Three studies included the use of SAF teledermoscopy;38,40,43 two studies relied on patient ability to perform skin self-examination when using teledermoscopy;44,46 two explored the use of teledermoscopy as a triage system;38,40 and two compared teledermoscopy to the asymmetry-colour rule.44,46 Piccolo et al. was the only study found to mention the use of multiple colleagues with varying levels of experience in dermoscopy in the evaluation of cancerous lesions.39
Table 1.
Summary of results.
| Author(s), year/purpose | Design | Sample | Instrument/device | Key findings |
|---|---|---|---|---|
| Börve et al.,45 2015 Smartphone TDS referrals vs. Paper-based referrals for faster skin cancer mgmt. |
Prospective observational study, quasi-experimental | TDS: 772; Paper-based: 746 Patients referred via TDS (474 female; 61.4%). |
PHC-iDoc24 PRO (iDoc24 AB) app, handheld DS compatible with smartphone | • Triage more reliable with TDS • Waiting times receiving surgical treatment shorter in TDS group (p < 0.001) • Smartphone TDS faster mgmt for skin cancer patients. |
| Börve et al.,32 2013 1. Mobile TDS app accuracy 2. IO concordance b/n two TDs & FTF dermatologist. |
Prospective observational study, quasi-experimental | N= 62 (24 females, 38 male); Total Lesions included = 69 | Smartphone (iPhone 4, Apple); customized DS attachment (FotoFinder, Handyscope, FotoFinder Systems GmbH); web-based TD platform (Tele- Dermis, iDoc24, AB, & iPhone (iDoc24, AB) | • FTF diagnostic accuracy statistically higher than TD 1 (p < 0.04), similar to TD 2. • IO concordance moderate agreement. • Image quality rated excellent/suffi- cient by TDs in 94%/84% cases, respectively. |
| Di Stefani et al.,33 2007 Feasibility of TD for patients with multiple PSL |
Cross-sectional design, no control groups | Patients (N= 18) with 465 PSLs (11 males,7 females) | Photo (Model 990, Nikon); JPEG images (resolution 2048 × 1536 pixels, 24-bit colour depth); stored dig VD (Videocap, DS Medica) | • FTF and two TDs moderate agree-ment (FTF 65 lesions clinically equivocal) • TD 1 and TD2 requested DS evaluation (18.1%, 13.5%) • Two-step clinical and TD approach recommended |
| Fabbrocini et al.,34 2008 Telediagnosis vs. FTF diagnosis for skin lesions. |
Cross-sectional, non-experimental | N = 44 pigmented lesions Age and gender not reported | Photo (SAF) Nikon 4500 Coolpix camera post oil immersion or US gel. | • FTF diagnosis correct (66%, p < 0.05)and better observation detected. • TDS did not show similar diagnostic accuracy as FTF in teleconsultation. |
| Griffiths,47 2010 TeleDerm service in providing GPs with rapid assessment in diagnosis & mgmt |
Cross-sectional, non-experimental |
N = 660 lesions, number and type of per-sons unspecified. Age and gender not reported |
TeleDerm service; GP record patient history & images in EMR. | • Patients discharged by GP post TDS referral = 75% • GPs identified 7 lesions with TDS referral confirmed melanomas. • No melanomas misdiagnosed using TeleDerm service. |
| Janda et al.,46 2014 Mobile TDS + AC rule or AC rule alone in identifying lesions during SSE |
Experimental randomized controlled trial | Male 60%; female 40% SSE: 107 lesions identified Intervention (N = 66), control (N = 41) | Smartphones with DS attach-ment and the asymmetry- colour variation rule. | • Additional lesions noted by clinical skin exam (N = 42) • Both intervention and control groups missed two lesions by SSE |
| Ishioka et al.,35 2009 FTF vs. web images in diagnostic analysis | Case control study; non- experimental | N = 64 (61 % female; 39% male) | Clinical photos (Nikon Coolpix 4300); DS photos (image resolution of 2272 × 1704 pixels) with DS attachment (Heine Delta 20), post 24 months SAF with JPEG images. | • 72% agreement b/n in-person diagnosis and biopsy result • 66% agreement b/n telediagnosis and biopsy results • Web-based diagnostic service feasible; four false negatives |
