Abstract
Background
Platinum‐based therapy, including cisplatin, carboplatin, oxaliplatin or a combination of these, is used to treat a variety of paediatric malignancies. Unfortunately, one of the most important adverse effects is the occurrence of hearing loss or ototoxicity. There is a wide variation in the reported prevalence of platinum‐induced ototoxicity and the associated risk factors. More insight into the prevalence of and risk factors for platinum‐induced hearing loss is essential in order to develop less ototoxic treatment protocols for the future treatment of children with cancer and to develop adequate follow‐up protocols for childhood cancer survivors treated with platinum‐based therapy.
Objectives
To evaluate the existing evidence on the association between childhood cancer treatment including platinum analogues and the occurrence of hearing loss.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8), MEDLINE (PubMed) (1945 to 23 September 2015) and EMBASE (Ovid) (1980 to 23 September 2015). In addition, we searched reference lists of relevant articles and the conference proceedings of the International Society for Paediatric Oncology (2008 to 2014), the American Society of Pediatric Hematology/Oncology (2008 to 2015) and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer (2010 to 2015). Experts in the field provided information on additional studies.
Selection criteria
All study designs, except case reports, case series (i.e. a description of non‐consecutive participants) and studies including fewer than 100 participants treated with platinum‐based therapy who had an ototoxicity assessment, examining the association between childhood cancer treatment including platinum analogues and the occurrence of hearing loss.
Data collection and analysis
Two review authors independently performed the study selection. One review author performed data extraction and risk of bias assessment, which was checked by another review author.
Main results
We identified 13 eligible cohort studies including 2837 participants with a hearing test after treatment with a platinum analogue for different types of childhood cancers. All studies had methodological limitations, with regard to both internal (risk of bias) and external validity. Participants were treated with cisplatin, carboplatin or both, in varying doses. The reported prevalence of hearing loss varied considerably between 0% and 90.1%; none of the studies provided data on tinnitus. Three studies reported a prevalence of 0%, but none of these studies provided a definition for hearing loss and there might be substantial or even complete overlap in included participants between these three studies. When only studies that did provide a definition for hearing loss were included, the prevalence of hearing loss still varied widely between 1.7% and 90.1%. All studies were very heterogeneous with regard to, for example, definitions of hearing loss, used diagnostic tests, participant characteristics, (prior) anti‐tumour treatment, other ototoxic drugs and length of follow‐up. Therefore, pooling of results was not possible.
Only two studies included a control group of people who had not received platinum treatment. In one study, the prevalence of hearing loss was 67.1% (95% confidence interval (CI) 59.3% to 74.1%) in platinum‐treated participants, while in the control participants it was 7.4% (95% CI 6.2% to 8.8%). However, hearing loss was detected by screening in survivors treated with platinum analogues and by clinical presentation in control participants. It is uncertain what the effect of this difference in follow‐up/diagnostic testing was. In the other study, the prevalence of hearing loss was 20.1% (95% CI 17.4% to 23.2%) in platinum‐treated participants and 0.4% (95% CI 0.12% to 1.6%) in control participants. As neither study was a randomized controlled trial or controlled clinical trial, the calculation of a risk ratio was not feasible as it is very likely that both groups differed more than only the platinum treatment.
Only two studies evaluated possible risk factors using multivariable analysis. One study identified a significantly higher risk of hearing loss in people treated with cisplatin 400 mg/m2 plus carboplatin 1700 mg/m2 as compared to treatment with cisplatin 400 mg/m2 or less, irrespective of the definition of hearing loss. They also identified a significantly higher risk of hearing loss in people treated with non‐anthracycline aminoglycosides antibiotics (using a surrogate marker) as compared to people not treated with them, for three out of four definitions of hearing loss. The other study reported that age at treatment (odds ratio less than 1 for each single‐unit increase) and single maximum cisplatin dose (odds ratio greater than 1 for each single‐unit increase) were significant predictors for hearing loss, while gender was not.
Authors' conclusions
This systematic review shows that children treated with platinum analogues are at risk for developing hearing loss, but the exact prevalence and risk factors remain unclear. There were no data available for tinnitus. Based on the currently available evidence we can only advise that children treated with platinum analogues are screened for ototoxicity in order to make it possible to diagnose hearing loss early and to take appropriate measures. However, we are unable to give recommendations for specific follow‐up protocols including frequency of testing. Counselling regarding the prevention of noise pollution can be considered, such as the use of noise‐limiting equipment, avoiding careers with excess noise and ototoxic medication. Before definitive conclusions on the prevalence and associated risk factors of platinum‐induced ototoxicity can be made, more high‐quality research is needed. Accurate and transparent reporting of findings will make it possible for readers to appraise the results of these studies critically.
Plain language summary
Hearing loss after treatment including platinum analogues for childhood cancer
Review question
We reviewed the evidence on the association between childhood cancer treatment including platinum analogues and the occurrence of hearing loss.
Background
Platinum‐based therapy, such as cisplatin, carboplatin and oxaliplatin, is used to treat a variety of cancers in children. Unfortunately, one of the most important side effects is hearing loss or ototoxicity. There is a wide variation in the reported frequency of platinum‐induced ototoxicity and associated risk factors (a condition, lifestyle or environment that affects the probability of occurrence of hearing loss). More insight into frequency and risk factors is essential to improve treatment for children with cancer and to develop better ways of monitoring (called follow‐up) survivors already treated with platinum‐based therapy.
Study characteristics
The evidence is current to September 2015.
We found 13 studies including 2837 participants with a hearing test after platinum‐based therapy for different types of childhood cancers. Participants were treated with cisplatin, carboplatin or both, in varying doses. All studies were very different with regard to definitions of hearing loss, used diagnostic tests, participant characteristics, (prior) anti‐cancer treatment, other ototoxic drugs and length of follow‐up.
Key results
The reported frequency of hearing loss varied between 0% and 90.1%; none of the studies provided data on tinnitus (that is, ringing in the ears). Three studies reported a frequency of 0%, but none of these studies provided a definition for hearing loss and there might be substantial or even complete overlap in included participants between these three studies. When only studies that did provide a definition for hearing loss were included, the frequency of hearing loss still varied between 1.7% and 90.1%.
Only two studies included people who had not received platinum treatment (called control group). In one study, the frequency of hearing loss was 67.1% in people treated with platinum, while in the control group it was 7.4%. In the other study, the frequency of hearing loss was 20.1% in people treated with platinum and 0.44% in the control group. But due to methodological problems of these studies, it is unclear how reliable these results are.
Only two studies evaluated possible risk factors. One study found a higher risk of hearing loss in people treated with cisplatin 400 mg/m2 plus carboplatin 1700 mg/m2 compared to treatment with cisplatin 400 mg/m2 or less, irrespective of the definition of hearing loss. They also found a higher risk of hearing loss in people treated with non‐anthracycline aminoglycosides antibiotics (that is, a certain type of antibiotics) as compared to people not treated with these antibiotics, for three out of four definitions of hearing loss. The other study reported that age at treatment (lower risk in older children) and single maximum cisplatin dose (higher risk with an increasing dose) were significant predictors for hearing loss, while gender was not.
Based on the currently available evidence, we can only advise that children treated with platinum analogues are screened for ototoxicity in order to make it possible to diagnose hearing loss early and to take appropriate measures. However, we are unable to give recommendations for specific follow‐up methods including how often hearing is tested. Counselling regarding the prevention of noise pollution can be considered, like the use of noise‐limiting equipment, avoiding careers with excess noise and ototoxic medicines. Before definitive conclusions on how often hearing loss happens (called prevalence) and associated risk factors of platinum‐induced ototoxicity can be made, more high‐quality research is needed.
Quality of the evidence
All studies had problems relating to quality of the evidence.
Background
Platinum‐based therapy, including cisplatin, carboplatin, oxaliplatin or a combination, is used to treat a variety of paediatric malignancies. One of the most important adverse effects is the occurrence of hearing loss (ototoxicity). It usually manifests as bilateral, symmetrical, sensorineural hearing loss first affecting the higher frequencies (6000 Hz or greater) (McHaney 1983) and it is often accompanied by tinnitus (Reddel 1982).
The hearing loss not only develops during platinum‐based therapy but also years after completion of the therapy (Bertolini 2004; Knight 2005). This might be explained by the prolonged retention of platinum in the body; up to 20 years after treatment circulating platinum is still detectable in the plasma (Gietema 2000). Platinum‐induced hearing loss seems to be irreversible and worsening of hearing loss occurs during follow‐up (Bertolini 2004; McHaney 1983).
There is a wide variation in the reported frequency of platinum‐induced hearing loss; frequencies as high as 88% have been described (McHaney 1983). Several risk factors have been mentioned in the literature, such as the type of platinum analogue used. Cisplatin seems to cause substantially more hearing loss than carboplatin and the highest incidence of hearing loss has been found in people who received both cisplatin and carboplatin (Bertolini 2004; Dean 2008); the ototoxicity of oxaliplatin as compared to the other platinum analogues is not as well established but oxaliplatin seems to be the least ototoxic (Eloxatin SPC). Furthermore, the incidence of platinum‐induced hearing loss seems to be dose‐dependent, increasing with higher cumulative doses (Bertolini 2004; Li 2004; McHaney 1983; Schell 1989), and with higher individual doses (Li 2004; Reddel 1982). Different dosing formulas, like dose per body surface area or per kilogram bodyweight, can influence the platinum doses actually received, especially in infants (Leahey 2012; Qaddoumi 2012). In addition, bolus injections seem to be more ototoxic than longer infusion durations (Reddel 1982), although this was not confirmed in a Cochrane systematic review (Van As 2014a). Cranial radiotherapy (Schell 1989), younger age (Li 2004; Qaddoumi 2012; Schell 1989), genetic variants (Grewal 2010; Ross 2009) and other host‐specific factors (Veal 2001), impaired renal function at the time of platinum treatment (Skinner 2004) and other ototoxic drugs, such as aminoglycosides (Cancer in Children 2005; Skinner 2004), and furosemide (Gallagher 1979), have been reported as additional risk factors.
Although platinum‐induced hearing loss is not life‐threatening, loss of hearing, especially during the first three years of life and even when only borderline to mild, can have important implications. It can negatively impact speech and language development, which may lead to difficulties with school performance and psychosocial functioning (Dean 2008; Gregg 2004; Skinner 2004). This is even more true for children who experience dual sensory loss, like people with retinoblastoma or optic pathway glioma.
One systematic review and its update have shown that at the moment there is no evidence that underscores the use of medical interventions, such as amifostine, to prevent the occurrence of platinum‐induced ototoxicity (Van As 2012a; Van As 2014b). More insight into the prevalence of and risk factors for platinum‐induced hearing loss is essential in order to develop less ototoxic treatment protocols for the future treatment of children with cancer and to develop adequate follow‐up protocols for childhood cancer survivors treated with platinum‐based therapy. This is, to our knowledge, the first systematic review on this important topic.
Objectives
To evaluate the existing evidence on the association between childhood cancer treatment including platinum analogues and the occurrence of hearing loss.
Methods
Criteria for considering studies for this review
Types of studies
All study designs, except case reports, case series (i.e. a description of non‐consecutive participants) and studies including fewer than 100 participants treated with platinum‐based therapy who had an ototoxicity assessment, examining the association between childhood cancer treatment including platinum analogues and the occurrence of hearing loss.
We defined cohort studies as studies in which a group of consecutive participants were followed from a similar well‐defined point in the course of the disease. The described study group could be the original cohort or a subgroup of the original cohort based on well‐defined inclusion criteria.
Types of participants
Participants (aged 0 to 18 years at tumour diagnosis) treated with platinum‐based therapy for any type of childhood malignancy. All participants should have finished platinum treatment. Studies including both children and adults were only eligible for inclusion in this review if the majority of participants were children (i.e. either more than 90% children or the maximal age did not exceed 22 years).
Types of interventions
Treatment including one or more platinum analogues. Studies also including people who did not receive platinum‐based therapy were only eligible for inclusion in this review if separate data were available for the people treated with platinum‐based therapy.
Types of outcome measures
Hearing loss, tinnitus or both (as defined by the authors of the original studies).
Search methods for identification of studies
We did not impose language restrictions.
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (2015, Issue 8), MEDLINE in PubMed (from 1945 to 23 September 2015) and EMBASE in Ovid (from 1980 to 23 September 2015). The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are in the appendices (Appendix 1; Appendix 2; Appendix 3).
Searching other resources
We located information about trials not registered in CENTRAL, MEDLINE or EMBASE, either published or unpublished, by searching the reference lists of included articles and review articles. We handsearched the conference proceedings of the International Society for Paediatric Oncology (SIOP) (from 2008 to 2014), the American Society of Pediatric Hematology/Oncology (ASPHO) (from 2008 to 2015) and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer (from 2010 to 2015). Experts in the field provided information on additional studies.
Data collection and analysis
Selection of studies
After employing the search strategy described, two review authors independently identified studies meeting the inclusion criteria for this review. Discrepancies between review authors were resolved by discussion. Third‐party arbitration was not needed. We obtained in full any study that seemed to meet the inclusion criteria on the grounds of the title, abstract or both, for closer inspection. We clearly stated details of the reasons for exclusion of any study considered for the review. We included a flow chart of the selection of studies in the review.
Data extraction and management
One review author performed data extraction using standardized forms, which was checked by another review author. We extracted data on study characteristics (such as study design, number of patients enrolled in the study, number of patients fulfilling the review's inclusion criteria), patient characteristics (such as age, sex, type of malignancy, prior hearing loss and renal function at time of platinum treatment), interventions (such as information on the received antineoplastic treatment including cumulative doses, possible other ototoxic drugs like aminoglycosides, furosemide and vincristine, and the use of otoprotective medical interventions), outcome measures (including definition used and method of detection), risk factors and length of follow‐up. We resolved discrepancies between authors by discussion. We needed no third‐party arbitration.
Assessment of risk of bias in included studies
One review author performed assessment of the risk of bias of the included studies, which another review author checked. We based the assessment of risk of bias in observational studies on previously described checklists according to evidence‐based medicine criteria (Grimes 2002; Laupacis 1994). See Table 1 for the definitions of the different 'Risk of bias' criteria. We resolved discrepancies between review authors by discussion. We needed no third‐party arbitration. We took the risk of bias in included studies into account in the interpretation of the review's results.
1. Risk of bias assessment criteria for observational studies.
| Internal validity | External validity | |
| Study group |
Selection bias (representative: yes/no):
|
Reporting bias (well‐defined: yes/no):
|
| Follow‐up |
Attrition bias (adequate: yes/no):
|
Reporting bias (well‐defined: yes/no):
|
| Outcome |
Detection bias (blind: yes/no):
|
Reporting bias (well‐defined: yes/no):
|
| Risk assessment |
Confounding (adjustment for other factors: yes/no):
|
Analyses (well‐defined: yes/no):
|
Measures of treatment effect
If a control group from a randomized controlled trial (RCT) or controlled clinical trial (CCT) had been available we would have analyzed hearing loss, tinnitus or both using risk ratios (RR). As this was not the case, we used prevalences to analyze hearing loss, tinnitus or both. We presented all results with the corresponding 95% confidence interval (CI).
Dealing with missing data
When relevant data regarding study selection, data extraction and 'Risk of bias' assessment were missing, we attempted to contact the study authors to retrieve the missing data.
Assessment of heterogeneity
We assessed heterogeneity by visual inspection of the forest plots. If we identified heterogeneity, we explored possible reasons for the occurrence of heterogeneity and took appropriate measures.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studies section, we assessed reporting bias by constructing a funnel plot where there was a sufficient number of included studies (i.e. at least 10 studies included in a meta‐analysis). When there were fewer studies, the power of the tests was too low to distinguish chance from real asymmetry (Higgins 2011). Since pooling of results was not possible, this was not applicable.
Data synthesis
We entered data into the Review Manager 5 software as provided by Cochrane (RevMan 2014). We included outcome measures only if it was the intention of the study to perform the necessary assessments in all included participants (i.e. not optional only or only performed in some centres). When the results of a particular outcome measure were available for less than 50% of the participants of a study, due to the associated high risk of attrition bias, we did not report the results of this outcome measure. We performed pooling of results only if studies were comparable, including the definition of ototoxicity that was used. We used the Wilson method to calculate the corresponding 95% CIs of the prevalences. As this was not possible in Review Manager 5 we used the following tool: EpiTools epidemiological calculator; we prepared forest plots in Excel software. If a study presented the results of hearing tests at different time points, we used the final test result for our calculations. We took different study designs into account in the analyses. We summarized studies for which pooling of results was not possible descriptively.
Sensitivity analysis
Since pooling of results was not possible, sensitivity analyses for 'Risk of bias' items (i.e. excluding studies with a high risk of bias and studies for which the risk of bias was unclear, and comparing the results of studies with a low risk of bias with the results of all available studies) were not applicable.
Results
Description of studies
Results of the search
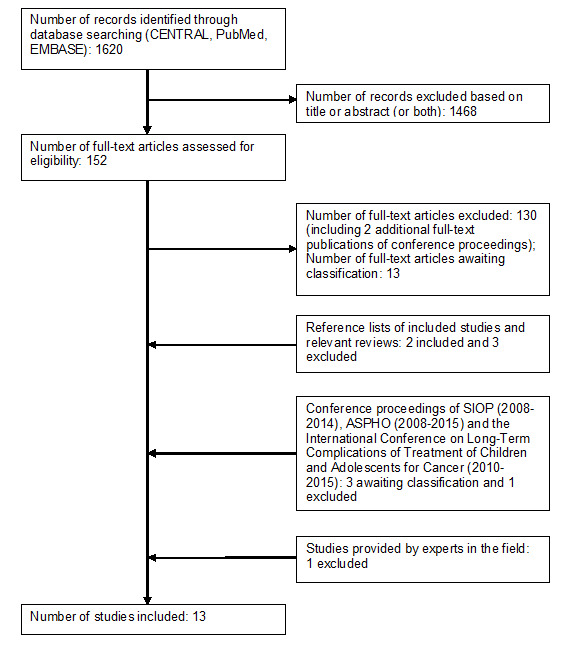
Running the searches in the electronic databases of CENTRAL, MEDLINE (PubMed) and EMBASE (Ovid) yielded 1620 references. Following initial screening of the titles, abstracts or both, we excluded 1468 references that clearly did not meet all criteria required for considering studies for this review. We assessed the remaining 152 references in full, of which 11 fulfilled all the criteria for considering studies for this review and were thus eligible for inclusion. Thirteen studies are awaiting further classification. We excluded the remaining 128 references. For two of the conference proceedings identified in this part of the search, we were able to obtain the full‐text articles published after the search date; we excluded both.
By scanning the reference lists of included studies and reviews, we identified five additional studies, of which two were eligible for inclusion and three were excluded. By scanning the conference proceedings of SIOP, ASPHO and the International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer, we identified four additional studies that had not been published yet; three are awaiting further classification and we excluded one.
An expert in the field provided a reference to an additional study, which we excluded.
In summary (see also Figure 1), the number of included studies was 13. We also identified 16 studies awaiting further classification (for reasons and more information see the Characteristics of studies awaiting classification table) and excluded 135 studies for the reasons described in the Characteristics of excluded studies table. We identified no ongoing studies.
1.
Included studies
The characteristics of the included studies are summarized below. For more detailed information, see the Characteristics of included studies table.
All 13 included studies were cohort studies; some studies were RCTs, but as participants in both treatment groups received cisplatin for this systematic review, we considered these as cohort studies (Cushing 2004; Kennedy 2014; Mandell 1999; Perilongo 2009). Eleven studies mentioned the time periods of treatment/enrolment, which varied between 1987 and 2012; two studies did not mention time periods (Hudson 2013; Simon 2002). Participants had hepatoblastoma in one study (Perilongo 2009), medulloblastoma in one (Kennedy 2014), different types of tumours arising from the pons in one (Mandell 1999), extracranial high‐risk malignant germcell tumours in one (Cushing 2004), retinoblastoma in four (Jehanne 2009; Lambert 2008; Shields 2002; Shields 2006), neuroblastoma in two (Landier 2014; Simon 2002), and different types of childhood cancers in three (Bertolini 2004; Hudson 2013; Peleva 2014).
The total number of participants with a hearing test after treatment with a platinum analogue was 2837 (range 103 to 715 participants per study). The age at tumour diagnosis of these participants ranged between 0 and 22 years; eight studies did not report age at tumour diagnosis (Cushing 2004; Hudson 2013; Kennedy 2014; Lambert 2008; Mandell 1999; Perilongo 2009; Shields 2006; Simon 2002). Only one study reported the age at outcome assessment/follow‐up, which ranged between 1 and 24 years (Landier 2014).
In four studies, participants received cisplatin (Cushing 2004; Kennedy 2014; Mandell 1999; Perilongo 2009), in four studies, carboplatin (Jehanne 2009; Lambert 2008; Shields 2002; Shields 2006), and in five studies cisplatin, carboplatin or both (Bertolini 2004; Hudson 2013; Landier 2014; Peleva 2014; Simon 2002). The cumulative platinum doses, if mentioned, varied widely between studies; for detailed information on the cumulative platinum doses, individual platinum doses and platinum infusion durations see the Characteristics of included studies table. Other treatment, including other ototoxic drugs, varied widely between the studies; see the Characteristics of included studies table for more information.
In seven studies, participants had no prior ototoxic treatment (i.e. platinum analogues, radiotherapy to the head/neck and/or cranial surgery) (Cushing 2004; Jehanne 2009; Landier 2014; Mandell 1999; Perilongo 2009; Shields 2002; Shields 2006). One study reported that participants did not receive cranial irradiation, but the authors provided no information on platinum treatment and surgery (Bertolini 2004). The other five studies did not report prior ototoxic treatment (Hudson 2013; Kennedy 2014; Lambert 2008; Peleva 2014; Simon 2002). In three studies, participants did not have prior hearing dysfunction (Peleva 2014; Shields 2002; Shields 2006), in one study this was only clear for some of the participants (Bertolini 2004), in one study 12% of the participants had prior hearing dysfunction (Lambert 2008) (for diagnostic criteria, see Characteristics of included studies table). The other eight studies did not report prior hearing dysfunction. In two studies, participants did not have pretreatment renal impairment (Shields 2002; Shields 2006). The other 11 studies did not report pretreatment renal impairment. None of the studies stated if there was impaired renal function at the time of platinum treatment.
Eight studies provided information on follow‐up for the eligible patients, which varied: maximal follow‐up was 13 years (Bertolini 2004), range 0.13 to 11 years (Jehanne 2009; Lambert 2008; Landier 2014; Shields 2002; Shields 2006; for both studies by Shields and colleagues, it was unclear if it was based on the timing of hearing assessment), at least eight weeks post‐therapy (Mandell 1999), or at least one year after diagnosis (Simon 2002).
Two studies had a control group without platinum treatment (Hudson 2013; Simon 2002); for more information, see the Characteristics of included studies table.
