Abstract
Introduction
Only a minority of patients with a positive fecal occult blood test (FOBT) undergo a follow-up second diagnostic procedure, thus minimizing its contribution for colorectal cancer (CRC) prevention. We aimed to obtain a precise estimation of this problem and also assess the diagnostic yield of CRC and adenomas by colonoscopy in these patients.
Methods
Literature searches were conducted for “compliance” OR “adherence” AND “fecal occult blood test” OR “fecal immunohistochemical test” AND “colonoscopy.” Comprehensive meta-analysis software was used.
Results
The search resulted in 42 studies (512,496 patients with positive FOBT), published through December 31, 2017. A funnel plot demonstrates a moderate publication bias. Compliance with any second procedure, colonoscopy, or combination of double-contrast barium enema with or without sigmoidoscopy in patients with a positive FOBT was 0.725 with 95% confidence interval (CI) 0.649–0.790 (p = 0.000), 0.804 with 95% CI 0.740–0.856 (p = 0.000) and 0.197 with 95% CI 0.096–0.361 (p = 0.000), respectively. The diagnostic yield for CRC, advanced adenoma and simple adenoma was 0.058 with 95% CI 0.050–0.068 (p = 0.000), 0.242 with 95% CI 0.188–0.306 (p = 0.000) and 0.147 with 95% CI 0.116–0.184 (p < 0.001), respectively.
Discussion
Compliance with diagnostic evaluation after a positive FOBT is still suboptimal. Therefore, measures to increase compliance need to be taken given the increased risk of CRC in these patients.
Keywords: Compliance rate, CRC prevention, diagnostic yield, fecal occult blood test, follow-up colonoscopy
Current knowledge
Worldwide, colorectal cancer (CRC) screening programs have been implemented and resulted in CRC incidence and mortality reduction.
Choosing fecal occult blood test (FOBT) as a screening strategy requires subsequent intervention, usually by full colonoscopy examination.
Only a minority of positive FOBTs are followed by an adequate evaluation, a suboptimal condition that undermines the benefits of undergoing the screening test itself.
New findings in this study
Compliance rates with any second procedure, colonoscopy, or combination of double-contrast barium enema with or without sigmoidoscopy in patients with a positive fecal occult blood test (FOBT) are 0.725, 0.804 and 0.197, respectively.
Worldwide, over the past 25 years, the compliance rate for positive FOBT has improved. Still, incomplete follow-up after positive FOBT has been and remains a major problem.
Measures need to be taken in each national screening program to increase this specific compliance rate thus reducing the incidence and mortality rates from colorectal cancer.
Introduction
Colorectal cancer (CRC) is the second leading cause of death from malignancies in developed countries. In the United States (US), about 50,000 people die from the disease each year.1 Fortunately, however, in contrast to other malignancies, CRC can be prevented when premalignant polyps are removed following their detection by an efficient screening. Therefore, CRC screening programs have been implemented worldwide in the last two decades,2 resulting in a steady reduction in the incidence and mortality from CRC.3 Despite these beneficial effects, only 60% to 65% of people older than 50 years actually participate in CRC screening.4 There are multiple screening modalities. As opposed to the European guidelines on CRC screening that recommend screening by fecal immunochemical test (FIT), recently, based on the results of a modeling study the US Preventive Services Task Force updated its guidelines and listed eight optional screening strategies. The purpose of this update was to encourage testing, assuming that offering a variety of screening programs would make it easier for patients to choose their preferred program, thus improving compliance rates. The problem is that improvement in participation rate of CRC screening is not enough. Choosing fecal occult blood test (FOBT) as a screening strategy requires subsequent intervention, usually by full colon examination.3 However, according to several studies, only 25%5 to 55%6 of positive FOBTs are followed by an adequate evaluation, suggesting a suboptimal condition. Failure to complete an appropriate evaluation after a positive result can undermine the benefits of undergoing the screening test itself and minimize its potential contribution to the reduction of incidence and mortality. While many studies had already investigated the compliance rate for colorectal screening with FOBT, the second compliance rate, for the colonoscopy itself, in patients with a positive FOBT, has never been thoroughly studied. Therefore, to obtain a precise estimation of this problem, our study represents the first quantitative meta-analysis of the compliance rate for the second diagnostic evaluation after a positive FOBT (primary aim). In addition, we explored the diagnostic yield of CRC and adenomas in patients with a positive FOBT (intention-to-screen analysis) by colonoscopy (per protocol analysis) (secondary aim).
Methods
Preferred reporting items for systematic reviews and meta-analyses guidelines were strictly followed.7 A local protocol for conducting a meta-analysis was applied based on previous descriptions.8 A data-extraction table was used (available on request).
Data sources and searches
We conducted a comprehensive search of PubMed, MEDLINE, Scopus, EMBASE, and CENTRAL through December 31, 2017. We used the following keywords and medical heading terms: “compliance” OR “adherence” AND “fecal occult blood test” OR “fecal immunohistochemical test” AND “colonoscopy.” Manual searches of articles’ bibliographies were also performed. Only fully published, controlled studies in English were included. The exclusion criteria were duplications, review articles, mathematical models, opinion papers and editorials, high-risk patients or screening of other malignancies, data presented in percentages, and articles based on questionnaires or not related to screening.
Study selection
Three authors (R.G.B., H.L. and D.B.) independently reviewed the pertinent studies to determine their eligibility. Only studies that met all the following criteria were included: reported the precise compliance rate of second diagnostic evaluation of patients with a positive FOBT, reported which diagnostic evaluation was applied, evaluated population without risk factors for CRC, and evaluated adult patients older than 18 years.
Data extraction and quality assessment
Three authors (R.G.B., H.L. and D.B.) independently evaluated and extracted relevant information from each included study. The extracted data were summarized into an included standardized table: name of the first author, year of the study publication, FOBT used, including the cutoff value for positive result when FIT was used (if specified), number of participants, including participation rate (if detailed), number of patients with positive FOBTs, and number of patients with a positive FOBT who underwent a second diagnostic evaluation. The advantages and disadvantages of each included study were briefly abstracted and are included too (Table 1). When reported, the authors also extracted data about the numbers of CRC, advanced adenoma or simple adenoma cases, diagnosed by the second diagnostic evaluation. Advanced adenoma was defined as adenomas ≥10 mm, adenoma with a villous component >20%, or adenomas with severe/high-grade dysplasia, while adenomas <10 mm without a villous component or severe/high-grade dysplasia were classified as simple adenoma, based on the World Health Organization criteria.9
Table 1.