| Kroemer et al.,42 2011 Diagnostic accuracy of clinical and dermoscopic images using tele-evaluation for mobile skin screening |
Quasi-experimental, non-randomized controlled trial | Patients (N = 80), tumours (N =113) (male 41; female 47) | Mobile phone (Nokia N73 built-in 3.2 Mpix; Nokia), camera lens pocket DS device (DermLite II PRO HR; 3Gen LLC), storage JPEG format, saved USB, Transmitted VI via e-derm consult GmbH) |
• Tele-evaluation strong agreementdifferentiating benign from malignant • Clinical image tele-evaluation superior to TDS with 16 vs. 22 opposing cases |
| Lamel et al.,36 2012 1. Mobile phone use for diagnoses and mgmt in skin cancer screening 2. Diagnosis/concordance b/n in-person & TD evaluations at screening event. |
Cross-Sectional design, non-experimental | N = 86 (137 skin lesions); male 36; female 50 | Mobile Google Android G1 (HTC Corporation); 3.2 Mpix autofocus cam; enabled with ClickDerm (Click Diagnostics). | • Mobile phone technology yielded high mgmt agreement b/n TD and in- person evaluation • Mgmt agreement b/n dermatologists high • Primary diagnostic agreement b/n dermatologists high |
| Manahan et al.,44 2015 1. Clinical accuracy of SSE +mobile TDS vs CSE. 2. SSE instructions improve accuracy. |
Randomized controlled trial | N = 58 (309 lesions), age 50–60 years | iPhone, Handyscope FotoFinder DS attachment (FotoFinder Systems GmbH) | • SSE + Mobile TDS at 69% with one lesion requiring follow-up • CSE found additional 40 lesions on 25 participants • Overall concordance with clinical diagnosis and TDS show substantial agreement |
| Massone et al.,37 2007 Melanoma screening feasibility with clinical and dermoscopic images via cellular phone. |
Cross-sectional; non-experi- mental, comparative | N = 18 (male 12, female 6) | Sony Ericsson K 750i (2 Mpix camera); pocket DS device with 25 mm 10 × lens (DermLite II PRO HR -3Gen, LLC); images 1632 × 1224 pixels JPEG format | • Two teleconsultants both agreed with FTF diagnosis in 89% of cases • Clinical image alone insufficient to correctly diagnose skin lesions and determine value of DS contribution |
| Massone et al.,38 2014 Feasibility of a SAF TD triage system |
Cross-sectional, Non-experi-mental comparative | N = 962 (lesions) N = 690 (patients); male 642; female 48 | Clinical images taken with Canon Powershot dig cam (Canon); DS images (DermLite Photo; 3Gen LLC) adjusted from MoleMax System (Derma Medical Systems) used (SAF) | • Image quality TDS vs DS: 88% excellent • Diagnostic accuracy among teleconsultants: 94% excellent • TD determined as suitable in triage of skin cancer |
| Piccolo et al.,39 2000 Agreement b/n direct clinical diagnosis vs. telediagnosis |
Cross-sectional; non-experi-mental, comparative | N = 40 (total patients); male 21; female 19 N = 43 (pigmented lesions) | FTF Dermoscope (Heine Delta 10, Heine Optotechnik), dig cam DCS 460, Kodak), with Nikon body (N90, Nikon). Storage (Digital ELM TeleDerm Workstation, Vanguard Imaging Ltd). Image size: 2036 × 3060 pixels RGB colour mode (32 bit/pixel) PEG 511 × 768 pixels in RGB colour mode (24 bit/pixel) |
• Concordance of observers:Telediagnosis performed better than FTF. • Melanomas diagnosed correctly (95%, 77% and 52%) at low, medium and high. • TDS reliable technique for cancerous skin lesion diagnosis |
| Tan et al.,40 2010 TDS as triage tool for skin lesions |
Cross-sectional; non-experi-mental, comparative | N = 200 (patients); male 74; female 126 N = 49l (lesions) | Nikon D50 digital SLR cam; HP Photosmart 912 or Canon Photoshot G6 1/1.8” 7 megapixel CCD dig cam, DS lighting unit (DermLite Foto; 3Gen LLC) with macro adapter for DS images (1600 × 1200 JPEG; 24-bit colour depth MoleMap point of diagnosis software (MoleMap). | • FTF and TD concordance 74% • FTF and TD consultation concord-anee (k = 0.90 in all categories) • TDS successful triage tool for patients referred to lesion diagnosis clinic |