It should be noted that there might be substantial or even complete overlap in included participants between Lambert 2008, Shields 2002, and Shields 2006. All three studies treated people with retinoblastoma in two hospitals in Philadelphia (USA). This was according to the same study protocol in two studies, the third study did not mention the name of the study protocol. In addition, time periods overlapped. Between Cushing 2004, Hudson 2013, and Mandell 1999 there might be a small overlap in included participants: Cushing 2004 and Mandell 1999 included people treated at St. Jude Children's Research Hospital, but it was unclear if these participants were all included in the survivor cohort of Hudson 2013; there was no overlap between Cushing 2004 and Mandell 1999.
Risk of bias in included studies
See the 'Risk of bias' section of the Characteristics of included studies table and Figure 2 for the exact scores per study and the support for the judgements made. We have looked both at internal and external validity.
2.
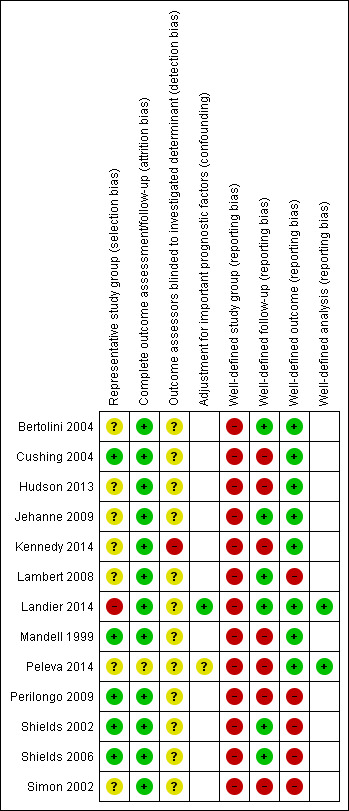
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Internal validity
Selection bias
For evaluating selection bias, we assessed if there was a representative study group. In five studies (38.5%), the risk of selection bias was low (Cushing 2004; Mandell 1999; Perilongo 2009; Shields 2002; Shields 2006), in one study (7.7%), it was high (Landier 2014), and in the seven remaining studies (53.8%), it was unclear (Bertolini 2004; Hudson 2013; Jehanne 2009; Kennedy 2014; Lambert 2008; Peleva 2014; Simon 2002).
Attrition bias
For evaluating attrition bias, we assessed the completeness of follow‐up. In 12 studies (92.3%), the risk of attrition bias was low (Bertolini 2004; Cushing 2004; Hudson 2013; Jehanne 2009; Kennedy 2014; Lambert 2008; Landier 2014; Mandell 1999; Perilongo 2009; Shields 2002; Shields 2006; Simon 2002), while in one study (7.7%), it was unclear (Peleva 2014).
Detection bias
For evaluating detection bias, we assessed if the outcome assessors were blinded to the investigated determinant. In one study (7.7%), the risk of detection bias was high (Kennedy 2014), while in 12 studies (92.3%), it was unclear (Bertolini 2004; Cushing 2004; Hudson 2013; Jehanne 2009; Lambert 2008; Landier 2014; Mandell 1999; Peleva 2014; Perilongo 2009; Shields 2002; Shields 2006; Simon 2002).
Confounding
For evaluating confounding, we assessed if there was adjustment for important prognostic factors. Two of the 13 (15.4%) included studies conducted multivariable analyses of potential risk factors. In one of these studies, there was a low risk of confounding (Landier 2014), while in the other study, it was unclear (Peleva 2014).
External validity
Reporting bias
None of the 13 included studies defined the study group well.
In six studies (46.2%), follow‐up was well‐defined (Bertolini 2004; Jehanne 2009; Lambert 2008; Landier 2014; Shields 2002; Shields 2006), while in the other seven studies (53.8%), it was not (Cushing 2004; Hudson 2013; Kennedy 2014; Mandell 1999; Peleva 2014; Perilongo 2009; Simon 2002).
In eight studies (61.5%), the outcome was well‐defined (Bertolini 2004; Cushing 2004; Hudson 2013; Jehanne 2009; Kennedy 2014; Landier 2014; Mandell 1999; Peleva 2014), while in the other five studies (38.5%), it was not (Lambert 2008; Perilongo 2009; Shields 2002; Shields 2006; Simon 2002).
In both studies that conducted multivariable analyses of potential risk factors, these analyses were well‐defined (Landier 2014; Peleva 2014).
Overall, none of the studies scored good on all applicable reporting bias items: two studies (15.4%) scored bad on all applicable items (Perilongo 2009; Simon 2002), while the other 11 studies (84.6%) had a combination of good and bad scores (Bertolini 2004; Cushing 2004; Hudson 2013; Jehanne 2009; Kennedy 2014; Lambert 2008; Landier 2014; Mandell 1999; Peleva 2014; Shields 2002; Shields 2006).
Effects of interventions
Prevalence of hearing loss
All 13 studies reported the prevalence of hearing loss, which varied widely between 0% and 90.1% (see Characteristics of included studies table). Three studies, in which there might be substantial or even complete overlap in included participants, did not provide a definition of hearing loss (Lambert 2008; Shields 2002; Shields 2006). However, when we included only studies that provided a definition for hearing loss, the prevalence of hearing loss still varied greatly between 1.7% and 90.1%. However, studies used different definitions of hearing loss (for detailed information on the different definitions see Table 2). In addition, studies used different diagnostic tests to assess hearing loss; in five studies (38.5%), the diagnostic test was not reported (Landier 2014 (only for one of the outcomes: use of hearing aids); Perilongo 2009; Shields 2002; Shields 2006; Simon 2002).
2. Used criteria for hearing loss.
| Brock criteria | Chang criteria | WHO criteria | NCI CTCAEv3 criteria | NCI CTCAEv2 criteria | NCI CTCAEv1 | POG criteria | ASHA criteria |
|
Grade 0: < 40 dB at all frequencies |
Grade 0: ≤ 20 dB at 1, 2 and 4 kHz |
Grade 0: none or no change |
Grade 0: does not meet criteria for grades 1‐4 |
Grade 0: none or no change |
Grade 0: none or no change |
Grade 0: does not meet criteria for grades 1‐4 |
Sensorineural hearing loss between baseline and postchemotherapy audiogram: ≥ 20 dB decrease in pure‐tone threshold at a single test frequency or ≥ 10 dB decrease in pure‐tone threshold at 2 adjacent frequencies or loss of response at 3 consecutive frequencies where responses were previously obtained |
|
Grade 1: ≥ 40 dB at 8000 Hz only (< 40 dB at all lower frequencies) |
Grade 1a: ≥ 40 dB at any frequency 6‐12 kHz Grade 1b: > 20 and < 40 dB at 4 kHz |
Grade 1: asymptomatic hearing loss on audiometry only |
Grade 1: threshold shift or loss of 15‐25 dB relative to baseline, averaged at ≥ 2 contiguous test frequencies in at least 1 ear, or subjective change in the absence of grade 1 threshold shift |
Grade 1: hearing loss on audiometry only |
Grade 1: asymptomatic hearing loss on audiometry only |
Grade 1: 20‐40 dB loss > 4 kHz |
‐ |
|
Grade 2: ≥ 40 dB at 4000 Hz and above (< 40 dB at all lower frequencies) |
Grade 2a: ≥ 40 dB at 4 kHz and above Grade 2b: 20 and < 40 dB at any frequency below 4 kHz |
Grade 2: tinnitus |
Grade 2: threshold shift or loss of > 25‐90 dB, averaged at 2 contiguous test frequencies in at least 1 ear |
Grade 2: tinnitus or hearing loss not requiring hearing aid or treatment |
Grade 2: tinnitus |
Grade 2: > 40 dB loss > 4 kHz |
‐ |
|
Grade 3: ≥ 40 dB at 2000 Hz and above (< 40 dB at all lower frequencies) |
Grade 3: ≥ 40 dB at 2 or 3 kHz and above |
Grade 3: hearing loss interfering with function, but correctable with hearing aid |
Grade 3: hearing loss sufficient to indicate therapeutic intervention, including hearing aids (e.g. ≥ 20 dB bilateral HL in the speech frequencies; ≥ 30 dB unilateral HL), and requiring additional speech‐language related services |
Grade 3: tinnitus or hearing loss correctable with hearing aid or treatment |
Grade 3: hearing loss interfering with function, but correctable with aid |
Grade 3: > 40 dB loss > 2‐4 kHz |
‐ |
|
Grade 4: ≥ 40 dB at 1000 Hz and above (< 40 dB at all lower frequencies) |
Grade 4: ≥ 40 dB at ≥ 1 kHz |
Grade 4: deafness not correctable |
Grade 4: audiological indication for cochlear implant and requiring additional speech‐language related services |
Grade 4: severe unilateral or bilateral hearing loss (deafness) not correctable |
Grade 4: deafness not correctable |
Grade 4: > 40 dB loss < 2 kHz |
‐ |
ASHA: American Speech‐Language‐Hearing Association; CTCAEv3: Common Terminology Criteria Adverse Effects version 3; dB: decibel; HL: hearing level; Hz: hertz; kHz: kilohertz; NCI: National Cancer Institute; POG: Pediatric Oncology Group; WHO: World Health Organization.
Furthermore, all studies were very heterogeneous with regard to, for example, participant characteristics, (prior) anti‐tumour treatment, other ototoxic drugs and length of follow‐up (for detailed information see the Characteristics of included studies table). As a result of this very heterogeneous nature of the included studies, pooling was not possible; we described each study separately.
Hearing loss defined as Brock grade 1 or higher
We could extract data on hearing loss defined as Brock grade 1 or higher from three studies; the number of participants with a hearing test after platinum treatment in the different studies ranged from 168 to 247 (Jehanne 2009; Landier 2014; Perilongo 2009). The prevalence of hearing loss varied between 3.4% and 87% (see Figure 3).
3.
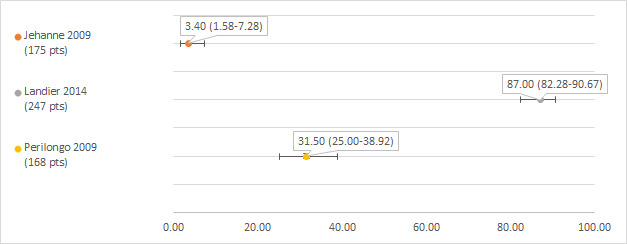
Prevalence and 95% confidence interval (%) of hearing loss defined as Brock grade 1 or higher. pt: participant.
It should be noted that in the study of Jehanne 2009, two of the 175 participants (1.1%) had grade 0 hearing loss (i.e. bilateral hearing loss, but not at 40 dB or greater bilaterally, so not corresponding to grade 1). Although the authors counted these people as having hearing loss, we omitted them from our analyses.
Hearing loss defined as Brock grade 2 or higher
We could extract data on hearing loss defined as Brock grade 2 or higher from four studies; the number of participants with a hearing test after platinum treatment in the different studies ranged from 120 to 247 (Bertolini 2004; Jehanne 2009; Landier 2014; Perilongo 2009). The prevalence of hearing loss varied between 1.7% and 66% (see Figure 4).
4.
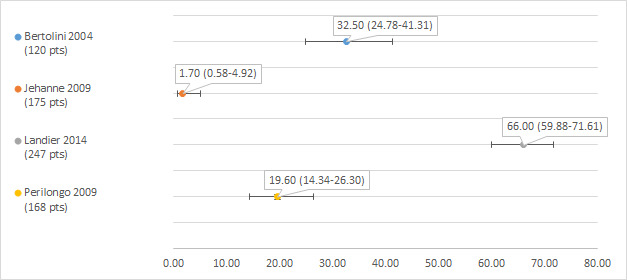
Prevalence and 95% confidence interval (%) of hearing loss defined as Brock grade 2 or higher. pt: participant.
Hearing loss defined as Chang grade 1a or higher
We could extract data on hearing loss defined as Chang grade 1a or higher from two studies including 152 and 243 participants with a hearing test after platinum treatment (Hudson 2013; Landier 2014). The prevalence of hearing loss was 67.1% (Hudson 2013) and 90.10% (Landier 2014) (see Figure 5).
5.
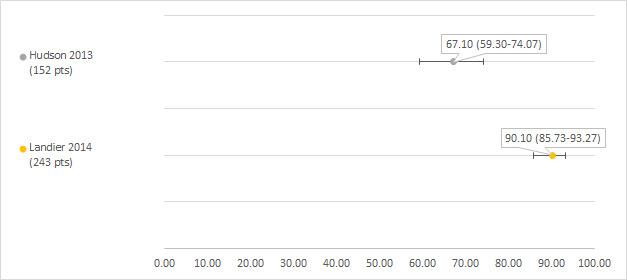
Prevalence and 95% confidence interval (%) of hearing loss defined as Chang grade 1a or higher. pt: participant.
One of the studies included 1561 control participants who received no platinum treatment; 116 of these participants developed hearing loss (prevalence 7.4%; 95% CI 6.2% to 8.8%) (Hudson 2013). It should be noted that hearing loss was detected by screening of survivors with specific cancer treatment‐related risk factors or those (mostly) diagnosed by clinical presentation in survivors without cancer treatment‐related risks.
Hearing loss defined as Chang grade 2a or higher
We could extract data on hearing loss defined as Chang grade 2a or higher from two studies including 243 and 306 participants with a hearing test after platinum treatment (Landier 2014; Peleva 2014). The prevalence of hearing loss was 69.1% (Landier 2014) and 29.7% (Peleva 2014) (see Figure 6).
6.
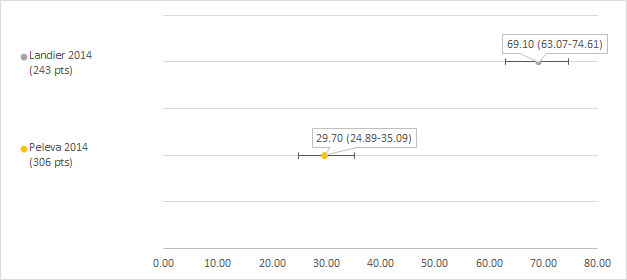
Prevalence and 95% confidence interval (%) of hearing loss defined as Chang grade 2a or higher. pt: participant.
Hearing loss defined as WHO grade 3 or higher
We could extract data on hearing loss defined as WHO (World Health Organization) grade 3 or higher from one study including 715 participants with a hearing test after platinum treatment (Simon 2002). The prevalence of hearing loss was 20.1% (see Figure 7).
7.
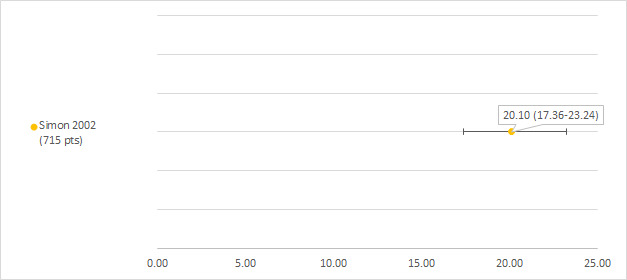
Prevalence and 95% confidence interval (%) of hearing loss defined as World Health Organization (WHO) grade 3 or higher. pt: participant.
This study also included 453 control participants who received no chemotherapy; two of these participants developed hearing loss (prevalence 0.44%; 95% CI 0.12% to 1.6%). One of the control participants with hearing loss had a family history of hearing impairments, the other had combined renal ectopia and hearing impairment.
Hearing loss defined as NCI CTCAEv3 grade 1 or higher
We could extract data on hearing loss defined as NCI CTCAEv3 grade 1 or higher from one study including 242 participants with a hearing test after platinum treatment (Landier 2014). The prevalence of hearing loss was 86% (see Figure 8).
8.
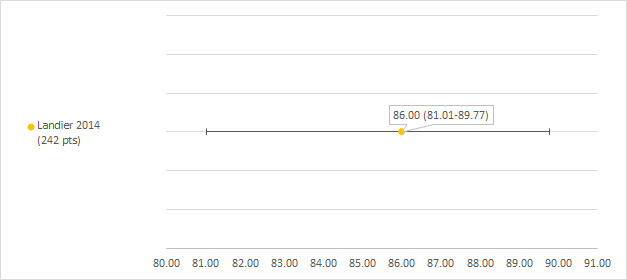
Prevalence and 95% confidence interval (%) of hearing loss defined as National Cancer Institute Common Terminology Criteria Adverse Effects (NCI CTCAEv3) grade 1 or higher. pt: participant.
Hearing loss defined as NCI CTCAE (version unclear) subjective grade 3 or 4
We could extract data on hearing loss defined as NCI CTCAE (version unclear; see notes section of the Characteristics of included studies table) subjective grade 3 or 4 from one study including 295 participants with a hearing test after platinum treatment (Cushing 2004). The prevalence of hearing loss was 1.7% (see Figure 9).
9.
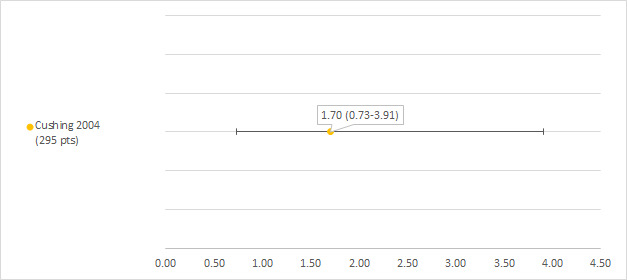
Prevalence and 95% confidence interval (%) of hearing loss defined as National Cancer Institute Common Terminology Criteria Adverse Effects (NCI CTCAE) (version unclear) subjective grade 3 or 4. pt: participant.
Hearing loss defined as NCI CTCAE (version unclear) objective grade 3 or 4
We could extract data on hearing loss defined as NCI CTCAE (version unclear; see notes section of the Characteristics of included studies table) objective grade 3 or 4 from one study including 295 participants with a hearing test after platinum treatment (Cushing 2004). The prevalence of hearing loss was 7.1% (see Figure 10).
10.

Prevalence and 95% confidence interval (%) of hearing loss defined as National Cancer Institute Common Terminology Criteria Adverse Effects (NCI CTCAE) (version unclear) objective grade 3 or 4. pt: participant.
Hearing loss defined as POG subjective grade 1 or higher
We could extract data on hearing loss defined as POG (Pediatric Oncology Group) subjective grade 1 or higher (see notes section of the Characteristics of included studies table) from one study including 113 participants with a hearing test after platinum treatment (Mandell 1999). The prevalence of hearing loss was 2.7% (see Figure 11).
11.
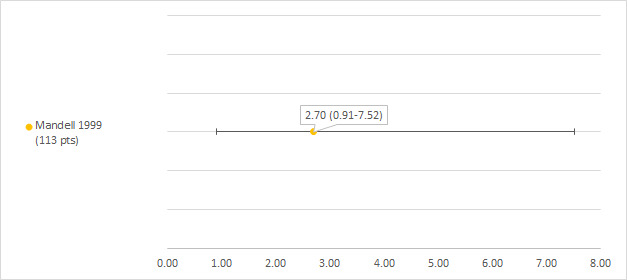
Prevalence and 95% confidence interval (%) of hearing loss defined as Pediatric Oncology Group (POG) subjective grade 1 or higher. pt: participant.
Hearing loss defined as POG objective grade 1 or higher
We could extract data on hearing loss defined as POG objective grade 1 or higher (see notes section of the Characteristics of included studies table) from one study including 113 participants with a hearing test after platinum treatment (Mandell 1999). The prevalence of hearing loss was 15% (see Figure 12).
12.
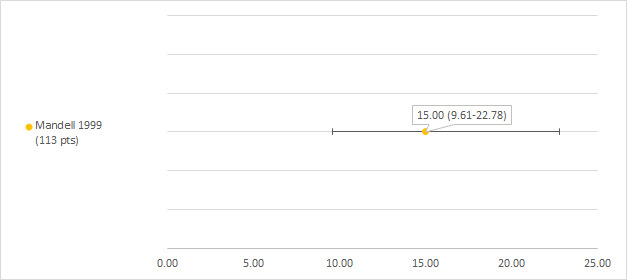
Prevalence and 95% confidence interval (%) of hearing loss defined as Pediatric Oncology Group (POG) objective grade 1 or higher. pt: participant.
Hearing loss defined as use of hearing aids
We could extract data on hearing loss defined use of hearing aids from two studies including 144 and 259 participants after platinum treatment (Kennedy 2014; Landier 2014). The prevalence of hearing loss was 16% (Kennedy 2014) and 59.8% (Landier 2014) (see Figure 13).
13.
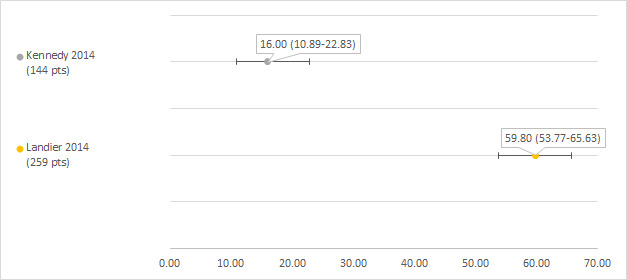
Prevalence and 95% confidence interval (%) of hearing loss defined as use of hearing aids. pt: participant.
Hearing loss defined as ASHA
We could extract data on hearing loss defined as ASHA (American Speech‐Language‐Hearing Association) from one study including 306 participants with a hearing test after platinum treatment (Peleva 2014). The prevalence of hearing loss was 48.4% (see Figure 14).
14.
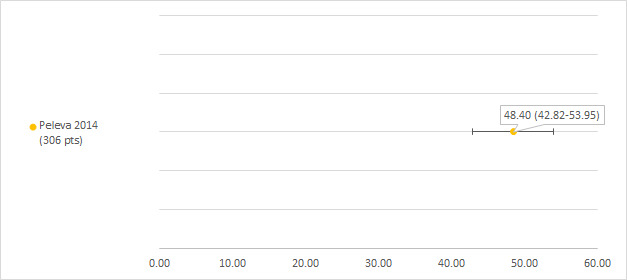
Prevalence and 95% confidence interval (%) of hearing loss defined as American Speech‐Language‐Hearing Association (ASHA). pt: participant.
Hearing loss for which no definition was provided
Three studies did not state how they defined hearing loss (Lambert 2008; Shields 2002; Shields 2006). In all these studies, the identified prevalence was 0%. However, there might be substantial or even complete overlap in included participants between these three studies (see Included studies for further details).
Prevalence of tinnitus
There was no information on tinnitus.
For the Cushing 2004 study, it was unclear which version of the NCI CTCAE criteria were used to define grade 3 or 4 toxicity, it could be either version 1 or 2. In version 2, grade 3 is defined as tinnitus or hearing loss. However, as the authors specifically used the term 'hearing loss' in the manuscript, we assumed that none of the participants developed tinnitus.
Risk factors for hearing loss, tinnitus or both
Two studies investigated possible risk factors for hearing loss after platinum treatment for childhood cancer in a multivariable analysis (Landier 2014; Peleva 2014).
The study of Landier 2014 reported that the risk of developing severe hearing loss for people treated with cisplatin 400 mg/m2 plus carboplatin 1700 mg/m2 was significantly (P < 0.05) higher than for people treated with cisplatin 400 mg/m2 or less irrespective of the used definition for hearing loss (i.e. Brock grade 3 or 4, Chang grade 2b to 4, CTCAEv3 grade 3 or 4 and requiring a hearing aid). The risk of developing severe hearing loss for people hospitalized at least once for infection during induction (used as a surrogate marker for exposure to non‐anthracycline aminoglycoside antibiotics) was significantly higher than for people never hospitalized for infection during induction for Brock grade 3 or 4, Chang grade 2b to 4 and requiring a hearing aid; for CTCAEv3, it was not significantly different. It should be noted that it is likely that also people who were not eligible for this review were included in the analyses.
The study of Peleva 2014 reported that age at treatment (odds ratio (OR) less than 1 for each single‐unit increase) and single maximum cisplatin dose (OR greater than 1 for each single‐unit increase) were significant predictors for hearing loss (defined as Chang grade 2a and higher), while gender was not.
See Table 3 for more detailed information.
3. Risk factors from multivariable analyses for platinum‐induced ototoxicity after childhood cancer treatment.
| Study | Analysis | Results |
| Landier 2014 | Unconditional multivariable logistic regression considering age at diagnosis, sex, race/ethnicity, cumulative platinum exposure (exposure 1: cisplatin ≤ 400 mg/m2 and exposure 2: cisplatin 400 mg/m2 plus carboplatin 1700 mg/m2), time interval between platinum and testing, preconsolidation glomerular filtration rate, chemotherapy dose reduction during induction therapy and hospitalization for infection during induction therapy (surrogate marker for exposure to non‐anthracycline aminoglycoside antibiotics) It is likely that also participants not eligible for this review were included in the analyses |
1) Risk of developing severe hearing loss for exposure 2 participants compared with exposure 1 participants: Brock grade 3 or 4: OR 3.2 (95% CI 1.1 to 9.8; P = 0.0038) Chang grade 2b to 4: OR 3.7 (95% CI approximately 1.7 to 8.0; P < 0.01) CTCAEv3 grade 3 or 4: OR 3.8 (95% CI 1.7 to 8.6; P = 0.002) 2) Risk of developing severe hearing loss for participants hospitalized at least once for infection during induction compared with participants never hospitalized for infection during induction: Brock grade 3 or 4: OR 5.1 (95% CI 1.7 to 14.9; P = 0.004) Chang grade 2b‐4: OR 2.2 (95% CI approximately 1.1 to 4.5; P < 0.05) CTCAEv3 grade 3 or 4: OR 1.8 (95% CI 0.86 to 3.7; P = 0.124) 3) Risk of requiring a hearing aid: 3.7 × more likely for exposure 2 participants than for exposure 1 participants (95% CI 1.8 to 7.9; P = 0.001) 4) Risk of requiring a hearing aid: 2.3 × more likely for participants hospitalized at least once for infection during induction compared with participants never hospitalized for infection during induction (95% CI 1.2 to 4.4; P = 0.01) |
| Peleva 2014 | Standard binary logistic regression model controlling for gender, single maximum cisplatin dose and/or age at treatment (in months). Chang grade 2a or higher was used to define hearing loss | Age at treatment (OR 0.994, 95% CI 0.990 to 0.999) and single maximum cisplatin dose (OR 1.017, 95% CI 1.005 to 1.029) were significant predictors for hearing loss, while gender was not (OR 0.958, 95% CI 0.551 to 1.668) |
CI: confidence interval; CTCAEv3: Common Terminology Criteria Adverse Effects version 3; OR: odds ratio.
Discussion
Summary of main results
One of the most important adverse effects of treatment with platinum analogues is the occurrence of hearing loss or ototoxicity and, although it is not life‐threatening, loss of hearing, especially during the first three years of life and even when only borderline to mild, can have important implications (Dean 2008; Gregg 2004; Gurney 2007; Skinner 2004), and early intervention is important (Bass 2014a). More insight into the prevalence of platinum‐induced hearing loss and associated risk factors is essential in order to develop less‐ototoxic treatment protocols for future treatment of children with cancer and to develop adequate follow‐up protocols for childhood cancer survivors treated with platinum‐based therapy. This is, to our knowledge, the first systematic review on this important topic.
We identified 13 eligible cohort studies including 2837 participants with a hearing test after treatment with a platinum analogue for different types of childhood cancers. Participants were treated with cisplatin, carboplatin or both, in varying doses. The reported prevalence of hearing loss varied considerably between 0% and 90.1%; none of the studies provided data on tinnitus. Three studies reported a prevalence of 0%, but none of these studies provided a definition for hearing loss and there might be substantial or even complete overlap in included participants between these three studies. When we included only studies that did provide a definition for hearing loss, the prevalence of hearing loss still varied widely between 1.7% and 90.1%. All studies were very heterogeneous with regard to, for example, definitions of hearing loss, used diagnostic tests, participant characteristics, (prior) anti‐tumour treatment, other ototoxic drugs and length of follow‐up. Therefore, pooling of results was not possible.
Only two studies included control participants who had not received platinum treatment. In one study, the prevalence of hearing loss defined as Chang grade 1a or higher was 67.1% (95% CI 59.3% to 74.07%) in platinum‐treated participants, while in the control participants it was 7.4% (95% CI 6.2% to 8.8%). In the other study, the prevalence of hearing loss defined as WHO grade 3 or higher was 20.1% (95% CI 17.36% to 23.24%) in platinum‐treated participants and 0.44% (95% CI 0.12% to 1.6%) in the control participants.
Only two studies evaluated possible risk factors for developing hearing loss after treatment with a platinum analogue using multivariable analysis. One study identified a significantly higher risk of hearing loss in people treated with cisplatin 400 mg/m2 plus carboplatin 1700 mg/m2 as compared to treatment with cisplatin 400 mg/m2 or less, irrespective of the definition of hearing loss. They also identified a significantly higher risk of hearing loss in people treated with non‐anthracycline aminoglycosides antibiotics (using a surrogate marker) as compared to people not treated with them, for three out of four definitions of hearing loss. The other study reported that age at treatment (OR less than 1 for each single‐unit increase) and single maximum cisplatin dose (OR greater than 1 for each single‐unit increase) were significant predictors for hearing loss, while gender was not.
Overall completeness and applicability of evidence
The wide variation in the prevalence of hearing loss in the included studies could be a reflection of the large heterogeneity of included studies with regard to, for example, participant characteristics, (prior) anti‐tumour treatment including different platinum analogues and dosing schedules, other ototoxic drugs, definition of hearing loss and length of follow‐up. However, we were unable to identify specific explanations for the variation. And since only two studies evaluated possible risk factors using multivariable analysis, there is only a limited amount of evidence regarding which people are at highest risk for developing hearing loss after treatment with a platinum analogue. As both studies had methodological problems related to these analyses (as explained elsewhere in the Discussion section), the exact risk factors are currently unclear.
The two studies that included control participants who had not received platinum treatment were not RCTs/CCTs so the calculation of an RR was not feasible as it is very likely that both groups differed not only with regard to platinum treatment, but also with regard to other prognostic factors such as cranial irradiation. Due to a lack of reporting, this remains unclear. Furthermore, in one of these studies, hearing loss was detected by screening in survivors treated with platinum analogues and by clinical presentation in control participants. It is uncertain what the effect of this difference in follow‐up/diagnostic testing is.
It should be noted that not for all outcomes of interest data were available. As none of the studies provided data on tinnitus, we could not draw conclusions regarding this outcome, but it is of course important for clinical practice.
The external validity of a study indicates how well its results can be extrapolated to individual participants treated with platinum analogues. It includes the following issues: well‐defined study group, well‐defined follow‐up, well‐defined outcome and, if risk assessment was performed, a well‐defined analysis. It varied in the included studies, on many occasions due to a lack of reporting. Overall, none of the 13 included studies scored 'good' on all applicable items: 15.4% scored 'bad' on all applicable items, while 84.6% had a combination of 'good' and 'bad' scores. If important information is missing regarding the exact treatment that participants received, the follow‐up duration, the outcome and the analyses, it is difficult to interpret the results correctly and extrapolate them to individual participants. In all studies, important information with regard to prior and current treatment was missing. Follow‐up was only reported in 62% of the included studies and varied widely. As hearing loss not only develops during platinum‐based therapy but also years after completion of the therapy (Bertolini 2004; Knight 2005), the length of follow‐up in some studies could have been too short for participants to develop hearing loss. In 39% of the studies, the outcome was not well‐defined, so either the method of detection, the definition of an abnormal outcome used in the study or both were not provided. But even if this information is provided there are still uncertainties with regard to the appropriateness of the used diagnostic tests, for example, if age‐specific tests were used or if participants were checked for otitis media, common in this age group (Bertolini 2004; Brock 1991). Monitoring hearing for children receiving potentially ototoxic therapy presents special issues and challenges for audiologists that are unique for this population (Bass 2014a). Development of standardized monitoring protocols is necessary and also, there is a need for a standardized, widely accepted ototoxicity grading scale; the current scales each have strengths and weaknesses (Bass 2014a) and prevalences of, for example, severe hearing loss differ by scale (Landier 2014). In both studies that conducted multivariable analyses of potential risk factors these analyses were well‐defined. However, in one of the studies it was likely that participants not eligible for this review were also included in the analysis, so it is unclear how useful the results are for our study population.
Other items that are important for the extrapolation of study results to individual participants, although not included in our external validity assessment, are, for example, age at diagnosis, renal function at time of platinum treatment, prior hearing dysfunction, and the use of other ototoxic drugs such as aminoglycosides and furosemide. Many studies (62%) did not mention the age at tumour diagnosis, none of the studies stated if there was impaired renal function at the time of platinum treatment, prior hearing dysfunction was not (completely) reported in 70% of the studies and other ototoxic drugs were often not mentioned. In addition, the time periods of treatment/enrolment varied between 1987 and 2012 (not reported in two studies). Supportive care, such as antibiotic use, and anti‐cancer treatments have changed substantially within this 25‐year period, so consequently, the results may not all be applicable to people who are treated today.
Children treated with platinum analogues are at risk for developing hearing loss, but the exact prevalence and risk factors remain unclear. However, it is important to realize that the real problem might be even larger: noisy environments make hearing even worse than expected from hearing tests, which often are performed in relatively noise‐free environments. Furthermore, at 40‐years of age natural hearing loss begins (NHS Information). Even though only one study mentioned the age at outcome assessment/follow‐up, in which it ranged between 1 and 24 years (Landier 2014), it is unlikely that many participants included in this systematic review were already 40 years or older, meaning that with longer follow‐up the effect of normal ageing likely will further increase the problem.
Quality of the evidence
The quality of the included studies varied, on many occasions due to a lack of reporting. The internal validity gives an indication of the bias present in a study and thus how valid the results of a certain study are. It includes the following issues: selection bias, attrition bias, detection bias and, if a risk assessment is performed, confounding. In 61.5% of the studies included in this systematic review, selection bias could not be ruled out. This may lead to an overestimation of the prevalence of hearing loss if people with a higher risk of hearing loss were included in the study or to an underestimation when people with a lower risk were selected. The risk of attrition bias was low in almost all studies; the risk was unclear in only one study (7.7%). So an over‐ or underestimation of the risk of hearing loss due to this type of bias is small. In all studies, the risk of detection bias could not be ruled out. This can lead to an overestimation of the prevalence of hearing loss, since knowledge of prognostic factors can increase the possibility of classifying a person as having hearing loss. Finally, two studies performed a multivariable risk assessment and in one of those studies (50%) the risk of confounding could not be ruled out, which could lead to an over‐ or underestimation of the real effect of the risk factors.
Potential biases in the review process
This systematic review used a very broad search strategy for identifying eligible studies. However, although it is unlikely that eligible studies were missed, it is never possible to rule out reporting bias.
Authors' conclusions
Implications for practice.
This systematic review shows that children treated with platinum analogues are at risk for developing hearing loss, but the exact prevalence and risk factors remain unclear. No data were available for the other outcome of interest, tinnitus. Based on the currently available evidence we can only advise that children treated with platinum analogues are screened for ototoxicity in order to make it possible to diagnose hearing loss early and to take appropriate measures. However, we are unable to give recommendations for specific follow‐up protocols including frequency of testing. Counselling regarding the prevention of noise pollution can be considered, such as the use of noise‐limiting equipment, avoiding careers with excess noise and ototoxic medication.
Implications for research.
Before definitive conclusions on the prevalence and associated risk factors of platinum‐induced ototoxicity can be made, more high‐quality research is needed. Future trials should preferably be prospective cohort studies with a long and complete follow‐up that longitudinally assess the risk of ototoxicity. They should include a control population, for example, siblings. Not only hearing loss, but also tinnitus should be evaluated. Appropriate age‐specific hearing tests should be used to assess ototoxicity and it should be described how exactly these tests are performed. In addition, valid outcome definitions for ototoxicity should be used. To assess risk factors adequately multivariable analyses should be performed. The number of included children should be sufficient to obtain the power needed for the results to be reliable. Accurate and transparent reporting of findings will make it possible for readers to appraise the results of these studies critically.
What's new
| Date | Event | Description |
|---|---|---|
| 16 April 2019 | Amended | Contact details updated. |
Acknowledgements
Guillermo Chantada, Birgitta Lannering, Claudia Lanvers‐Kominsky, Carol Shields, Henk Visscher, Shahla Ansari, Kaukab Rajput, Melanie Hagleitner, Brian Kushner, Amulya Nageswara Rao and Michele Lambert provided additional information regarding their studies, which is greatly appreciated. We also thank Dr P Bertolini and an undisclosed person who kindly agreed to peer review our manuscript. We would like to acknowledge the Editorial Base of Cochrane Childhood Cancer for their advice and support. The Editorial Base of Cochrane Childhood Cancer is funded by ‘Stichting Kinderen Kankervrij’ (KiKa), the Netherlands.
Appendices
Appendix 1. Search strategy for the Cochrane Central Register of Controlled Trials (CENTRAL)
1. For Hearing loss the following text words were used:
Deafness OR hearing loss OR Loss, Hearing OR hearing disorders OR auditory OR hearing impairment OR hearing impairments OR hearing impairment* OR audiologic OR audiometry OR audiometr* OR audiogram OR ototoxicology OR ototoxic* OR hypoacusis OR hypoacuses OR hypoacus* OR ototoxicity OR deaf* OR cochleotoxicity
2. For Cisplatin the following text words were used:
Cisplatin OR cis‐Diamminedichloroplatinum(II) OR Platinum Diamminodichloride OR Diamminodichloride, Platinum OR cis‐Platinum OR cis Platinum OR Dichlorodiammineplatinum OR cis‐Diamminedichloroplatinum OR cis Diamminedichloroplatinum OR cis‐Dichlorodiammineplatinum(II) OR Platinol OR Platidiam OR Platino OR NSC‐119875 OR Biocisplatinum OR CDDP OR CACP OR cisplatin* OR abiplatin OR neoplatin OR cis‐DDP
3. For Carboplatin the following text words were used:
Carboplatin OR cis‐Diammine(cyclobutanedicarboxylato)platinum II OR CBDCA OR Carbosin OR Pharmachemie Brand of Carboplatin OR Carbotec OR Columbia Brand of Carboplatin OR Ercar OR Almirall Brand of Carboplatin OR JM‐8 OR JM 8 OR JM8 OR Neocarbo OR Neocorp Brand of Carboplatin OR NSC‐241240 OR NSC 241240 OR NSC241240 OR Paraplatin OR Carboplat OR Paraplatine OR Bristol‐Myers Squibb Brand of Carboplatin OR Platinwas OR Chiesi Brand of Carboplatin OR Ribocarbo OR ribosepharm Brand of Carboplatin OR Blastocarb OR Lemery Brand of Carboplatin OR Nealorin OR Prasfarma Brand of Carboplatin OR carboplatin* OR Platinum OR Platinum Compounds OR platinum*
4. For Oxaliplatin and other platinum compounds the following text words were used:
Oxaliplatin OR oxaliplatin* OR oxaliplatine OR platinum(II)‐1,2‐cyclohexanediamine oxalate OR 1,2‐diaminocyclohexane platinum oxalate OR oxalato‐(1,2‐cyclohexanediamine)platinum II OR cis‐oxalato‐(trans‐l)‐1,2‐diaminocyclohexane‐platinum(II) OR Eloxatine OR Eloxatin OR oxaliplatin, (SP‐4‐2‐(1S‐trans))‐isomer OR oxaliplatin, (SP‐4‐3‐(cis))‐isomer OR ACT 078 OR ACT‐078 OR oxaliplatin, (SP‐4‐2‐(1R‐trans))‐isomer OR 63121‐00‐6 OR 61825‐94‐3 OR dacotin OR dacplat OR jm‐83 OR l‐ohp OR oxalatoplatinum OR rp 54780 OR sr‐96669 OR Platinum OR Platinum Compounds OR platinum* OR organoplatinum compounds
5. For Childhood cancer the following text words were used:
(leukemia OR leukemi* OR leukaemi* OR (childhood ALL) OR AML OR lymphoma OR lymphom* OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR neuroectodermal tumors, primitive OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR cancer or neoplasms or tumor or cancers or neoplasm or tumors)
Final search 1 AND (2 OR 3 OR 4) AND 5
The search was performed in title, abstract or keywords
[* = zero or more characters]
Appendix 2. Search strategy for MEDLINE (PubMed)
1. ForHearing loss the following MeSH headings and text words were used:
Deafness OR hearing loss OR Loss, Hearing OR hearing disorder OR hearing disorders OR auditory OR hearing impairment OR hearing impairments OR hearing impairment* OR audiology OR audiologic OR audiometry OR audiometr* OR audiogram OR audiography OR ototoxicology OR ototoxic* OR hypoacusis OR hypoacuses OR hypoacus* OR ototoxicity OR deaf* OR cochleotoxicity
2. For Cisplatin the following MeSH headings and text words were used:
Cisplatin OR cis‐Diamminedichloroplatinum(II) OR Platinum Diamminodichloride OR Diamminodichloride, Platinum OR cis‐Platinum OR cis Platinum OR Dichlorodiammineplatinum OR cis‐Diamminedichloroplatinum OR cis Diamminedichloroplatinum OR cis‐Dichlorodiammineplatinum(II) OR Platinol OR Platidiam OR Platino OR NSC‐119875 OR Biocisplatinum OR CDDP OR CACP OR cisplatin* OR abiplatin OR (neoplatin) OR cis‐DDP
3. ForCarboplatin the following MeSH headings and text words were used:
Carboplatin OR cis‐Diammine(cyclobutanedicarboxylato)platinum II OR CBDCA OR Carbosin OR Pharmachemie Brand of Carboplatin OR Carbotec OR Columbia Brand of Carboplatin OR Ercar OR Almirall Brand of Carboplatin OR JM‐8 OR JM 8 OR JM8 OR Neocarbo OR Neocorp Brand of Carboplatin OR NSC‐241240 OR NSC 241240 OR NSC241240 OR Paraplatin OR Carboplat OR Paraplatine OR Bristol‐Myers Squibb Brand of Carboplatin OR Platinwas OR Chiesi Brand of Carboplatin OR Ribocarbo OR ribosepharm Brand of Carboplatin OR Blastocarb OR Lemery Brand of Carboplatin OR Nealorin OR Prasfarma Brand of Carboplatin OR carboplatin*
4. For Oxaliplatin and other platinum compounds the following MeSH headings and text words were used:
Oxaliplatin OR oxaliplatin* OR 1,2‐diamminocyclohexane(trans‐1)oxolatoplatinum(II) OR oxaliplatine OR platinum(II)‐1,2‐cyclohexanediamine oxalate OR 1,2‐diaminocyclohexane platinum oxalate OR oxalato‐(1,2‐cyclohexanediamine)platinum II OR cis‐oxalato‐(trans‐l)‐1,2‐diaminocyclohexane‐platinum(II) OR Eloxatine OR Eloxatin OR oxaliplatin, (SP‐4‐2‐(1S‐trans))‐isomer OR oxaliplatin, (SP‐4‐3‐(cis))‐isomer OR ACT 078 OR ACT‐078 OR oxaliplatin, (SP‐4‐2‐(1R‐trans))‐isomer OR 63121‐00‐6 OR 61825‐94‐3 OR dacotin OR dacplat OR jm‐83 OR l‐ohp OR oxalatoplatinum OR rp 54780 OR sr‐96669 OR Platinum OR Platinum Compounds OR platinum* OR organoplatinum compounds [mh]
5. For Childhood cancer the following MeSH headings and text words were used:
leukemia OR leukemi* OR leukaemi* OR childhood ALL OR AML OR lymphoma OR lymphom* OR hodgkin OR hodgkin* OR T‐cell OR B‐cell OR non‐hodgkin OR sarcoma OR sarcom* OR Ewing* OR osteosarcoma OR osteosarcom* OR wilms tumor OR wilms* OR nephroblastom* OR neuroblastoma OR neuroblastom* OR rhabdomyosarcoma OR rhabdomyosarcom* OR teratoma OR teratom* OR hepatoma OR hepatom* OR hepatoblastoma OR hepatoblastom* OR PNET OR medulloblastoma OR medulloblastom* OR PNET* OR primitive neuroectodermal tumors OR retinoblastoma OR retinoblastom* OR meningioma OR meningiom* OR glioma OR gliom* OR pediatric oncology OR paediatric oncology OR childhood cancer OR childhood tumor OR childhood tumors OR brain tumor* OR brain tumour* OR brain neoplasms OR central nervous system neoplasm OR central nervous system neoplasms OR central nervous system tumor* OR central nervous system tumour* OR brain cancer* OR brain neoplasm* OR intracranial neoplasm* OR acute lymphocytic leukemia
Final search 1 AND (2 OR 3 OR 4) AND 5
[tw = text word; mh = MeSH term; * = zero or more characters]
Appendix 3. Search strategy for EMBASE (Ovid)
1. For Hearing loss the following Emtree terms and text words were used:
1. exp hearing impairment/ 2. (deafness or deaf$ or hearing impairment or hearing impairments or hearing impairment$).mp. 3. hearing loss.mp. or exp hearing loss/ 4. exp hearing disorder/ 5. (hearing disorder or hearing disorders).mp. 6. auditory.mp. 7. exp audiology/ or audiologic$.mp. 8. exp audiometry/ 9. (audiometry or audiometr$ or audiogram).mp. 10. exp audiography/ 11. (ototoxicology or ototoxic$ or ototoxicity).mp. 12. exp OTOTOXICITY/ 13. exp HYPOACUSIS/ 14. (hypoacusis or hypoacuses or hypoacus$).mp. 15. cochleotoxicity.mp. 16. or/1‐15
2. For Cisplatin the following Emtree terms and text words were used:
1. exp CISPLATIN DERIVATIVE/ or exp CISPLATIN/ or cisplatin.mp. 2. cis‐Diamminedichloroplatinum.mp. 3. Platinum Diamminodichloride.mp. 4. (cis‐Platinum or cis Platinum or Dichlorodiammineplatinum or cis‐Diamminedichloroplatinum or cis Diamminedichloroplatinum or cis‐Dichlorodiammineplatinum).mp. 5. (Platinol or Platidiam or Platino or NSC‐119875 or Biocisplatinum or CDDP or CACP).mp. 6. (cisplatin$ or abiplatin or neoplatin or cis‐DDP).mp. 7. or/1‐6
3. For Carboplatin the following Emtree terms and text words were used:
1. carboplatin.mp. or exp CARBOPLATIN/ 2. (CBDCA or Carbosin or Carbotec or Ercar).mp. 3. (JM‐8 or JM 8 or JM8).mp. 4. (NSC‐241240 or NSC 241240 or NSC241240).mp. 5. (Neocarbo ot Paraplatin or Carboplat or Paraplatine).mp. 6. (Platinwas or Ribocarbo or Blastocarb or nealorin).mp. 7. (carboplatin$ or Platinum or Platinum Compounds or platinum$).mp. 8. or/1‐7
4. For Oxaliplatin and other platinum compounds the following Emtree terms and text words were used:
1. Oxaliplatin.mp. or exp OXALIPLATIN/ 2. (oxaliplatin$ or oxaliplatine).mp. 3. 1,2‐diaminocyclohexane platinum oxalate.mp. or exp platinum 1,2 diaminocyclohexane/ 4. (Eloxatine or Eloxatin).mp. 5. ("ACT 078" or ACT‐078).mp. 6. (dacotin or dacplat or jm‐83 or l‐ohp or oxalatoplatinum or rp 54780 or sr‐96669).mp. 7. (oxalato 1,2 cyclohexanediamine platinum or platinum 1,2 cyclohexanediamine oxalate or platinum 1,2 diaminocyclohexane oxalate or platinum oxalate 1,2 diaminocyclohexane).mp. 8. transplastin.mp. 9. Organoplatinum Compounds.mp. or exp platinum complex/ 10. 61825‐94‐3.rn. 11. or/1‐10
5. For Childhood cancer the following Emtree terms and text words were used:
1. (leukemia or leukemi$ or leukaemi$ or (childhood adj ALL) or acute lymphocytic leukemia).mp. 2. (AML or lymphoma or lymphom$ or hodgkin or hodgkin$ or T‐cell or B‐cell or non‐hodgkin).mp. 3. (sarcoma or sarcom$ or Ewing$ or osteosarcoma or osteosarcom$ or wilms tumor or wilms$).mp. 4. (nephroblastom$ or neuroblastoma or neuroblastom$ or rhabdomyosarcoma or rhabdomyosarcom$ or teratoma or teratom$ or hepatoma or hepatom$ or hepatoblastoma or hepatoblastom$).mp. 5. (PNET or medulloblastoma or medulloblastom$ or PNET$ or neuroectodermal tumors or primitive neuroectodermal tumor$ or retinoblastoma or retinoblastom$ or meningioma or meningiom$ or glioma or gliom$).mp. 6. (pediatric oncology or paediatric oncology).mp. 7. ((childhood adj cancer) or (childhood adj tumor) or (childhood adj tumors) or childhood malignancy or (childhood adj malignancies) or childhood neoplasm$).mp. 8. ((pediatric adj malignancy) or (pediatric adj malignancies) or (paediatric adj malignancy) or (paediatric adj malignancies)).mp. 9. ((brain adj tumor$) or (brain adj tumour$) or (brain adj neoplasms) or (brain adj cancer$) or brain neoplasm$).mp. 10. (central nervous system tumor$ or central nervous system neoplasm or central nervous system neoplasms or central nervous system tumour$).mp. 11. intracranial neoplasm$.mp. 12. LEUKEMIA/ or LYMPHOMA/ or brain tumor/ or central nervous system tumor/ or teratoma/ or sarcoma/ or osteosarcoma/ 13. nephroblastoma/ or neuroblastoma/ or rhabdomyosarcoma/ or hepatoblastoma/ or medulloblastoma/ or neuroectodermal tumor/ or retinoblastoma/ or meningioma/ or glioma/ or childhood cancer/ 14. or/1‐13
Final search 1 AND (2 OR 3 OR 4) AND 5
[mp = title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name; / = Emtree term; $ = one or more characters; rn = registry number]
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bertolini 2004.
| Methods | Design: retrospective cohort study with an update of the audiometric assessment for long‐term survivors Time period: treatment between 1987 and 1997 Setting: single centre study in France Control group without platinum treatment: no |
|
| Participants | Original cohort: nm; study group of interest: 120; participants with a hearing test: 120 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: median 2.6 years, range 0‐17 years Age at outcome assessment/follow‐up: nm Gender: 59 female (49%); 61 male (51%) Type of malignancy; primary disease or recurrence: n = 90 neuroblastoma, n = 11 hepatoblastoma, n = 10 germcell tumour, n = 9 osteosarcoma; nm Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no cranial radiotherapy; for other items nm Prior hearing dysfunction: no for 34 tested participants (28%; using Brock's grading system, grades nm); unclear for the other 86 participants (72%) Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: different SFOP protocols; no further information provided All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: n = 52 cisplatin, n = 24 carboplatin, n = 44 cisplatin plus carboplatin Cumulative platinum dose: cisplatin median 400 mg/m2, range 80‐800 mg/m2; carboplatin median 1600 mg/m2, range 400‐8000 mg/m2 Individual platinum dose: nm Platinum infusion duration: different infusion durations, at least 1‐3 hours and continuous over 5 days; no further information provided Other chemotherapy: yes, but no further information provided Radiotherapy: no cranial radiotherapy; no further information provided Surgery: nm Other treatment: nm Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, anthracyclines nm, furosemide nm, vincristine: nm Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss according to Brock criteria (Brock 1991; grade 2 or higher); method of detection: different audiometric and behavioural techniques depending on age. Participants with hearing loss: 39/120 (32.5%) Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: hearing evaluation median 7 years, maximal 13 years after the last platinum course (82 participants ≥ 2 years after the end of platinum treatment) Partial overlap with other included studies: no Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: nm |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Number of participants in the original cohort unclear; to be included in this study, participants needed to have a post‐treatment hearing evaluation |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for all participants in the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors nm |
| Well‐defined study group (reporting bias) | High risk | Other (prior) treatment nm |
| Well‐defined follow‐up (reporting bias) | Low risk | Follow‐up duration mentioned |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
Cushing 2004.
| Methods | Design: prospective cohort study (see notes) Time period: enrolment between March 1990 and February 1996 Setting: multicentre study in USA Control group without platinum treatment: no |
|
| Participants | Original cohort: 299; study group of interest: 299; participants with a hearing test: 295 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: nm (for the 299 eligible participants: range 0‐20.1 years) Age at outcome assessment/follow‐up: nm Gender: nm (for the 299 eligible participants: 183 female (61%); 116 male (39%)) Type of malignancy; primary disease or recurrence: extracranial high‐risk malignant germcell tumours; (immature) teratoma without malignant elements were not included (for 299 eligible participants: n = 60 testicular, n = 74 ovarian, n = 165 extragonadal); both eligible, no further information provided Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no prior therapy other than surgical resection was allowed, no further information provided Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: POG‐9049 and Children's Cancer Group 8882 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: cisplatin Cumulative platinum dose: nm (according to protocol 800‐1200 mg/m2 in the high‐dose group and 400‐600 mg/m2 in the standard‐dose group) Individual platinum dose: 40 mg/m2 in high‐dose group and 20 mg/m2 in standard‐dose group Platinum infusion duration: nm Other chemotherapy: bleomycin, cumulative dose nm (according to protocol 60‐90 units/m2); etoposide, cumulative dose nm (according to protocol 2000‐3000 mg/m2). Vigorous pre‐ and postchemotherapy hydration with mannitol and continuous oral magnesium supplementation were recommended Chemotherapy dose adjustments were made for children < 12 months of age Radiotherapy: no Surgery: if possible all gonadal tumours completely resected; for extragonadal tumours surgery depended on primary tumour site; no further information provided Other treatment: no Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, anthracyclines no; furosemide nm, vincristine no Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Subjective and objective hearing loss according to NCI criteria (version nm: see notes; grade 3 and 4); method of detection: audiogram. Participants with subjective hearing loss: 5/295 (1.7%) Participants with objective hearing loss: 21/295 (7.1%) Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: nm This study was an RCT comparing high‐dose (n = 149) and standard‐dose (n = 150) cisplatin; however, as participants in both treatment groups received cisplatin, for this systematic review, we considered it a prospective cohort study This manuscript did not state which version of the NCI criteria was used and they did not provide a reference, so it could be either version 1 (Common Toxicity Criteria Version 1) or version 2 (Common Toxicity Criteria Version 2). However, both versions do not include a statement on subjective or objective hearing loss Partial overlap with other included studies: possible with Hudson 2013; this study included people treated at St. Jude Children's Research Hospital, unclear if these people were included in the survivor cohort of Hudson 2013 Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | Complete original cohort included in the study |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for 98.7% of the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
Hudson 2013.
| Methods | Design: cohort study (SJLIFE) of at least 10‐year survivors Time period: nm (all study participants including participants not eligible for this review were diagnosed and treated between 1962 and 2001) Setting: single‐centre study in USA Control group: yes (n = 1561 no platinum treatment) |
|
| Participants | Original cohort: nm; study group of interest: 152; participants with a hearing test: 152 All information provided below is for participants with a hearing test in the platinum group and the control group unless otherwise stated Age at diagnosis: nm (for all participants: mean 7.5 years, range 0‐24 years) Age at outcome assessment/follow‐up: nm (for all participants: age at recruitment mean: 33.1 years, median 32 years, range 18‐60 years) Gender: nm (for all participants: 880 female (51%); 833 male (49%)) Type of malignancy; primary disease or recurrence: different solid and haematological tumours; nm Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: nm Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: different protocols (no names provided) All information provided below is for participants with a hearing test in the platinum group and the control group unless otherwise stated Type of platinum analogue: cisplatin or carboplatin (or both); not applicable Cumulative platinum dose as cisplatin equivalent dose, i.e. cisplatin*1 and carboplatin/4: mean 556.8 mg/m2, median 403 mg/m2, range 64‐2764.6 mg/m2; not applicable Individual platinum dose: nm; not applicable Platinum infusion duration: nm; not applicable Other chemotherapy: nm Radiotherapy: some of the participants received radiotherapy to the ear, no further information provided; nm Surgery: nm Other treatment: nm Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, anthracyclines: nm; furosemide nm, vincristine: nm Otoprotective medical interventions: nm Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss according to Chang (Chang 2010; grade 1a or higher, i.e. ≥ 40 dB at any frequency 6‐12 kHz); method of detection: otoscopy, tympanometry, conventional pure tone audiometry Hearing loss was detected by screening of survivors with specific cancer treatment‐related risk factors or those (mostly) diagnosed by clinical presentation in survivors without cancer treatment‐related risks Participants with hearing loss: 102/152 in platinum‐treated participants (67.1%); 116/1561 control patients (7.4%; 95% CI 6.2‐8.8%). Multivariable risk factor analysis: no. |
|
| Notes | Follow‐up duration: nm (for all patients mean 25.6 years after diagnosis, median 25.1 years, range 10.9 to 47.9 years). Partial overlap with other included studies: unclear, but possible (Cushing 2004 and Mandell 1999 included people treated at St. Jude Children's Research Hospital, unclear if these participants were included in this survivor cohort) Inappropriate influence of funders: no role of funders Declaration of interest primary investigators: 3 authors reported being a consultant or board member of a pharmaceutical company |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Number of eligible platinum‐treated participants unclear |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for all participants in the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessor not reported |
| Well‐defined study group (reporting bias) | High risk | Only information available for platinum treatment, not for other treatment |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
Jehanne 2009.
| Methods | Design: retrospective review of audiometric follow‐up Time period: treatment between December 1994 and December 2002 Setting: single centre study in France Control group without platinum treatment: no |
|
| Participants | Original cohort: nm; study group of interest: 192; participants with a hearing test: 175 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: median 8 months, range 0‐60 months Age at outcome assessment/follow‐up: nm Gender: 93 female (53%); 82 male (47%) Type of malignancy; primary disease or recurrence: retinoblastoma; primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: nm All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: carboplatin Cumulative platinum dose: median 2880 mg/m2, range 560‐6160 mg/m2 Individual platinum dose: 200 mg/m2 (for local chemothermotherapy of 560 mg/m2 total nm) Platinum infusion duration: nm Other chemotherapy: etoposide, cumulative dose nm (according to protocol usually 900 mg/m2); postenucleation chemotherapy adapted to histological risks: etoposide, cumulative dose nm (according to protocol 500 mg/m2), vincristine, cumulative dose nm (according to protocol 7.5 mg/m2), cyclophosphamide, cumulative dose nm (according to protocol 1500 mg/m2), or a combination of these Dose adjustments were made for children under the age of 1 year or weighing < 10 kg, or both (at least for chemotherapy, possibly also for other treatments) Radiotherapy: n = 45 external beam radiotherapy (no further information provided); some participants iodine125 brachytherapy (no further information provided) Surgery: n = 96 enucleation Other treatment: some participants cryotherapy or laser thermotherapy (no further information provided) Other ototoxic drugs (aminoglycosides, furosemide, vincristine): glycopeptides: 27/160 participants and aminoglycosides 56/161 participants, anthracyclines no; furosemide no, vincristine: see 'Other chemotherapy' above Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm (1/175 participants had renal failure after first course of etoposide/carboplatin, but no further information provided on recovery etc.) |
|
| Outcomes | Hearing loss according to Brock criteria (Brock 1991; grade 1 and higher; see notes); method of detection: different audiometric and behavioural techniques depending on age and cooperation Participants with hearing loss: 6/175 (3.4%) of whom 3/175 (1.7%) grade 1, 1/175 (0.6%) grade 2 and 2/175 (1.1%) grade 4; none of the participants developed grade 3 hearing loss Multivariable risk factor analysis: no |
|
| Notes | 7/175 participants had a history of prematuritya 2/175 participants (1.1%) had grade 0 hearing loss (i.e. bilateral hearing loss, but not at least 40 dB bilaterally, so not corresponding to grade 1). Although the authors counted these as hearing loss, we omitted these participants from our analyses Follow‐up duration: median 5 years, range 1.8‐11 years between last carboplatin dose and hearing assessment Partial overlap with other included studies: no Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: unclear (no information provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Number of participants in the original cohort unclear; 192 participants fulfilled inclusion criteria |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for 91.1% of the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | Only cumulative carboplatin dose available, other relative items nm |
| Well‐defined follow‐up (reporting bias) | Low risk | Follow‐up duration mentioned |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
Kennedy 2014.
| Methods | Design: prospective cohort study with cross‐sectional follow‐up in childhood cancer survivors (see notes) Time period: allocation between 2001 and 2006 Setting: multicentre study in different European countries Control group without platinum treatment: no |
|
| Participants | Original cohort: 244; study group of interest: 151; participants with a hearing test: 144 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: nm Age at outcome assessment/follow‐up: nm Gender: nm Type of malignancy; primary disease or recurrence: medulloblastoma; nm, but most likely primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: nm Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: HIT‐SIOP PNET 4 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: cisplatin Cumulative platinum dose: nm (according to protocol 560 mg/m2) Individual platinum dose: 70 mg/m2 Platinum infusion duration: nm Other chemotherapy: vincristine, cumulative dose nm (according to protocol 48 mg/m2); lomustine, cumulative dose nm, according to protocol 600 mg/m2) Radiotherapy: yes, according to protocol 23.4 Gy craniospinal axis/54 Gy posterior fossa in the conventional group (n = 74) and 36 Gy craniospinal axis/60 Gy posterior fossa and 68 Gy tumour bed in the hyperfractionated group (n = 70) Surgery: nm Other treatment: no Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm; anthracyclines no; furosemide nm; vincristine: see 'Other chemotherapy' above Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss defined as use of hearing aids; method of detection: age appropriate questionnaires/HUI3 hearing attribute Participants with hearing loss: 23/144 (16%) Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: nm (for 151/244 participants, the median interval from diagnosis was 5.8 years, range 4.2‐9.9 years) This study was an RCT comparing conventional radiotherapy and hyperfractionated radiotherapy; however, as participants in both treatment groups received cisplatin, for this systematic review, we considered it a prospective cohort study Partial overlap with other included studies: no Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: no conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Described study group consisted of 62% of the original cohort; unclear if this was a random sample |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for 95% of the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | High risk | Outcome assessors not blinded (i.e. self reported outcome) |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | Low risk | All relevant items reported |
Lambert 2008.
| Methods | Design: cohort study Time period: treatment between 1993 and 2003 Setting: multicentre study in Philadelphia (2 hospitals) Control group without platinum treatment: no |
|
| Participants | Original cohort: nm; study group of interest: 116; participants with a hearing test: 116 (the first author confirmed that all participants finished their platinum treatment) All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: nm (at start therapy median 10 months, range <1‐87 months) Age at outcome assessment/follow‐up: nm Gender: nm Type of malignancy; primary disease or recurrence: retinoblastoma; nm Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: nm Prior hearing dysfunction: 14/116 participants (12%); no definition provided Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: n =110 CHP‐582; n = 6 nm (no treatment data available) All information provided below is for 110 participants treated on CHP‐582 with a hearing test unless otherwise stated Type of platinum analogue: carboplatin Cumulative platinum dose: nm (according to protocol 111.6 mg/kg); n = 4 also subconjunctival carboplatin; no further information provided Individual platinum dose: 18.6 mg/kg Platinum infusion duration: nm Other chemotherapy: n = 105 etoposide, cumulative dose nm (according to protocol 60 mg/kg); n = nm vincristine, cumulative dose nm (according to protocol 0.3 mg/kg) Radiotherapy: n = 30 external beam radiotherapy (dose nm); n = 10 plaque radiotherapy (dose nm) Surgery: nm Other treatment: nm Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin 10/116 participants at least 1 dose (no further information provided; none of these participants developed hearing loss), anthracyclines no; furosemide nm, vincristine: see 'Other chemotherapy' above Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss, no definition provided; method of detection: brainstem auditory‐evoked response, otoacoustic emissions, pure tone audiometry, and soundfield testing were seen as appropriate tests; sometimes only clinical evaluation by parents or clinician Participants with hearing loss: 0/116 (0%; 95% CI 0% to 3.2%) after platinum treatment (3 participants already had hearing loss prior to carboplatin treatment; all these hearing tests were done after treatment, as confirmed by the authors) Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: median 40 months, range 3‐127 months Partial overlap with other included studies: very likely with Shields 2002 and Shields 2006 Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: unclear (no information provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Number of participants in the original cohort unclear |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for the complete study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | Low risk | Duration of follow‐up reported |
| Well‐defined outcome (reporting bias) | High risk | Definition nm |
Landier 2014.
| Methods | Design: cross‐sectional cohort study Time period: enrolled between February 2001 and February 2006 Setting: multicentre study in North America Control group: no |
|
| Participants | Original cohort: 489; study group of interest: 333; participants with a hearing test: for 267 participants, it was certain that the hearing test was done after finishing platinum treatment (but not all of them could be included for all different grading systems; see information at 'Outcomes') All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: mean 3.92 years, median 3.31 years, range 0.3‐22.78 years Age at outcome assessment/follow‐up: mean 5.73 years, median 5.16 years, range 1.37‐24.05 years Gender: 110 female (41%); 157 male (59%) Type of malignancy; primary disease or recurrence: neuroblastoma; primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: COG A3973 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: cisplatin/carboplatin Cumulative platinum dose: nm (according to protocol cisplatin 400 mg/m2 and carboplatin 1700 mg/m2) Individual platinum dose: cisplatin 50 mg/m2 and carboplatin 425 mg/m2 Platinum infusion duration: cisplatin 1 hour and carboplatin not clearly mentioned, but possibly 24 hours Other chemotherapy:
Radiotherapy: 261 Gy/ 12 fractions to primary tumour and persistently active metastatic sites after myeloablative phase (no further information) Surgery: yes (no further information) Other treatment: stemcell transplant; 13‐cisretinoic acid with or without chimeric anti‐GD2 Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm (in the induction phase hospitalization was used as a surrogate marker for gentamycin use: for 263 participants data available: 216/263 hospitalized (82.1%); no surrogate marker available in other treatment phases), doxorubicin: see 'Other chemotherapy' above; furosemide nm, vincristine: see 'Other chemotherapy' above Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss according to different criteria, i.e. Brock (Brock 1991), Chang (Chang 2010), CTCAEv3 (CTCAEv3; grade 1 or higher) and use of hearing aids; method of detection: behavioural audiometry or auditory brainstem response testing based on participant's age, developmental and clinical status and ability to cooperate; for hearing aids nm Participants with hearing loss using Brock criteria: 215/247 (87%) of whom 52/247 (21%) grade 1, 89/247 (36%) grade 2 and 74/247 (30%) grade 3 or 4; 163/247 participants (66%) had grade 2 and higher hearing loss Participants with hearing loss using Chang criteria: 219/243 (90.1%) of whom 51/243 (21%) grade 1a or 1b, 24/243 (10%) grade 2a and 144/243 (59%) grade 2b, 3 or 4 Participants with hearing loss using CTCAEv3 criteria: 208/242 (86%) of whom 2/242 (1%) grade 1, 34/242 (14%) grade 2 and 172/242 (71%) grade 3 or 4 Participants with hearing aids: 155/259 (59.8%) Multivariable risk factor analysis: yes; see Table 3 for more information |
|
| Notes | This study also reported hearing loss according to the ASHA criteria; these results are not reported as < 50% of participants underwent this test Follow‐up duration: mean 480.1 days, median 273 days, range 47‐2517 days Partial overlap with other included studies: very unlikely (see Peleva 2014 for more information) Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: no potential conflict of interest relevant to this article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | High risk | Described study group consisted of 68.1% of the original cohort |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for 80.2% of the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Adjustment for important prognostic factors (confounding) | Low risk | Although not all our prespecified prognostic factors were taken into account, most of them were and, therefore, we judged the risk of bias as low |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | Low risk | Duration of follow‐up reported |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
| Well‐defined analysis (reporting bias) | Low risk | OR calculated |
Mandell 1999.
| Methods | Design: prospective cohort study (see notes) Time period of treatment (initiated within 28 days of diagnosis): June 1992 and March 1996 Setting: multicentre study in USA Control group without platinum treatment: no |
|
| Participants | Original cohort: 130; study group of interest: 130; participants with a hearing test: 113 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: nm (for the 130 eligible participants: age at treatment 37‐266 months) Age at outcome assessment/follow‐up: nm Gender: nm (for the 130 eligible participants: 73 female (56%); 57 male (44%)) Type of malignancy; primary disease or recurrence: different types of tumours arising in the pons; primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no Prior hearing dysfunction: nm (results of baseline hearing test not reported) Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: POG‐9239 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: cisplatin Cumulative platinum dose: nm (according to protocol 300 mg/m2) Individual platinum dose: nm (according to protocol 100 mg/m2) Platinum infusion duration: 120 hours continuous infusion Other chemotherapy: no Radiotherapy: yes, local field radiotherapy; according to protocol 5400 cGy in the conventional group (n = 58) and 7020 cGy in the hyperfractionated group (n = 55) Surgery: nm Other treatment: all participants received steroids during radiotherapy; no further information provided Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, anthracyclines: no; furosemide nm, vincristine: no Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Subjective and objective hearing loss according to the POG toxicity criteria (Kadota 1994; grade 1 or higher; see notes); method of detection: audiograms were study of choice, otherwise brain auditory evoked response was used Participants with subjective hearing loss: 3/113 (2.7%) of whom 2/113 (1.8%) grade 2 and 1/113 (0.9%) grade 3; none of the participants developed grade 1, 4 or 5 hearing loss Participants with objective hearing loss: 17/113 (15%) of whom 11/113 (9.7%) grade 1, 5/113 (4.4%) grade 2, 1/113 (0.9%) grade 3; none of the participants developed grade 4 or 5 hearing loss Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: hearing tests were done 8 weeks post‐therapy and thereafter as clinically indicated; no further information provided This study was an RCT comparing conventional radiotherapy plus cisplatin and hyperfractionated radiotherapy plus cisplatin; however, as participants in both treatment groups received cisplatin, for this systematic review we considered it a prospective cohort study In this publication, it was stated that NCI toxicity criteria were used (version nm); however, in the accompanying reference, the POG toxicity criteria were explained and, therefore, we assume that the POG criteria were used. In addition, this study stated that they assessed subjective and objective hearing loss, however, in the criteria, this is not mentioned Partial overlap with other included studies: possible with Hudson 2013; this study included people treated at St. Jude Children's Research Hospital, unclear if these participants were included in the survivor cohort of Hudson 2013 Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: unclear (no information provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | All eligible participants included |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for 87% of the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | All relevant items not or only partially provided |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
Peleva 2014.
| Methods | Design: retrospective cohort study Time period: treatment between January 2000 and either July 2011 or January 2012 (depending on hospital) Setting: multicentre study in Quebec, Canada (2 hospitals) Control group: no |
|
| Participants | Original cohort: 466; study group of interest: unclear (nm how many participants finished platinum treatment); participants with a hearing test: 306 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: mean 7.8 years, range 2 months to 21.4 years Age at outcome assessment/follow‐up: nm Gender: 144 female (47%); 162 male (53%) Type of malignancy; primary disease or recurrence: different childhood cancers; nm Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: nm Prior hearing dysfunction: no (hearing loss at baseline was an exclusion criterion for this study) Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: nm All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: cisplatin and carboplatin (n = 147 cisplatin, n = 88 carboplatin, n = 71 cisplatin plus carboplatin) Cumulative platinum dose: cisplatin mean 380 mg/m2 (range 20‐720 mg/m2); carboplatin mean 2581 mg/m2 (range 450‐14,820 mg/m2) Individual platinum dose: cisplatin mean 64 mg/m2 (range 16‐120 mg/m2); carboplatin 444 mg/m2 (range 35‐840 mg/m2) Platinum infusion duration: nm Other chemotherapy: at least cyclophosphamide (no further information provided) Dose adjustments were made in 63 participants for the following reasons: ototoxicity (n = 25), nephrotoxicity (n = 10), infection/neutropenia (n = 4), carboplatin allergy (n = 1), low weight (n = 1), myelosuppression (n = 1) and unknown reasons (n = 21) Radiotherapy: n = 105 radiotherapy to head or neck (no further information provided) Surgery: nm Other treatment: nm Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, vancomycin or tobramycin n = 231 (no further information), anthracyclines nm; furosemide or mannitol (or both) n = 247 (no further information), vincristine n = 201 (no further information) Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm (but at least n = 10 dose reduction due to nephrotoxicity) |
|
| Outcomes | Hearing loss according to different criteria, i.e. ASHA criteria (ASHA) and Chang (Chang 2010); method of detection: determined by age, physical status, cooperation of participant. It included visual reinforcement audiometry, conditional play audiometry and conventional audiometry; unaided audiograms in people using hearing aids Participants with hearing loss using ASHA criteria: 148/306 (48.4%) Participants with hearing loss using Chang criteria: 91/306 (29.7%) grade ≥ 2a Multivariable risk factor analysis: yes; see Table 3 for more information |
|
| Notes | Follow‐up duration: nm Partial overlap with other included studies: 1 of the hospitals contributed to Landier 2014, but only 69 people with neuroblastoma were included in this study (from both hospitals), so we judged the possible overlap to be very low Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: nothing to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Number of eligible participants treated with platinum unclear |
| Complete outcome assessment/follow‐up (attrition bias) | Unclear risk | Number of participants in study group of interest unclear |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Adjustment for important prognostic factors (confounding) | Unclear risk | Since only a small part of our prespecified prognostic factors were taken into account, we judged the risk of bias as unclear |
| Well‐defined study group (reporting bias) | High risk | Other (prior) treatment not reported |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | Low risk | Method of detection and definition of hearing loss both provided |
| Well‐defined analysis (reporting bias) | Low risk | OR calculated |
Perilongo 2009.
| Methods | Design: prospective cohort study (see notes) Time period: open for registration between June 1998 and December 2006 Setting: multicentre study in 24 countries Control group without platinum treatment: no |
|
| Participants | Original cohort: 255; study group of interest: 255; participants with a hearing test: 168 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: nm (for the 255 eligible participants: median 13.5 months, range 0‐134 months) Age at outcome assessment/follow‐up: nm Gender: nm (for the 255 eligible participants: 100 female (39%); 155 male (61%)) Type of malignancy; primary disease or recurrence: hepatoblastoma; primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: SIOPEL 3 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: cisplatin Cumulative platinum dose: nm (according to protocol 480 mg/m2) Individual platinum dose: 80 mg/m2 Platinum infusion duration: 24 hours Other chemotherapy: number = nm (131/255 eligible participants received doxorubicin and 14/255 eligible participants received other chemotherapy; no further information on other chemotherapy available), doxorubicin, cumulative dose nm (according to protocol 300 mg/m2) Chemotherapy dose adjustments were made for children < 10 kg and for haematological and organ toxicity Radiotherapy: no Surgery: yes (of primary tumour) Other treatment: no Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, doxorubicin: see 'Other chemotherapy' above; furosemide nm, vincristine: see 'Other chemotherapy' above (possibly in other chemotherapy) Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss according to Brock criteria (Brock 1991; grade 1‐4); method of detection nm Participants with hearing loss: 53/168 (31.5%) of whom 20/168 (11.9%) grade 1, 21/168 (12.5%) grade 2, 7/168 (4.2%) grade 3 and 5/168 (3%) grade 4 Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: nm This study was an RCT comparing cisplatin and cisplatin plus doxorubicin; however, as participants in both treatment groups received cisplatin, for this systematic review, we considered it a prospective cohort study Partial overlap with other included studies: no Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: no potential conflict of interest relevant to this article |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | Described study group consisted of 97% of the original cohort |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for 65.9% of the study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | High risk | Method of detection nm |
Shields 2002.
| Methods | Design: prospective cohort study Time period: treatment between June 1994 and August 1999 Setting: multicentre study in Philadelphia (2 hospitals) Control group without platinum treatment: no |
|
| Participants | Original cohort: 103; study group of interest: 103; participants with a hearing test: 103 All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: mean 11 months, median 8 months, range 0.2‐72 months Age at outcome assessment/follow‐up: nm Gender: 44 female (43%); 59 male (57%) Type of malignancy; primary disease or recurrence: retinoblastoma; primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no Prior hearing dysfunction: no (inadequate auditory function was an exclusion criterion for this study; no further information provided) Pretreatment renal impairment: no (inadequate renal function was an exclusion criterion for this study; no further information provided) Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: CHP‐582 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: carboplatin Cumulative platinum dose: nm (according to protocol 3360 mg/m2) Individual platinum dose: 560 mg/m2 Platinum infusion duration: nm Other chemotherapy: vincristine, cumulative dose nm (according to protocol 9 mg/m2), etoposide, cumulative dose nm (according to protocol 1800 mg/m2). Dose adjustments were made for children aged ≤ 36 months (at least for chemotherapy, possibly also for other treatments) Radiotherapy and surgery: enucleation or external beam radiotherapy in 50% of the participants (no further information provided); see also 'Other treatment'. Other treatment: at least some of the participants had focal therapy, i.e. thermotherapy or cryotherapy (all participants), laser photocoagulation or plaque radiotherapy; no further information provided Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, anthracyclines no; furosemide nm, vincristine: see 'Other chemotherapy' above Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss, definition nm; method of detection nm Participants with hearing loss: 0/103 (0%; 95% CI 0% to 3.6%) Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: mean 29 months, median 28 months, range 2‐63 months. Unclear if follow‐up was based on timing of hearing assessment Partial overlap with other included studies: very likely with Shields 2006 and Lambert 2008 Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: unclear (no information provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | Complete original cohort included in the study |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for the complete study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | Low risk | Duration of follow‐up reported |
| Well‐defined outcome (reporting bias) | High risk | Definition and method of detection nm |
Shields 2006.
| Methods | Design: prospective cohort study Time period: treatment between July 1994 to June 2004 Setting: multicentre study in Philadelphia (2 hospitals) Control group without platinum treatment: no |
|
| Participants | Original cohort: 163; study group of interest: 163; participants with a hearing test: 163 (based on additional information provided by authors) All information provided below is for participants with a hearing test unless otherwise stated Age at diagnosis: nm Age at outcome assessment/follow‐up: nm Gender: nm Type of malignancy; primary disease or recurrence: retinoblastoma; primary disease Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: no Prior hearing dysfunction: no Pretreatment renal impairment: no Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: CHP‐582 All information provided below is for participants with a hearing test unless otherwise stated Type of platinum analogue: carboplatin Cumulative platinum dose: nm Individual platinum dose: nm Platinum infusion duration: nm Other chemotherapy: etoposide, cumulative dose nm; vincristine, cumulative dose nm Radiotherapy, surgery and other treatment: at least some of the participants received thermotherapy, cryotherapy, enucleation, external beam radiotherapy, plaque radiotherapy, or a combination; no further information provided Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm, anthracyclines no; furosemide nm, vincristine: see 'Other chemotherapy' above Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss, definition nm; method of detection nm (we received no response from our author enquiry) Participants with hearing loss: 0/163 (0%; confirmed by the authors; 95% CI 0% to 2.3%) Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: mean/median 6.2 years, range 1‐10.6 years. Unclear if follow‐up was based on timing of hearing assessment Partial overlap with other included studies: very likely with Shields 2002 and Lambert 2008 Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: unclear (no information provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Low risk | Complete original cohort included in the study |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Hearing test available for the complete study group of interest |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | All relevant items nm |
| Well‐defined follow‐up (reporting bias) | Low risk | Duration of follow‐up reported |
| Well‐defined outcome (reporting bias) | High risk | Definition and method of detection nm |
Simon 2002.
| Methods | Design: retrospective data from 2 cohort studies Time period: nm Setting: multicentre study in Germany Control group without platinum treatment: yes (n = 453 no chemotherapy) |
|
| Participants | Original cohort: nm; study group of interest: 717; participants with a hearing test: 715 All information provided below is for participants with a hearing test in the platinum group and the control group unless otherwise stated Age at diagnosis: nm Age at outcome assessment/follow‐up: nm Gender: nm Type of malignancy; primary disease or recurrence: neuroblastoma; nm Prior platinum treatment, radiotherapy to head or neck (or both), cranial surgery: nm Prior hearing dysfunction: nm Pretreatment renal impairment: nm Tested for genetic variants of platinum ototoxicity: no |
|
| Interventions | Name of study protocol: NB90 and NB97 All information provided below is for participants with a hearing test in the platinum group and the control group unless otherwise stated Type of platinum analogue: n = 717 cisplatin, at least n = 188 also carboplatin; not applicable Cumulative platinum dose: cisplatin range 1‐800 mg/m2, carboplatin nm (according to protocol 1500 mg/m2); not applicable Individual platinum dose: nm; not applicable Platinum infusion duration: varying, in at least some of the participants 96 hours for cisplatin and 1‐2 or 4‐8 hours for carboplatin; not applicable Other chemotherapy: yes, at least n = 188 melphalan and etoposide (no further information provided), at least n = 217 cyclophosphamide (no further information provided); no Radiotherapy: nm Surgery: nm Other treatment: at least n = 188 autologous stemcell rescue; nm Other ototoxic drugs (aminoglycosides, furosemide, vincristine): gentamycin nm (but it was reported that it was used more often during megatherapy for stemcell transplant than during maintenance chemotherapy), doxorubicin: nm; no, furosemide nm, vincristine: nm; no Otoprotective medical interventions: no Impaired renal function at time of platinum treatment: nm |
|
| Outcomes | Hearing loss according to WHO criteria (no reference provided, but we assume: WHO Toxicity Criteria; ≥ grade 3); method of detection nm Participants with hearing loss: 144/715 participants in platinum group (20.1%); 2/453 participants in control group (0.44%; 95% CI 0.12% to 1.6%). 1 participant in control group with hearing loss had a family history of hearing impairments and 1 had combined renal ectopia and hearing impairment Multivariable risk factor analysis: no |
|
| Notes | Follow‐up duration: at least 1 year after diagnosis; no further information provided Partial overlap with other included studies: presumably not Inappropriate influence of funders: unclear (no information provided) Declaration of interest primary investigators: unclear (no information provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Representative study group (selection bias) | Unclear risk | Number of participants in the original cohort unclear |
| Complete outcome assessment/follow‐up (attrition bias) | Low risk | Only 2 participants lost to follow‐up |
| Outcome assessors blinded to investigated determinant (detection bias) | Unclear risk | Blinding of outcome assessors not reported |
| Well‐defined study group (reporting bias) | High risk | Only cumulative cisplatin dose available, other relative items nm |
| Well‐defined follow‐up (reporting bias) | High risk | Duration of follow‐up nm |
| Well‐defined outcome (reporting bias) | High risk | Method of detection nm |
ASHA: American Speech‐Language‐Hearing Association; cGy: centigray; CI: confidence interval; COG: Children's Oncology Group; CTCAE: Common Terminology Criteria Adverse Effects; dB: decibel: Gy: gray; HUI3: Health Utilities Index Mark 3; min: minute; kHz: kilohertz; n: number of participants; NCI: National Cancer Institute; nm: not mentioned; OR: odds ratio; POG: Pediatric Oncology Group; RCT: randomized controlled trial; SFOP: French Society of Pediatric Oncology; WHO: World Health Organization.
a In the other studies prematurity was not reported.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aksnes 2009 | Children were not the majority of participants; no separate data on children reported |
| Altaf 2013 | Participants had not finished their platinum treatment |
| Ansari 2010 | < 100 children treated with a platinum analogue (additional information provided by the authors) |
| Armstrong 2010 | Conference proceeding; full‐text manuscript currently published and excluded from this review (< 100 children treated with a platinum analogue) |
| Bacci 2005 | Children were not the majority of participants; no separate data on children reported |
| Baker 2010 | Not all participants had finished their platinum treatment at time of hearing assessment |
| Bass 2014b | Not all participants had finished their platinum treatment |
| Batra 2015 | < 100 children treated with a platinum analogue |
| Berthold 2005 | Platinum‐induced ototoxicity not reported |
| Bostrom 1984 | < 100 children treated with a platinum analogue |
| Bramwell 1979 | Adults |
| Brinkman 2015 | No distinction between participants treated with platinum analogues and other therapies; very likely overlap with Hudson 2013 |
| Brock 1988 | < 100 children treated with a platinum analogue |
| Brock 1991 | < 100 children treated with a platinum analogue |
| Buckner 2006 | Adults |
| Calvo 1979 | < 100 participants treated with a platinum analogue |
| Carleton 2009 | Incidence and possible risk factors of platinum‐induced hearing loss not mentioned |
| Carleton 2014a | No original research |
| Carleton 2014b | No original research |
| Carr 2010 | Adults |
| Castel 1995 | < 100 participants |
| Chang 2010 | Ototoxicity assessed in < 100 participants treated with a platinum analogue |
| Chantada 2004 | < 100 children treated with a platinum analogue (additional information provided by the authors) |
| Chen 2014 | Children were not the majority of participants |
| Cohen 1991 | < 100 children treated with a platinum analogue |
| Corder 1979 | < 100 participants treated with a platinum analogue |
| Coze 1997 | Ototoxicity assessed in < 100 participants treated with a platinum analogue; data on ototoxicity available for < 50% of participants |
| Di Pinto 2012 | < 100 children treated with a platinum analogue |
| Diez 1985 | < 100 children treated with a platinum analogue |
| Dominici 1989 | < 100 children treated with a platinum analogue |
| Einhorn 2006 | Adults |
| Einhorn 2007 | Children were not the majority of participants; no separate data on children reported |
| Ekhart 2008 | Children were not the majority of participants; < 100 children treated with platinum analogues |
| Ettinger 1994 | Ototoxicity assessed in < 100 participants treated with a platinum analogue; data on ototoxicity available for < 50% of the participants |
| Flege 2004 | Review |
| Fosså 2003 | Children were not the majority of participants; no separate data on children reported |
| Fouladi 2005 | No distinction between participants treated with platinum analogues and other therapies |
| Fox 2009 | No original research |
| Fuchs 1998 | Children were not the majority of participants; no separate data on children reported |
| Fuchs 1999 | < 100 children treated with a platinum analogue |
| Gaynon 1979 | < 100 participants treated with a platinum analogue |
| Germà Lluch 1984 | < 100 children treated with a platinum analogue; children were not the majority of participants |
| Gnekow 2004 | Not all participants had finished their platinum treatment at time of hearing assessment |
| Gobel 1989 | No distinction between participants treated with platinum analogues and other therapies; prevalence of hearing loss not reported |
| Green 2008 | No original research |
| Grewal 2010 | Review (1 additional eligible study identified: Cushing 2004) |
| Grill 2006 | No original research |
| Gurney 2007 | No distinction between participants treated with platinum analogues and other therapies |
| Gurney 2014 | Not all participants had finished their platinum treatment; same study population as Bass 2014b |
| Göbel 1990 | Ototoxicity assessed in < 100 participants; not all participants treated with a platinum analogue; unclear which participants received a platinum analogue |
| Hagleitner 2011 | Children were not the majority of participants; no separate data on children reported |
| Hagleitner 2012a | Ototoxicity assessed in < 100 children treated with a platinum analogue |
| Hagleitner 2012b | Conference proceeding of Hagleitner 2014 (additional information provided by from the authors) |
| Hagleitner 2014 | Children were not the majority of participants; no separate data on children reported; < 100 children treated with a platinum analogue |
| Hill 1975 | < 100 participants treated with a platinum analogue; age not mentioned, but mainly adult cancer types |
| Hishiki 2011 | Ototoxicity assessed in < 100 participants treated with a platinum analogue; data on ototoxicity available for < 50% of participants |
| Hiyama 2010a | Conference proceeding; full‐text manuscript currently published and excluded from this review (Hishiki 2011) |
| Hiyama 2010b | Conference proceeding; full‐text manuscript currently published and excluded from this review (Hishiki 2011) |
| Hiyama 2013b | No original research on ototoxicity (it refers to Hishiki 2011 for ototoxicity data, which was excluded from this review) |
| Hovi 2003 | < 100 children treated with a platinum analogue |
| Jakacki 2012 | Ototoxicity was only assessed during platinum treatment |
| Kahn 1979 | < 100 participants treated with a platinum analogue |
| Kamalakar 1976 | < 100 participants treated with a platinum analogue |
| Kingston 1986 | < 100 children treated with a platinum analogue |
| Kortmann 2000 | Participants had not finished their platinum treatment at time of hearing assessment |
| Kreissman 2013 | Participants had not finished their platinum treatment |
| Kremers 2003 | Review |
| Landier 2011 | Conference proceeding; full‐text manuscript currently published and included in this review (Landier 2014) |
| Landier 2012 | Ototoxicity assessed in < 100 children; unclear if all participants were treated with a platinum analogue |
| Lanvers‐Kaminsky 2014 | < 100 children treated with a platinum analogue |
| Laverdiere 2009 | No distinction between participants treated with platinum analogues and other therapies |
| Le Deley 2007 | < 100 participants treated with a platinum analogue |
| Lewis 1991 | < 100 participants |
| Lewis 2007 | Children were not the majority of participants; no separate data on children reported |
| Li 2004 | Not consecutive participants |
| Lippman 1973 | Only 1 child treated with a platinum analogue; other participants were adults |
| Liu 2014 | < 100 children treated with a platinum analogue; not all participants had finished their platinum treatment at time of hearing assessment |
| Mahoney 1982 | Conference proceeding; full‐text manuscript currently published and excluded from this review (Mahoney 1983) |
| Mahoney 1983 | < 100 children treated with a platinum analogue |
| Manfredini 1996 | < 100 children; unclear if/which participants received a platinum analogue |
| Mann 2000 | Not all participants had finished their platinum treatment at time of hearing assessment |
| Marshall 2006 | Adults |
| Mbue 2007 | Review (no additional studies identified) |
| McHaney 1983 | < 100 children treated with a platinum analogue |
| Meyers 2005 | Children were not the majority of participants; no separate data on children reported |
| Montero 2005 | Review |
| Nageswara 2011 | Conference proceeding; full‐text manuscript currently published and included in this review (Nageswara Rao 2014) |
| Nageswara Rao 2011 | Conference proceeding; full‐text manuscript currently published and excluded from this review (Nageswara Rao 2014) |
| Nageswara Rao 2014 | Not all participants had finished their platinum treatment (additional information provided by the authors) |
| Nichols 1991 | Children were not the majority of participants; no separate data on children reported |
| Packer 1991 | < 100 children treated with a platinum analogue |
| Packer 2006 | Participants had not finished their platinum treatment at time of hearing assessment |
| Pearson 2008 | Hearing tests performed during platinum treatment |
| Pendergrass 1987 | At least part of the participants had not finished their platinum treatment at time of hearing assessment |
| Perilongo 2004 | Ototoxicity assessed in < 100 participants treated with a platinum analogue; data on ototoxicity available for < 50% of the participants |
| Pritchard 2000 | Ototoxicity assessed in < 100 participants treated with a platinum analogue |
| Punyko 2005 | No distinction between participants treated with platinum analogues and other therapies |
| Pussegoda 2013 | Not consecutive participants |
| Raney 1999 | No distinction between participants treated with platinum analogues and other therapies |
| Rassekh 2009 | Conference proceeding; full‐text manuscript currently published and excluded from this review (Ross 2009) |
| Rednam 2012 | Conference proceeding; full‐text manuscript currently published and excluded from this review (Rednam 2013) |
| Rednam 2013 | Ototoxicity assessed in < 100 participants treated with a platinum analogue |
| Roark 2003 | No original research |
| Rosen 1984 | < 100 children treated with a platinum analogue |
| Ross 2009 | Not consecutive participants (additional information provided by the authors) |
| Rutledge 2007 | Review (ototoxicity not reported) |
| Sanz 1994 | No original research |
| Sawaguchi 1990 | Not all participants had finished their platinum treatment |
| Sawamura 1998 | Children were not the majority of participants; no separate data on children reported |
| Schell 1989 | Not all participants had finished their platinum treatment at time of hearing assessment |
| Schreiber 2014 | Not all participants had finished their platinum treatment at time of hearing assessment; all participants received possible otoprotective interventions |
| Sefi 2013 | Participants had not finished their platinum treatment at time of hearing assessment (additional information provided by the authors) |
| Singh Chauhan 2011 | < 100 participants treated with a platinum analogue |
| Soomal 2003 | No original research |
| Souhami 1997 | Children were not the majority of participants; no separate data on children reported |
| Spracklen 2014 | < 100 children treated with a platinum analogue; children were not the majority of participants |
| Steinherz 1977 | < 100 participants treated with a platinum analogue |
| Stewart 1981 | Conference proceeding; full‐text manuscript excluded from review based on title and abstract |
| Suita 1994 | Ototoxicity assessed in < 100 participants treated with a platinum analogue |
| Tseng 1987 | Adults |
| Umeda 1986 | < 100 children treated with a platinum analogue |
| Van Maldegem 2015 | Children were not the majority of participants; toxicity data were incomplete |
| Veal 2012 | No original research |
| Von Heyden 1982 | Adults |
| Von Hoff 2009 | Data on ototoxicity available for < 50% of the participants |
| Voskens 2012 | Case report; adult |
| Whelan 2011 | No distinction between participants treated with platinum analogues and other therapies |
| Whitehorn 2014 | Children were not the majority of participants; no separate data on children reported |
| Winkler 1990 | Children were not the majority of participants; no separate data on children reported |
| Winkler 1993 | Review (no eligible studies identified) |
| Xu 2015 | Many participants received possible otoprotective interventions; no separate data for participants treated without possible otoprotective interventions |
| Yancey 2010 | Conference proceeding; full‐text manuscript currently published and included in this review (Yancey 2012) |
| Yancey 2012 | Not all participants had finished their platinum treatment at time of hearing assessment |
| Yang 2013 | No consecutive participants |
| Zage 2008 | Ototoxicity assessed in < 100 participants who finished their platinum treatment |
Characteristics of studies awaiting assessment [ordered by study ID]
Baum 1981.
| Methods | Phase II trial |
| Participants | 114 participants with different refractory solid tumours (aged 1‐26 years) |
| Interventions | Cisplatin |
| Outcomes | Symptomatic hearing problems defined as hearing loss or tinnitus |
| Notes | It is unclear if the ototoxicity assessment was done during or after the end of platinum treatment. We were unable to obtain additional information from the authors |
Clemens 2015.
| Methods | Multicentre cohort study |
| Participants | 240 long‐term childhood cancer survivors (types of malignancies nm) |
| Interventions | Platinum‐based chemotherapy (cisplatin, carboplatin or combination); no cranial radiotherapy |
| Outcomes | Severe hearing loss defined as Munster grade 2b or higher and Brock grade 2 or higher |
| Notes | On 27 September 2015 no full‐text publication available |
Clerico 2010.
| Methods | Nm |
| Participants | Children with medulloblastoma |
| Interventions | Carboplatin, etoposide and radiotherapy |
| Outcomes | Nm |
| Notes | Only a title was available in the conference abstract book. On 27 September 2015 no full‐text publication available. We were unable to obtain additional information from the authors. It remains unclear if this study is eligible for inclusion in this review |
Finlay 2009.
| Methods | 3 sequential prospective non‐randomized feasibility studies |
| Participants | Children with newly diagnosed primary CNS embryonal tumours |
| Interventions | Cisplatin‐ and carboplatin‐containing chemotherapy followed by rescue with autologous haematopoietic progenitor cells |
| Outcomes | Hearing loss after long‐term follow‐up |
| Notes | On 27 September 2015 no full‐text publication including at least 100 participants was available |
Geyer 2005.
| Methods | Randomized controlled trial (for this review: cohort study) |
| Participants | 284 infants (< 36 months) with newly diagnosed malignant brain tumours |
| Interventions | Carboplatin‐ versus cisplatin‐containing induction chemotherapy, carboplatin‐containing maintenance chemotherapy, surgery and in some cases radiotherapy |
| Outcomes | Grade 3 or 4 hearing loss |
| Notes | It is unclear if the ototoxicity assessment was done during or after the end of platinum treatment. We were unable to obtain additional information from the authors |
Hiyama 2013a.
| Methods | Cohort study |
| Participants | 254 children (< 15 years) with hepatoblastoma |
| Interventions | Cisplatin, pirarubicin and surgery |
| Outcomes | Late ototoxicity |
| Notes | On 27 September 2015 no full‐text publication with relevant ototoxicity data available |
Knight 2014.
| Methods | Retrospective cohort study |
| Participants | 128 childhood cancer survivors; various malignancies |
| Interventions | Platinum chemotherapy (cisplatin, carboplatin or combination); 52 also received cranial radiotherapy |
| Outcomes | Hearing loss |
| Notes | On 27 September 2015 no full‐text publication available |
Korzeniewska 2009.
| Methods | Prospective study, no further information provided |
| Participants | Malignant childhood brain tumour survivors |
| Interventions | Neurosurgery, radiotherapy and chemotherapy |
| Outcomes | Hearing loss |
| Notes | On 27 September 2015 no full‐text publication available. We were unable to obtain additional information from the authors. It remains unclear if this study is eligible for inclusion in this review |
Kuhl 1998.
| Methods | Neoadjuvant phase II and single‐arm pilot trial |
| Participants | 147 children and young adults (aged 3‐29.9 years) with newly diagnosed malignant brain tumours |
| Interventions | Cisplatin‐containing chemotherapy, surgery and radiotherapy |
| Outcomes | Ototoxicity (according to WHO criteria) |
| Notes | It is unclear if the ototoxicity assessment was done during or after the end of platinum treatment. We were unable to obtain additional information from the authors |
Kushner 2006.
| Methods | Cohort |
| Participants | 173 neuroblastoma participants |
| Interventions | Cisplatin‐ with or without carboplatin‐containing chemotherapy |
| Outcomes | Ototoxicity (according to Brock criteria) |
| Notes | Part of the results were from participants still receiving platinum treatment. We were unable to obtain all necessary additional information needed to be able to include this study in the review from the authors There is possibly overlap with the included study of Landier 2014 |
Lannering 2012.
| Methods | Randomized controlled trial (for this review: cohort study) |
| Participants | 340 children and young adults (aged 4‐21 years) with medulloblastoma |
| Interventions | Radiotherapy, surgery and cisplatin‐containing chemotherapy |
| Outcomes | Hearing loss (according to HIT and Brock criteria) |
| Notes | It is unclear if the ototoxicity assessment was done during or after the end of platinum treatment and not for all participants ototoxicity data were available in the manuscript. We were unable to obtain all necessary additional information needed to be able to include this study in the review from the authors |
Merchant 2011.
| Methods | Retrospective review |
| Participants | 140 children with brain tumours |
| Interventions | Radiotherapy and cisplatin or carboplatin |
| Outcomes | Hearing loss |
| Notes | On 27 September 2015 no full‐text publication available |
Nirenberg 1981.
| Methods | Nm |
| Participants | Participants with osteogenic sarcoma (age nm) |
| Interventions | Cisplatin |
| Outcomes | Auditory toxicity |
| Notes | On 27 September 2015 no full‐text publication available. We were unable to obtain additional information from the authors. It remains unclear if this study is eligible for inclusion in this review |
Ohnuma 1995.
| Methods | Controlled clinical trial |
| Participants | 110 children with neuroblastoma |
| Interventions | Cisplatin‐containing chemotherapy, surgery with or without bone marrow transplantation (with or without total body irradiation) |
| Outcomes | Auditory disturbances |
| Notes | Part of the participants had not finished treatment yet and no separate results were available for ototoxicity assessments after end of platinum treatment. We were unable to obtain additional information from the authors |
Vos 2014.
| Methods | Nm |
| Participants | Osteosarcoma participants; age nm and number treated with platinum analogues nm |
| Interventions | Cisplatin |
| Outcomes | Ototoxicity |
| Notes | On 27 September 2015 no full‐text publication available. Unclear if at least 100 children treated with platinum analogues; unclear if participants were consecutive |
Weiss 2015.
| Methods | Cohort study |
| Participants | Long‐term childhood cancer survivors; number treated with platinum analogues nm; different types of malignancies |
| Interventions | Platinum analogues |
| Outcomes | Hearing loss and tinnitus reported in a questionnaire |
| Notes | On 27 September 2015 no full‐text publication available. Unclear if at least 100 participants treated with platinum analogues, but based on the fact that almost 2400 childhood cancer survivors were included this is very likely |
CNS: central nervous system; nm: not mentioned; WHO: World Health Organization.
Differences between protocol and review
Types of studies: as opposed to what was stated in the protocol we did not use a cutoff point of 50 participants to be eligible for this review, but a cutoff point of 100 participants. We clarified that this cutoff point related to participants treated with platinum‐based therapy who had an ototoxicity assessment.
Types of participants: we clarified that with "all participants should have finished treatment" we meant that all participants should have finished platinum treatment.
Search methods for identification of studies: we added experts in the field as a source for possible eligible studies. In addition, the Information Specialist of Cochrane Childhood Cancer optimalized the search strategy as described in the appendices.
Data extraction and management and 'Risk of bias' assessment in included studies: instead of data extraction and 'Risk of bias' assessment by two independent review authors, this was done by one review author and checked by another review author.
Measures of treatment effect: we clarified that only for control groups from a randomized controlled trial or controlled clinical trial it would be feasible to calculate a risk ratio.
Data synthesis: after the publication of our protocol, Cochrane Childhood Cancer changed its policy regarding meta‐regression analyses and advised us not to perform these; also they advised that we include only multivariable risk factor analyses. Cochrane Childhood Cancer also changed its policy regarding the calculation of prevalences and the corresponding 95% confidence intervals. Therefore, instead of using the generic inverse variance function of Review Manager 5 to calculate the 95% confidence intervals we were advised to use the Wilson method. As this was not possible in Review Manager 5 we used the following tool: EpiTools epidemiological calculator. Forest plots were prepared in Excel software. As it was not possible to calculate the I2 statistic or use either a fixed‐effect or random‐effect model, these had to be omitted from the heterogeneity assessment of included studies.
All changes between protocol and review have been made in consultation with Cochrane Childhood Cancer.
Contributions of authors
Jorrit van As wrote the protocol. He identified the studies meeting the inclusion criteria. He checked the data extraction and risk of bias assessment of the included studies. He analyzed the data and interpreted the results. He wrote and revised the manuscript.
Henk van den Berg critically reviewed the protocol. He contributed to the interpretation of the results. He critically reviewed the manuscript.
Elvira van Dalen designed the study and critically reviewed the protocol. She developed the search strategy in collaboration with the Information Specialist of Cochrane Childhood Cancer. She identified the studies meeting the inclusion criteria. She searched for unpublished and ongoing studies. She performed the data extraction and 'Risk of bias' assessment of the included studies. She analyzed the data and interpreted the results. She wrote and revised the manuscript.
All authors approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
Stichting Kinderen Kankervrij (KiKa), Netherlands.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bertolini 2004 {published data only}
- Bertolini P, Lassalle M, Mercier G, Raquin MA, Izzi G, Corradini N, et al. Platinum compound‐related ototoxicity in children: long‐term follow‐up reveals continuous worsening of hearing loss. Journal of Pediatric Hematology/Oncology 2004;26(10):649‐55. [DOI] [PubMed] [Google Scholar]
Cushing 2004 {published data only}
- Cushing B, Giller R, Cullen JW, Marina NM, Lauer SJ, Olson TA, et al. Randomized comparison of combination chemotherapy with etoposide, bleomycin, and either high‐dose or standard‐dose cisplatin in children and adolescents with high‐risk malignant germ cell tumors: a pediatric intergroup study ‐ Pediatric Oncology Group 9049 and Children's Cancer Group 8882. Journal of Clinical Oncology 2004;22(13):2691‐700. [DOI] [PubMed] [Google Scholar]
Hudson 2013 {published data only}
- Hudson MM, Ness KK, Gurney JG, Mulrooney DA, Chemaitilly W, Krull KR, et al. Clinical ascertainment of health outcomes among adults treated for childhood cancer. JAMA 2013;309(22):2371‐81 Erratum in: JAMA (2013);310(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jehanne 2009 {published data only}
- Jehanne M, Lumbroso‐Le Rouic L, Savignoni A, Aerts I, Mercier G, Bours D, et al. Analysis of ototoxicity in young children receiving carboplatin in the context of conservative management of unilateral or bilateral retinoblastoma. Pediatric Blood & Cancer 2009;52(5):637‐43. [DOI] [PubMed] [Google Scholar]
Kennedy 2014 {published data only}
- Kennedy C, Bull K, Chevignard M, Culliford D, Dörr HG, Doz F, et al. Quality of survival and growth in children and young adults in the PNET4 European controlled trial of hyperfractionated versus conventional radiation therapy for standard‐risk medulloblastoma. International Journal of Radiation Oncology Biology Physics 2014;88(2):292‐300. [DOI] [PubMed] [Google Scholar]
Lambert 2008 {published and unpublished data}
- Lambert MP, Shields C, Meadows AT. A retrospective review of hearing in children with retinoblastoma treated with carboplatin‐based chemotherapy. Pediatric Blood & Cancer 2008;50(2):223‐6. [DOI] [PubMed] [Google Scholar]
Landier 2014 {published data only}
- Landier W, Knight K, Wong FL, Lee J, Thomas O, Kim H, et al. Ototoxicity in children with high‐risk neuroblastoma: prevalence, risk factors, and concordance of grading scales ‐ a report from the Children's Oncology Group. Journal of Clinical Oncology 2014;32(6):527‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 1999 {published data only}
- Mandell LR, Kadota R, Freeman C, Douglass EC, Fontanesi J, Cohen ME, et al. There is no role for hyperfractionated radiotherapy in the management of children with newly diagnosed diffuse intrinsic brainstem tumors: results of a Pediatric Oncology Group phase III trial comparing conventional vs. hyperfractionated radiotherapy. International Journal of Radiation Oncology, Biology, Physics 1999;43(5):959‐64. [DOI] [PubMed] [Google Scholar]
Peleva 2014 {published data only}
- Peleva E, Emami N, Alzahrani M, Bezdjian A, Gurberg J, Carret AS, et al. Incidence of platinum‐induced ototoxicity in pediatric patients in Quebec. Pediatric Blood & Cancer 2014;61(11):2012‐7. [DOI] [PubMed] [Google Scholar]
Perilongo 2009 {published data only}
- Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, et al. Cisplatin versus cisplatin plus doxorubicin for standard‐risk hepatoblastoma. New England Journal of Medicine 2009;361:1662‐70. [DOI] [PubMed] [Google Scholar]
Shields 2002 {published data only}
- Shields CL, Honavar SG, Meadows AT, Shields JA, Demirci H, Singh A, et al. Chemoreduction plus focal therapy for retinoblastoma: factors predictive of need for treatment with external beam radiotherapy or enucleation. American Journal of Ophthalmology 2002;133(5):657‐64. [DOI] [PubMed] [Google Scholar]
Shields 2006 {published and unpublished data}
- Shields CL, Mashayekhi A, Au AK, Czyz C, Leahey A, Meadows AT, et al. The International Classification of Retinoblastoma Predicts Chemoreduction Success. Ophthalmology 2006;113:2276‐80. [DOI] [PubMed] [Google Scholar]
Simon 2002 {published data only}
- Simon T, Hero B, Dupuis W, Selle B, Berthold F. The incidence of hearing impairment after successful treatment of neuroblastoma. Klinische Padiatrie 2002;214(4):149‐52. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aksnes 2009 {published data only}
- Aksnes LH, Bauer HCF, Dahl AA, Fossa SD, Hjorth L, Jebsen N, et al. Health status at long‐term follow‐up in patients treated for extremity localized Ewing sarcoma or osteosarcoma: a Scandinavian Sarcoma Group Study. Pediatric Blood & Cancer 2009;53:84‐9. [DOI] [PubMed] [Google Scholar]
Altaf 2013 {published data only}
- Altaf S, Enders F, Jeavons E, Krailo M, Barkauskas DA, Meyers P, et al. High‐BMI at diagnosis is associated with inferior survival in patients with osteosarcoma: a report from the Children's Oncology Group. Pediatric Blood & Cancer 2013;60(12):2042‐6. [DOI] [PubMed] [Google Scholar]
Ansari 2010 {published and unpublished data}
- Ansari S, Parhyscar J. Hearing loss in pediatric oncology patients cisplatin regimens. Pediatric Blood & Cancer 2010:1008. [Google Scholar]
Armstrong 2010 {published data only}
- Armstrong GT, Conklin H, Huang S, Gajjar A, Merchant TE, Hudson M, et al. Long‐term survival & health outcomes after diagnosis of low grade glioma (ii30). International Symposium on Pediatric Neuro‐Oncology. 2010.
Bacci 2005 {published data only}
- Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P (2005). Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. European Journal of Cancer 2005;41:2836‐45. [DOI] [PubMed] [Google Scholar]
Baker 2010 {published data only}
- Baker DL, Schmidt ML, Cohn SL, Maris JM, London WB, Buxton A, et al. Outcome after reduced chemotherapy for intermediate‐risk neuroblastoma. New England Journal of Medicine 2010;363:1313‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bass 2014b {published data only}
- Bass JK, Huang J, Onar‐Thomas A, Chang KW, Bhagat SP, Chintagumpala M, et al. Concordance between the Chang and the International Society of Pediatric Oncology (SIOP) ototoxicity grading scales in patients treated with cisplatin for medulloblastoma. Pediatric Blood & Cancer 2014;61(4):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Batra 2015 {published data only}
- Batra A, Thakar A, Bakhshi S. Ototoxicity in retinoblastoma survivors treated with carboplatin based chemotherapy: a cross‐sectional study of 116 patients. Pediatric Blood & Cancer 2015;62(11):2060. [DOI] [PubMed] [Google Scholar]
Berthold 2005 {published data only}
- Berthold F, Boos J, Burdach S, Erttmann R, Henze G, Hermann J, et al. Myeloablative megatherapy with autologous stem‐cell rescue versus oral maintenance chemotherapy as consolidation treatment in patients with high‐risk neuroblastoma: a randomised controlled trial. Lancet Oncology 2005;6:649‐58. [DOI] [PubMed] [Google Scholar]
Bostrom 1984 {published data only}
- Bostrom B, Woods WG, Ramsay NK, Krivit W, Levine P, Nesbit ME Jr. Cisplatin, vinblastine, and bleomycin (CVB) therapy for relapsed disseminated neuroblastoma. Cancer Treatment Reports 1984;68(9):1157‐8. [PubMed] [Google Scholar]
Bramwell 1979 {published data only}
- Bramwell VH, Brugarolas A, Mouridsen HT, Cheix F, Jager R, Oosterom AT, et al. E.O.R.T.C. phase II study of cisplatin in cyvadic‐resistant soft tissue sarcoma. European Journal of Cancer 1979;15(12):1511‐3. [DOI] [PubMed] [Google Scholar]
Brinkman 2015 {published data only}
- Brinkman TM, Bass JK, Li Z, Ness KK, Gajjar A, Pappo AS, et al. Treatment‐induced hearing loss and adult social outcomes in survivors of childhood CNS and non‐CNS solid tumors: results from the St. Jude Lifetime Cohort Study. Cancer 2015;121(22):4053‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brock 1988 {published data only}
- Brock P, Pritchard J, Bellman S, Pinkerton CR. Ototoxicity of high‐dose cis‐platinum in children. Medical and Pediatric Oncology 1988;16(5):368‐9. [DOI] [PubMed] [Google Scholar]
Brock 1991 {published data only}
- Brock PR, Bellman SC, Yeomans EC, Pinkerton CR, Pritchard J. Cisplatin ototoxicity in children: a practical grading system. Medical and Pediatric Oncology 1991;19:295‐300. [DOI] [PubMed] [Google Scholar]
Buckner 2006 {published data only}
- Buckner JC, Ballman KV, Michalak JC, Burton GV, Cascino TL, Schomberg PJ, et al. Phase III trial of carmustine and cisplatin compared with carmustine alone and standard radiation therapy or accelerated radiation therapy in patients with glioblastoma multiforme: North Central Cancer Treatment Group 93‐72‐52 and Southwest Oncology Group 9503 Trials. Journal of Clinical Oncology 2006;24(24):3871‐9. [DOI] [PubMed] [Google Scholar]
Calvo 1979 {published data only}
- Calvo D, Pat YZ, Wallace S, Hersch EM, Benjamin RS, Pritchard J, et al. Phase I trial of percutaneous intra‐arterial (IA) cis‐diammine dichloride platinum II (CDDP) for regionally confined malignancies (C‐558). Proceedings of the American Association for Cancer Research. 1979.
Carleton 2009 {published data only}
- Carleton B, Poole R, Smith M, Leeder J, Ghannadan R, Ross C, et al. Adverse drug reaction active surveillance: developing a national network in Canada's children's hospitals. Pharmacoepidemiology and Drug Safety 2009;18(8):713‐21. [DOI] [PubMed] [Google Scholar]
Carleton 2014a {published data only}
- Carleton BC, Ross CJ, Bhavsar AP, Lee JW, Visscher H, Rassekh SR, et al. Response to "Evaluation of pharmacogenetic markers to predict the risk of cisplatin‐induced ototoxicity". Clinical Pharmacology and Therapeutics 2014;96(2):158. [DOI] [PubMed] [Google Scholar]
Carleton 2014b {published data only}
- Carleton BC, Ross CJ, Bhavsar AP, Amstutz U, Pussegoda K, Visscher H, et al. Role of TPMT and COMT genetic variation in cisplatin‐induced ototoxicity. Clinical Pharmacology and Therapeutics 2014;95(3):253. [DOI] [PubMed] [Google Scholar]
Carr 2010 {published data only}
- Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two‐cohort study. Cancer 2010;116:1305‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Castel 1995 {published data only}
- Castel V, Badal MD, Bezanilla JL, Llombart A, Ruiz‐Jimenez JI, Sanchez de TJ, et al. Treatment of stage III neuroblastoma with emphasis on intensive induction chemotherapy: a report from the Neuroblastoma Group of the Spanish Society of Pediatric Oncology. Medical and Pediatric Oncology 1995;24(1):29‐35. [DOI] [PubMed] [Google Scholar]
Chang 2010 {published data only}
- Chang KW, Chinosornvatana N. Practical grading system for evaluating cisplatin ototoxicity in children. Journal of Clinical Oncology 2010;28:1788‐95. [DOI] [PubMed] [Google Scholar]
Chantada 2004 {published and unpublished data}
- Chantada G, Fandino A, Davila MTG, Manzitti J, Raslawski E, Casak S, et al. Results of a prospective study for the treatment of retinoblastoma. Cancer 2004;100:834‐42. [DOI] [PubMed] [Google Scholar]
Chen 2014 {published data only}
- Chen CA, Lin H, Weng CS, Wen KC, Lu CH, Chou HH, et al. Outcome of 3‐day bleomycin, etoposide and cisplatin chemotherapeutic regimen for patients with malignant ovarian germ cell tumours: a Taiwanese Gynecologic Oncology Group study. European Journal of Cancer 2014;50(18):3161‐7. [DOI] [PubMed] [Google Scholar]
Cohen 1991 {published data only}
- Cohen BH, Zweidler P, Goldwein JW, Molloy J, Packer RJ. Ototoxic effect of cisplatin in children with brain tumors. Pediatric Neurosurgery 1991;16:292‐6. [DOI] [PubMed] [Google Scholar]
Corder 1979 {published data only}
- Corder MP, Leimert JT, Panther SK, Elliott TE. A phase II study of cis‐platinum II diamminedichloride (CPDD) in lymphoma (C‐38). Proceedings of the American Association for Cancer Research. 1979.
Coze 1997 {published data only}
- Coze C, Hartmann O, Michon J, Frappaz D, Dusol F, Rubie H, et al. NB87 induction protocol for stage 4 neuroblastoma in children over 1 year of age: a report from the French Society of Pediatric Oncology. Journal of Clinical Oncology 1997;15(12):3433‐40. [DOI] [PubMed] [Google Scholar]
Diez 1985 {published data only}
- Diez B, Monges J, Muriel FS. Evaluation of cisplatin in children with recurrent brain tumors. Cancer Treatment Reports 1985;69:911‐3. [PubMed] [Google Scholar]
Di Pinto 2012 {published data only}
- Pinto M, Conklin HM, Li C, Merchant TE. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low‐grade glioma. International Journal of Radiation Oncology Biology Physics 2012;84(3):e363‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dominici 1989 {published data only}
- Dominici C, Clerico A, Castello MA. Chemotherapy of regional or metastatic neuroblastoma with high‐dose cisplatin by continuous intravenous infusion and etoposide. Rivista Italiana di Pediatria 1989;15:373‐8. [Google Scholar]
Einhorn 2006 {published data only}
- Einhorn LH, Foster RS. Bleomycin, etoposide, and cisplatin for three cycles compared with etoposide and cisplatin for four cycles in good‐risk germ cell tumors: is there a preferred regimen?. Journal of Clinical Oncology 2006;24:2597‐98. [DOI] [PubMed] [Google Scholar]
Einhorn 2007 {published data only}
- Einhorn LH, Williams SD, Chamness A, Brames MJ, Perkins SM, Abonour R. High‐dose chemotherapy and stem‐cell rescue for metastatic germ‐cell tumors. New England Journal of Medicine 2007;357(4):340‐8. [DOI] [PubMed] [Google Scholar]
Ekhart 2008 {published data only}
- Ekhart C, Rodenhuis S, Smits PHM, Beijnen JH, Huitema ADR. Relations between polymorphisms in drug‐metabolising enzymes and toxicity of chemotherapy with cyclophosphamide, thiotepa and carboplatin. Pharmacogenetics and Genomics 2008;18:1009‐15. [DOI] [PubMed] [Google Scholar]
Ettinger 1994 {published data only}
- Ettinger LJ, Gaynon PS, Krailo MD, Ru N, Baum ES, Siegel SE, et al. A phase II study of carboplatin in children with recurrent or progressive solid tumors. A report from the Childrens Cancer Group. Cancer 1994;73(4):1297‐301. [DOI] [PubMed] [Google Scholar]
Flege 2004 {published data only}
- Flege S, Bielack S. Goal and results of the COSS study. Handchirurgie, Mikrochirurgie, Plastische Chirurgie 2004;36(5):282‐8. [DOI] [PubMed] [Google Scholar]
Fosså 2003 {published data only}
- Fosså SD, Wit R, Roberts JT, Wilkinson PM, Mulder PH, Mead GM, et al. Quality of life in good prognosis patients with metastatic germ cell cancer: a prospective study of the European Organization for Research and Treatment of Cancer Genitourinary Group/Medical Research Council Testicular Cancer Study Group (30941/TE20). Journal of Clinical Oncology 2003;21(6):1107‐18. [DOI] [PubMed] [Google Scholar]
Fouladi 2005 {published data only}
- Fouladi M, Gilger E, Kocak M, Wallace D, Buchanan G, Reeves C, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. Journal of Clinical Oncology 2005;23:7152‐60. [DOI] [PubMed] [Google Scholar]
Fox 2009 {published data only}
- Fox E, Citrin D, Balis FM. The legacy of cancer therapy in children. Journal of the National Cancer Institute 2009;101(16):1105‐7. [DOI] [PubMed] [Google Scholar]
Fuchs 1998 {published data only}
- Fuchs N, Bielack SS, Epler D, Bieling P, Delling G, Korholz D, et al. Long‐term results of the co‐operative German‐Austrian‐Swiss osteosarcoma study group's protocol COSS‐86 of intensive multidrug chemotherapy and surgery for osteosarcoma of the limbs. Annals of Oncology 1998;9:893‐99. [DOI] [PubMed] [Google Scholar]
Fuchs 1999 {published data only}
- Fuchs J, Bode U, Schweinitz DV, Weinel P, Erttmann R, Harms D, et al. Analysis of treatment efficiency of carboplatin and etoposide in combination with radical surgery in advanced and recurrent childhood hepatoblastoma: a report of the German cooperative pediatric liver tumor study HB 89 and HB 94. Klinische Padiatrie 1999;211:305‐9. [DOI] [PubMed] [Google Scholar]
Gaynon 1979 {published data only}
- Gaynon P, Baum E, Greenberg L, Krivit W, Hammond D, Taylor S. A phase II trial of cis‐platinum diammine dichloride (DDP) (NSC 119825) in refractory childhood tumors: a CCSG trial (C‐424). Proceedings of the American Association for Cancer Research. 1979.
Germà Lluch 1984 {published data only}
- Germà Lluch JR, Izquierdo Font A, Barnadas Molins A, Arcusa Lanza MA, Ojeda González B. Delayed platinum diamminodichloride toxicity in patients with germ tumors of the gonads. Medicina Clinica 1984;82(10):442‐5. [PubMed] [Google Scholar]
Gnekow 2004 {published data only}
- Gnekow AK, Kortmann RD, Pietsch T, Emser A. Low grade chiasmatic‐hypothalamic glioma ‐ carboplatin and vincristin chemotherapy effectively defers radiotherapy within a comprehensive treatment strategy: Report from the multicenter treatment study for children and adolescents with a low grade glioma ‐ HIT‐LGG 1996 ‐ of the Society of Pediatric Oncology and Hematology (GPOH). Klinische Pädiatrie 2004;216:331‐42. [DOI] [PubMed] [Google Scholar]
Gobel 1989 {published data only}
- Gobel U, Bamberg M, Haas RJ, Bokkerink JPM, Bramswig G, Calaminus G, et al. Non‐testicular germ cell tumors: analysis of the MAKEI 83/86 therapy study and protocol changes for the follow‐up study. Klinische Padiatrie 1989;201:247‐60. [DOI] [PubMed] [Google Scholar]
Göbel 1990 {published data only}
- Göbel U, Haas RJ, Calaminus G, Bamberg M, Bökkerink EB, Engert J, et al. Treatment of germ cell tumors in children: results of European trials for testicular and non‐testicular primary sites. Critical Reviews in Oncology/Hematolology 1990;10(2):89‐98. [DOI] [PubMed] [Google Scholar]
Green 2008 {published data only}
- Green DM. Chemotherapy for the treatment of children and adolescents with malignant germ cell tumors. Journal of Clinical Oncology 2008;26(20):3297‐8. [DOI] [PubMed] [Google Scholar]
Grewal 2010 {published data only}
- Grewal S, Merchant T, Reymond R, McInerney M, Hodge C, Shearer P. Auditory late effects of childhood cancer therapy: a report from the Children's Oncology Group. Pediatrics 2010;125(4):e938‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grill 2006 {published data only}
- Grill J, Dufour C, Kalifa C. High‐dose chemotherapy in children with newly‐diagnosed medulloblastoma. Lancet Oncology 2006;7(10):787‐9. [DOI] [PubMed] [Google Scholar]
Gurney 2007 {published data only}
- Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long‐term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics 2007;120:e1229‐36. [DOI] [PubMed] [Google Scholar]
Gurney 2014 {published data only}
- Gurney JG, Bass JK, Onar‐Thomas A, Huang J, Chintagumpala M, Bouffet E, et al. Evaluation of amifostine for protection against cisplatin‐induced serious hearing loss in children treated for average‐risk or high‐risk medulloblastoma. Neuro‐Oncology 2014;16(6):848‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagleitner 2011 {published data only}
- Hagleitner MM, Hoogerbrugge PM, Graaf WTA, Flucke U, Schreuder HWB, Loo DMWM. Age as prognostic factor in patients with osteosarcoma. Bone 2011;49:1173‐7. [DOI] [PubMed] [Google Scholar]
Hagleitner 2012a {published data only}
- Hagleitner MM, Bont ES, Loo DM. Survival trends and long‐term toxicity in pediatric patients with osteosarcoma. Sarcoma 2012;2012:636405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hagleitner 2012b {published and unpublished data}
- Hagleitner MM, Coenen MJH, Gelderblom H, Hoogerbrugge P, Guchelaar HJ, Loo DMWM. Association of the genetic variants in the nucleotide excision repair genes XPA and XPC with cisplatin‐induced hearing loss in patients with osteosarcoma (10077). Annual Meeting of the American Society of Clinical Oncology 2012. [Google Scholar]
Hagleitner 2014 {unpublished data only}
- Hagleitner MM, Coenen MJ, Patino‐Garcia A, Bont ES, Gonzalez‐Neira A, Vos HI, et al. Influence of genetic variants in TPMT and COMT associated with cisplatin induced hearing loss in patients with cancer: two new cohorts and a meta‐analysis reveal significant heterogeneity between cohorts. PLoS One 2014;9(12):e115869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hill 1975 {published data only}
- Hill JM, Loeb E, MacLellan A, Hill NO, Khan A, King JJ. Clinical studies of platinum coordination compounds in the treatment of various malignant diseases. Cancer Chemotherapy Reports 1975;59(3):647‐59. [PubMed] [Google Scholar]
Hishiki 2011 {published data only}
- Hishiki T, Matsunaga T, Sasaki F, Yano M, Ida K, Horie H, et al. Outcome of hepatoblastomas treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol‐2: report from the JPLT. Pediatric Surgery International 2011;27:1‐8. [DOI] [PubMed] [Google Scholar]
Hiyama 2010a {published data only}
- Hiyama E, Kamimatsuse A, Kamei N, Watanabe K, Hishiki T, Tajiri T, et al. Outcome of hepatoblastoma treated with the JPLT‐2 protocol from the experience of JPLT (Japanese Study Group for Pediatric Liver Tumor) study. Pediatric Blood & Cancer 2010:816. [Google Scholar]
Hiyama 2010b {published data only}
- Hiyama E, Kamimatsuse A, Kamei N, Watanabe K, Hishiki T, Tajiri T, et al. Cisplatin plus pirarubicin chemotherapy and combination ifomide, etoposide, pirarubicin, and carboplatin chemotherapy for hepatoblastoma. Annual Meeting of the American Society of Clinical Oncology. 2010.
Hiyama 2013b {published data only}
- Hiyama E, Ueda Y, Onitake Y, Kurihara S, Watanabe K, Hishiki T, et al. A cisplatin plus pirarubicin‐based JPLT2 chemotherapy for hepatoblastoma: experience and future of the Japanese Study Group for Pediatric Liver Tumor (JPLT). Pediatric Surgery International 2013;29(10):1071‐5. [DOI] [PubMed] [Google Scholar]
Hovi 2003 {published data only}
- Hovi L, Wikstrom S, Vettenranta K, Heikkila P, Saarinen‐Pihkala UM. Adrenocortical carcinoma in children: a role for etoposide and cisplatin adjuvant therapy? Preliminary report. Medical and Pediatric Oncology 2003;40:324‐6. [DOI] [PubMed] [Google Scholar]
Jakacki 2012 {published data only}
- Jakacki RI, Burger PC, Zhou T, Holmes EJ, Kocak M, Onar A, et al. Outcome of children with metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy: a children's oncology group phase I/II study. Journal of Clinical Oncology 2012;30(21):2648‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahn 1979 {published data only}
- Khan A, McCullough D. Use of cis‐platinum in CNS malignancy (C‐144). Proceedings of the American Association for Cancer Research. 1979.
Kamalakar 1976 {published data only}
- Kamalakar P, Wang JJ, Higby D, Freeman AI, Wallace HJ. Clinical experience with CIS diammine dichloroplatinum (DDP) in children (C‐188). Proceedings of the American Association for Cancer Research. 1976.
Kingston 1986 {published data only}
- Kingston JE, Abramovich S, Billings RJ. Assessment of the effect of chemotherapy and radiotherapy on the auditory function of children with cancer. Clinical Otolaryngology and Allied Sciences 1986;11:403‐9. [DOI] [PubMed] [Google Scholar]
Kortmann 2000 {published data only}
- Kortmann RD, Kuhl J, Timmermann B, Mittler U, Urban C, Budach V, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. International Journal of Radiation Oncology, Biology, Physics 2000;46(2):269‐79. [DOI] [PubMed] [Google Scholar]
Kreissman 2013 {published data only}
- Kreissman SG, Seeger RC, Matthay KK, London WB, Sposto R, Grupp SA, et al. Purged versus non‐purged peripheral blood stem‐cell transplantation for high‐risk neuroblastoma (COG A3973): a randomised phase 3 trial. Lancet Oncology 2013;14(10):999‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kremers 2003 {published data only}
- Kremers A, Langer T, Stohr W, Beck JD, Rascher W. Late effects of oncological therapy in children. Padiatrische Praxis 2003;64:47‐64. [Google Scholar]
Landier 2011 {published data only}
- Landier W, Knight KR, Wong FL, Lee JK, Thomas O, Kim H, et al. Ototoxicity in children with high‐risk neuroblastoma: prevalence, risk factors, and concordance of grading scales ‐ a report from the Children's Oncology Group (COG) (abstract number 9515). ASCO Annual Meeting. 2011. [DOI] [PMC free article] [PubMed]
Landier 2012 {published data only}
- Landier W, Armenian SH, Lee J, Thomas O, Wong FL, Francisco L, et al. Yield of screening for long‐term complications using the children's oncology group long‐term follow‐up guidelines. Journal of Clinical Oncology 2012;30(35):4401‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lanvers‐Kaminsky 2014 {published data only}
- Lanvers‐Kaminsky C, Malath I, Deuster D, Ciarimboli G, Boos J, Am Zehnhoff‐Dinnesen AG. Evaluation of pharmacogenetic markers to predict the risk of cisplatin‐induced ototoxicity. Clinical Pharmacology and Therapeutics 2014;96(2):156‐7. [DOI] [PubMed] [Google Scholar]
Laverdiere 2009 {published data only}
- Laverdiere C, Liu Q, Yasui Y, Nathan PC, Gurney JG, Stovall M, et al. Long‐term outcomes in survivors of neuroblastoma: a report from the childhood cancer survivor study. Journal of the National Cancer Institute 2009;101:1131‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Le Deley 2007 {published data only}
- Deley MC, Guinebretière JM, Gentet JC, Pacquement H, Pichon F, Marec‐Bérard P, et al. SFOP OS94: a randomised trial comparing preoperative high‐dose methotrexate plus doxorubicin to high‐dose methotrexate plus etoposide and ifosfamide in osteosarcoma patients. European Journal of Cancer 2007;43(4):752‐61. [DOI] [PubMed] [Google Scholar]
Lewis 1991 {published data only}
- Lewis IJ, Stevens MC, Pearson AD, Pinkerton CR, Barnes JM. Carboplatin activity in cisplatin treated neuroblastoma. Advances in Neuroblastoma Research 1991;366:553‐9. [PubMed] [Google Scholar]
Lewis 2007 {published data only}
- Lewis IJ, Nooij MA, Whelan J, Sydes MR, Grimer R, Hogendoorn PCW, et al. Improvement in histologic response but not survival in osteosarcoma patients treated with intensified chemotherapy: a randomized phase III trial of the European Osteosarcoma Intergroup. Journal of the National Cancer Institute 2007;99:112‐28. [DOI] [PubMed] [Google Scholar]
Li 2004 {published data only}
- Li Y, Womer RB, Silber JH. Predicting cisplatin ototoxicity in children: the influence of age and the cumulative dose. European Journal of Cancer 2004;40(16):2445‐51. [DOI] [PubMed] [Google Scholar]
Lippman 1973 {published data only}
- Lippman AJ, Helson C, Helson L, Krakoff IH. Clinical trials of cis‐diamminedichloroplatinum (NSC‐119875). Cancer Chemotherapy Reports Part 1 1973;57(2):191‐200. [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu W, Tang Y, Gao L, Huang X, Luo J, Zhang S, et al. Nasopharyngeal carcinoma in children and adolescents ‐ a single institution experience of 158 patients. Radiation Oncology 2014;9:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahoney 1982 {published data only}
- Mahoney DH Jr, Weaver T, Steuber CP, Starling KA. Cis‐platinum (CDDP) ototoxicity in pediatric patients. Proceedings of the American Society of Clinical Oncology. 1982.
Mahoney 1983 {published data only}
- Mahoney DH Jr, Weaver T, Steuber CP, Starling KA. Ototoxicity with cisplatin therapy. Journal of Pediatrics 1983;103(6):1006‐7. [DOI] [PubMed] [Google Scholar]
Manfredini 1996 {published data only}
- Manfredini L, Garaventa A, Barra S, Caro E, Picco P, Faraci M, et al. Health status of long term survivors after myeloablative therapy and bone marrow transplantation in children. Bone Marrow Transplantation 1996;18 Suppl 2:163‐4. [PubMed] [Google Scholar]
Mann 2000 {published data only}
- Mann JR, Raafat F, Robinson K, Imeson J, Gornall P, Sokal M, et al. The United Kingdom Children's Cancer Study Group's second germ cell tumor study: carboplatin, etoposide, and bleomycin are effective treatment for children with malignant extracranial germ cell tumors, with acceptable toxicity. Journal of Clinical Oncology 2000;18(22):3809‐18. [DOI] [PubMed] [Google Scholar]
Marshall 2006 {published data only}
- Marshall NE, Ballman KV, Michalak JC, Schomberg PJ, Burton GV, Sandler HM, et al. Ototoxicity of cisplatin plus standard radiation therapy vs. accelerated radiation therapy in glioblastoma patients. Journal of Neuro‐Oncology 2006;77(3):315‐20. [DOI] [PubMed] [Google Scholar]
Mbue 2007 {published data only}
- Mbue JE, Solimando DA Jr, Waddell JA. Doxorubicin, cisplatin, high‐dose methotrexate, and ifosfamide for osteosarcoma. Hospital Pharmacy 2007;42:801‐15. [Google Scholar]
McHaney 1983 {published data only}
- McHaney VA, Thibadoux G, Hayes FA, Green AA. Hearing loss in children receiving cisplatin chemotherapy. Journal of Pediatrics 1983;102(2):314‐7. [DOI] [PubMed] [Google Scholar]
Meyers 2005 {published data only}
- Meyers PA, Schwartz CL, Krailo M, Kleinerman ES, Betcher D, Bernstein ML, et al. Osteosarcoma: a randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high‐dose methotrexate. Journal of Clinical Oncology 2005;23:2004‐11. [DOI] [PubMed] [Google Scholar]
Montero 2005 {published data only}
- Montero A, Hervas A, Morera R, Sancho S, Cordoba S, Corona JA, et al. Control of chronic symptoms. Secondary effects of treatments with radiotherapy and chemotherapy. Oncologia 2005;28:41‐50. [Google Scholar]
Nageswara 2011 {published data only}
- Nageswara RAA, Wallace D, Boyett J, Gajjar A, Packer RJ. Cumulative cisplatin dose (CCD) does not correlate with event free (EFS) and overall survival (OS) outcomes in children with newly diagnosed average‐risk medulloblastoma (ARMB) treated with cisplatin based adjuvant chemotherapy. Annals of Neurology Conference. 2011:S118‐9.
Nageswara Rao 2011 {published data only}
- Nageswara Rao AA, Wallace D, Boyett J, Gajjar A, Packer RJ. Cumulative cisplatin dose does not correlate with event‐free and overall survival outcomes in children with newly diagnosed average‐risk medulloblastoma treated with cisplatin‐based adjuvant chemotherapy (PC‐07). Annual Scientific Meeting of the Society for Neuro‐Oncology. 2011:iii96. [DOI] [PMC free article] [PubMed]
Nageswara Rao 2014 {published and unpublished data}
- Nageswara Rao AA, Wallace DJ, Billups C, Boyett JM, Gajjar A, Packer RJ. Cumulative cisplatin dose is not associated with event‐free or overall survival in children with newly diagnosed average‐risk medulloblastoma treated with cisplatin based adjuvant chemotherapy: report from the Children's Oncology Group. Pediatric Blood & Cancer 2014;61(1):102‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nichols 1991 {published data only}
- Nichols CR, Williams SD, Loehrer PJ, Greco FA, Crawford ED, Weetlaufer J, et al. Randomized study of cisplatin dose intensity in poor‐risk germ cell tumors: a Southeastern Cancer Study Group and Southwest Oncology Group protocol. Journal of Clinical Oncology 1991;9(7):1163‐72. [DOI] [PubMed] [Google Scholar]
Packer 1991 {published data only}
- Packer RJ, Sutton LN, Goldwein JW, Perilongo G, Bunin G, Ryan J, et al. Improved survival with the use of adjuvant chemotherapy in the treatment of medulloblastoma. Journal of Neurosurgery 1991;74:433‐40. [DOI] [PubMed] [Google Scholar]
Packer 2006 {published data only}
- Packer RJ, Gajjar A, Vezina G, Rorke‐Adams L, Burger PC, Robertson PL, et al. Phase III study of craniospinal radiation therapy followed by adjuvant chemotherapy for newly diagnosed average‐risk medulloblastoma. Journal of Clinical Oncology 2006;24:4202‐8. [DOI] [PubMed] [Google Scholar]
Pearson 2008 {published data only}
- Pearson AD, Pinkerton CR, Lewis IJ, Imeson J, Ellershaw C, Machin D. High‐dose rapid and standard induction chemotherapy for patients aged over 1 year with stage 4 neuroblastoma: a randomised trial. Lancet Oncology 2008;9:247‐56. [DOI] [PubMed] [Google Scholar]
Pendergrass 1987 {published data only}
- Pendergrass TW, Milstein JM, Geyer JR, Mulne AF, Kosnik EJ, Morris JD, et al. Eight drugs in one day chemotherapy for brain tumors: experience in 107 children and rationale for preradiation chemotherapy. Journal of Clinical Oncology 1987;5(8):1221‐31. [DOI] [PubMed] [Google Scholar]
Perilongo 2004 {published data only}
- Perilongo G, Shafford E, Maibach R, Aronson D, Brugières L, Brock P, et al. Risk‐adapted treatment for childhood hepatoblastoma. final report of the second study of the International Society of Paediatric Oncology‐SIOPEL 2. European Journal of Cancer 2004;40(3):411‐21. [DOI] [PubMed] [Google Scholar]
Pritchard 2000 {published data only}
- Pritchard J, Brown J, Shafford E, Perilongo G, Brock P, Dicks‐Mireaux C, et al. Cisplatin, doxorubicin, and delayed surgery for childhood hepatoblastoma: a successful approach ‐ results of the first prospective study of the International Society of Pediatric Oncology. Journal of Clinical Oncology 2000;18:3819‐28. [DOI] [PubMed] [Google Scholar]
Punyko 2005 {published data only}
- Punyko JA, Mertens AC, Gurney JG, Yasui Y, Donaldson SS, Rodeberg DA, et al. Long‐term medical effects of childhood and adolescent rhabdomyosarcoma: a report from the childhood cancer survivor study. Pediatric Blood & Cancer 2005;44:643‐53. [DOI] [PubMed] [Google Scholar]
Pussegoda 2013 {published data only}
- Pussegoda K, Ross CJ, Visscher H, Yazdanpanah M, Brooks B, Rassekh SR, et al. Replication of TPMT and ABCC3 genetic variants highly associated with cisplatin‐induced hearing loss in children. Clinical Pharmacology and Therapeutics 2013;94(2):243‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raney 1999 {published data only}
- Raney RB, Asmar L, Vassilopoulou‐Sellin R, Klein MJ, Donaldson SS, Green J, et al. Late complications of therapy in 213 children with localized, nonorbital soft‐tissue sarcoma of the head and neck: a descriptive report from the Intergroup Rhabdomyosarcoma Studies (IRS)‐II and ‐ III. IRS Group of the Children's Cancer Group and the Pediatric Oncology Group. Medical and Pediatric Oncology 1999;33(4):362‐71. [DOI] [PubMed] [Google Scholar]
Rassekh 2009 {published data only}
- Rassekh R, Ross C, Brooks B, Katzov H, Dube MP, Carleton B, et al. Identification of genetic markers of severe hearing loss in children receiving cisplatin. Pediatric Blood & Cancer 2009:733. [Google Scholar]
Rednam 2012 {published data only}
- Rednam S, Scheurer M, Adesina A, Lau C, Okcu M. Glutathione s‐transferase P1 single nucleotide polymorphism predicts permanent ototoxicity in children with medulloblastoma (i125). International Symposium on Pediatric Neuro‐Oncology. 2012. [DOI] [PMC free article] [PubMed]
Rednam 2013 {published data only}
- Rednam S, Scheurer ME, Adesina A, Lau CC, Okcu MF. Glutathione S‐transferase P1 single nucleotide polymorphism predicts permanent ototoxicity in children with medulloblastoma. Pediatric Blood & Cancer 2013;60(4):593‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roark 2003 {published data only}
- Roark KM, Waddell JA, Solimando DA Jr. Bleomycin, etoposide, and cisplatin (BEP) regimen for testicular cancer. Hospital Pharmacy 2003;38:638‐44. [Google Scholar]
Rosen 1984 {published data only}
- Rosen EM, Cassady JR, Frantz CN. Improved survival in neuroblastoma using multimodality therapy. Radiotherapy and Oncology 1984;2:189‐200. [DOI] [PubMed] [Google Scholar]
Ross 2009 {published and unpublished data}
- Ross CJ, Katzov‐Eckert H, Dubé MP, Brooks B, Rassekh SR, Barhdadi A, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nature Genetics 2009;41(12):1345‐9. [DOI] [PubMed] [Google Scholar]
Rutledge 2007 {published data only}
- Rutledge MR, Solimando DA Jr, Waddell JA. Doxorubicin and cisplatin regimen for high‐grade osteosarcoma or malignant fibrous histiocytoma (MFH) of the bone. Hospital Pharmacy 2007;42:605‐16. [Google Scholar]
Sanz 1994 {published data only}
- Sanz MA, Bonanad S, Sanz GF, Martín G. Carboplatin and etoposide in acute myeloid leukemia. Leukemia 1994;8(9):1599‐600. [PubMed] [Google Scholar]
Sawaguchi 1990 {published data only}
- Sawaguchi S, Kaneko M, Uchino J, Takeda T, Iwafuchi M, Matsuyama S, et al. Treatment of advanced neuroblastoma with emphasis on intensive induction chemotherapy. A report from the Study Group of Japan. Cancer 1990;66(9):1879‐87. [DOI] [PubMed] [Google Scholar]
Sawamura 1998 {published data only}
- Sawamura Y, Ikeda J, Shirato H, Tada M, Abe H. Germ cell tumours of the central nervous system: treatment consideration based on 111 cases and their long‐term clinical outcomes. European Journal of Cancer 1998;34:104‐10. [DOI] [PubMed] [Google Scholar]
Schell 1989 {published data only}
- Schell MJ, McHaney VA, Green AA, Kun LE, Hayes FA, Horowitz M, et al. Hearing loss in children and young adults receiving cisplatin with or without prior cranial irradiation. Journal of Clinical Oncology 1989;7(6):754‐60. [DOI] [PubMed] [Google Scholar]
Schreiber 2014 {published data only}
- Schreiber JE, Gurney JG, Palmer SL, Bass JK, Wang M, Chen S, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro‐Oncology 2014;16(8):1129‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sefi 2013 {published and unpublished data}
- Sefi E, Brock P, Chang K, Neuwelt E, Knight K, Rajput K. Validation of the SIOP Boston ototoxicity scale ‐ an international scale for the evaluation of platinum‐induced ototoxicity in children (P‐0486). Pediatric Blood & Cancer. 2013:167.
Singh Chauhan 2011 {published data only}
- Singh Chauhan R, Saxena RK, Varshey S. The role of ultrahigh‐frequency audiometry in the early detection of systemic drug‐induced hearing loss. Ear Nose & Throat Journal 2011;90(5):218‐22. [DOI] [PubMed] [Google Scholar]
Soomal 2003 {published data only}
- Soomal R, Saran F, Brada M. In regard to Huang et al.: intensity‐modulated radiation therapy for pediatric medulloblastoma: early report on the reduction of ototoxicity. IJROBP 2002;52:599‐605. International Journal of Radiation Oncology Biology Physics 2003;55(3):853. [DOI] [PubMed] [Google Scholar]
Souhami 1997 {published data only}
- Souhami RL, Craft AW, Eijken JW, Nooij M, Spooner D, Bramwell VHC, et al. Randomised trial of two regimens of chemotherapy in operable osteosarcoma: a study of the European Osteosarcoma Intergroup. Lancet 1997;350:911‐7. [DOI] [PubMed] [Google Scholar]
Spracklen 2014 {published data only}
- Spracklen TF, Whitehorn H, Vorster AA, Ramma L, Dalvie S, Ramesar RS. Genetic variation in Otos is associated with cisplatin‐induced ototoxicity. Pharmacogenomics 2014;15(13):1667‐76. [DOI] [PubMed] [Google Scholar]
Steinherz 1977 {published data only}
- Steinherz P, Tan C, Ghavimi F, Helson L, Rosen G, Wollner N. VAB III combination chemotherapy in childhood malignancies (C‐231). Proceedings of the American Association for Cancer Research. 1977.
Stewart 1981 {published data only}
- Stewart D, Wallace S, Leavens M. Phase I study of intracarotid (IC) cis‐diamminedichloroplatinum (DDP) in patients with intracerebral tumors (ICT). Proceedings of the American Association for Cancer Research. 1981; Vol. 22:749.
Suita 1994 {published data only}
- Suita S, Zaizen Y, Kaneko M, Uchino J, Takeda T, Iwafuchi M, et al. What is the benefit of aggressive chemotherapy for advanced neuroblastoma with N‐myc amplification? A report from the Japanese study group for the treatment of advanced neuroblastoma. Journal of Pediatric Surgery 1994;29:746‐50. [DOI] [PubMed] [Google Scholar]
Tseng 1987 {published data only}
- Tseng A Jr, Jacobs C, Coleman CN, Horning SJ, Lewis BJ, Rosenberg SA. Treatment of refractory non‐Hodgkin's lymphomas of unfavorable histology with teniposide, cytarabine, and cisplatin. Cancer Treatment Reports 1987;71:659‐60. [PubMed] [Google Scholar]
Umeda 1986 {published data only}
- Umeda T, Takada N, Hodaka E. Toxic effects of cisplatin in treatment of malignant bone and soft tissue tumors. Japanese Journal of Cancer and Chemotherapy 1986;13:1857‐61. [PubMed] [Google Scholar]
Van Maldegem 2015 {published data only}
- Maldegem AM, Benson C, Rutkowski P, Blay JY, Berg H, Placzke J, et al. Etoposide and carbo‐ or cisplatin combination therapy in refractory or relapsed Ewing sarcoma: a large retrospective study. Pediatric Blood & Cancer 2015;62(1):40‐4. [DOI] [PubMed] [Google Scholar]
Veal 2012 {published data only}
- Veal GJ, Boddy AV. Carboplatin dosing in infants with retinoblastoma: a case for therapeutic drug monitoring. Journal of Clinical Oncology 2012;30(27):3424. [DOI] [PubMed] [Google Scholar]
Von Heyden 1982 {published data only}
- Heyden HW, Scherpe A, Nagel GA. Cis‐dichlorodiammineplatinum (II) (cis‐platinum) and etoposide for patients with refractory lymphomas. Cancer Treatment Reviews 1982;9:45‐52. [DOI] [PubMed] [Google Scholar]
Von Hoff 2009 {published data only}
- Hoff K, Hinkes B, Gerber NU, Deinlein F, Mittler U, Urban C, et al. Long‐term outcome and clinical prognostic factors in children with medulloblastoma treated in the prospective randomised multicentre trial HIT'91. European Journal of Cancer 2009;45:1209‐17. [DOI] [PubMed] [Google Scholar]
Voskens 2012 {published data only}
- Voskens C, Cavallaro A, Erdmann M, Dippel O, Kaempgen E, Schuler G, et al. Anti‐cytotoxic T‐cell lymphocyte antigen‐4‐induced regression of spinal cord metastases in association with renal failure, atypical pneumonia, vision loss, and hearing loss. Journal of Clinical Oncology 2012;30(33):e356‐7. [DOI] [PubMed] [Google Scholar]
Whelan 2011 {published data only}
- Whelan K, Stratton K, Kawashima T, Leisenring W, Hayashi S, Waterbor J, et al. Auditory complications in childhood cancer survivors: a report from the childhood cancer survivor study. Pediatric Blood & Cancer 2011;57(1):126‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitehorn 2014 {published data only}
- Whitehorn H, Sibanda M, Lacerda M, Spracklen T, Ramma L, Dalvie S, et al. High prevalence of cisplatin‐induced ototoxicity in Cape Town, South Africa. South African Medical Journal 2014;104(4):288‐91. [DOI] [PubMed] [Google Scholar]
Winkler 1990 {published data only}
- Winkler K, Bielack S, Delling G, Salzer‐Kuntschik M, Kotz R, Greenshaw C, et al. Effect of intraarterial versus intravenous cisplatin in addition to systemic doxorubicin, high‐dose methotrexate, and ifosfamide on histologic tumor response in osteosarcoma (study COSS‐86). Cancer 1990;66(8):1703‐10. [DOI] [PubMed] [Google Scholar]
Winkler 1993 {published data only}
- Winkler K, Bielack SS, Delling G, Jurgens H, Kotz R, Salzer‐Kuntschik M. Treatment of osteosarcoma: experience of the Cooperative Osteosarcoma Study Group (COSS). Cancer Treatment and Research 1993;62:269‐77. [DOI] [PubMed] [Google Scholar]
Xu 2015 {published data only}
- Xu H, Robinson GW, Huang J, Lim JY, Zhang H, Bass JK, et al. Common variants in ACYP2 influence susceptibility to cisplatin‐induced hearing loss. Nature Genetics 2015;47(3):263‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yancey 2010 {published data only}
- Yancey A, Harris M, Egbelakin A, Gilbert J, Pisoni D, Renbarger J. Gender is a predictor of cisplatin ototoxicity. Pediatric Blood & Cancer 2010:851. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yancey 2012 {published data only}
- Yancey A, Harris MS, Egbelakin A, Gilbert J, Pisoni DB, Renbarger J. Risk factors for cisplatin‐associated ototoxicity in pediatric oncology patients. Pediatric Blood & Cancer 2012;59(1):144‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2013 {published data only}
- Yang JJ, Lim JY, Huang J, Bass J, Wu J, Wang C, et al. The role of inherited TPMT and COMT genetic variation in cisplatin‐induced ototoxicity in children with cancer. Clinical Pharmacology and Therapeutics 2013;94(2):252‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zage 2008 {published data only}
- Zage PE, Kletzel M, Murray K, Marcus R, Castleberry R, Zhang Y, et al. Outcomes of the POG 9340/9341/9342 trials for children with high‐risk neuroblastoma: a report from the children's oncology group. Pediatric Blood & Cancer 2008;51:747‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baum 1981 {published data only}
- Baum ES, Gaynon P, Greenberg L, Krivit W, Hammond D. Phase II trial of cisplatin in refractory childhood cancer: Children's Cancer Study Group Report. Cancer Treatment Reports 1981;65(9‐10):815‐22. [PubMed] [Google Scholar]
Clemens 2015 {published data only}
- Clemens E, Vries AC, Dulmen‐den Broeder E, Grotel M, Pluijm SF, Tissing WJ, et al. The influence of co‐medication on platinum‐related ototoxicity in long‐term survivors of childhood cancer: a descriptive DCOG study. 14th International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer. 2015:69 (abstract 111).
Clerico 2010 {published data only}
- Clerico A, Robert M, Marucci G, D'Attilia FR, Dionne V, Pecoraro R, et al. Preirradiation carboplatin and etoposide in the treatment of paediatric medulloblastoma in a single institution. Pediatric Blood & Cancer 2010:1005. [Google Scholar]
Finlay 2009 {published data only}
- Finlay JL, Haley K, Dhall G, Fangusaro J, Chi S, Allen J, et al. Management of young children newly diagnosed with CNS embryonal tumors: 18 years of three sequential irradiation‐avoiding chemotherapy studies ‐ The "Head Start" protocols. Neuro‐Oncology Conference 2009:875‐6. [Google Scholar]
Geyer 2005 {published data only}
- Geyer JR, Sposto R, Jennings M, Boyett JM, Axtell RA, Breiger D, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: a report from the Children's Cancer Group. Journal of Clinical Oncology 2005;23:7621‐31. [DOI] [PubMed] [Google Scholar]
Hiyama 2013a {published data only}
- Hiyama E, Kamimatsuse A, Onitake Y, Ueda Y, Watanabe K, Hishiki T, et al. Survival, surgical resectability, and late effects in the hepatoblastoma patients treated by cisplatin plus pirarubicin (CITA) chemotherapy (10038). Annual Meeting of the American Society of Clinical Oncology 2013. [Google Scholar]
Knight 2014 {published data only}
- Knight K, Middaugh J, Fu R, Neuwelt E, Winter C. Long term audiologic outcomes in children treated with platinum chemotherapy. Pediatric Blood & Cancer 2014:S196. [Google Scholar]
Korzeniewska 2009 {published data only}
- Korzeniewska J, Dembowska‐Baginska B, Drogosiewicz M, Perek‐Polnik M, Perek D. Sensory status of childhood brain tumors survivors (PQ.002). Pediatric Blood & Cancer 2009:853. [Google Scholar]
Kuhl 1998 {published data only}
- Kuhl J, Muller HL, Berthold F, Kortmann RD, Deinlein F, Maass E, et al. Preradiation chemotherapy of children and young adults with malignant brain tumors: results of the German pilot trial HIT'88/'89. Klinische Padiatrie 1998;210(4):227‐33. [DOI] [PubMed] [Google Scholar]
Kushner 2006 {published and unpublished data}
- Kushner BH, Budnick A, Kramer K, Modak S, Cheung NK. Ototoxicity from high‐dose use of platinum compounds in patients with neuroblastoma. Cancer 2006;107(2):417‐22. [DOI] [PubMed] [Google Scholar]
Lannering 2012 {published and unpublished data}
- Lannering B, Rutkowski S, Doz F, Pizer B, Gustafsson G, Navajas A, et al. Hyperfractionated versus conventional radiotherapy followed by chemotherapy in standard‐risk medulloblastoma: results from the randomized multicenter HIT‐SIOP PNET 4 trial. Journal of Clinical Oncology 2012;30(26):3187‐93. [DOI] [PubMed] [Google Scholar]
Merchant 2011 {published data only}
- Merchant TE, Hua C, Bass JK, Wu S, Xiong X, Gajjar A. The impact of ototoxic chemotherapy on hearing loss after radiation therapy in children with brain tumors. International Journal of Radiation Oncology, Biology, Physics 2011:S113‐4. [Google Scholar]
Nirenberg 1981 {published data only}
- Nirenberg A, Cacavio A, Bru G, Gaparros B, Rosen G. Ambulatory high dose cisplatinum (HD‐DDP) with mannitol diuresis: a treatment system without renal, auditory or biochemical toxicity C‐766. Proceedings of the American Association for Cancer Research. 1981.
Ohnuma 1995 {published data only}
- Ohnuma N, Takahashi H, Kaneko M, Uchino J‐I, Takeda T, Iwafuchi M, et al. Treatment combined with bone marrow transplantation for advanced neuroblastoma: an analysis of patients who were pretreated intensively with the protocol of the study group of Japan. Medical and Pediatric Oncology 1995;24:181‐7. [DOI] [PubMed] [Google Scholar]
Vos 2014 {published data only}
- Vos HI, Coenen MJ, Gelderblom H, Straaten T, Bont ES, Graaf WT, et al. Association of genetic variants involved in drug metabolism and transport with efficacy and toxicity of chemotherapeutic treatment in osteosarcoma patients. Journal of Clinical Oncology 2014;32: 5s:abstract 10038. [Google Scholar]
Weiss 2015 {published data only}
- Weiss A, Kasteler R, Kuonen R, Wengenroth L, Scheinemann K, Grotzer M, et al. Hearing impairment in Swiss childhood cancer survivors. 14th International Conference on Long‐Term Complications of Treatment of Children and Adolescents for Cancer. 2015:69‐70 (abstract 112).
Additional references
Bass 2014a
- Bass JK, Bhagat SP. Challenges in ototoxicity monitoring in the pediatric oncology population. Journal of the American Academy of Audiology 2014;25(8):760‐74. [DOI] [PubMed] [Google Scholar]
Cancer in Children 2005
- Jenney MEM. Late effects of cancer treatment and current protective measures. In: Voûte PA, Barett A, Stevens MCG, Caron HN editor(s). Cancer in Children. 5th Edition. Oxford: Oxford University Press, 2005:123‐37. [Google Scholar]
Dean 2008
- Dean JB, Hayashi SS, Albert CM, King AA, Karzon R, Hayashi RJ. Hearing loss in pediatric oncology patients receiving carboplatin‐containing regimens. Journal of Pediatric Hematology/Oncology 2008;30(2):130‐4. [DOI] [PubMed] [Google Scholar]
Eloxatin SPC
- Eloxatin Summary of Product Characteristics. www.sanofi‐aventis.co.uk/products/Eloxatin_SPC.pdf (accessed 2 March 2010).
Gallagher 1979
- Gallagher KL, Jones JK. Furosemide‐induced ototoxicity. Annals of Internal Medicine 1979;91(1):744‐5. [DOI] [PubMed] [Google Scholar]
Gietema 2000
- Gietema JA, Meinardi MT, Messerschmidt J, Gelevert T, Alt F, Uges DR, et al. Circulating plasma platinum more than 10 years after cisplatin treatment for testicular cancer. Lancet 2000;355(9209):1075‐6. [DOI] [PubMed] [Google Scholar]
Gregg 2004
- Gregg RB, Wiorek LS, Arvedson JC. Pediatric audiology: a review. Pediatrics in Review 2004;25(7):224‐33. [DOI] [PubMed] [Google Scholar]
Grimes 2002
- Grimes DA, Schulz KF. Cohort studies: marching towards outcomes. Lancet 2002;359(9303):341‐5. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kadota 1994
- Kadota RP, Mandell LR, Fontanesi J, Kovnar EH, Krischer J, Kun LE, et al. Hyperfractionated irradiation and concurrent cisplatin in brain stem tumors: a Pediatric Oncology Group pilot study (9139). Pediatric Neurosurgery 1994;20(4):221‐5. [DOI] [PubMed] [Google Scholar]
Knight 2005
- Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. Journal of Clinical Oncology 2005;23(34):8588‐96. [DOI] [PubMed] [Google Scholar]
Laupacis 1994
- Laupacis A, Wells G, Richardson WS, Tugwell P. Users' guides to the medical literature. V. How to use an article about prognosis. Evidence‐Based Medicine Working Group. JAMA 1994;272(3):234‐7. [DOI] [PubMed] [Google Scholar]
Leahey 2012
- Leahey A. A cautionary tale: dosing chemotherapy in infants with retinoblastoma. Journal of Clinical Oncology 2012;30(10):1023‐4. [DOI] [PubMed] [Google Scholar]
NHS Information
- National Health Service. Hearing loss ‐ causes. www.nhs.uk/Conditions/hearing‐impairment/Pages/causes.aspx (accessed 18 July 2016).
Qaddoumi 2012
- Qaddoumi I, Bass JK, Wu J, Billups CA, Wozniak AW, Merchant TE, et al. Carboplatin‐associated ototoxicity in children with retinoblastoma. Journal of Clinical Oncology 2012;30(10):1034‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reddel 1982
- Reddel RR, Kefford RF, Grant JM, Coates AS, Fox RM, Tattersall MH. Ototoxicity in patients receiving cisplatin: importance of dose and method of drug administration. Cancer Treatment Reports 1982;66(1):19‐23. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Skinner 2004
- Skinner R. Best practice in assessing ototoxicity in children with cancer. European Journal of Cancer 2004;40(16):2352‐4. [DOI] [PubMed] [Google Scholar]
Van As 2012a
- As JW, Berg H, Dalen EC. Medical interventions for the prevention of platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD009219.pub2] [DOI] [PubMed] [Google Scholar]
Van As 2014a
- As JW, Berg H, Dalen EC. Different infusion durations for preventing platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD010885.pub2] [DOI] [PubMed] [Google Scholar]
Van As 2014b
- As JW, Berg H, Dalen EC. Medical interventions for the prevention of platinum‐induced hearing loss in children with cancer. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009219.pub3] [DOI] [PubMed] [Google Scholar]
Veal 2001
- Veal GJ, Dias C, Price L, Parry A, Errington J, Hale J, et al. Influence of cellular factors and pharmacokinetics on the formation of platinum‐DNA adducts in leukocytes of children receiving cisplatin therapy. Clinical Cancer Research 2001;7(8):2205‐12. [PubMed] [Google Scholar]
References to other published versions of this review
Van As 2012b
- As JW, Berg H, Dalen EC. Platinum‐induced hearing loss after treatment for childhood cancer. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD010181] [DOI] [PMC free article] [PubMed] [Google Scholar]