Summary of included studies.
| First author, year, countryreference | Number of participants (participation rate) | Positive FOBT (%) | Follow-up diagnostic evaluation (%) | Advantage | Disadvantage |
|---|---|---|---|---|---|
| gFOBT | |||||
| Population-based clinical trials | |||||
| Kronborg, 1996, Denmark (first round)10 | 20,672 (67%) | 215 (1.04) | 180a (83.72) | Randomized study comparing mortality rates after FOBTs every two years during 10-year period with unscreened controls (representing general population). | Used biennial Hemoccult-II test without rehydration; thus a low rate of positivity tests and high rate of interval cancers. |
| Kronborg, 1996, Denmark (first round)10 | 20,672 (67%) | 35 (0.17) | 12b (34.29) | g | g |
| Kronborg, 1996, Denmark (second round)10 | 18,781 (93%) | 159 (0.85) | 142a (89.31) | g | g |
| Kronborg, 1996, Denmark (second round)10 | 18,781 (93%) | 17 (0.09) | 5b (29.41) | g | g |
| Kronborg, 1996, Denmark (third round)10 | 17,279 (94%) | 151 (0.87) | 124a (82.12) | g | g |
| Kronborg, 1996, Denmark (third round)10 | 17,279 (94%) | 27 (0.16) | 15b (55.56) | g | g |
| Kronborg, 1996, Denmark (fourth round)10 | 15,845 (94%) | 200 (1.26) | 168a (84) | g | g |
| Kronborg, 1996, Denmark (fourth round)10 | 15,845 (94%) | 32 (0.2) | 16b (50) | g | g |
| Kronborg, 1996, Denmark (fifth round)10 | 14,203 (92%) | 261 (1.84) | 213a (81.61) | g | g |
| Kronborg, 1996, Denmark (fifth round)10 | 14,203 (92%) | 48 (0.34) | 17b (35.42) | g | g |
| Mandel, 2000, USA11 | 31,082 | 13,833 (44.5) | 11,532c (83.37) | Large sample size; comparison of two screening programs strategies; long follow-up. | Individuals in screening groups may have participated in some screening beyond that offered in study. |
| Niv, 2002, Israel12 | 2538 (71.5%) | 231 (9.1) | 231a (100) | All participants were from small and stable communities of northern Israel. First time that screening with FOBT proved successful in reducing mortality for CRC in a relatively small but well-defined population. | No difference in incidence of CRC in FOBT group compared with refusers and controls. |
| Ko, 2003, USA13 | 1369 (47%) | 122 (8.91) | 64a (52.46) | Compared patient compliance and characteristics of gFOBT vs FIT. | Unexpectedly demonstrated similar positivity rate and positive predictive value for two types of tests. |
| van Rossum, 2008, Netherlands14 | 4836 (46.9%) | 117 (2.42) | 103a (88.03) | Random comparison of two screening program strategies. | Method used for estimating specificity slightly overestimates true specificity. |
| Singh, 2009, USA15 | – | 749 (–) | 271a (36.18) | Implemented quality improvement activities to improve follow-up of FOBT-positive results. | Colonoscopy not indicated in approximately one-third of patients with positive FOBT. |
| Singh, 2009, USA15 | – | 478h (–) | 13e (2.72) | g | g |
| Singh, 2009,USA15 | – | 465 (–) | 1f (0.22) | g | g |
| Levi, 2011, Israel16 | 2266 (28.8%) | 88 (3.88) | 63a (71.59) | Prospective, controlled study; follow-up of two years after end of study. | Randomization by clinic site, with only nine clinics studied, and not by patients. |
| Moss, 2012, UK17 | 49,311 (61.8%) | 651 (1.32) | 512a (78.65) | Population-based study on performance of repeated FIT screening with three rounds. | No access to patient-level identifiable data; colonoscopy uptake may be underestimated in analyses because of lack of information on reasons for nonreferral or attendance in those with positive FOBT. |
| Inadomi, 2012, USA18 | 353 | 8 (2.27) | 5a (62.5) | Randomized clinical trial comparing three screening programs in racially/ethnically diverse population. | Potential generalizability bias; single-point observation of adherence and potential residual system barriers to colonoscopy performance. |
| Scholefield, 2012, UK19 | 43,585 (57%) | 2050 (4.7) | 1474a (71.9) | Twenty-year follow-up of a large, randomized trial demonstrated sustained reduction in CRC mortality in individuals invited for FOBT screening. | Did not demonstrate significant reduction in CRC incidence. More than 1000 interval cancers occurred, reflecting limited sensitivity of FOB screening. |
| Pitkäniemi, 2015, Finland20 | 301,900 (68.8%) | 10,743 (3.56) | 9024a (84) | First results on mortality of an individually randomized community-based CRC screening program in Finland. | Observed 33% increase in CRC mortality in females; can be related to poor sensitivity of gFOBT in Finland. |
| Population screening program | |||||
| Myres, 1993, USA5 | 2201 | 166 (7.54) | 42a (25.3) | Prospective study in a health maintenance organization. | Data not collected about factors associated with physicians’ reluctance to recommend colonoscopy to patients with positive FOBT. |
| Levin, 1997, USA21 | 8293 (13%) | 1337 (16.12) | 553c (41.36) | Mass community-based screening study in urban setting comparing three FOBTs. | Could not determine sensitivity and specificity of each guaiac test. |
| Baig, 2003, USA6 | – | 554 (–) | 296a (53.43) | Demonstrates reasons for nonperformance of colonoscopy after positive FOBT. | Retrospective study based on physicians’ reports. |
| Etzioni, 2006, USA22 | 13,337 (33.45%) | 313 (2.35) | 158a (50.48) | Examines patterns of colorectal screening and follow-up in USA’s largest health care system: VA | Retrospective study based on data from VA chart review. |
| Etzioni, 2006, USA22 | 13,337 (33.45%) | 155h (1.16) | 27e (17.42) | g | g |
| Manfredi, 2008, France4 | 96,048 (52.4%) | 2,477 (2.58) | 2,246a (90.67) | Compliance rate for colonoscopy one of best valued reported. | Participation rate in this first round of CRC mass screening slightly lower than values reported previously. |
| Seeff, 2013, USA23 | 2252 (53%) | 225 (9.99) | 185a (82.22) | Documents successful implementation of four-year CRC screening demonstration program. | Cohort too small and number of years too few to detect whether this low-income population had a higher burden of disease than general screening. |
| Rabeneck, 2014, Canada24 | 778,480 (29.8%) | 20,740 (2.66) | 15,472a (74.6) | Wide CRC screening program. | No detailed information regarding colonoscopies performed outside a collaborating hospital. |
| Stock, 2015, Canada25 | – | 39,105 (–) | 13,229a (33.83) | First study assessing interventions to improve follow-up rates; large-scale study. | Short-term follow-up; comparison of two strategies, but only a short study interval implementing strategy number two. |
|
First author, year, countryr
e
f
e
r
e
n
c
e
|
Number of participants (participation rate)
|
Positive FOBT (%) (positive cutoff value)
|
Follow-up diagnostic evaluation (%)
|
Advantage
|
Disadvantage
|
| FIT | |||||
| Population-based clinical trials | |||||
| Sieg, 1998, Germany26 | 2284 (82%) | 224 (9.81) (10 µg Hb/g) | 184d (82.14) | Used new immunological human fecal Hb and albumin test with high sensitivity. | Fifteen percent of screened population younger than 45 years |
| Ko, 2003, USA13 | 1410 (48%) | 128 (9.08) | 69a (53.91) | Compared patient compliance and characteristics of gFOBT vs FIT. | Unexpectedly demonstrated similar positivity rate and positive predictive value for two types of tests |
| Segnan, 2007, Italy9 | 1965 (32.3%) | 92 (4.68) | 81a (88.04) | First large trial comparing uptake and neoplasia yield of colonoscopy with sigmoidoscopy and FIT. | Used only single screening round with FIT, resulting in low yield of advanced neoplasia compared with other tests |
| van Rossum, 2008, Netherlands14 | 6157 (59.6%) | 339 (5.51) (100 ng/ml) | 280a (82.6) | Random comparison of two screening program strategies. | Method used for estimating specificity slightly overestimates true specificity |
| Janda, 2010, Australia27 | 1181 (34.7%) | 150 (12.7) | 138a (92) | Population-based study on performance of repeated FIT screening with two rounds. | Study conducted in a rural, real-world setting; potential recruitment and generalizability bias; study measured only small proportion of factors likely associated with FOBT adherence |