| Warshaw et al.,43 2009 Accuracy of SAF TD vs. in-person clinical diagnosis |
Cross-sectional, experimen-tal, repeated measures trial | N = 542 (pigmented lesions); male 96%; female 4% | Macro images (Nikon Coolpix 4500 SL-I ring flash, (Nikon); PLD images (Nikon Coolpix 4500 3Gen DermLite lens (3Gen), DS image (35-mm Minolta × 370 with Heine dermphot lens attachment, (Heine); 35-mm kodachrome (Nikon Cool Scan LS-4000ED, Konica Minolta) | • No significant differences in accuracy b/n macro images alone and macro + PLD • No significant differences in primary diagnosis or mgmt plan • Diagnostic accuracy of TDS inferior to clinical dermatology • Patient receptivity: TD significantly |
| Wu et al.,41 2015 Feasibility, efficacy and receptivity of TDS for atypical nevi monitoring |
Prospective, cohort study | N = 29; male 16; female 18 | Images (Nikon D90 SLR dig cam with 60-mm Macro Nikkor lens with derm attachment (Epilume; Canfield Imaging Systems, Inc.); 1 by patient with mobile DS (DermScope; Canfield Imaging Systems, Inc.) attached to mobile phone with 8-Mpix cam (iPhone 4S; Apple, Inc.) Standard & routing taken under contact, polarized light with × 10 magnification. | • Patient receptivity: TD significantly higher in agreement compared to negative attitude toward TD(p < 0.01) • Findings suggest TDS both feasible and effective for short-term monitoring of clinically atypical nevi |
Abbreviations listed in alphabetical order: AC = asymmetry-colour; b/n = between; CSE = clinical skin examination; DS = dermoscopic; EMR = electronic medical record; FTF = face-to-face; GP= General Practitioner; IO = interobserver; mgmt = management; PLD = polarized light dermoscopy; PSL = pigmented skin lesions; SAF = store-and-forward; SSE = skin self-examination; TD = teledermatology; TDS = teledermoscopy; TDs = teledermoscopists).
Clinical diagnostic accuracy
Four studies reported on the clinical accuracy of diagnosis using teledermoscopy.35,44–46 Ishioka et al. conducted a 24-month case-controlled study of 64 cases to determine agreement between the clinical specialist making the diagnosis in person and the clinician’s diagnosis by examining data and images via teledermoscopy with the same two dermatologists for pre-selection and post diagnosis.35 Results of this study demonstrated agreement between in-person examinations and biopsy results, and between levels of teledermoscopy and biopsy results. Janda et al. compared teledermoscopy to skin self-examination using a randomized controlled trial design with 22 participants.46 The intervention group utilized smartphones with a dermoscope attachment plus the asymmetry-colour variation rule and the control group used only the asymmetry-colour variation rule. During skin self-examination, 107 lesions were identified and during clinical evaluation, 42 lesions were noted but there was no difference between groups. Börve et al. conducted a prospective study for one year to compare teledermoscopy referral via smartphone to paper-based referrals.45 The sample of 772 patients referred with smartphone teledermoscopy, and 746 patients referred by a paper-based system were compared to face-to-face observations. The teledermoscopy group showed 22.6% more referrals than the paper-based group and 35 invasive melanocytic melanomas were prioritized correctly. Manahan et al. used a randomized controlled trial over a 3 month time frame (N=49, 309 lesions submitted) to compare skin self-examination with mobile teledermoscopy compared to clinical skin examination.44 Skin self-examination plus mobile teledermoscopy showed high sensitivity and moderate specificity. The clinical skin evaluation submitted by mobile teledermoscopy was highly sensitive and highly specific, indicating concordance with the clinical diagnosis and telediagnosis.
Diagnostic reliability
Eight studies reported on the reliability of teledermoscopy for diagnosing skin lesions.32,34,36,39,40,42,43,47 Piccolo et al. determined that telediagnosis of melanomas was correct at rates of 95%, 77% and 52% at low, medium and high diagnostic difficulty and dependent on the experience of the observer.39 Fabbrocini et al. studied 44 lesions and determined that irregular pigmentation and regression structures had a higher frequency for diagnosis in teledermoscopic observations34 but in all other criteria, face-to face observations were better. Warshaw et al. reported no significant differences in accuracy rates between macro images alone and macro plus polarized light dermoscopy, primary diagnoses and management plans.43 Griffiths compared patients using images via teledermoscopy to assess diagnostic accuracy of 660 lesions in a crosssectional study and reported that no melanomas were misdiagnosed using a TeleDerm service;47 net cost savings of 50% were projected due to decreased hospital admissions/routine referrals. Tan et al. reported concordance between face-to-face diagnosis and teledermoscopy at 74% (285/ 385 lesions),40 and 74% (219/296 lesions) for two dermatologists performing both exams for each patient. Kroemer et al. demonstrated strong agreement between clinical image tele-evaluation and teledermoscopy with biopsy,42 differentiating benign from malignant (90%; k=0.84). Both methods were equally high on sensitivity for all diagnostic categories and specificity for benign non-melanocytic (97%) and melanocytic lesions (99%). Lamel et al. reported overall diagnostic agreement between mobile phone images and TeleDerm consultation and high agreement between dermatologists.36 Börve et al. conducted a second study and concluded that there is high interobserver agreement between face-to-face consultation and teledermoscopy.32
Feasibility
Five studies reported on feasibility of using teledermoscopy for assessing skin lesions in addition to clinical diagnostic accuracy. Di Stefani et al. demonstrated that it was feasible to achieve clinical diagnostic accuracy with agreement between face-to-face and two remote teledermatologists.33 Massone et al. completed two studies, the first of which examined the feasibility to perform a melanoma screening using cell phone images versus dermoscope images.37 They determined that the quality of cell phone images was poorer than the quality of dermoscopic images. The second study by Massone et al. investigated the feasibility of a SAF teledermoscopy triage system over a 24-month time frame.38 The image quality was excellent and diagnostic accuracy among teleconsultants was excellent with malignant and benign lesions being correctly diagnosed by teleconsultants. Wu et al. evaluated the feasibility of mobile dermoscopy by patients, diagnostic concordance of teledermoscopy with conventional office-based visits and patient’s receptiveness to teledermoscopy for short-term monitoring of nevi.41 On 30 image pairs evaluated by the teledermatologists, there was 97% agreement with the decision made by the clinical dermatologist and patients were receptive to teledermoscopy. Finally, Ishioka et al. reported that a web-based teledermoscopic diagnosis is feasible and could potentially meet the patient care access needs in rural areas.35
Limitations
In this systematic review, there is some potential for selection bias due to the limited number of available abstracts found as a result of key term search criteria. Conclusions were interpreted with caution due to the lack of randomized controlled trials performed or accessible. Cost was not considered as part of this literature review due to lack of reporting. Finally, the variance of instrumentation could contribute to outcome bias due to identified lack of consistency with image capture and quality. Additional selection bias may exist due to individual selection of suspicious lesions for skin self-examinations, restriction of lesion selection, and only primary body areas selection (arms, legs, face, backs and shoulders) and lack of blinding was not possible in several studies due to face-to-face examinations. External validity is limited in some studies due to small sample sizes. In addition, lack of diversity in sample population (primarily male) is a consideration for generalizability. Finally, the potential for recall bias using the same dermatologists for teledermoscopic and face-to-face diagnosis exists as well as the reliability and reproducibility could be dependent upon the level of dermatological training and experience.
Discussion
Teledermoscopy is an identified modality with both mobile patient skin self-examination and diagnosis or monitoring via teledermatologist. The American Telemedicine Association has recommendations for best practices that delineate appropriate image acquisition, storage retrieval and transmission and image display.29 These include both technical and clinical specifications. However, practice guidelines for teledermatology have no current recommendation for standard lesion comparison.29
The results of this review revealed varying types of instrumentation used to capture skin lesion images. The majority of the studies focused on digital cameras with attachable dermoscopic devices, while others were built-in mobile phone applications. This difference between types of image capture and storage can alter the appearance of the lesion, emphasizing a need for consistent use of valid and reliable instrumentation that maximizes image quality and contributes to accuracy of image interpretation.
Future implications for research and practice
There are significant gaps remaining in the research methodological approaches that have been used to study teledermoscopy. There is a continued need for future comparative effectiveness studies aiming to identify the premier method for image capture, storage, and review so that evidence can be provided for clinical practice guidelines. A more immediate clinical practice change could include the integration of teledermoscopic images into the patient electronic medical record (EMR). Automatic inclusion of patient images in the patient’s record would enhance efficiency of care and access to specialty care. Ensuring that image data is easily retrievable for patients and providers would be consistent with the most recent emphasis on meaningful use of electronic health records. This would require that both acute and chronic care facilities have access to picture archiving and communication systems (PACS), which are key to digital integration for diagnostic management. As EMRs continue to transform into meaningful use stage 3, image technology advancement is an essential focus for clinical practice.
Conclusion
With the escalation of malignant melanoma diagnoses on a national level, access to care is essential and should be placed as a high priority on legislative agendas. The ability to take mobile diagnostic preventive screening to the community has proved to be effective, and incorporating teledermoscopy for suspected cancerous lesions could improve access to care.
Teledermoscopic evaluation of potentially cancerous skin lesions can be useful in the prevention, diagnosis, and treatment of malignant melanoma. Higher level evidence such as randomized controlled trials, comparative effectiveness trials, and meta-analyses are needed to build evidence for clinical practice changes in both the acute care and community settings.
Acknowledgement
Dr. Mallow’s time on this project was supported by the Robert Wood Johnson Foundation Nurse Faculty Scholars Program.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interestwith respect to the research, authorship, and/or publication of this article.
References
- 1.Cancer.org. Skin cancer facts, www.cancer.org/cancer/cancercauses/sunanduvexposure/skin-cancer-facts (2016, accessed 1 July 2016).
- 2.Skincancer.org. Skin cancer facts & statistics, www.skincancer.org/skin-cancer-information/skin-cancer-facts (2016, accessed 1 July 2016).
- 3.Cancer.org. Key statistics for melanoma skin cancer, www.cancer.org/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-key-statistics (2016, accessed 1 July 2016).
- 4.Guy G, Machlin S, Ekwueme D, et al. Prevalence and costs of skin cancer treatment in the US, 2002–2006 and 2007–2011. Am J Prev Med 2015; 48: 183–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer.org. Learn about cancer: American Cancer Society,www.cancer.org/cancer/index (2016, accessed 1 July 2016).
- 6.US Preventive Services Task Force. Final update summary: skin cancer screening, www.uspreventiveservicestaskforce.org/Page/Document/UpdateSummaryFinal/skin-cancerscreening2?ds¼1&s¼skin (2016, accessed 19 August 2016).
- 7.Romero G, Sánchez P, García M, et al. Randomized controlled trial comparing store-and-forward teledermatology alone and in combination with web-camera videoconferencing. Clin Exp Dermatol 2010; 35: 311–317. [DOI] [PubMed] [Google Scholar]
- 8.Massone C, Brunasso A, Hofmann-Wellenhof R, et al. Teledermoscopy: education, discussion forums, teleconsulting and mobile teledermoscopy. G Ital Dermatol Venereol 2010; 145: 127–132. [PubMed] [Google Scholar]
- 9.Liberati A The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009; 151: W–65. [DOI] [PubMed] [Google Scholar]
- 10.Rosswurm M and Larrabee J. A model for change to evidence-based practice. Image J Nurs Scholarship 1999; 31: 317–322. [DOI] [PubMed] [Google Scholar]
- 11.Eedy D and Wootton R. Teledermatology: a review. Br J Dermatol 2001; 144: 696–707. [DOI] [PubMed] [Google Scholar]
- 12.High W, Houston M, Calobrisi S, et al. Assessment of the accuracy of low-cost store-and-forward teledermatology consultation. J Am Acad Dermatol 2000; 42: 776–783. [DOI] [PubMed] [Google Scholar]
- 13.Hsiao J and Oh D. The impact of store-and-forward teledermatology on skin cancer diagnosis and treatment. J Am Acad Dermatol 2008; 59: 260–267. [DOI] [PubMed] [Google Scholar]
- 14.Hu S, Foong H and Elpern D. Virtual grand rounds in dermatology: an 8-year experience in web-based teledermatology. Int J Dermatol 2009; 48: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 15.Kahn E, Sossong S, Goh A, et al. Evaluation of skin cancer in Northern California Kaiser Permanente’s store-and-forward teledermatology referral program. Telemed eHealth 2013; 19: 780–785. [DOI] [PubMed] [Google Scholar]
- 16.Kanthraj G A longitudinal study of consistency in diagnostic accuracy of teledermatology tools. Indian J Dermatol Venereol Leprol 2013; 79: 668. [DOI] [PubMed] [Google Scholar]
- 17.Lim A, Egerton I, See A, et al. Accuracy and reliability of store-and-forward teledermatology: preliminary results from the St George Teledermatology Project. Australas J Dermatol 2001; 42: 247–251. [DOI] [PubMed] [Google Scholar]
- 18.Mahendran R, Goodfield M and Sheehan-Dare R. An evaluation of the role of a store-and-forward teledermatology system in skin cancer diagnosis and management. Clin Exp Dermatol 2005; 30: 209–214. [DOI] [PubMed] [Google Scholar]
- 19.Massone C, Lozzi G, Wurm E, et al. Personal digital assistants in teledermatology. Br J Dermatol 2006; 154: 801–802. [DOI] [PubMed] [Google Scholar]
- 20.Osei-tutu A, Shih T, Rosen A, et al. Mobile teledermatology in Ghana: sending and answering consults via mobile platform. J Am Acad Dermatol 2013; 69: e90–e91. [DOI] [PubMed] [Google Scholar]
- 21.Williams CM, Qureshi A, Geller A, et al. Skin cancer education program via teledermatology: is it effective? J Am Acad Dermatol 2007; 56: AB100. [Google Scholar]
- 22.Warshaw E, Gravely A and Nelson D. Accuracy of teledermatology/teledermoscopy and clinic-based dermatology for specific categories of skin neoplasms. J Am Acad Dermatol 2010; 63: 348–352. [DOI] [PubMed] [Google Scholar]
- 23.Weinstock M Evaluation of in-person dermatology versus teledermatology. J Am Acad Dermatol 2009; 61: 902–903. [DOI] [PubMed] [Google Scholar]
- 24.Kanthraj G A teledermatology practice model for a national healthcare system: proposal and recommendations. J Eur Acad Dermatol Venereol 2010; 24: 616–619. [Google Scholar]
- 25.Lipozencic J, Zrinjka P and Shahbaz J. Teledermotology. Acta Dermatovenerol Croatica 2007; 15: 199–201. [PubMed] [Google Scholar]
- 26.Van der Heijden J Teledermatology integrated in the Dutch national healthcare system. J Eur Acad Dermatol Venereol 2010; 24: 615–616. [DOI] [PubMed] [Google Scholar]
- 27.Federman D, Kirsner R and Viola K. Skin cancer screening and primary prevention: facts and controversies. Clin Dermatol 2013; 31: 666–670. [DOI] [PubMed] [Google Scholar]
- 28.Wurm E, Hofmann-Wellenhof R, Wurm R, et al. Telemedicine and teledermatology: past, present and future. JDDG 2008; 6: 106–112. [DOI] [PubMed] [Google Scholar]
- 29.Americantelemed.org. Practice guidelines for teledermatology, www.americantelemed.org/resources/telemedicinepractice-guidelines/telemedicine-practice-guidelines/practice-guidelines-for-teledermatology#.VjtatrerSUk (2007, accessed 1 July 2016).
- 30.Gulia A, Giovanna Brunasso A and Massone C. Dermoscopy: distinguishing malignant tumors from benign. Expert Rev Dermatol 2012; 7: 439–458. [Google Scholar]
- 31.Janda M, Youl P, Marshall A, et al. The HealthyTexts study: a randomized controlled trial to improve skin cancer prevention behaviors among young people. Contemp Clin Trials 2013; 35: 159–167. [DOI] [PubMed] [Google Scholar]
- 32.Börve A, Terstappen K, Sandberg C, et al. Mobile teledermoscopy—there’s an app for that! Dermatol Pract Conceptual 2013; 3: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Di Stefani A, Zalaudek I, Argenziano G, et al. Feasibility of a two-step teledermatologic approach for the management of patients with multiple pigmented skin lesions. Dermatol Surg 2007; 33: 686–692. [DOI] [PubMed] [Google Scholar]
- 34.Fabbrocini G, Balato A, Rescigno O, et al. Telediagnosis and face-to-face diagnosis reliability for melanocytic and non-melanocytic ‘pink’ lesions. J Eur Acad Dermatol Venereol 2008; 22: 229–234. [DOI] [PubMed] [Google Scholar]
- 35.Ishioka P, Tenorio J, Lopes P, et al. A comparative study of teledermatoscopy and face-to-face examination of pigmented skin lesions. J Telemed Telecare 2009; 15: 221–225. [DOI] [PubMed] [Google Scholar]
- 36.Lamel S, Haldeman K, Ely H, et al. Application of mobile teledermatology for skin cancer screening. J Am Acad Dermatol 2012; 67: 576–581. [DOI] [PubMed] [Google Scholar]
- 37.Massone C, Hofmann-Wellenhof R, Ahlgrimm-Siess V, et al. Melanoma screening with cellular phones. PLoS One 2007; 2: e483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massone C, Maak D, Hofmann-Wellenhof R, et al. Teledermatology for skin cancer prevention: an experience on 690 Austrian patients. J Eur Acad Dermatol Venereol 2014; 28: 1103–1108. [DOI] [PubMed] [Google Scholar]
- 39.Piccolo D, Smolle J, Argenziano G, et al. Teledermoscopy: results of a multicentre study on 43 pigmented skin lesions. J Telemed Telecare 2000; 6: 132–137. [DOI] [PubMed] [Google Scholar]
- 40.Tan E, Yung A, Jameson M, et al. Successful triage of patients referred to a skin lesion clinic using teledermoscopy (IMAGE IT trial). Br J Dermatol 2010; 162: 803–811. [DOI] [PubMed] [Google Scholar]
- 41.Wu X, Oliveria S, Yagerman S, et al. Feasibility and efficacy of patient-initiated mobile teledermoscopy for short-term monitoring of clinically atypical nevi. JAMA Dermatol 2015; 151: 489–496. [DOI] [PubMed] [Google Scholar]
- 42.Kroemer S, Frühauf J, Campbell T, et al. Mobile teledermatology for skin tumour screening: diagnostic accuracy of clinical and dermoscopic image tele-evaluation using cellular phones. Br J Dermatol 2011; 164: 973–979. [DOI] [PubMed] [Google Scholar]
- 43.Warshaw E, Lederle F, Grill J, et al. Accuracy of teledermatology for pigmented neoplasms. J Am Acad Dermatol 2009; 61: 753–765. [DOI] [PubMed] [Google Scholar]
- 44.Manahan M, Soyer H, Loescher L, et al. A pilot trial of mobile, patient-performed teledermoscopy. Br J Dermatol 2015; 172: 1072–1080. [DOI] [PubMed] [Google Scholar]
- 45.Börve A, Gyllencreutz J, Terstappen K, et al. Smartphone teledermoscopy referrals: a novel process for improved triage of skin cancer patients. Acta Dermatol Venereol 2015; 95: 186–190. [DOI] [PubMed] [Google Scholar]
- 46.Janda M, Loescher L, Banan P, et al. Lesion selection by melanoma high-risk consumers during skin self-examination using mobile teledermoscopy. JAMA Dermatol 2014; 150: 656–658. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths W Improving melanoma diagnosis in primary care: a tele-dermatoscopy project. J Telemed Telecare 2010; 16: 185–186. [DOI] [PubMed] [Google Scholar]