Abstract
Poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) micelles affect drug solubilization, and a paclitaxel (PTX) loaded-PEG-b-PLA micelle (PTX-PM) is approved for cancer treatment due to injection safety and dose escalation (Genexol-PM®) compares to Taxol®. However, PTX-PM is unstable in blood, has rapid clearance, and causes dose-limiting toxicity. We have synthesized a prodrug for PTX (7-OH), using oligo(lactic acid) as a novel pro-moiety (o(LA)8-PTX) specifically for PEG-b-PLA micelles, gaining higher loading and slower release of o(LA)8-PTX over PTX. Notably, o(LA)8-PTX prodrug converts into PTX by a backbiting reaction in vitro, without requiring esterases. We hypothesize that o(LA)8-PTX-loaded PEG-b-PLA micelles (o(LA)8-PTX-PM) has a lower Cmax and higher plasma AUC than PTX-PM for improved therapeutic effectiveness. In Sprague-Dawley rats at 10 mg/kg, compared to o(LA)8-PTX-PM (10% w/w loading) and PTX-PM (10%), o(LA)8-PTX-PM (50% w/w loading) produced a 2- and 3-fold higher plasma AUC0–24 of PTX, lactic acid-PTX, and o(LA)2-PTX (o(LA)0–2-PTX), respectively. For o(LA)8-PTX-PM at 10 and 50% w/w loading, PTX and lactic acid-PTX are major bioactive metabolites, respectively. Fast prodrug conversion of o(LA)8-PTX in vivo versus in vitro (by backbiting) suggests that o(LA)8 is a good substrate for esterases. At 60 mg/kg (qwx3), o(LA)8-PTX-PM (50%) has higher antitumor activity than o(LA)8-PTX-PM (10%) and PTX-PM (10%) in a syngeneic 4T1-luc breast tumor model based on measurements of tumor volume, 4T1-luc breast tumor bioluminescence, and survival. Importantly, intravenous administration of o(LA)8-PTX-PM was well tolerated by BALB/c mice. In summary, oligo(lactic acid)8-PTX is more compatible than PTX with PEG-b-PLA micelles, more stable, and may expand the role of PEG-b-PLA micelles from “solubilizer” into “nanocarrier” for PTX as a next-generation taxane for cancer.
Keywords: Block copolymer, oligo(lactic acid), PEG, polymeric micelle, prodrug, taxanes
Graphical Abstract

1. Introduction
Paclitaxel (PTX) for injection (Taxol®) is used to treat advanced breast, non-small cell lung, and ovarian cancers, as well as Kaposi’s sarcoma, stabilizing microtubules as an antineoplastic agent [1–4]. It interferes with the late G-2 mitotic phase, thereby causing cell cycle arrest, inhibition of replication, and cell death [5–6]. However, PTX has poor water solubility (< 0.3 μg/mL) and requires Cremophor EL and ethanol for IV injection/infusion [7]. Taxol® causes dose-limiting toxicities such as neutropenia and peripheral neurotoxicity and life-threatening hypersensitivity reactions despite pre-medication with corticosteroids [8–10]. Omitting Cremophor EL and ethanol, a PTX-loaded poly(ethylene glycol)-block-poly(D,L-lactic acid) (PEG-b-PLA) polymeric micelle (PTX-PM or Genexol-PM®) has a higher maximum tolerated dose (MTD) in humans (300 versus 175 mg/m2 qwx3) and evidence of higher antitumor efficacy [11–13]. However, PTX-PM acts primarily as a “solubilizer” for PTX having fast release, evidenced by a high fraction of PTX being associated with serum lipoprotein in 5 min after incubation in human plasma in vitro [14]. Fast in vivo drug release is often associated with undesirable pharmacokinetic parameters: high Cmax and low plasma AUC, which leads to dose-limiting toxicity and reduced antitumor efficacy, respectively [10, 15–16]. Thus, Genexol-PM® requires a higher dose than Taxol® to achieve equal tumor accumulation in murine tumor models [11–12, 17–18].
Hydrophobic prodrugs have been proposed for PEG-b-PLA micelles, aiming for stability in blood and increased plasma AUC, as a strategy to gain higher drug exposure at solid tumors. Acyl ester prodrugs of PTX are more stable than PTX in polymeric micelles and are long-circulating in blood [19]. A small library of hydrophobic pro-moieties has been tested for 7-ethyl-10-hydroxycamptothecin and PEG-b-PLA nanoparticles, affecting drug loading and release kinetics [20]. Besides hydrophobicity, augmenting drug compatibility for PEG-b-PLA micelles has been realized by oligo(lactic acid) (o(LA)n) as a novel pro-moiety, attaining evidence of higher stability in vitro and in vivo and improved antitumor efficacy [21–22]. We have synthesized an o(LA)8-PTX prodrug (7-OH) and it has higher loading in PEG-b-PLA micelles than PTX and is more stable in vitro [22]. In vitro conversion of o(LA)8-PTX prodrug into PTX occurs at a preset rate by an intramolecular backbiting mechanism: Attack of chain end hydroxyl of o(LA)8 on penultimate ester bond, forming PTX, lactic acid-PTX, and o(LA)2-PTX (o(LA)0–2-PTX) as major metabolites [22–23]. In an A549 NSCLC tumor model, an o(LA)8-PTX prodrug-loaded PEG-b-PLA micelle (o(LA)8-PTX-PM) has higher antitumor efficacy than PTX-PM and less toxicity based on body weight changes [22].
Based on this o(LA)8-PTX prodrug design, PEG-b-PLA micelles may be more stable than PTX-PM in blood after IV injection, resulting in a lower Cmax and higher plasma AUC (Figure 1). Key findings include a “nanocarrier” effect on o(LA)8-PTX prodrug by PEG-b-PLA micelles, manifested at early times after injection, versus primarily a “solubilizer” effect, i.e. fast release for PTX. Upon release, conversion of o(LA)8-PTX prodrug is faster in vivo than anticipated based on backbiting of o(LA)8-PTX prodrug, indicating a surprising finding that o(LA)n is a good substrate for esterases. Lastly, o(LA)8-PTX-PM has higher antitumor efficacy than PTX-PM in a syngeneic 4T1-luc breast tumor model, attaining a higher therapeutic index for cancer therapy.
Figure 1.
An o(LA)8-PTX prodrug-loaded PEG-b-PLA micelle (o(LA)8-PTX-PM). Relative to a PTX-loaded PEG-b-PLA micelle (PTX-PM), o(LA)8-PTX-PM may be more stable in blood. After release, o(LA)8-PTX converts into PTX, lactic acid-PTX, and o(LA)2-PTX (o(LA)0–2-PTX) as metabolites for a lower Cmax and higher plasma AUC relative to PTX-PM, aiming for reduced toxicity and higher antitumor efficacy.
2. Materials and methods
2.1. Materials
Paclitaxel (PTX) was purchased from LC Laboratories (Woburn, MA). Oligo(lactic acid)8 paclitaxel (o(LA)8-PTX) was synthesized according to our previous report [22]. PEG-b-PLA was purchased from Advanced Polymer Materials Inc. (Montreal, Canada): Mn of PEG and PLA was 4,000 and 2,200 g/mol, respectively; PDI 1.05. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) and used as received. Analytical grade organic solvents and all other reagents were purchased from Fisher Scientific (Pittsburgh, PA). 4T1 human breast cancer cell lines were purchased from ATCC (Manassas, VA). 4T1 cells were stably transfected with a luciferase-expressing plasmid pGL4.51 containing the neomycin-resistance gene (Promega, Madison, WI) using lipofectamine 2000 from Invitrogen (Carlsbad, CA) according to the manufacturer’s instructions and our previous report [24]. D-Luciferin was purchased from Caliper Life Science (Hopkinton, MA). Heparinized Sprague-Dawley rat plasma and human pool plasma were purchased from Innovative Research Inc. (Novi, MI).
2.2. Reverse-phase high performance liquid chromatography (RP-HPLC) of o(LA)0–8-PTX prodrug
Samples were analyzed by RP-HPLC using a Shimadzu Prominence HPLC system (Shimadzu, Japan), consisting of a LC-20AT pump, a SIL-20AC HT autosampler, a CTO-20AC column oven, and a SPD-M20A diode array detector. A Waters Symmetry Shield™ RP18 column (4.6 mm × 250 mm, 5 μm, 100 Å) was used for analyte separation. 10 μL of sample was injected at a flow rate of 0.8 mL/min, column temperature at 25 °C, and UV detection wavelength for PTX and o(LA)n-PTX at 227 nm. The separation of o(LA)0–8-PTX (refers to all metabolites with the number of lactic acid oligomers (n) of o(LA)n-PTX from 0–8) was done in gradient mode with organic phase containing 100% acetonitrile as solvent A, and aqueous phase containing 100% milliQ water as solvent B. Gradient elution was employed as follows: 0 min, 50% solvent A and 50% solvent B; 35 min, 95% solvent A and 5% solvent B; and 40 min for equilibration.
2.3. Preparation of o(LA)8-PTX-PM and PTX-PM
Briefly, o(LA)8-PTX-PM was prepared and characterized as previously described [22]. For 10 and 50% w/w loading, 17.0 mg of o(LA)8-PTX prodrug (10.0 mg PTX equivalent (-eq)) and 170.0 mg (10% w/w loading) or 17.0 mg (50% w/w loading) of PEG-b-PLA were dissolved in 2.0 mL of acetonitrile and transferred to a 10.0 mL round-bottom flask. Acetonitrile was removed under reduced pressure using a rotary evaporator to form a thin film at 60 °C. 1.0 mL of pre-warmed 0.9% saline or 10 mM phosphate buffer saline (PBS) solution at 60 °C was added to the polymer film for rehydration. The aqueous solution containing o(LA)8-PTX-PM was centrifuged at 13,000 rpm for 5 min, followed by sterile filtration (0.22 μm (Corning, NY)). Similarly, for PTX-PM at 10% w/w loading, 10.0 mg of PTX and 100.0 mg of PEG-b-PLA were used. The final level of o(LA)8-PTX or PTX was quantified by RP-HPLC and level adjusted before IV injection into mice. Prodrug or drug loading efficiency was calculated from the level of o(LA)8-PTX prodrug- or PTX-loaded in PEG-b-PLA micelles divided by the initial level of o(LA)8-PTX prodrug or PTX, respectively. Actual drug loading content was calculated based on the weight of o(LA)8-PTX prodrug or PTX divided by the total weight of o(LA)8-PTX prodrug or PTX plus PEG-b-PLA micelles.
2.4. Particle size analysis
Dynamic light scattering (DLS) was used to measure hydrodynamic diameters of PEG-b-PLA micelles using a Zetasizer Nano-ZS (Malvern Instruments Inc., UK) at 25 °C with a detection angle of 173° and a He-Ne ion laser as the light source (4 mW, 633 nm). Prior to measurements, PEG-b-PLA micelle solutions were diluted with milliQ water or PBS solution (10 mM, pH 7.4) to ca. 0.10 mg/mL of PEG-b-PLA, and 1.0 mL of each sample was placed into a disposable sizing cuvette (BrandTech Scientific Inc., Essex, CT). The cumulant method was used to curve-fit the correlation function, and the z-average diameter and polydispersity index (PDI) of PEG-b-PLA micelles were calculated from the Stokes-Einstein equation and slope of the correlation function, respectively. All measurements were performed in triplicate. The morphology of o(LA)8-PTX-PM was observed using an atomic force microscope (AFM) in AC mode after adsorption of the polymer at 5–10 mg/mL on mica. Micelles were imaged in MilliQ water using an AC40 biolever on an Infinity Bioscope (Asylum Research, Santa Barbara, CA).
2.5. In vitro plasma stability
In vitro stability of o(LA)8-PTX prodrug and o(LA)8-PTX-PM (10 or 50%) in plasma were determined using heparinized Sprague-Dawley rat plasma (Innovative Research Inc., Novi, MI) and pooled human plasma (Innovative Research Inc., Novi, MI). Frozen plasma samples were incubated at 37 °C for 5 min before use. Stock solution of o(LA)8-PTX was prepared in DMSO at 1 mM. In 1.0 mL plasma samples, 10 μL of stock solution was added to reach a concentration of o(LA)8-PTX at 10 μM (< 1% DMSO in the mixture). Alternatively, 100 μL of o(LA)8-PTX-PM (10%) or o(LA)8-PTX-PM (50%) in PBS solution (10 mM, pH 7.4) was added to plasma (900 μL) to reach a final concentration of 10 μM. Samples were incubated at 37 °C in a temperature adjusted water bath (GCA Corporation, IL). At predetermined time intervals (0, 0.25, 0.5, 1, 2, 4, 6, 9, and 24 h), 50 μL of plasma samples were withdrawn and diluted with 100 μL of acetonitrile containing 0.1% formic acid. Precipitated samples were centrifuged at 13,000 rpm for 10 min and the supernatant containing solubilized o(LA)8-PTX and its metabolites (o(LA)0–8-PTX) were analyzed by RP-HPLC. The relative amount of o(LA)8-PTX and its metabolites (o(LA)0–8-PTX) was calculated as a percentage relative to the starting level of o(LA)8-PTX.
2.6. In vitro drug release
PTX-PM or o(LA)8-PTX-PM was diluted to 0.5 mg/mL in 10 mM PBS solution at pH 7.4 and 2.5 mL added into a Slide-A-Lyzer™ dialysis cassette with MWCO 20K (ThermoFisher, Waltham, MA). The dialysis cassette was placed in a 4 L PBS solution (10 mM, pH 7.4) on a Corning Hotplate Stirrer (Corning, NY) at 37 °C (n=3). At 0, 1, 2, 3, 6, 9, 24, 48, 72, 120, and 168 h after incubation, 100 μL of sample was withdrawn from the dialysis cassette and the cassette was replenished with 100 μL of PBS solution (10 mM, pH 7.4). The external medium was replaced with 4 L of fresh buffer at 2, 6, 24, and 72 h to approximate sink conditions. The quantification of PTX or o(LA)8-PTX remaining in PEG-b-PLA micelles was accomplished by RP-HPLC analysis, and cumulative percent release of PTX or o(LA)8-PTX was calculated, plotting % release versus time and estimating in vitro release half-life (t1/2), assuming first-order kinetics.
2.7. In vitro cytotoxicity
The cytotoxicity of PTX and o(LA)8-PTX (free and micelle) against a 4T1-luc breast carcinoma cell lines was investigated by the CellTiter-Blue® Cell Viability Assay (Promega, WI). The cells were seeded into a 96-well plate at a seeding density of 4,000 cells/100 μL/well and cultured in Dulbecco’s Modified Eagle Medium (DMEM) at 37 °C in 5% CO2 incubator for 24 h. PTX, o(LA)2-PTX or o(LA)8-PTX was dissolved in DMSO. The final level of DMSO in the medium was < 0.3%. Each was added into the wells to attain levels of 0.001 – 10 μM. While PTX-PM or o(LA)8-PTX-PM in PBS solution (10 mM, pH 7.4) was added into the wells to attain levels of 0.01 – 100 μM. Cells cultured with diluted DMSO or PBS in medium were used as controls. Treated cells were placed in an incubator at 5% CO2 at 37 °C for 72 h. The medium in each well was aspirated, and 100 μL of 20% (v/v) CellTiter-Blue® reagent in serum free DMEM was added, followed by incubation at 37 °C in 5% CO2 atmosphere for 1.5 h. Fluorescence intensity of viable cells was measured by a SpectraMax M2 plate reader (Molecular Devices, CA) with excitation and emission at 560 and 590 nm, respectively. The half maximal inhibitory drug concentration (IC50) was determined by using GraphPad Prism version 5.00 for Windows (GraphPad Software, CA).
2.8. Plasma profile in rats
All animal studies were approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee and conducted in accordance with institutional and NIH guidance. Male Sprague-Dawley rats (200–240 g) with a jugular vein catheter were purchased from Charles River Laboratories (Wilmington, MA). Rats were individually housed in ventilated cages with free access of food and water and acclimatized for at least 1 week prior to beginning of study. PTX-PM (10%), o(LA)8-PTX-PM (10%), or o(LA)8-PTX-PM (50%) was injected into rats at 10 mg/kg PTX-eq (n=4). Serial blood samples (ca. 400 μl) were collected from the jugular vein cannula at 5, 30 min, then 1, 2, 4, 6, 9, and 24 h after IV injection. Each blood sample was collected in lithium heparin w/plasma separator tube (BD Biosciences, NJ), followed by centrifugation at 2,000 rpm for 10 min. Plasma was collected and stored at −80 °C until analyses.
2.9. Bioanalysis of o(LA)0–8-PTX in plasma
The level of o(LA)8-PTX and its metabolites (o(LA)0–8-PTX) in plasma was determined by a liquid-liquid extraction method using RP-HPLC [25]. Total levels of o(LA)0–8-PTX were measured in plasma: o(LA)8-PTX in PEG-b-PLA micelles, released o(LA)8-PTX, and its metabolites. In brief, 100 μL of plasma samples were spiked with 10 μL of methyl-p-hydroxybenzoate (25 μg/mL in acetonitrile containing 0.1% formic acid) as internal standard (IS). Tert-butyl methyl ether (t-BME, 0.50 mL) was added to the mixture and vortexed for 2 min, followed by centrifugation at 13,000 rpm for 5 min. Clear supernatant was collected and placed in a separate microcentrifuge tube. The residual plasma precipitate was re-extracted with another 0.5 mL of t-BME, vortexed for 2 min, and centrifuged at 13,000 rpm for 5 min. Clear supernatants were combined and solvent evaporated under reduced pressure using a Savant™ SpeedVac™ concentrator (Waltham, MA). Dried samples were reconstituted with 150 μL of acetonitrile containing 0.1% formic acid, vortexed for 1 min, and centrifuged at 13,000 rpm for 5 min. 100 μL of each sample was injected into RP-HPLC for plasma analysis. The range of linearity tested for o(LA)8-PTX prodrug and PTX was 0.25 – 25 μg/mL (r2 = 0.99 and 0.97, respectively).
2.10. Plasma AUC
The plasma area under the concentration-time curve from 0 to 24 hours (AUC0–24) of PTX, o(LA)0–2-PTX, and o(LA)0–8-PTX was calculated using the linear trapezoidal rule:
The term (tt+1 − ti) is the time interval between two time points, including 0, 5, 30 min, and 1, 2, 4, 6, 9, and 24 h. While Ci and Ci+1 are the corresponding plasma concentrations. The relative intensity (%) of PTX, o(LA)0–2-PTX, and o(LA)0–8-PTX was calculated with respect to the total intensity of all species at each time point.
2.11. Antitumor efficacy
Six- to eight-week-old female BALB/c mice (15–25 g) were acquired from Harlan Laboratories (Envigo, Indianapolis, IN). Mice were housed in ventilated cages with free access to water and food and acclimated for 1-week prior tumor cell injection. 4T1-luc cells (1 × 106 cells in 100 μL of serum-free DMEM) were harvested from sub-confluent cultures after trypsinization and were injected subcutaneously into the right flank of each mouse. Mice were randomly divided into 4 treatment groups (n = 3–4/group) 14 days post tumor inoculation and injected via tail vein (qwx3) with a saline control, PTX-PM (10%) at 60 mg/kg, o(LA)8-PTX-PM (10%) at 60 mg/kg PTX-eq, and o(LA)8-PTX-PM (50%) at 60 mg/kg PTX-eq. Body weight and tumor volume were monitored over the course of study. Tumor volume was calculated using the formula: V = (a × b2)/2, where V is tumor volume, a is tumor length, and b is tumor width. Whole-body bioluminescence images were obtained using Xenogen IVIS® 200 Series and Living Image® software was used for image acquisition/quantification of total photon counts in a region of interest (ROI). Whole-body bioluminescence of anesthetized mice was recorded on 0, 4, 7, 14, and 21 days post treatment. D-Luciferin (Caliper Life Science, Hopkinton, MA) at 100 mg/kg was injected subcutaneously into the tumor of 4T1-luc breast cancer model 10 min prior to whole-body imaging. The long-term survival of each treatment group post tumor inoculation was monitored. The maximum tumor burden was set at > 1200 mm3 endpoint tumor volume. Any animal that was noticeably ill, as manifested by hunched posture, respiratory distress, absence of fur grooming, lack of food/water intake, or >15% body weight loss were euthanized by CO2 asphyxiation.
2.12. Acute toxicity
Six- to eight-week-old female BALB/c mice (15 – 25 g) were used to examine the acute toxicity of PTX-PM and o(LA)8-PTX-PM. In a single dose study, mice were randomly divided into five treatment groups (n=3) and injected via tail vein with a saline control, PTX-PM (10%) at 100 mg/kg, o(LA)8-PTX-PM (10%) at 100 mg/kg PTX-eq, and o(LA)8-PTX-PM (50%) at higher doses: 100, 150, and 200 mg/kg PTX-eq, respectively. For a repeat dose toxicity study, mice were randomly divided into four treatment groups (n=5) and injected via the tail vein (daily injection for three days) with a saline control, PTX-PM (10%) at 50 mg/kg, o(LA)8-PTX-PM (10%) at 50 mg/kg PTX-eq, and o(LA)8-PTX-PM (50%) at 50 mg/kg PTX-eq. Generally, animals were observed daily over the course of an experiment including assessment of activity levels (feeding, movement, and posture), signs of infection, and weight loss. Any animal that was noticeably ill, as manifested by hunched posture, respiratory distress, absence of fur grooming, lack of food/water intake, or >15% body weight loss were euthanized by CO2 asphyxiation [26]. Body weight changes were normalized by dividing animal weights at time t by the initial body weight of each animal.
2.13. Statistical analysis
Non-parametric student’s t-test at 5% significance level was performed for statistical analysis. Log-rank test was used for survival analysis. All data analyses were performed using GraphPad Prism version 5.00 for Windows (GraphPad Software, CA).
3. Results and discussion
3.1. Physicochemical properties of o(LA)8-PTX loaded PEG-b-PLA micelles for injection
For evaluation of in vivo stability, prodrug conversion, and antitumor activity, o(LA)8-PTX-PM at 10 and 50% w/w loading (o(LA)8-PTX-PM (10%) and o(LA)8-PTX-PM (50%)) have been prepared (Table 1). At 25 °C, o(LA)8-PTX-PM was stable in PBS, indicating thermodynamic stability, whereas PTX-PM at 10% w/w loading (PTX-PM (10%)) precipitated <24 h (Figure 2A). The hydrodynamic diameter of o(LA)8-PTX-PM (10%) and o(LA)8-PTX-PM (50%) was ca. 30 and 100 nm, respectively, showing a monomodal particle size distribution with a polydispersity index (PDI) < 0.2 (Table 1, Figure 2B). Despite increased particle size for o(LA)8-PTX-PM (50%), a spherical morphology was observed by atomic force microscopy (AFM) (Figure 2C), akin to o(LA)8-PTX-PM (10%) (Figure S1). Notably, the release rate of o(LA)8-PTX from PEG-b-PLA micelles was dependent on loading: In vitro release t1/2 of o(LA)8-PTX-PM (10%) and o(LA)8-PTX-PM (50%) was 14 and 120 h, respectively, (Figure 2D). The prolonged release of o(LA)8-PTX-PM (50%) was unexpected, given instability often associated with high loading [27–28]. Presumably, o(LA)8-PTX resides intimately in cores of PEG-b-PLA micelles, and diffusion of o(LA)8-PTX prodrug from an enlarged core at 50% w/w loading accounts for prolonged release relative to o(LA)8-PTX-PM (10%).
Table 1.
Physicochemical properties of PTX-PM (10%), o(LA)8-PTX-PM (10%), and o(LA)8-PTXPM (50%) at 10.0 mg/mL PTX-eq (mean ± SD, n=3) a
| Size (nm) | PDI | Loading efficiency (%) | Actual drug loading (%) | |
|---|---|---|---|---|
| PEG-b-PLA empty PM | 32.2 ± 0.2 | 0.15 ± 0.01 | -- | -- |
| PTX-PM (10%) | 28.2 ± 0.8 | 0.07 ± 0.03 | 23.5 ± 13.7 | 2.3 ± 1.3 |
| o(LA)8-PTX-PM (10%) | 30.7 ± 1.0 | 0.07 ± 0.01 | 68.4 ± 9.1 | 6.5 ± 0.7 |
| o(LA)8-PTX-PM (50%) | 95.2 ± 6.4 | 0.15 ± 0.01 | 81.1 ± 25.8 | 44.7 ± 8.2 |
Mn of PEG=4000 g/mol; Mn of PLA=2200 g/mol
Figure 2.
(A) Images of PTX-PM (10%), o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%) at 2 mg/mL drug level at 25 °C after 24 hours, (B) Particle size analysis by dynamic light scattering (DLS) of PTX-PM (10%), o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%), (C) Representative AFM image of o(LA)8-PTX-PM (50%), and (D) In vitro drug release profiles of PTX from PTX-PM (10%) (drug precipitation < 4 hours), o(LA)8-PTX from o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%) in 10 mM PBS at 37 °C and pH 7.4 (mean ± SD, n=3).
3.2. In vitro plasma stability
This is the first report that suggests that oligo(lactic acid) as a pro-moiety is a good substrate for plasma esterases. In contrast, a linoleic acid ester prodrug of PTX in rat plasma converted into 4.7% of PTX at 48 h [29]. The in vitro stability of o(LA)8-PTX, o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%) was tested in rat plasma, quantifying conversion by reverse-phase HPLC analysis (Figure 3). The level of o(LA)8-PTX quickly decreased, t1/2 = 1 h, forming even and odd numbered o(LA)n-PTX species and mostly lactic acid-PTX as early as 4 h (Figure 3A). The rapid loss of o(LA)8-PTX and presence of odd number of o(LA)n-PTX suggest that conversion occurs primarily by degradation of o(LA)n by plasma esterases. By contrast, conversion of o(LA)8-PTX in a 1:1 mixture of PBS (pH 7.4, 10 mM) and acetonitrile by backbiting has a t1/2 = 7.3 h, even numbered o(LA)n-PTX, and primarily o(LA)2-PTX as the major product [22]. In human plasma, o(LA)8-PTX degraded with t1/2 = 2 h, forming o(LA)2-PTX, lactic acid-PTX, and PTX as early as 4 h (Figure S2), consistent with higher esterase activity in plasma of rats over humans [30]. o(LA)8-PTX-PM (10%) produced o(LA)2-PTX at ca. 1 h, followed by lactic acid-PTX (Figure 3B). Conversion of o(LA)8-PTX-PM (50%) was noticeably slower, noting a gradual decline in o(LA)8-PTX that reflects slower in vitro release of o(LA)8-PTX prodrug relative to o(LA)8-PTX-PM (10%) (Figure 3C).
Figure 3.
In vitro rat plasma stability of (A) o(LA)8-PTX, (B) o(LA)8-PTX-PM (10%), and (C) o(LA)8-PTX-PM (50%) at a final concentration of 10 μM at 37 °C (mean ± SEM, n=3).
3.3. In vitro cytotoxicity
The in vitro cytotoxicity of o(LA)8-PTX prodrug, o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%) was tested against syngeneic 4T1-luc breast cancer cells (Figure 4). The IC50 value of PTX and o(LA)8-PTX was 3.8 and 20.2 μM, respectively. The micromolar IC50 value of PTX for 4T1-luc cells indicates low potency, noting nanomolar values for many human cancer cell lines. The higher value of o(LA)8-PTX prodrug versus PTX reflects incomplete backbiting conversion during cell incubation. The IC50 value of o(LA)8-PTX-PM (10%) and o(LA)8-PTX-PM (50%) was 13.2 and 30.5 μM, respectively, reflecting o(LA)8-PTX prodrug release from PEG-b-PLA micelles during cell incubation. The in vitro cytotoxicity of PTX and o(LA)2-PTX was equivalent, indicating that full conversion of o(LA)8-PTX (7-OH) into PTX is not a requirement for cytotoxicity. Thus, two lactic acids at the 7-OH position of PTX does not appear to affect microtubule binding, and o(LA)2-PTX, lactic acid-PTX, and PTX (o(LA)0–2-PTX) are bioactive metabolites of o(LA)8-PTX.
Figure 4.
In vitro cytotoxicity of PTX, o(LA)2-PTX, o(LA)8-PTX, PTX-PM (10%), o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%) for 4T1-luc cells (mean ± SEM, n=4) (**. p < 0.01; *, p < 0.05).
3.4. Plasma AUC and conversion in plasma
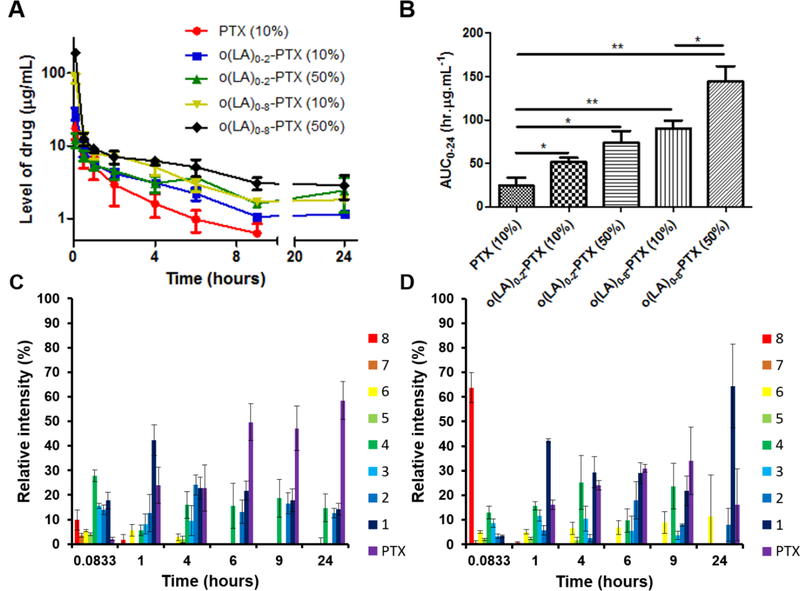
After injection of Sprague-Dawley rats (10 mg/kg), o(LA)8-PTX-PM (50% w/w loading) had the highest Cmax, 120 ± 2.6 μg/mL for o(LA)0–8-PTX and a biphasic profile for o(LA)0–8-PTX, characterized by rapid distribution and a slower elimination phase (Figure 5A). The plasma area under the concentration-time curve (AUC0–24) of o(LA)0–8-PTX for o(LA)8-PTX-PM (50%) was 2- and 6-fold higher than o(LA)8-PTX-PM (10%) and PTX-PM (10%), respectively (Figure 5B). The in vivo conversion of o(LA)8-PTX-PM (10%) and o(LA)8-PTX-PM (50%) is depicted in Figures 5C and 5D. For o(LA)8-PTX-PM (10%), loss of o(LA)8-PTX was evident at 5 min, consistent with rapid release of o(LA)8-PTX from PEG-b-PLA micelles, followed by conversion into lactic acid-PTX and PTX over 24 h (Figure 5C). By contrast, ca. 60% of o(LA)8-PTX of o(LA)8-PTX-PM (50%) was present at 5 min after injection, consistent with the presence of o(LA)8-PTX inside PEG-b-PLA micelles, followed by release in 1 h and then conversion of released o(LA)8-PTX into lactic acid-PTX (Figure 5D). This in vivo release profile for o(LA)8-PTX-PM (50%) was not expected, given t1/2 of 120 h in vitro, but may reflect dilution of PEG-b-PLA micelle beneath its CMC. Nonetheless, o(LA)8-PTX-PM (50%) produced a plasma AUC0–24 for o(LA)0–2-PTX that is 3-fold higher than PTX-PM (10%). Cmax of o(LA)0–2-PTX was 12.1 ± 2.6 μg/mL; lower, but not statistically different than PTX-PM (10%). Notably, high Cmax of PTX is associated with dose-limiting peripheral neurotoxicity; we anticipate that o(LA)8-PTX prodrug will have a lower Cmax in humans due to slower conversion [29].
Figure 5.
(A) Concentration-time profile in rat plasma and (B) area under the concentration-time curve (AUC0–24) of PTX from PTX-PM (10%) at 10 mg/kg, o(LA)0–2-PTX and o(LA)0–8-PTX from o(LA)8-PTX-PM (10%) at 10 mg/kg PTX-eq and o(LA)0–2-PTX and o(LA)0–8-PTX from o(LA)8-PTX-PM (50%) at 10 mg/kg PTX-eq in male Sprague-Dawley rats. (mean ± SEM, n=4; ***, p < 0.001; **, p < 0.01; *, p < 0.05). Relative intensity of o(LA)0–8-PTX over time in rat plasma after IV injection of (C) o(LA)8-PTX-PM (10%) at 10 mg/kg PTX-eq, and (D) o(LA)8-PTX-PM (50%) at 10 mg/kg PTX-eq in male Sprague-Dawley rats (Digits in Figures 5C and 5D represent the number of lactic acid oligomers (n) of o(LA)n-PTX; mean ± SEM, n=4).
3.5. Antitumor efficacy
In BALB/c mice with syngeneic 4T1-luc breast tumors, IV injection of o(LA)8-PTX-PM (50%) at 60 mg/kg of PTX (qwx3) reduced the bioluminescence of 4T1-luc tumors, markedly inhibited tumor growth and prolonged survival relative to PTX-PM (10%) and o(LA)8-PTX-PM (10%) (Figures 6A - 6E) despite PTX having low potency against 4T1-luc breast cancer cells (Figure 4). Tumor growth of mice receiving o(LA)8-PTX-PM (50%) was significantly inhibited at day 9, whereas PTX-PM (10%) and o(LA)8-PTX-PM (10%) showed no significant difference compared to saline control (Figure 6B). Mice treated with o(LA)8-PTX-PM (50%) prolonged median overall survival time (> 45 days) versus PTX-PM (10%) at 28 days (Figure 6E). Bioluminescence of 4T1-luc tumors was monitored in the ROI of tumor-bearing mice (Figure 6A and 6D), and % bioluminescence for o(LA)8-PTX-PM (50%) was 30% at day 21. By contrast, PTX-PM (10%) and o(LA)8-PTX-PM (10%) a rapid increase in bioluminescence signal was observed from 4T1-luc tumors at day 7 and reaching to ca. 190 and 220%, respectively at day 21, indicating proliferation despite ongoing treatment. In terms of toxicity, all mice dosed at 60 mg/kg (qwx3) had minimal body weight loss and no deaths, contrasting starkly with the MTD of Taxol® at 20 mg/kg in mice [11].
Figure 6.
In vivo antitumor efficacy in BALB/c mice bearing s.c. 4T1-luc breast tumors. Treatments were initiated 14 days post tumor implantation: PTX-PM (10%), o(LA)8-PTX-PM (10%), and o(LA)8-PTX-PM (50%) IV injection at 60 mg/kg PTX-eq (qwx3), indicated by arrows. (A) Whole-body bioluminescence images of 4T1-luc mice, (B) Relative tumor volume (mean ± SEM, n=3–4; *: p < 0.05 (o(LA)8-PTX-PM (50%) vs. o(LA)8-PTX-PM (10%), PTX-PM (10%), or saline control), (C) Relative body weight change, (D) % bioluminescence intensity of region of interest (ROI) (mean ± SEM, n=3; *: p < 0.05 (o(LA)8-PTX-PM (50%) vs. o(LA)8-PTX-PM (10%), PTX-PM (10%), or saline control), and (E) Kaplan-Meier analysis for survival (> 1200 mm3 endpoint tumor volume; **: p < 0.01 (o(LA)8-PTX-PM (50%) vs. PTX-PM (10%)).
3.6. Acute toxicity
BALB/c mice tolerated a single IV dose of o(LA)8-PTX-PM (50%) > 150 mg/kg with < 15% body weight loss and no deaths over 14 days (Figure 7A). In contrast, severe toxicity was observed including loss of consciousness, flushed skin, and dyspnea after a single dose of PTX-PM at 100 mg/kg (data not shown). In a repeat dose study, a significant loss in body weight of mice receiving daily doses of PTX-PM (10%) at 50 mg/kg for 3 days was observed by day 5. Notably, no substantial change in body weight was caused by o(LA)8-PTX-PM (50%) at 50 mg/kg (Figure 7B). Importantly, the MTD of Genexol-PM® in mice is 60 mg/kg, indicating improved tolerability of o(LA)8-PTX-PM (50%) and the potential of o(LA)8-PTX-PM (50%) for dose escalation to gain antitumor efficacy.
Figure 7.
(A) Relative body weight of female BALB/c mice over time after single tail-vein injection: PTX-PM (10%) at 100 mg/kg, o(LA)8-PTX-PM (10%) at 100 mg/kg PTX-eq, and o(LA)8-PTX-PM (50%) at 100, 150, and 200 mg/kg PTX-eq, respectively (mean ± SEM, n=3). Two mice suffered from loss of consciousness, flushed skin, and dyspnea were euthanized on day 0 and day 1 for PTX-PM (10%) at 100 mg/kg. (B) Relative body weight of female BALB/c mice over time after daily injection on days 0, 1 and 2 of PTX-PM (10%) at 50 mg/kg, o(LA)8-PTX-PM (10%) at 50 mg/kg PTX-eq, and o(LA)8-PTX-PM (50%) at 50 mg/kg PTX-eq (mean ± SEM, n=5; *: p < 0.05 (o(LA)8-PTX-PM (50%) vs. PTX-PM (10%)); **: p < 0.01 (saline vs. PTX-PM (10%)).
4. Conclusions
In summary, o(LA)8-PTX-PM (50%) has higher plasma exposure than PTX-PM (10%), increasing plasma AUC0–24 of o(LA)0–2-PTX by 3-fold, resulting in higher antitumor efficacy and lower acute toxicity. Notably, o(LA)8-PTX is a good substrate for plasma esterases and converts into o(LA)2-PTX in vivo in 24 hrs. While intratumoral accumulation of polymeric micelles depends on the size of the micelles and permeability of the tumor [31], biodistribution studies on o(LA)8-PTX-PM are needed to prove that there are higher tumor levels o(LA)0–2-PTX that are associated with elevated plasma AUC. This data supports the development of o(LA)8-PTX-PM (50%) for cancer treatment, combining o(LA)n prodrug and PEG-b-PLA micelle nanotechnology to potentially improve the therapeutic index of PTX over Genexol-PM® and Taxol®.
Supplementary Material
Highlights.
Oligo(lactic acid)8-PTX prodrug is more stable than PTX in a PEG-b-PLA micelle.
Major metabolites are oligo(lactic acid)2-PTX, lactic acid-PTX and PTX.
Major metabolites have a 3-fold higher plasma AUC than PTX.
Oligo(lactic acid)8-PTX has higher antitumor efficacy than PTX.
Oligo(lactic acid)8-PTX is less toxic than PTX.
Acknowledgments
This work was supported by the National Institutes of Health (R01AI01157). The authors acknowledge Dr. John Feltenberger and Dr. Zhi-xiong Ma of the Medicinal Chemistry Center (School of Pharmacy, UW-Madison), Ye Rim Jeong of the Kwon lab (School of Pharmacy, UW-Madison) for their support in PTX prodrug synthesis, and Michael Poellmann of the Hong lab (School of Pharmacy, UW-Madison) for his assistance in AFM analysis.
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).McGuire WP, Hoskins WJ, Brady MF, Kucera PR, Partridge EE, Look KY, Clarke-Pearson DL, Davidson M, Cyclophosphamide and cisplatin compared with paclitaxel and cisplatin in patients with stage III and stage IV ovarian cancer, N. Engl. J. Med 334 (1996) 1–6. [DOI] [PubMed] [Google Scholar]
- (2).Sparano JA, Wang M, Martino S, Jones V, Perez EA, Saphner T, Wolff AC, Sledge GW Jr, Wood WC, Davidson NE, Weekly paclitaxel in the adjuvant treatment of breast cancer, N. Engl. J. Med 358 (2008) 1663–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Murphy WK, Fossella FV, Winn RJ, Shin DM, Hynes HE, Gross HM, Davilla E, Leimert J, Dhingra H, Raber MN, Krakoff IH, Phase II study of taxol in patients with untreated advanced non-small-cell lung cancer, J. Natl. Cancer Inst 85 (1993) 384–388. [DOI] [PubMed] [Google Scholar]
- (4).Silvestris N, Galetta D, Colucci G, Successful treatment with three-weekly paclitaxel of an anthracycline-refractory classical Kaposi’s sarcoma, Anticancer Res. 29 (2009) 675–676. [PubMed] [Google Scholar]
- (5).Arnal I, Wade RH, How does taxol stabilize microtubules?, Curr. Biol 5 (1995) 900–908. [DOI] [PubMed] [Google Scholar]
- (6).Xiao H, Verdier-Pinard P, Fernandez-Fuentes N, Burd B, Angeletti R, Fiser A, Horwitz SB, Orr GA, Insights into the mechanism of microtubule stabilization by Taxol, Proc. Natl. Acad. Sci. U. S. A 103 (2006) 10166–10173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Hennenfent KL, Govindan R, Novel formulations of taxanes: a review. Old wine in a new bottle?, Ann. Oncol 17 (2005) 735–749. [DOI] [PubMed] [Google Scholar]
- (8).Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, Baker JR Jr, Van Echo DA, Von Hoff DD, Leyland-Jones B, Hypersensitivity reactions from taxol, J. Clin. Oncol. 8 (1990) 1263–1268. [DOI] [PubMed] [Google Scholar]
- (9).Bookman MA, Kloth DD, Kover PE, Smolinsk S, Ozols RF Short-course intravenous prophylaxis for paclitaxel-related hypersensitivity reactions. Ann. Oncol 8 (1997) 611–614. [DOI] [PubMed] [Google Scholar]
- (10).Scripture CD, Figg WD, Sparreboom A, Peripheral neuropathy induced by paclitaxel: recent insights and future perspectives., Curr. Neuropharmacol 4 (2006) 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Kim SC, Kim DW, Shim YH, Bang JS, Oh HS, Kim SW, Seo MH, In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy, J. Control. Release 72 (2001) 191–202. [DOI] [PubMed] [Google Scholar]
- (12).Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, Kim NK, Bang YJ, Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies, Clin. Cancer Res 10 (2004) 3708–3716. [DOI] [PubMed] [Google Scholar]
- (13).Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, Rha SY, Lee MY, Ro J, Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer., Breast Cancer Res. Treat 108 (2008) 241–250. [DOI] [PubMed] [Google Scholar]
- (14).Ramaswamy M, Zhang X, Burt HM, Wasan KM, Human plasma distribution of free paclitaxel and paclitaxel associated with diblock copolymers, J. Pharm. Sci 86 (1997) 460–464. [DOI] [PubMed] [Google Scholar]
- (15).Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, Park K, Lee MY, Heo DS, Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer, Ann. Oncol 18 (2007) 2009–2014. [DOI] [PubMed] [Google Scholar]
- (16).Owen SC, Chan DP, Shoichet MS, Polymeric micelle stability, Nano Today 7 (2012) 53–65. [Google Scholar]
- (17).Cho H, Gao J, Kwon GS, PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol–gels for drug delivery, J. Control. Release 240 (2016) 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Shin DH, Tam YT, Kwon GS, Polymeric micelle nanocarriers in cancer research, Front. Chem. Sci. Eng 10 (2016) 348–359. [Google Scholar]
- (19).Forrest ML, Yáñez JA, Remsberg CM, Ohgami Y, Kwon GS, Davies NM, Paclitaxel prodrugs with sustained release and high solubility in poly (ethylene glycol)-b-poly (ε-caprolactone) micelle nanocarriers: pharmacokinetic disposition, tolerability, and cytotoxicity, Pharm. Res 25 (2008) 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wang H, Xie H, Wu J, Wei X, Zhou L, Xu X, Zheng S, Structure-Based Rational Design of Prodrugs To Enable Their Combination with Polymeric Nanoparticle Delivery Platforms for Enhanced Antitumor Efficacy, Angew. Chem. Int. Ed 53 (2014), 11532–11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Zhao Y, Fay F, Hak S, Perez-Aguilar JM, Sanchez-Gaytan BL, Goode B, Duivenvoorden R, de Lange Davies C, Bjørkøy A, Weinstein H, Fayad ZA, Augmenting drug–carrier compatibility improves tumour nanotherapy efficacy, Nat. Commun 7 (2016) 11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Tam YT, Gao J, Kwon GS, Oligo (lactic acid)n-paclitaxel prodrugs for poly (ethylene glycol)-block-poly (lactic acid) micelles: loading, release, and backbiting conversion for anticancer activity, J. Am. Chem. Soc 138 (2016) 8674–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).van Nostrum CF, Veldhuis TF, Bos GW, Hennink WE, Hydrolytic degradation of oligo (lactic acid): a kinetic and mechanistic study, Polymer 45 (2004) 6779–6787. [Google Scholar]
- (24).Cho H, Lai TC, Kwon GS, Poly (ethylene glycol)-block-poly (ε-caprolactone) micelles for combination drug delivery: evaluation of paclitaxel, cyclopamine and gossypol in intraperitoneal xenograft models of ovarian cancer, J. Control. Release 166 (2013) 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Shin HC, Cho H, Lai TC, Kozak KR, Kolesar JM, Kwon GS, Pharmacokinetic study of 3-in-1 poly (ethylene glycol)-block-poly (D, L-lactic acid) micelles carrying paclitaxel, 17-allylamino-17-demethoxygeldanamycin, and rapamycin, J. Control. Release 163 (2012) 93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE, Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man, Cancer Chemother. Rep 50 (1966) 219. [PubMed] [Google Scholar]
- (27).Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K, Hydrotropic polymer micelle system for delivery of paclitaxel, J. Control. Release 101 (2005) 59–68. [DOI] [PubMed] [Google Scholar]
- (28).Liu J, Lee H, Allen C, Formulation of drugs in block copolymer micelles: drug loading and release, Curr. Pharm. Des 12 (2006) 4685–4701. [DOI] [PubMed] [Google Scholar]
- (29).Ke XY, Zhao BJ, Zhao X, Wang Y, Huang Y, Chen XM, Zhao BX, Zhao SS, Zhang X, Zhang Q, The therapeutic efficacy of conjugated linoleic acid–paclitaxel on glioma in the rat, Biomaterials 31 (2010) 5855–5864. [DOI] [PubMed] [Google Scholar]
- (30).Liederer BM, Borchardt RT, Enzymes involved in the bioconversion of ester-based prodrugs, J. Pharm. Sci 95 (2006) 1177–1195. [DOI] [PubMed] [Google Scholar]
- (31).Cabral H, Matsumoto Y, Mizuno K, Chen Q, Murakami M, Kimura M, Terada Y, Kano MR, Miyazono K, Uesaka M, Nishiyama N, Kataoka K, Accumulation of sub-100 nm polymeric micelles in poorly permeable tumours depends on size, Nat. Nanotechnol 6 (2011) 815–823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.