| Levi, 2011, Israel16 | 1224 (25.9%) | 153 (12.5) (70 ng/ml] | 108a (70.59) | Prospective, controlled study; follow-up of two years after study end. | Randomization by clinic site, with only nine clinics studied, and not by patients. |
| Gupta, 2013, USA28 | 748 (40.7%) | 60 (8.02) (50 mg Hb/ml buffer) | 49a (81.67) | Prospective, randomized trial comparing three screening programs; diverse population. | Only one round of invitation; study not designed to allow for meaningful statistical comparisons of neoplasia outcomes; potential generalizability bias. |
| Kapidzic, 2015, Netherlands (first round)29 | 4523 (62.6%) | 380 (8.4) (10 µg Hb/g) | 364a (95.79) | Population-based study on performance of repeated FIT screening with three rounds; high uptake by reinvitation of previous nonattenders. | Recruitment in population that at baseline had limited knowledge of CRC and CRC screening. May have positively affected participation rate in second and third screening rounds. |
| Kapidzic, 2015, Netherlands (second round)29 | 3864 (63.2%) | 230 (5.95) (10 µg Hb/g) | 223a (96.96) | Population-based study on performance of repeated FIT screening with three rounds; high uptake by reinvitation of previous non-attenders. | Recruitment in population that at baseline had limited knowledge on CRC and CRC screening. May have positively affected participation rate in the second and third screening rounds. |
| Kapidzic, 2015, Netherlands (third round)29 | 3704 (68.3%) | 211 (5.7) (10 µg Hb/g) | 200a (94.79) | ||
| Stegeman, 2015, Netherlands (first round)30 | 2871 (57%) | 233 (8.12) (10 µg Hb/g) | 186a (79.83) | Population-based study on performance of biennial FIT based screening with three rounds; possibility to merge data with major Netherlands cancer registry. | Absolute numbers of screen-detected and nonscreen-detected cancer cases were small, which limits power of statistical tests for comparisons of cancers in different groups. |
| Stegeman, 2015, Netherlands (second round)30 | 2359 (56%) | 186 (7.88) (10 µg Hb/g) | 156a (83.87) | g | g |
| Stegeman, 2015, Netherlands (third round)30 | 2142 (60%) | 153 (7.14) (10 µg Hb/g) | 123a (80.39) | g | g |
| Wong, 2015, Hong Kong31 | 5863 (97.3%) | 382 (6.52) | 343a (89.79) | Comparison of real-life differences in cost effectiveness between two screening strategies. | Potential selection bias; short-term follow-up. |
| Panic, 2015, Montenegro32 | 920 (33.3%) | 95 (10.33) (50 mg/ml) | 92a (96.84) | Comparison of two screening program strategies. | Generalizability bias (screening limited to only one municipality in Montenegro); possible CRC underestimation because of archive system limitations. |
| Grobbee, 2016, Holland (OC-Sensor)33 | 6014 (63%) | 390 (6.48) (10 µg Hb/g) | 353a (90.51) | For a fair comparison on diagnostic yield between FITs, positivity rate rather than Hb concentration should be used. | Included fourth round of FIT screening, so majority of population not screening naïve. |
| Grobbee, 2016, Holland (FOB-Gold)33 | 6040 (63%) | 479 (7.93) (10 µg Hb/g) | 436a (91.02) | g | g |
| Jensen, 2016, USA (first round)34 | 323,349 (48.2%) | 16,037 (4.96) (20 µg Hb/g) | 12,113a (75.53) | Largest evaluation of FIT performance to date; up to four rounds of annual testing. | Does not compare FIT outreach to usual care or other screening methods. Censoring patients at termination of health plan membership may have led to under-ascertainment of CRC. |
| Jensen, 2016, USA (second round)34 | 183,988 (75.3%) | 7143 (3.88) (20 µg Hb/g) | 5747a (80.46) | g | g |
| Jensen, 2016, USA (third round)34 | 163,281 (83.4%) | 6102 (3.74) (20 µg Hb/g) | 4912a (80.5) | g | g |
| Jensen, 2016, USA (fourth round)34 | 145,452 (86.1%) | 6211 (4.27) (20 µg Hb/g) | 5039a (81.13) | g | g |
| Teixeira, 2017, Brazil35 | 948 (91.2%) | 92 (9.7) (10 µg Hb/g) | 73a (79.35) | First CRC screening study with OC-Light (i-FOBT) performed in a Brazilian population. | Relatively small number of volunteers evaluated by colonoscopy. |
| Arana-Arri, 2017, Spain36 | 296,378 (67%) | 18,273 (6.17) (20 µg Hb/g) | 17,387a (95.15) | Prospective, large-cohort study provided detailed outcomes in men and women of different ages at a range of fecal Hb concentration cutoffs. | Examines effects of sex and age on fecal Hb concentration cutoff without taking into account other confounders. |
| Corely, 2017, USA37 | 1,258,039 | 81,518 (6.48) (20 µg Hb/g) | 70,124a (86.02) | Multi–medical center, community-based, diverse population study. | Retrospective cohort study with observational design. |
| Population screening program | |||||
| Grazzini, 2000, Italy38 | 6418 (42.1%) | 268 (4.18) | 231a (86.19) | Population-based screening program. | Used a one-day screening test without any dietary restrictions. |
| Choi, 2012, Korea (first round)39 | 238,376 (10.5%) | 18,980 (7.96) | 3397a (17.9) | Large sample size; data document follow-up decision made by participants. | Possible missing data regarding participants undergoing tests outside nationwide population-based screening program; no data on reasons for absence of follow-up examinations. |
| Choi, 2012, Korea (first round)39 | 238,376 (10.5%) | 18,980 (7.96) | 8237e (43.4) | g | g |
| Choi, 2012, Korea (fifth round)39 | 976,901 (21.1%) | 66,870 (6.85) | 18,456a (27.6) | g | g |
| Choi, 2012, Korea (fifth round)39 | 976,901 (21.1%) | 66,870 (6.85) | 7355e (11) | g | g |
| Ferrari Bravo, 2012, Italy40 | 17,441 (41.3%) | 1739 (9.97) | 956a (54.97) | Stepwise screening approach starting at one district and extended to other districts after optimization of program. | Low compliance with FOBT; 33% of participants with positive FIT did not complete colonoscopy. |
| Parente, 2012, Italy (first round)41 | 38,807 (49.7%) | 2406 (6.2) (100 ng/ml) | 2213a (91.98) | One of the first accounts of initial two biennial rounds of operational population-based CRC screening program based on FIT outside trial setting; large sample size; cost evaluation performed | Reasons for higher rate of colonoscopy attendance in present program not specifically addressed. |
| Parente, 2012, Italy (second round)41 | 44,400 (54.4%) | 2575 (5.8) (100 ng/ml) | 2394a (92.97) | g | g |
| Wu, 2013, Taiwan42 | 10,563 | 1192 (11.28) | 669a (56.12) | Evaluation of two independent databases that used different screening protocols with FIT. | Possible population bias; different socioeconomic state between two groups; limited study power because of low number of participants. |
| Abu Hassan, 2016, Malaysia43 | 710 (94.7%) | 72 (10.14) | 49a (68.06) | Evaluation conducted in a multiracial setting. | Potential generalizability bias (data from only two districts); potential limitation in data collection could affect detection rate. |
| Portillo, 2017, Spain44 | 818,811 (68.6%) | 49,687 (6.07) (20 µg Hb/g) | 46,060a (92.7) | Screening program had a high participation rate and acceptance of colonoscopy, thus demonstrated valid results. | Retrospective observational study assessing interval cancer without taking into account patient-related factors. |
| Buron, 2017, Spain45 | 77,349 (44.7%) | 4764 (6.16) (20 µg Hb/g) | 4003a (84.03) | Shows by large cohort that socioeconomic deprivation has a significant impact at all stages of FIT-based CRC screening pathway. | Retrospective study. Patient socioeconomic level determined according to ecological deprivation index calculated nine years earlier. |
| Toes-Zoutendijk, 2017, Netherlands2 | 529,056 (71.3%) | 40,842 (7.72) (15 µg Hb/g) | 31,759a (77.76) | Real-time monitoring of results of CRC screening by FIT resulting in altered FIT cutoff levels. | Information for monitoring based on SceenIT national information system but not all data sources completely linked to it. |
| Issaka, 2017, USA | – | 2238 (–) (50 µg Hb/g) | 1245a (55.63) | Examined colonoscopy completion rate one year following abnormal FIT in an integrated safety-net system. | Retrospective cohort study in a dynamic population belonging to San Francisco Health Network. |
CRC: colorectal cancer; FIT: fecal immunohistochemical test; FOBT: fecal occult blood test; gFOBT: guaiac fecal occult blood test; Hb: hemoglobin; UK: United Kingdom; USA: United States of America; VA: Veterans Health Administration.
Colonoscopy.
Double-contrast barium enema (DCBE) with sigmoidoscopy.
Colonoscopy or DCBE with sigmoidoscopy.
Colonoscopy with albumin.
DCBE.
Sigmoidoscopy.
As mentioned above.
Number of positive FOBT after subtracting those who underwent colonoscopy.
, Missing data.
Data synthesis and analysis
Meta-analysis was performed by using Comprehensive meta-analysis software (Version 3, BioStat Inc, Englewood, NJ, USA). Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for the number of patients with each pathological diagnosis. Heterogeneity between studies was evaluated using the Cochran Q test, and it was considered to be present if the Q test p value was less than 0.10. I2 statistics were used to measure the proportion of inconsistency in individual studies. We also calculated a potential publication bias using a funnel plot of standard error by log odds ratio. Pooled meta-analysis data were presented as forest plots.
Our secondary outcome measures were the diagnostic yield of CRC, advanced adenoma and simple adenoma, defined as the proportion of participants undergoing colonoscopy after a positive FOBT and diagnosed with CRC, advanced adenoma or simple adenoma, relative to the number of participants with a positive test result, respectively.
Results
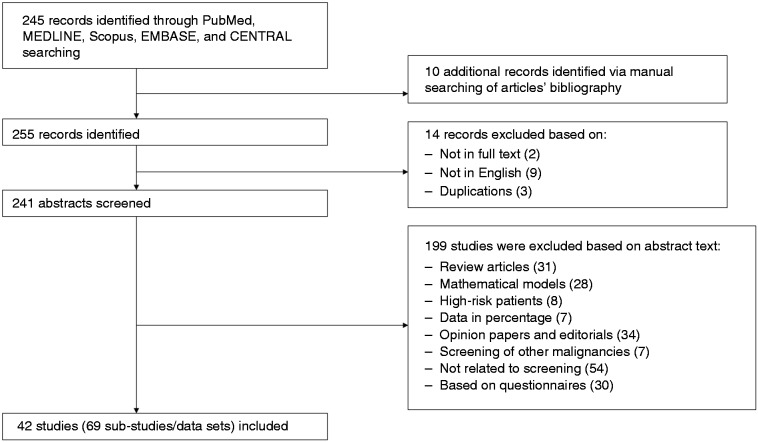
Search strategy and selection studies are shown in Figure 1. Database search identified 245 studies. Ten additional studies were identified after manual searches of articles’ bibliographies. We identified 255 articles. Fourteen articles were excluded because they were studies not in full text, not in English or duplications. A total of 199 studies were excluded based on abstract text: review articles, mathematical models, high-risk patients, data in percentage, opinion papers and editorials, screening of other malignancies, not related to screening, or based on questionnaires.
Figure 1.
Flowchart of articles identified for the meta-analysis.
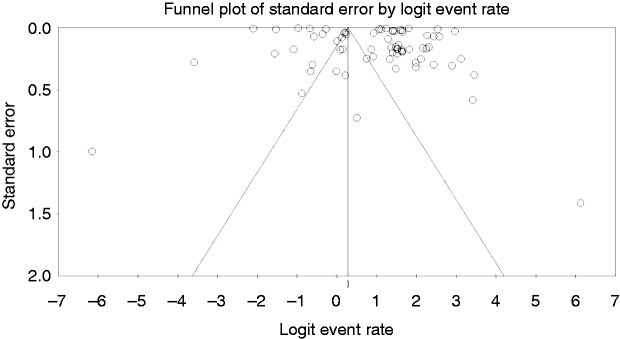
In total, we were left with 42 studies that fulfilled the inclusion criteria and were published through December 31, 2017 (Figure 1). The analysis itself included 69 substudies/data sets, which included 512,496 patients from 17 countries (US: 13, Spain: 3, Brazil: 1, Canada: 2, Denmark: 1, Germany: 1, Italy: 4, Israel: 2, France: 1, Netherland: 5, Finland: 2, Australia: 1, United Kingdom: 2, Korea: 1, Taiwan: 1, Montenegro: 1, and Malaysia: 1). Funnel plot demonstrates a moderate publication bias. Egger test p = 0.076 (NS) (Figure 2, Table 1).2,4–6,9–46
Figure 2.
Funnel plot for publication bias.
Therefore, a random-effects model was used in our meta-analysis. The event rate for the number of patients with a positive FOBT out of all screening participants was 0.0591 with 95% CI 0.059–0.059 (p < 0.001).
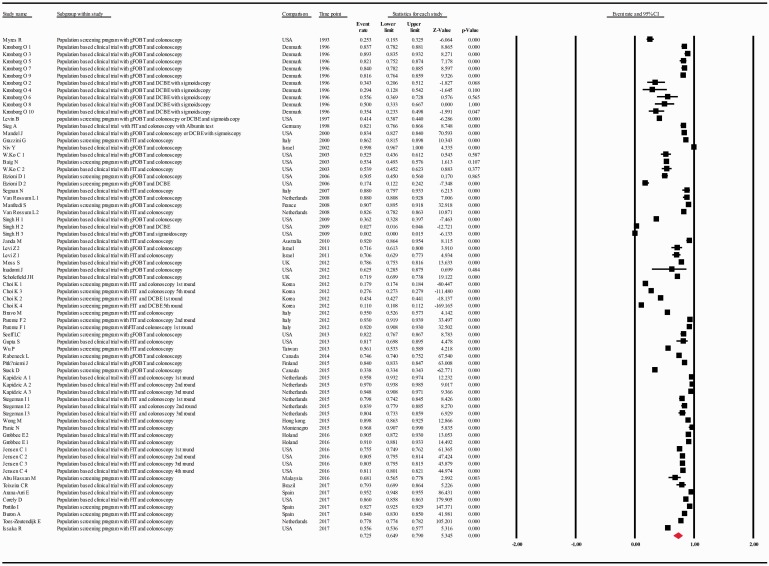
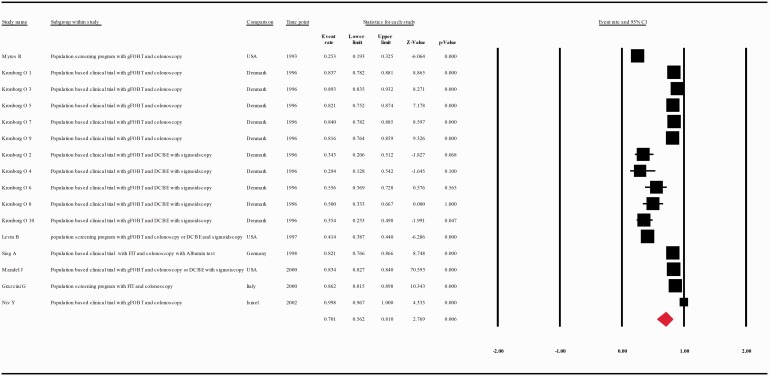
The compliance rate for any second procedure, for colonoscopy, or for combination of DCBE with or without sigmoidoscopy in patients with a positive FOBT was 0.725 with 95% CI 0.649–0.790 (p = 0.000), 0.804 with 95% CI 0.740–0.856 (p = 0.000), 0.197 with 95% CI 0.096–0.361(p = 0.000), respectively (Figure 3, Supplementary Figure 1, Supplementary Figure 2).
Figure 3.
Meta-analysis of descriptive studies looking at the compliance of patients with a positive FOBT for second procedure (all types): 42 studies (69 substudies/data sets); 302,197 procedures performed in 512,496 patients with a positive FOBT. CI: confidence interval; DCBE: double-contrast barium enema; FIT: fecal immunohistochemical test; FOBT: fecal occult blood test; gFOBT: guaiac fecal occult blood test; UK: United Kingdom; USA: United States of America.
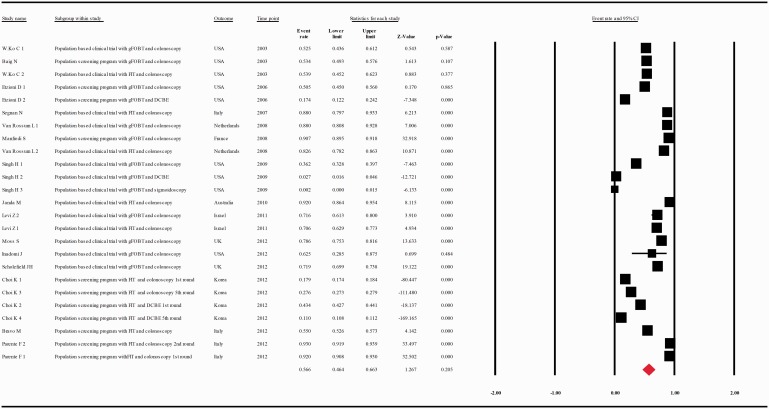
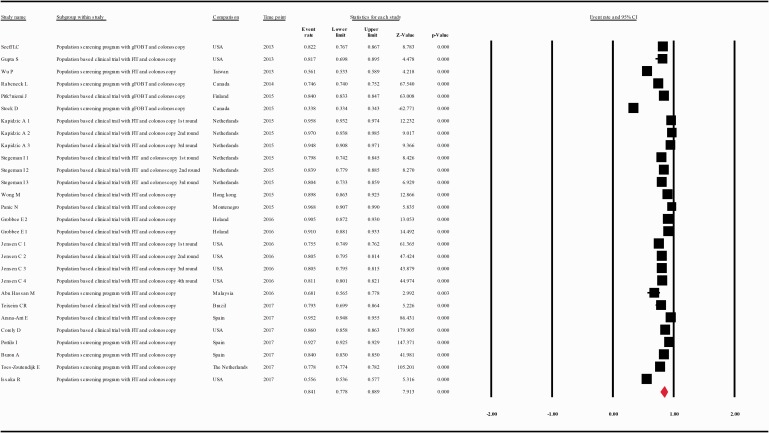
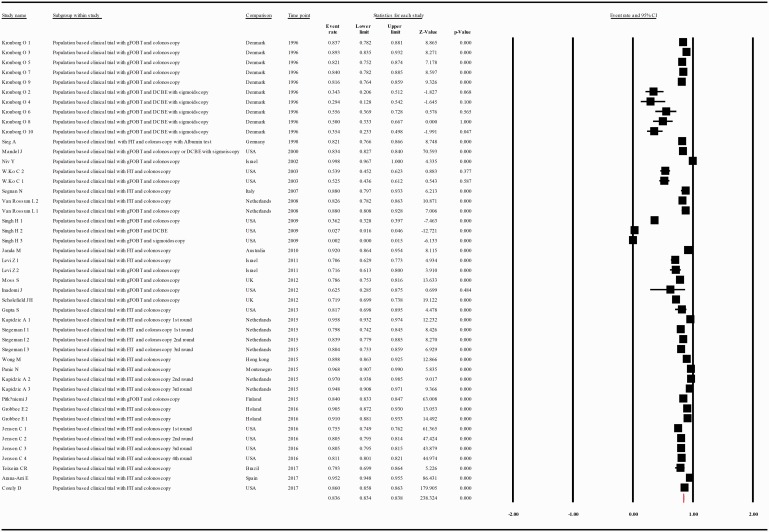
The compliance rate for any second procedure in patients with a positive FOBT after classification of the studies according to their publication year (at intervals of 10 years) was 0.701 with 95% CI 0.562–0.810 (p = 0.006), 0.566 with 95% CI 0.464–0.663 (p = 0.101), 0.841 with 95% CI 0.778–0.889, between the years 1993–2002, 2003–2012, and 2013–2017, respectively (Figure 4(a–c).
Figure 4.
(a) Meta-analysis of descriptive studies published 1993–2002, looking at the compliance of patients with a positive FOBT for second procedure: seven studies (16 substudies/data sets); 13,665 procedures performed in 17,204 patients with a positive FOBT. (b) Meta-analysis of descriptive studies published 2003–2012, looking at the compliance of patients with a positive FOBT for second procedure: 15 studies (25 substudies/data sets); 48,917 procedures performed in 187,509 patients with a positive FOBT. (c) Meta-analysis of descriptive studies published 2013–2017, looking at the compliance of patients with a positive FOBT for second procedure: 20 studies (28 substudies/data sets); 239,615 procedures performed in 307,783 patients with a positive FOBT. CI: confidence interval; DCBE: double-contrast barium enema; FIT: fecal immunohistochemical test; FOBT: fecal occult blood test; gFOBT: guaiac fecal occult blood test; USA: United States of America.
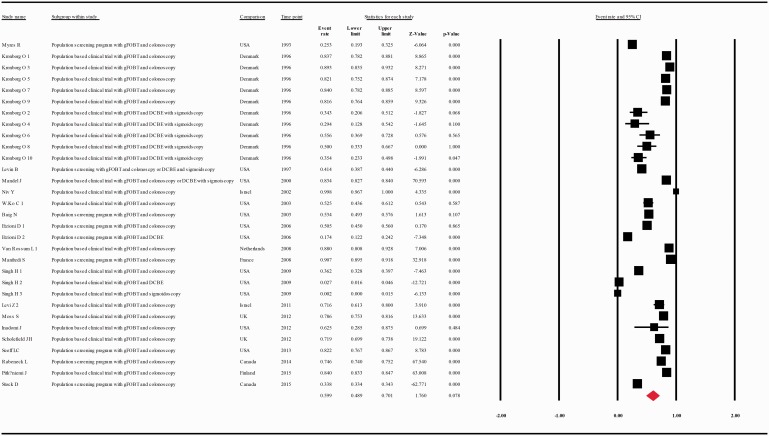
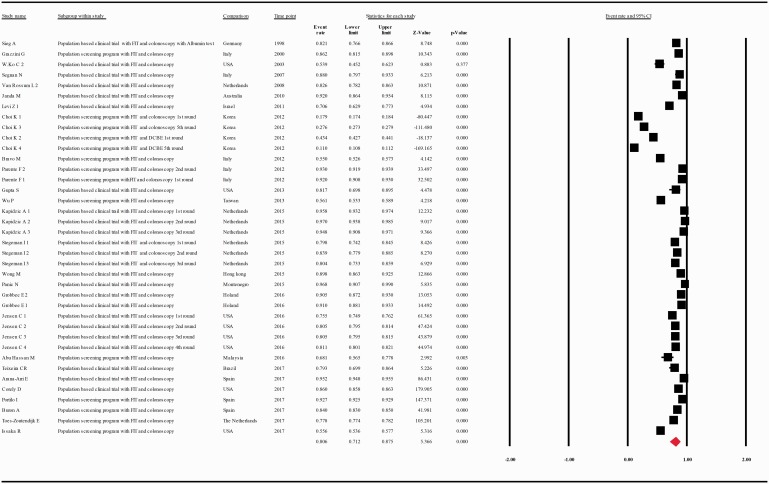
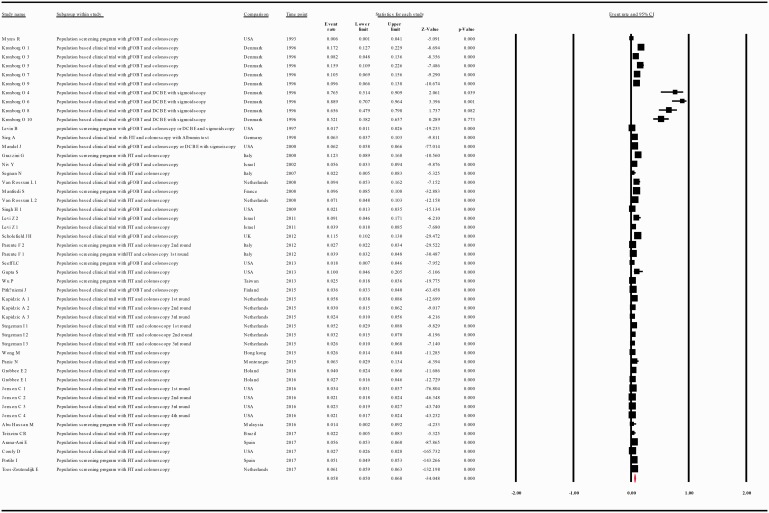
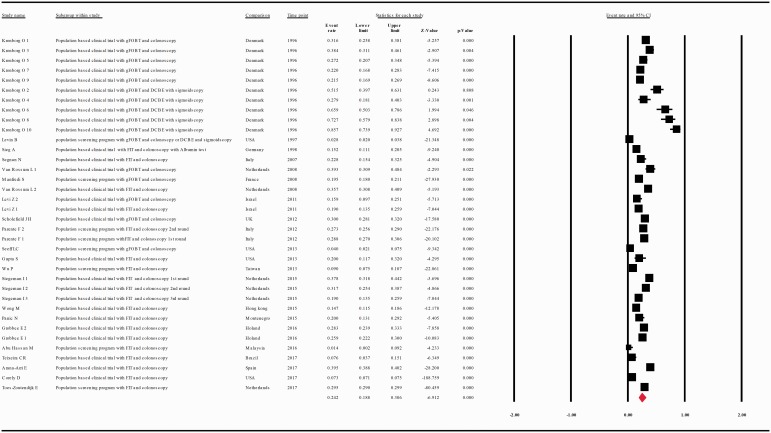
When data were analyzed according to the screening test used, the compliance rate for any second procedure in patients with a positive guaiac FOBT (gFOBT) was 0.599 with 95% CI 0.489–0.701 (p = 0.018) whereas in patients with a positive FIT it was 0.806 with 95% CI 0.712–0.875 (p = 0.000) (Figure 5(a and b)).
Figure 5.
(a) Meta-analysis of descriptive studies, looking at the compliance of patients with a positive gFOBT for second procedure: 19 studies (31 substudies/data sets); 56,393 procedures performed in 95,752 patients with a positive FOBT. (b) Meta-analysis of descriptive studies, looking at the compliance of patients with a positive FIT for second procedure: 26 studies (38 substudies/data sets); 245,804 procedures performed in 416,744 patients with a positive FOBT. (c) Meta-analysis of population-based clinical trials studies, looking at the compliance of patients with a positive FOBT for second procedure: 25 studies (46 substudies/data sets); 142,965 procedures performed in 169,941 patients with a positive FOBT. (d) Meta-analysis of population screening programs studies, looking at the compliance of patients with a positive FOBT for second procedure: 18 studies (23 substudies/data sets); 159,232 procedures performed in 342,555 patients with a positive FOBT. CI: confidence interval; DCBE: double-contrast barium enema; FIT: fecal immunohistochemical test; FOBT: fecal occult blood test; gFOBT: guaiac fecal occult blood test; UK: United Kingdom; USA: United States of America.
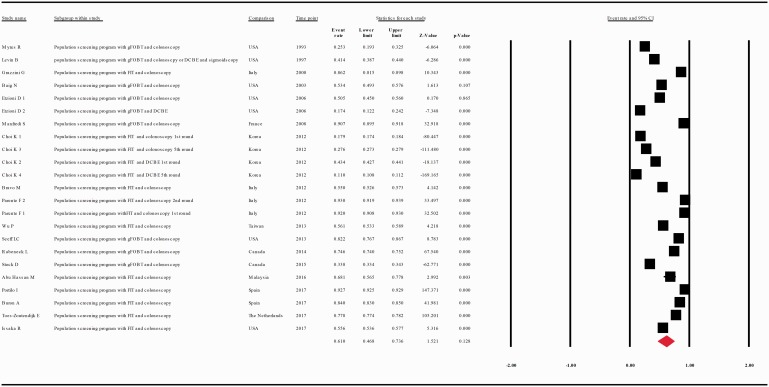
When we analyzed the results of population-based clinical trials studies separately from the results of population screening programs studies, the compliance rate for any second procedure in patients with a positive FOBT was 0.836 with 95% CI 0.834–0.838 (p = 0.000) and 0.616 with 95% CI 0.468–0.736 (p = 0.121), respectively (Figure 5(c and d)).
Heterogeneity (the proportion of inconsistency in individual studies) between studies was significant with Q = 150,038, degree of freedom(Q) = 68, p < 0.001 and I2 = 99.955%.
The diagnostic yield of CRC, advanced adenoma and simple adenoma, by colonoscopy, was provided in 30, 21 and 15 studies, respectively, and in 50, 36 and 18 substudies, respectively. Thus, the diagnostic yield for CRC, advanced adenoma and simple adenoma in patients with a positive FOBT (intention-to-screen analysis) was 0.058 with 95% CI 0.050–0.068(p < 0.001), 0.242 with 95% CI 0.188–0.306 (p < 0.001), 0.147 with 95% CI 0.116–0.184 (p < 0.001), respectively (Figures 6 and 7).
Figure 6.
Meta-analysis of descriptive studies looking at the diagnostic yield of CRC: 30 studies (50 substudies/data sets); 11,591 cases in 269,149 patients with a positive FOBT. CI: confidence interval; DCBE: double-contrast barium enema; FIT: fecal immunohistochemical test; FOBT: fecal occult blood test; gFOBT: guaiac fecal occult blood test; USA: United States of America.
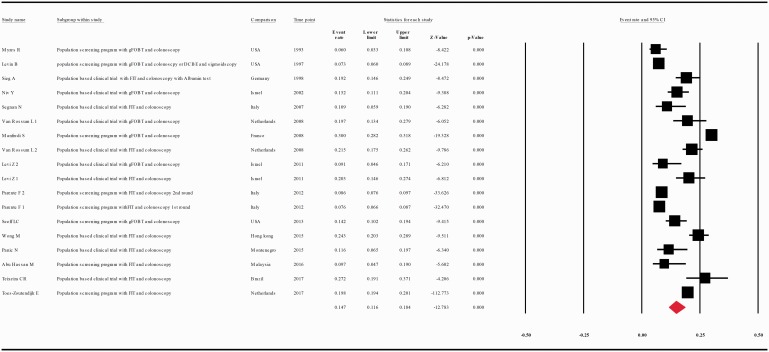
Figure 7.
(a) Meta-analysis of descriptive studies looking at the diagnostic yield of advanced adenoma: 21 studies (36 substudies/data sets) 29,140 cases in 157,158 patients with a positive FOBT. (b) Meta-analysis of descriptive studies looking at the diagnostic yield of simple adenoma: 15 studies (18 substudies/data sets); 9716 cases in 51,913 patients with a positive FOBT. CI: confidence interval; DCBE: double-contrast barium enema; FIT: fecal immunohistochemical test; FOBT: fecal occult blood test; gFOBT: guaiac fecal occult blood test; UK: United Kingdom; USA: United States of America.
When the compliance rate for colonoscopy and for DCBE, in patients with a positive FOBT, was compared, we found an OR of 2.996, in favor of colonoscopy, with 95% CI 0.571–15.729, (p < 0.001) (Supplementary Figure 3).
Discussion
There has been continuous debate over many years among gastroenterologists and public health experts regarding the best way to screen the average-risk population for prevention and early detection of CRC. Following the first randomized, controlled studies of screening with FOBT and many cost-effectiveness studies lacking studies reporting on screening with colonoscopy, the scientific community was convinced that annual FOBT during 25 years is comparable to colonoscopy every 10 years.47 There is no debate, though, that colonoscopy should be the second procedure in patients with a positive FOBT. Many studies examined the compliance rate for screening with FOBT or colonoscopy as the first procedure, but the second compliance rate for the colonoscopy itself in patients with a positive FOBT has never been thoroughly studied. Therefore, our systematic review and meta-analysis is the first that provides a precise, quantitative analysis of the compliance rate for colonoscopy, or for DCBE with or without sigmoidoscopy, after a positive result from fecal CRC screening tests.
In our meta-analysis, a positive FOBT was found in 5.91% of the participants. The compliance rate for colonoscopy, or for DCBE with or without sigmoidoscopy, after a positive test result was 80.4% and 19.7%, respectively. In addition, consistent with the reported rate in a study by Yabroff et al.,48 we have found that the calculated compliance rate for any second diagnostic evaluation was 72.5%. Thus, about 30% of participants with a positive FOBT did not undergo a second diagnostic evaluation and were at risk of having CRC. For example, Meester and colleagues, by using a microsimulation model of an average-risk screening cohort, estimated that a delay of 12 months in performing colonoscopy after a positive FIT can produce an increase of 4% in incidence of CRC and 16% in mortality.49 They did not include patients who refused colonoscopy, who are probably at higher risk. Meester’s estimation is supported by the work of Corley et al., which is based on a retrospective cohort study of Kaiser Permanente Northern California and Southern California health plan members showing that, compared with colonoscopy performed during the first 30 days, after a positive FIT result, each additional 30-day delay is associated with an average increased risk of approximately 3% for any CRC, and approximately 5% of advanced-stage disease, excluding follow-up examinations performed, within six months.37
When we calculated the compliance rate for any second procedure in patients with a positive FOBT, after classification of the studies according to their publication year (at intervals of 10 years) we found that between the first 10 years (1993–2002), it was 70.1%, while between the last five years (2013–2017) it was 84.1%, a result that can point at an improving trend in the last years, maybe due to an increase in the awareness of patients and clinicians of the importance of adherence to positive screening tests.1
When we separated the studies according to the screening test used, the calculated compliance rate for any second diagnostic evaluation was 59.9% for gFOBT and 80.6% for FIT.
In our study, meta-analysis of the studies according to type revealed that the calculated compliance rate for any second diagnostic evaluation of the population-based clinical trials studies was 22% higher than that of the population screening programs studies (83.6% vs 61.6%). This result can be explained by situations that can happen more often in screening programs and less frequently in controlled clinical trials, for example, absence of physician recommendation for further follow-up, incomplete communication of results between providers.48
It should be emphasized that the problem of incomplete diagnostic evaluation after screening programs has also been reported in screening for other types of cancer.48 In our meta-analysis, the diagnostic yield of CRC in patients with a positive FOBT was 5.8%. This result is 10-fold higher than that of screening colonoscopy among the average-risk population50 and reflects a significant number of patients who are at risk. Interestingly, in our meta-analysis, contrary to the adenoma diagnostic yield reported in screening colonoscopies (average-risk population),23,50 the diagnostic yield of advanced adenoma (containing severe/high-grade dysplasia, more than 20% villous component, or larger than 10 mm) was higher than that of simple adenoma (24.2% vs 14.7%). Since an advanced adenoma is associated with bleeding tendency more than a simple adenoma, this finding is expected and supports the effectiveness of screening by FOBT. The compliance rate for colonoscopy in patients with a positive FOBT was found to be almost 300% higher than that for DCBE.
Strengths of this meta-analysis reside in the inclusion of diverse studies from different countries and during a period of more than 20 years (1993–2017). Thus, our study emphasizes that incomplete follow-up after abnormal stool-based tests has been and remains a major worldwide problem that should be addressed.
The limitations of our study are the moderate heterogeneity among studies, the different FOBT brands used over the years and their performance’s heterogeneity, and the different approaches to second diagnostic evaluation. Nevertheless, our findings should alert health care providers to patients with a positive FOBT who do not undergo colonoscopy and are at risk of an undiagnosed CRC.
In conclusion, the second diagnostic evaluation after a positive FOBT is about 70%. Since at least 90% of the patients with a positive test should undergo diagnostic test to obtain an effective mass screening program,21 an effort should be made in each national screening program to increase this specific compliance rate, thus maximizing the positional contribution to reduce the incidence and mortality rates of CRC.
Supplemental Material
Supplemental Material for The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: A systematic review and meta-analysis by Rachel Gingold-Belfer, Haim Leibovitzh, Doron Boltin, Nidal Issa, Tsachi Tsadok Perets, Ram Dickman and Yaron Niv in United European Gastroenterology Journal
Acknowledgment
This study was presented as a poster at Digestive Disease Week, May 2018, in Washington, DC, USA, and was published as an abstract in Gastroenterology 2018; 154 (Suppl 1): S-785–S-786.
Declaration of conflicting interests
None declared.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics approval
Approval from ethics committee was not required for the study.
Informed consent
Informed consent was not required for this study.
References
- 1.Lieberman D, Ladabaum U, Cruz-Correa M, et al. Screening for colorectal cancer and evolving issues for physicians and patients: A review. JAMA 2016; 316: 2135–2145. [DOI] [PubMed] [Google Scholar]
- 2.Toes-Zoutendijk E, van Leerdam ME, Dekker E, et al. Real-time monitoring of results during first year of Dutch colorectal cancer screening program and optimization by altering fecal immunochemical test cut-off levels. Gastroenterology 2017; 152: 767–775.e2. [DOI] [PubMed] [Google Scholar]
- 3.Hardcastle JD, Chamberlain JO, Robinson MH, et al. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet 1996; 348: 1472–1477. [DOI] [PubMed] [Google Scholar]
- 4.Manfredi S, Piette C, Durand G, et al. Colonoscopy results of a French regional FOBT-based colorectal cancer screening program with high compliance. Endoscopy 2008; 40: 422–427. [DOI] [PubMed] [Google Scholar]
- 5.Myers RE, Balshem AM, Wolf TA, et al. Screening for colorectal neoplasia: Physicians’ adherence to complete diagnostic evaluation. Am J Public Health 1993; 83: 1620–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baig N, Myers RE, Turner BJ, et al. Physician-reported reasons for limited follow-up of patients with a positive fecal occult blood test screening result. Am J Gastroenterol 2003; 98: 2078–2081. [DOI] [PubMed] [Google Scholar]
- 7.Moher D, Liberati A, Tetzlaff J, et al. Reprint—Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. Phys Ther 2009; 89: 873–880. [PubMed] [Google Scholar]
- 8.Voorham QJ, Rondagh EJ, Knol DL, et al. Tracking the molecular features of nonpolypoid colorectal neoplasms: A systematic review and meta-analysis. Am J Gastroenterol 2013; 108: 1042–1056. [DOI] [PubMed] [Google Scholar]
- 9.Segnan N, Senore C, Andreoni B, et al. Comparing attendance and detection rate of colonoscopy with sigmoidoscopy and FIT for colorectal cancer screening. Gastroenterology 2007; 132: 2304–2312. [DOI] [PubMed] [Google Scholar]
- 10.Kronborg O, Fenger C, Olsen J, et al. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet 1996; 348: 1467–1471. [DOI] [PubMed] [Google Scholar]
- 11.Mandel JS, Church TR, Bond JH, et al. The effect of fecal occult-blood screening on the incidence of colorectal cancer. N Engl J Med 2000; 343: 1603–1607. [DOI] [PubMed] [Google Scholar]
- 12.Niv Y, Lev-El M, Fraser G, et al. Protective effect of faecal occult blood test screening for colorectal cancer: Worse prognosis for screening refusers. Gut 2002; 50: 33–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ko CW, Dominitz JA, Nguyen TD. Fecal occult blood testing in a general medical clinic: Comparison between guaiac-based and immunochemical-based tests. Am J Med 2003; 115: 111–114. [DOI] [PubMed] [Google Scholar]
- 14.van Rossum LG, van Rijn AF, Laheij RJ, et al. Random comparison of guaiac and immunochemical fecal occult blood tests for colorectal cancer in a screening population. Gastroenterology 2008; 135: 82–90. [DOI] [PubMed] [Google Scholar]
- 15.Singh H, Kadiyala H, Bhagwath G, et al. Using a multifaceted approach to improve the follow-up of positive fecal occult blood test results. Am J Gastroenterol 2009; 104: 942–952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levi Z, Birkenfeld S, Vilkin A, et al. A higher detection rate for colorectal cancer and advanced adenomatous polyp for screening with immunochemical fecal occult blood test than guaiac fecal occult blood test, despite lower compliance rate. A prospective, controlled, feasibility study. Int J Cancer 2011; 128: 2415–2424. [DOI] [PubMed] [Google Scholar]
- 17.Moss SM, Campbell C, Melia J, et al. Performance measures in three rounds of the English bowel cancer screening pilot. Gut 2012; 61: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inadomi JM, Vijan S, Janz NK, et al. Adherence to colorectal cancer screening: A randomized clinical trial of competing strategies. Arch Intern Med 2012; 172: 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scholefield JH, Moss SM, Mangham CM, et al. Nottingham trial of faecal occult blood testing for colorectal cancer: A 20-year follow-up. Gut 2012; 61: 1036–1040. [DOI] [PubMed] [Google Scholar]
- 20.Pitkäniemi J, Seppä K, Hakama M, et al. Effectiveness of screening for colorectal cancer with a faecal occult-blood test, in Finland. BMJ Open Gastroenterol 2015; 2: e000034–e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levin B, Hess K, Johnson C. Screening for colorectal cancer. A comparison of 3 fecal occult blood tests. Arch Intern Med 1997; 157: 970–976. [PubMed] [Google Scholar]
- 22.Etzioni DA, Yano EM, Rubenstein LV, et al. Measuring the quality of colorectal cancer screening: The importance of follow-up. Dis Colon Rectum 2006; 49: 1002–1010. [DOI] [PubMed] [Google Scholar]
- 23.Seeff LC, Royalty J, Helsel WE, et al. Clinical outcomes from the CDC’s Colorectal Cancer Screening Demonstration Program. Cancer 2013; 119(Suppl 15): 2820–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rabeneck L, Tinmouth JM, Paszat LF, et al. Ontario’s ColonCancerCheck: Results from Canada’s first province-wide colorectal cancer screening program. Cancer Epidemiol Biomarkers Prev 2014; 23: 508–515. [DOI] [PubMed] [Google Scholar]
- 25.Stock D, Rabeneck L, Baxter NN, et al. Mailed participant reminders are associated with improved colonoscopy uptake after a positive FOBT result in Ontario’s ColonCancerCheck program. Implement Sci 2015; 10: 35–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sieg A, Hertel A, John MR, et al. Screening for colorectal neoplasms with a new immunological human faecal haemoglobin and albumin test. Eur J Cancer Prev 1998; 7: 279–285. [DOI] [PubMed] [Google Scholar]
- 27.Janda M, Hughes KL, Auster JF, et al. Repeat participation in colorectal cancer screening utilizing fecal occult blood testing: A community-based project in a rural setting. J Gastroenterol Hepatol 2010; 25: 1661–1667. [DOI] [PubMed] [Google Scholar]
- 28.Gupta S, Halm EA, Rockey DC, et al. Comparative effectiveness of fecal immunochemical test outreach, colonoscopy outreach, and usual care for boosting colorectal cancer screening among the underserved: A randomized clinical trial. JAMA Intern Med 2013; 173: 1725–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapidzic A, Grobbee EJ, Hol L, et al. Attendance and yield over three rounds of population-based fecal immunochemical test screening. Am J Gastroenterol 2014; 109: 1257–1264. [DOI] [PubMed] [Google Scholar]
- 30.Stegeman I, van Doorn SC, Mundt MW, et al. Participation, yield, and interval carcinomas in three rounds of biennial FIT-based colorectal cancer screening. Cancer Epidemiol 2015; 39: 388–393. [DOI] [PubMed] [Google Scholar]
- 31.Wong MC, Ching JY, Chan VC, et al. The comparative cost-effectiveness of colorectal cancer screening using faecal immunochemical test vs. colonoscopy. Sci Rep 2015; 5: 13568–13568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panic N, Rösch T, Smolovic B, et al. Colorectal cancer screening in a low-incidence area: general invitation versus family risk targeting: A comparative study from Montenegro. Eur J Gastroenterol Hepatol 2015; 27: 1222–1225. [DOI] [PubMed] [Google Scholar]
- 33.Grobbee EJ, van der Vlugt M, van Vuuren AJ, et al. A randomised comparison of two faecal immunochemical tests in population-based colorectal cancer screening. Gut 2017; 66: 1975–1982. [DOI] [PubMed] [Google Scholar]
- 34.Jensen CD, Corley DA, Quinn VP, et al. Fecal immunochemical test program performance over 4 rounds of annual screening: A retrospective cohort study. Ann Intern Med 2016; 164: 456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Teixeira CR, Bonotto ML, