Abstract
Background
Nanomaterials that exhibit intrinsic enzyme-like characteristics have shown great promise as potential antibacterial agents. However, many of them exhibit inefficient antibacterial activity and biosafety problems that limit their usefulness. The development of new nanomaterials with good biocompatibility and rapid bactericidal effects is therefore highly desirable. Here, we show a new type of terbium oxide nanoparticles (Tb4O7 NPs) with intrinsic oxidase-like activity for in vitro and in vivo antibacterial application.
Results
We find that Tb4O7 NPs can quickly oxidize a series of organic substrates in the absence of hydrogen peroxide. The oxidase-like capacity of Tb4O7 NPs allows these NPs to consume antioxidant biomolecules and generate reactive oxygen species to disable bacteria in vitro. Moreover, the in vivo experiments showed that Tb4O7 NPs are efficacious in wound-healing and are protective of normal tissues.
Conclusions
Our results reveal that Tb4O7 NPs have intrinsic oxidase-like activity and show effective antibacterial ability both in vitro and in vivo. These findings demonstrate that Tb4O7 NPs are effective antibacterial agents and may have a potential application in wound healing.
Electronic supplementary material
The online version of this article (10.1186/s12951-019-0487-x) contains supplementary material, which is available to authorized users.
Keywords: Tb4O7 nanoparticles, Oxidase, Reactive oxygen species, Antibacterial, Wound healing
Background
Wound infection is an important cause of poor wound healing and its treatment often requires the use of antibiotics [1, 2]. However, excessive use of antibiotics may lead to the development of antibiotic-resistant bacteria, and may also cause side effects on human health, such as gastrointestinal disturbances. In recent years, developments in nanomaterial technology have provided an opportunity to develop novel antimicrobial agents. Due to the diversity in mechanisms of action against bacteria, bacterial cells are less likely to develop antibacterial resistance compared to existing antibiotics [3–5]. However, most of these nanomaterials have application limitations, such as cytotoxicity, not biocompatible for human use, and environmental concerns.
Nanozymes are nanomaterials that catalyze the same reactions originally catalyzed by natural enzymes in biological systems [6–8]. Over the past several years, a wide variety of nanomaterials, such as noble metals [9–11], metal oxides [12–14], and carbon nanomaterials [15–18], have been explored as potential nanozymes. Based on their intrinsic enzyme-like activity, several nanozymes have been used in antibacterial applications [19–23]. For instance, platinum nanomaterials have shown effective antibacterial activity in the presence of hydrogen peroxide (H2O2) [24]. The antibacterial activity of these nanozymes are attributed primarily to their oxidase- and peroxidase-like activities that catalyze the production of hydroxyl radicals (·OH) in the presence of exogenous H2O2 and enhance the cellular levels of reactive oxygen species (ROS) within bacteria cells. Fang et al. also showed that palladium nanomaterials with oxidase- and peroxidase-like activities displayed effective antibacterial activity in the presence of H2O2 [25]. Although many reported enzyme-like nanomaterials have been proposed as novel antibacterial agents, the high price and persistence in living tissues are still important issues. Moreover, the application of H2O2 in human wound disinfection is harmful to healthy tissue and may delay wound healing [26].
Terbium oxide nanoparticles (Tb4O7 NPs) have been extensively used as precursors for the synthesis of lanthanide nanophosphors and superconductor materials [27, 28]. For example, Tb4O7 complexed with reduced graphene oxide composite exhibit typical green emission of terbium ions as well as the blue self-luminescence of graphene [28]. In addition, it has been found that Tb4O7 NPs can be used as analytical reagents for food analysis [29]. Compared with noble metal nanomaterials, Tb4O7 NPs are easier to synthesize and are less expensive. However, a review of scientific literature was unable to find any studies that described the enzyme-like activity of Tb4O7 NPs and their applications as antibacterial agents. In this paper, we show that Tb4O7 NPs have an intrinsic oxidase-like activity at acidic pH values, as they quickly oxidize a series of organic substrates in the absence of H2O2. We then demonstrate the relationship between the oxidase-like property of Tb4O7 NPs and their antibacterial activity with in vitro studies. Finally, the effects of Tb4O7 NPs on wound disinfection and healing are evaluated in in vivo studies using a wound infection mouse model.
Materials and methods
Chemicals and materials
Tb4O7 NPs were purchased from US Research Nanomaterials, Inc. (TX, USA). 3,3,5,5-tetramethylbenzidinedihydrochloride (TMB), diammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate) (ABTS), o-phenylenediamine (OPD), and Lipid Peroxidation MDA Assay Kit were all purchased from Sigma-Aldrich (St. Louis, MO). The Live/Dead BacLight bacterial viability kit and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were obtained from Thermo Fisher Scientific, Inc. (MA, USA). 5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide (BMPO) and Cell Counting Kit-8 (CCK-8) were obtained from Dojindo Laboratories (Kumamoto, Japan). Escherichia coli (E. coli) and Staphylococcus aureus subsp. aureus (S. aureus) were obtained from the China General Microbiological Culture Collection Center (CGMCC, Beijing, China). Human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection (ATCC, MD, USA).
Characterization of Tb4O7 NPs
The hydrodynamic size and zeta potential of the Tb4O7 NPs were measured using a Zetasizer Nano-ZS (Malvern, UK). The morphology and size of the Tb4O7 NPs were characterized using a transmission electron microscopy (TEM, Tecnai G-20, FEI). The UV–vis absorption spectrum was recorded on a spectrophotometer (UV-3600, Shimadzu).
Electron spin resonance spectroscopic measurements
The electron spin resonance (ESR) measurements were carried out using a Bruker EMX ESR spectrometer according to our previous study [3, 9]. The final concentration of each component is described in each figure caption. All the ESR measurements were carried out at ambient temperature.
Measurement of intracellular ROS
After Tb4O7 NPs (100 μg/mL) treatment, bacteria (1 × 109 CFU/mL) were collected by centrifugation and incubated with DCFH-DA (10 µM) for 30 min at dark, and stained bacteria were visualized with a confocal laser microscopy.
Cytotoxicity experiments
HUVECs were employed for investigating the cytotoxicity of Tb4O7 NPs. HUVECs were seeded at a density of 1 × 105 cells/well in 96-well plates and incubated overnight. The HUVECs were then incubated with Tb4O7 NPs (0–100 μg/mL) for 24 h, and cell viability was measured by MTT assay.
Mice injury model
BALB/c mice (8 weeks) were purchased from Pengsheng. On the day 0, the mice were anesthetized using 10% chloral hydrate. Then, the dorsal hair of mouse was shaved, full-thickness skin wounds with the diameter of 10 mm were created on the back of each mouse. After 24 h (day 1), the mice were treated with 50 μL PBS or Tb4O7 NPs (100 μg/mL). The Tb4O7 NPs were dripping on the surface of the wound.
Hemolysis test
Fresh blood was collected under sterile conditions from healthy BALB/c mice (n = 5) into an anticoagulation tube. The red blood cells were precipitated by centrifugation at 2000 rpm for 10 min and washed three times with PBS buffer solution to obtain red blood cells. The appropriate amount of red blood cells was diluted five times with PBS buffer solution to prepare a red blood cell solution. 20 μL of the diluted red blood cell suspension was mixed with a series of different concentrations of Tb4O7 NPs (0–200 μg/mL); ultrapure water was used as control. All the above samples were incubated at 37 °C for 2 h, centrifuged at 2000 rpm for 10 min, imaged, and the supernatant after centrifugation was taken in a 96-well plate to measure the absorbance at 540 nm using a microplate reader. The hemolysis rate was calculated as follows:
Results and discussion
Characterization of Tb4O7 NPs
The Tb4O7 NPs used in the present study were purchased from US Research Nanomaterials, Inc. The physical characterization of Tb4O7 NPs is shown in Additional file 1: Figure S1 and included images of particle core size and shape captured by TEM, the mean and homogeneity of particle hydrodynamic size by dynamic light scattering (DLS), and particle absorption spectrum by UV–vis. According to TEM and DLS data, the dispersity of Tb4O7 NPs is poor. The mean core particle size of Tb4O7 NPs is approximately 200 nm (Additional file 1: Figure S1a, b); while the DLS result of Tb4O7 NPs is around 400 nm (Additional file 1: Figure S1c). This is mainly due to the size measured by DLS was a hydrodynamic size, and therefore the nanoparticles showed a larger hydrodynamic volume due to solvent effect in the hydrated state. The zeta potential value of Tb4O7 NPs is 31.6 mV in water. The UV–vis spectrum of Tb4O7 NPs is shown in Additional file 1: Figure S1d.
Catalytic activity of Tb4O7 NPs as oxidase mimetics
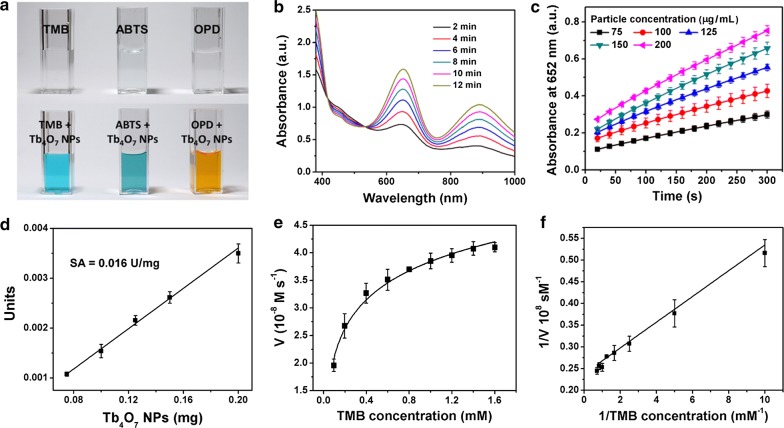
The oxidase-like activity of Tb4O7 NPs was evaluated using the substrate TMB. The UV–vis spectroscopy measurements show time-dependent increases in TMB oxidation catalyzed by Tb4O7 NPs, yielding a blue-colored product (Fig. 1a, b). In addition, Tb4O7 NPs can also catalyze the oxidation of ABTS and OPD (Fig. 1a).
Fig. 1.
Oxidase-like activity of Tb4O7 NPs. a A photograph showing the capability of the Tb4O7 NPs in catalyzing the oxidations of TMB, ABTS, and OPD that produce colored products. b Time-dependent absorption spectra of TMB catalyzed by Tb4O7 NPs. c Absorbance at 652 nm measured from samples containing TMB and different concentrations of Tb4O7 NPs. d The specific activities of Tb4O7 NPs. Steady-state kinetic assays of Tb4O7 NPs (e, f). e TMB concentration dependence of initial reaction velocity. f Double-reciprocal plot generated from (d)
We used the oxidation of TMB as a model reaction and found that the catalytic efficiency of the Tb4O7 NPs is dependent on TMB concentrations, pH and temperature (Fig. 1c and Additional file 1: Figure S2). As shown in Additional file 1: Figure S2a, Tb4O7 NPs exhibit excellent catalytic activity over a broad temperature range (25–60 °C). Moreover, an acidic condition (pH = 3.6) is conducive to the oxidase-like activity of Tb4O7 NPs (Additional file 1: Figure S2b). We adopted pH 3.6 and 25 °C (room temperature) as the standard conditions for subsequent studies.
Next, we determined the apparent steady-state kinetic parameters for the reaction of Tb4O7 NPs with TMB. Typical Michaelis–Menten curves were established (Fig. 1d). The curves were then fitted to the double-reciprocal Lineweaver–Burk plots (Fig. 1e), from which the kinetic parameters shown in Table 1 were determined.
Table 1.
The kinetic constants of Tb4O7 NPs
| [E] (M) | Km (M) | Vmax (M/s) | Kcat (s−1) | Kcat/Km (M−1 S−1) | |
|---|---|---|---|---|---|
| Tb4O7 NPs | 7.04 × 10−10 | 1.24 × 10−4 | 4.31 × 10−8 | 1.61 × 10−4 | 1.30 |
[E] is the concentration of Tb4O7 NPs. The particle number of Tb4O7 NPs is calculated using the density and diameter of Tb4O7 NPs. Dividing the particle number by the Avogadro constant is the molar concentration of Tb4O7 NPs
Effects of Tb4O7 NPs on the anti-oxidant defense system
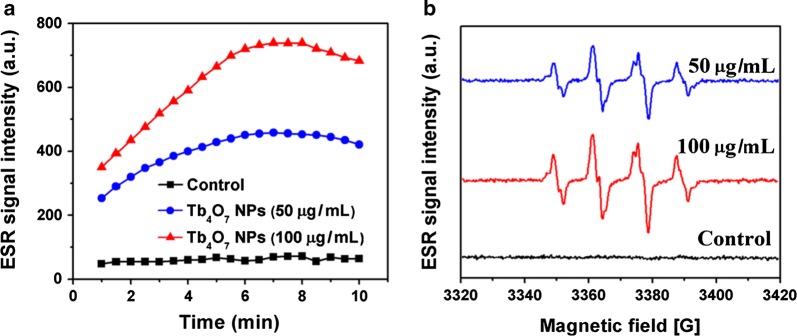
The above results show that Tb4O7 NPs have oxidase-like activity oxidizing TMB, ABTS, and OPD in the absence of H2O2. We predict that Tb4O7 NPs would deplete intracellular antioxidants and, eventually, disrupt the antioxidant defense systems of bacteria. To test this hypothesis, we examined the effects of Tb4O7 NPs on ascorbic acid (AA) oxidation in vitro. AA is an important endogenous bacterial antioxidant that prevents cellular damage from ROS. AA can be oxidized to form an intermediate ascorbyl radical (·AA), which is detectable by ESR spectroscopy [9]. As shown in Fig. 2a, the oxidation of AA was negligible within 10 min, while in the presence of Tb4O7 NPs the system showed a time-dependent increase in the ESR signal intensity in the first 8 min and then decreased over time. These results indicate that Tb4O7 NPs can accelerate AA oxidation.
Fig. 2.
a Oxidation of AA by Tb4O7 NPs. b ESR spectra of BMPO/·OH generated from a sample solution containing 25 mM BMPO, 1 mM H2O2 in the absence (control) and presence of different concentrations of Tb4O7 NPs
In the substrate oxidation mechanism of most oxidases in nature, oxygen acts as the electron acceptor and is reduced to H2O2. To gain a better understanding of the oxidation of AA by Tb4O7 NPs, we examined whether the catalytic oxidation product of Tb4O7 NPs and AA produced H2O2. As shown in Additional file 1: Figure S3, a marked increase of H2O2 was detected in the presence of Tb4O7 compared to control (AA alone). Moreover, the production of H2O2 was Tb4O7 concentration-dependent.
Previous studies have demonstrated that several kinds of nanoparticles are capable of catalyzing the production of hydroxyl radicals by H2O2 [30, 31]. Therefore, we determined whether Tb4O7 NPs would catalyze the production of hydroxyl radicals by H2O2 using ESR spectroscopy. We selected BMPO as the capture agent, since BMPO can capture hydroxyl radicals to form BMPO/·OH adducts indicated by the presence of four characteristic lines on the ESR spectrum. As shown in Fig. 2b, the characteristic ESR signals of BMPO/·OH were negligible in the absence of Tb4O7 NPs. However, the addition of Tb4O7 NPs resulted in a strong ESR spectrum that displayed the four characteristic lines (1:2:2:1) of BMPO/·OH. These results clearly show that Tb4O7 NPs can be used as a catalyst in the decomposition of H2O2 to produce hydroxyl radicals.
Taken together, our results confirm that Tb4O7 NPs are capable of catalyzing the oxidation of biologically relevant antioxidant agents, resulting in the production of H2O2. Moreover, Tb4O7 NPs can further catalyze the production of hydroxyl radicals via the decomposition of H2O2.
Antibacterial activity of Tb4O7 NPs
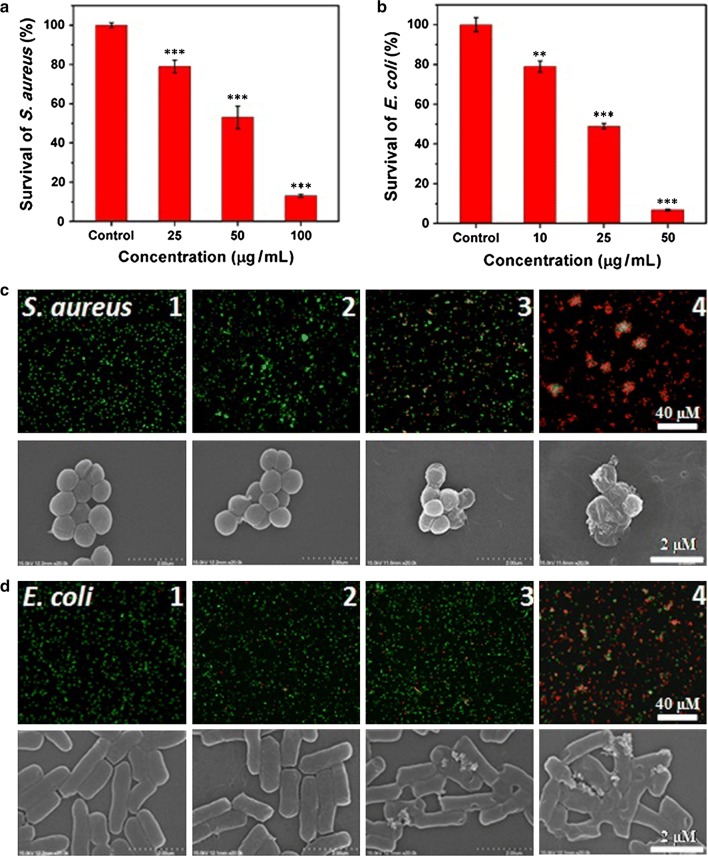
Both H2O2 and hydroxyl radicals have strong oxidizing ability and can oxidize biological macromolecules, such as proteins and phospholipids [32]. Our study found that Tb4O7 NPs with oxidase-like activity can catalyze the production of H2O2 and further produce hydroxyl radicals. Therefore, the oxidase-like activity of the Tb4O7 NPs makes them potentially useful as antibacterial agents. We evaluated the antibacterial activity of Tb4O7 NPs against E. coli and S. aureus. A colony-forming units plate counting method was used to determine the antibacterial ability (Fig. 3a, b). As compared to the PBS control group, Tb4O7 NPs exhibited potent antimicrobial activity against both S. aureus and E. coli in a concentration-dependent manner. At a concentration of 25 μg/mL, Tb4O7 NPs exhibited only modest antibacterial effects against S. aureus; more than 80% of the bacterial cells survived. However, when the concentration of Tb4O7 NPs was increased to 100 μg/mL, nearly 90% of the S. aureus were killed. A similar trend of antibacterial effects were observed towards the E. coli.
Fig. 3.
The effect of Tb4O7 NPs on survival rates of bacteria a CFUs of S. aureus following incubation with Tb4O7 NPs; b CFUs of E. coli following incubation with Tb4O7 NPs; c Representative fluorescence and SEM images of S. aureus after Tb4O7 NPs treatments. Bacterial cells were treated with 1) PBS as control, 2) 25 μg/mL Tb4O7 NPs, 3) 50 μg/mL Tb4O7 NPs or 4) 100 μg/mL Tb4O7 NPs; d Representative fluorescence and SEM images of E. coli after Tb4O7 NPs treatments. Bacterial cells were treated with 1) PBS as control, 2) 10 μg/mL Tb4O7 NPs, 3) 25 μg/mL Tb4O7 NPs or 4) 50 μg/mL Tb4O7 NPs. **p < 0.01 and ***p < 0.001 vs control
To further investigate the interaction between Tb4O7 NPs and bacteria, a fluorescent-based cell live/dead assay was conducted. As shown in Fig. 3c, d, Tb4O7 NPs exhibited significant antibacterial activity against both S. aureus and E. coli, which was consistent with the aforementioned results. The exposure of S. aureus cells to Tb4O7 NPs at a concentration of 100 μg/mL resulted in nearly 100% lethality, as evidenced by the dominant red fluorescent signal. At a concentration of 50 μg/mL, Tb4O7 NPs completely inhibited the bacterial growth of E. coli.
The morphology and membrane integrity of bacteria were then determined by SEM (Fig. 3c, d). Untreated S. aureus displayed a typical rod-shaped structure with a continuous, smooth surface. When exposure to the 50 μg/mL of Tb4O7 NPs, the S. aureus bacterial cell walls became partially wrinkled and discontinuance. Notably, after treatment with 100 μg/mL of Tb4O7 NPs, the S. aureus bacterial cell walls showed much more pronounced damage, indicating stronger antibacterial effects at higher concentrations of Tb4O7 NPs. A similar tendency was found for E. coli. The loss of membrane integrity of E. coli was observed at concentrations lower than 100 μg/mL of Tb4O7 NPs treatment. Moreover, Tb4O7 NPs were observed by SEM and SEM-energy dispersive X-ray spectroscopy (EDS) to aggregate on the surfaces of S. aureus and E. coli (Additional file 1: Figure S4).
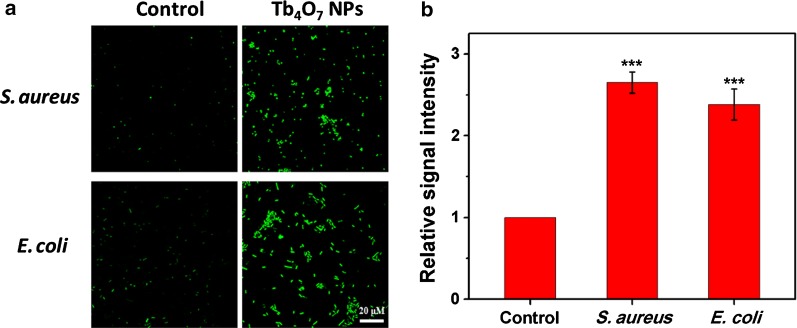
It is known that the proton motive force decreases the local pH (as low as pH 3.0) in the cytoplasm and membrane of bacteria cells [33, 34]. Since we found that Tb4O7 NPs exhibit oxidase-like activity under acidic conditions, we speculate that the antibacterial mechanism of Tb4O7 NPs may arise from their oxidase activity to accelerate the process of bacterial cell oxidation and consumption of antioxidant biomolecules, leading to a reduction of oxygen products including H2O2 along with other antibacterial activity from the accumulation of ROS. To confirm this hypothesis, the intracellular levels of ROS were determined using the florescent probe, DCFH-DA (Fig. 4). For both S. aureus and E. coli, the untreated cells showed extremely weak fluorescence, indicating low levels in the formation of intracellular ROS. In contrast, bacterial cells exposed to Tb4O7 NPs showed high levels of ROS formed within the cellular cytoplasm, as evidenced by the strong fluorescence signal. We also found that the generation of ROS is Tb4O7 NPs dose-dependent (Additional file 1: Figure S5).
Fig. 4.
a Fluorescence images of bacterial cells. b Analysis of the ROS levels by microplate reader. ***p < 0.001 vs control
In vivo wound disinfection effect of Tb4O7 NPs
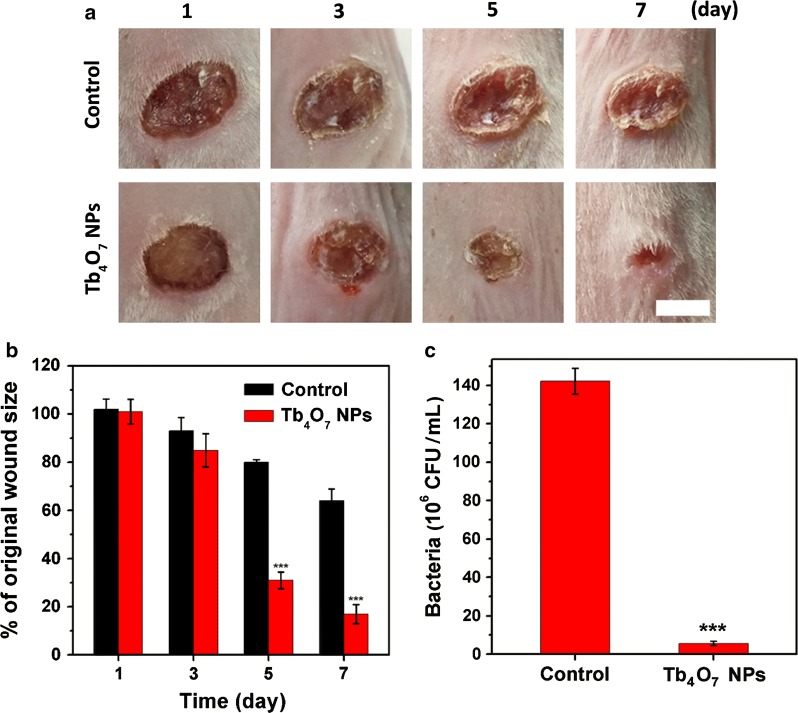
The above findings suggest that Tb4O7 NPs may have role as an antibacterial agent for bacterial infections in vivo. To assess the antibacterial capacity of Tb4O7 NPs in vivo, a wound infection model was constructed using BALB/c mice. A wound was introduced on the back of the mouse and an infection was established by implanting S. aureus into the wounded area. After infection was established, PBS or Tb4O7 NPs were applied to the infected wound. Figure 5a, b shows the progress of the wounds. Compared with the control (PBS treatment) group after 3 days, the wound area was reduced under Tb4O7 NPs treatment. After treating of 7 days with Tb4O7 NPs treatment, the wounds were nearly healed completely. In contrast, obvious scab was observed from the control group, indicating incomplete recovery.
Fig. 5.
a Photographs of wounds on the backs of mice in control (PBS) and Tb4O7 NPs treatment groups (n = 5). Scale bar: 5 mm. b Related wound size in each treatment group. c Bacterial number of infected wounds on the 7th day. ***p < 0.001 vs control
Biosafety of Tb4O7 NPs
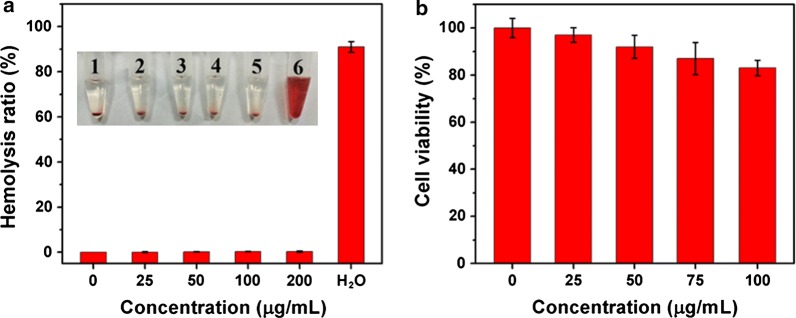
Biosafety is an important factor for antimicrobial agents designers. To assess the biosafety of Tb4O7 NPs, we first determined the effects of Tb4O7 NPs on red blood cells and HUVECs in vitro. The effect of Tb4O7 NPs on cell membrane disrupt was first determined by a red blood cell hemolysis assay. As shown in Fig. 6a, pure water can cause severe red blood cells hemolysis within 2 h. In contrast, the introduction of Tb4O7 NPs did not cause signs of hemolysis. In this experimental result, the hemolysis rate was still less than 1% at a concentration of 100 µg/mL, which fully demonstrated that Tb4O7 NPs have good blood compatibility. Meanwhile, the cytotoxicity tests on mammalian cells HUVECs further confirm the biosafety of Tb4O7 NPs (Fig. 6b).
Fig. 6.
a The hemolysis ratio of red blood cells. The insert images of tubes containing red blood cells solution show the direct observation of hemolysis. Tube 1: PBS buffer; Tube 2–5: 25, 50, 100, and 200 μg/mL Tb4O7 NPs; Tube 6: ultrapure water. b MTT assays determined cell viability of HUVECs after Tb4O7 NPs treatment
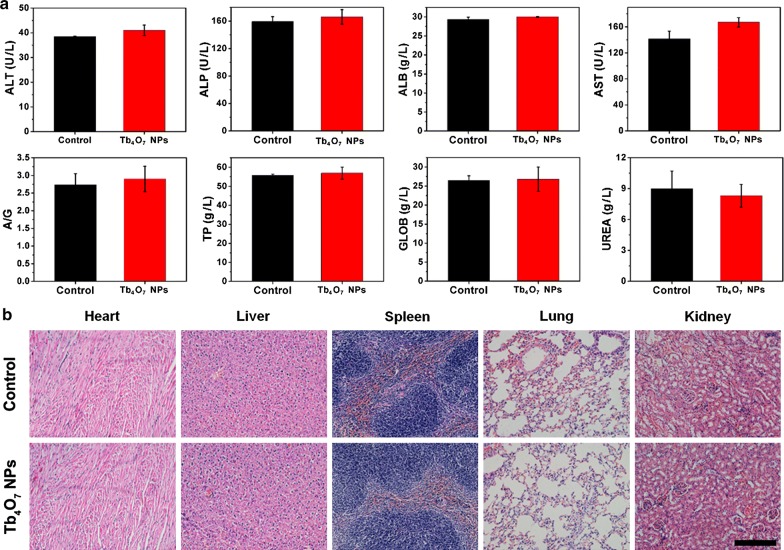
Then, the biosafety of Tb4O7 NPs in vivo were determined. As shown in Fig. 7a, the indicators in blood were within the normal range. The major mouse organs (heart, liver, spleen, lung, and kidney) were formalin-fixed and processed for the evaluation of H&E sections by histopathology (Fig. 7b). No obvious mouse organ damage was observed from Tb4O7 NPs treatment.
Fig. 7.
In vivo toxicity of Tb4O7 NPs. a The blood biochemistry data of the mice treated with Tb4O7 NPs after 7 d (n = 5). b Histological data (H&E staining images) are obtained from the major organs of mice treated with Tb4O7 NPs after 7 d. Scale bar = 100 μm
Conclusions
In summary, this study demonstrates for the first time that Tb4O7 NPs exhibit oxidase-like activity. In addition, the results of this study established a relationship between the oxidase-like enzyme activity of Tb4O7 NPs and their antibacterial properties. The data collected from this work revealed that the oxidase-like activity of Tb4O7 NPs was able to function with a variety of substrates, including biomolecules, and resulted in the generation of ROS, which further enhanced their antibacterial activity. The application of the antibacterial activities of Tb4O7 NPs were demonstrated in a wound infection mouse model. Our study provides evidence that Tb4O7 NPs can be utilized as an efficient antibacterial agent and the potential applications in wound healing are promising.
Additional file
Additional file 1: Figure S1. Characterization of Tb4O7 NPs. Figure S2. The oxidase-like catalytic activity of the Tb4O7 NPs. Figure S3. The concentration of H2O2 generated in the catalytic system. Figure S4. SEM-EDS elemental images. Figure S5. ROS levels of S. aureus.
Authors’ contributions
CL, YS, and XL prepared and performed the experiments. YS, XL and YL analyzed the data and interpreted the results. XT, SF and YP conceived and supervised the study. The manuscript was written by XT and XJ, and revised critically by JY and MB. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its additional file.
Consent for publication
All authors agree to be published.
Ethics approval and consent to participate
The protocols and the use of animals were approved by and in accordance with the animal welfare committee of Soochow University.
Funding
This work is partially supported by the National Natural Science Foundation of China (31400862), a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD), Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection, Guangdong Science and Technology Department (2017B030314026). This article is not an official U.S. FDA guidance or policy statement. No official support or endorsement by the U.S. FDA is intended or should be inferred.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- Tb4O7
terbium oxide
- NPs
nanoparticles
- H2O2
hydrogen peroxide
- ROS
reactive oxygen species
- ABTS
diammonium 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonate)
- OPD
o-phenylenediamine
- AA
ascorbic acid
- DCFH-DA
2′,7′-dichlorodihydrofluorescein diacetate
- BMPO
5-tert-butoxycarbonyl-5-methyl-1-pyrroline-N-oxide
- CCK-8
Cell Counting Kit-8
- E. coli
Escherichia coli
- S. aureus
Staphylococcus aureus subsp. aureus
- HUVEC
human umbilical vein endothelial cells
- TEM
transmission electron microscopy
- ESR
electron spin resonance
- CFUs
colony forming units
- PI
propidium iodide
- SEM
scanning electron microscope
- DLS
dynamic light scattering
- EDS
energy dispersive X-ray spectroscopy
Contributor Information
Sanhong Fan, Email: fsh729@sxu.edu.cn.
Yue Pan, Email: panyue@mail.sysu.edu.cn.
Xin Tian, Email: xtian@suda.edu.cn.
References
- 1.Fischbach MA, Walsh CT. Antibiotics for emerging pathogens. Science. 2009;325:1089–1093. doi: 10.1126/science.1176667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8:423–435. doi: 10.1038/nrmicro2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tian X, Jiang X, Welch C, Croley TR, Wong TY, Chen C, Fan S, Chong Y, Li R, Ge C, Chen C, Yin JJ. Bactericidal effects of silver nanoparticles on lactobacilli and the underlying mechanism. ACS Appl Mater Interfaces. 2018;10:8443–8450. doi: 10.1021/acsami.7b17274. [DOI] [PubMed] [Google Scholar]
- 4.Mahmoudi M, Serpooshan V. Silver-coated engineered magnetic nanoparticles are promising for the success in the fight against antibacterial resistance threat. ACS Nano. 2012;6:2656–2664. doi: 10.1021/nn300042m. [DOI] [PubMed] [Google Scholar]
- 5.Panacek A, Kvitek L, Smekalova M, Vecerova R, Kolar M, Roderova M, Dycka F, Sebela M, Prucek R, Tomanec O, Zboril R. Bacterial resistance to silver nanoparticles and how to overcome it. Nat Nanotechnol. 2018;13:65–71. doi: 10.1038/s41565-017-0013-y. [DOI] [PubMed] [Google Scholar]
- 6.Jiang B, Duan D, Gao L, Zhou M, Fan K, Tang Y, Xi J, Bi Y, Tong Z, Gao GF, Xie N, Tang A, Nie G, Liang M, Yan X. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat Protoc. 2018;13:1506–1520. doi: 10.1038/s41596-018-0001-1. [DOI] [PubMed] [Google Scholar]
- 7.Gao L, Fan K, Yan X. Iron oxide nanozyme: a multifunctional enzyme mimetic for biomedical applications. Theranostics. 2017;7:3207–3227. doi: 10.7150/thno.19738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang X, Hu Y, Wei H. Nanozymes in bionanotechnology: from sensing to therapeutics and beyond. Inorg Chem Front. 2016;3:41–60. doi: 10.1039/C5QI00240K. [DOI] [Google Scholar]
- 9.Chen C, Fan SH, Li C, Chong Y, Tian X, Zheng JW, Fu PP, Jiang XM, Wamer WG, Yin JJ. Platinum nanoparticles inhibit antioxidant effects of vitamin C via ascorbate oxidase-mimetic activity. J Mater Chem B. 2016;4:7895–7901. doi: 10.1039/C6TB02382G. [DOI] [PubMed] [Google Scholar]
- 10.Luo W, Zhu C, Su S, Li D, He Y, Huang Q, Fan C. Self-catalyzed self-limiting growth of glucose oxidase-mimicking gold nanoparticles. ACS Nano. 2010;4:7451–7458. doi: 10.1021/nn102592h. [DOI] [PubMed] [Google Scholar]
- 11.Xia X, Zhang J, Lu N, Kim M, Ghal K, Xu W, McKenzie E, Liu J, Ye H. Pd-Ir core-shell nanocubes: a type of highly efficient and versatile peroxidase mimic. ACS Nano. 2015;9:9994–10004. doi: 10.1021/acsnano.5b03525. [DOI] [PubMed] [Google Scholar]
- 12.Yao J, Cheng Y, Zhou M, Zhao S, Lin S, Wang X, Wu J, Li S, Wei H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem Sci. 2018;9:2927–2933. doi: 10.1039/C7SC05476A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh S, Roy P, Karmodak N, Jemmis ED, Mugesh G. Nanoisozymes: crystal-facet-dependent enzyme-mimetic activity of V2O5 nanomaterials. Angew Chem Int Ed Engl. 2018;57:4510–4515. doi: 10.1002/anie.201800681. [DOI] [PubMed] [Google Scholar]
- 14.Singh N, Savanur MA, Srivastava S, D’Silva P, Mugesh GA. Redox modulatory Mn3O4 nanozyme with multi-enzyme activity provides efficient cytoprotection to human cells in a Parkinson’s disease model. Angew Chem Int Ed Engl. 2017;56:14267–14271. doi: 10.1002/anie.201708573. [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Song X, Chen Y, Rong M, Zhao T, Wang Y, Jiang Y, Chen X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal Chim Acta. 2015;869:89–95. doi: 10.1016/j.aca.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 16.Zheng AX, Cong ZX, Wang JR, Li J, Yang HH, Chen GN. Highly-efficient peroxidase-like catalytic activity of graphene dots for biosensing. Biosens Bioelectron. 2013;49:519–524. doi: 10.1016/j.bios.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Liu C, Liu Z, Ren J, Qu X. Specific oxygenated groups enriched graphene quantum dots as highly efficient enzyme mimics. Small. 2018;14:e1703710. doi: 10.1002/smll.201703710. [DOI] [PubMed] [Google Scholar]
- 18.Hassanzadeh J, Khataee A. Ultrasensitive chemiluminescent biosensor for the detection of cholesterol based on synergetic peroxidase-like activity of MoS2 and graphene quantum dots. Talanta. 2018;178:992–1000. doi: 10.1016/j.talanta.2017.08.107. [DOI] [PubMed] [Google Scholar]
- 19.Wang Z, Dong K, Liu Z, Zhang Y, Chen Z, Sun H, Ren J, Qu X. Activation of biologically relevant levels of reactive oxygen species by Au/g-C3N4 hybrid nanozyme for bacteria killing and wound disinfection. Biomaterials. 2017;113:145–157. doi: 10.1016/j.biomaterials.2016.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Cai S, Jia X, Han Q, Yan X, Yang R, Wang C. Porous Pt/Ag nanoparticles with excellent multifunctional enzyme mimic activities and antibacterial effects. Nano Res. 2017;10:2056–2069. doi: 10.1007/s12274-016-1395-0. [DOI] [Google Scholar]
- 21.Tao Y, Ju E, Ren J, Qu X. Bifunctionalized mesoporous silica-supported gold nanoparticles: intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv Mater. 2015;27:1097–1104. doi: 10.1002/adma.201405105. [DOI] [PubMed] [Google Scholar]
- 22.Chen Z, Wang Z, Ren J, Qu X. Enzyme mimicry for combating bacteria and biofilms. Acc Chem Res. 2018;51:789–799. doi: 10.1021/acs.accounts.8b00011. [DOI] [PubMed] [Google Scholar]
- 23.Chen S, Quan Y, Yu YL, Wang JH. Graphene quantum dot/silver nanoparticle hybrids with oxidase activities for antibacterial application. ACS Biomater Sci Eng. 2017;3:313–321. doi: 10.1021/acsbiomaterials.6b00644. [DOI] [PubMed] [Google Scholar]
- 24.Ge C, Wu R, Chong Y, Fang G, Jiang X, Pan Y, Chen C, Yin JJ. Synthesis of Pt hollow nanodendrites with enhanced peroxidase-like activity against bacterial infections: implication for wound healing. Adv Funct Mater. 2018;28:1801484. doi: 10.1002/adfm.201801484. [DOI] [Google Scholar]
- 25.Fang G, Li W, Shen X, Perez-Aguilar JM, Chong Y, Gao X, Chai Z, Chen C, Ge C, Zhou R. Differential Pd-nanocrystal facets demonstrate distinct antibacterial activity against Gram-positive and Gram-negative bacteria. Nat Commun. 2018;9:129. doi: 10.1038/s41467-017-02502-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loo AE, Wong YT, Ho R, Wasser M, Du T, Ng WT, Halliwell B. Effects of hydrogen peroxide on wound healing in mice in relation to oxidative damage. PLoS ONE. 2012;7:e49215. doi: 10.1371/journal.pone.0049215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahm CW. Varistor characteristics of ZnO/V2O5/MnO2/Nb2O5 semiconducting ceramics with Tb4O7 addition. J Mater Sci Mater El. 2015;26:4144–4151. doi: 10.1007/s10854-015-2959-6. [DOI] [Google Scholar]
- 28.Gao H, Zhou Y, Chen KQ, Li XL. Synthesis of Tb4O7 complexed with reduced graphene oxide for Rhodamine-B absorption. Mater Res Bull. 2016;77:111–114. doi: 10.1016/j.materresbull.2016.01.016. [DOI] [Google Scholar]
- 29.Castillo-Garcia ML, Aguilar-Caballos MP, Gomez-Hens A. Application of Tb(4)O(7) nanoparticles for lasalocid and salicylate determination in food analysis. J Agric Food Chem. 2012;60:11741–11747. doi: 10.1021/jf303919q. [DOI] [PubMed] [Google Scholar]
- 30.Tian X, Sun Y, Fan S, Boudreau M, Chen C, Ge C, Yin JJ. Photogenerated charge carriers in molybdenum disulfide quantum dots with enhanced antibacterial activity. ACS Appl Mater Interfaces. 2019;11:4858–4866. doi: 10.1021/acsami.8b19958. [DOI] [PubMed] [Google Scholar]
- 31.Tao W, Zhang H, Chong Y, Wamer WG, Yin JJ, Wu XC. Probing hydroxyl radical generation from H2O2 upon plasmon excitation of gold nanorods using electron spin resonance: molecular oxygen-mediated activation. Nano Res. 2016;9:1663–1673. doi: 10.1007/s12274-016-1060-7. [DOI] [Google Scholar]
- 32.Sun HJ, Gao N, Dong K, Ren JS, Qu XG. Graphene quantum dots-band-aids used for wound disinfection. ACS Nano. 2014;8:6202–6210. doi: 10.1021/nn501640q. [DOI] [PubMed] [Google Scholar]
- 33.Koch AL. The pH in the neighborhood of membranes generating a protonmotive force. J Theor Biol. 1986;120:73–84. doi: 10.1016/S0022-5193(86)80018-2. [DOI] [PubMed] [Google Scholar]
- 34.Xiu ZM, Zhang QB, Puppala HL, Colvin VL, Alvarez PJ. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012;12:4271–4275. doi: 10.1021/nl301934w. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Characterization of Tb4O7 NPs. Figure S2. The oxidase-like catalytic activity of the Tb4O7 NPs. Figure S3. The concentration of H2O2 generated in the catalytic system. Figure S4. SEM-EDS elemental images. Figure S5. ROS levels of S. aureus.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional file.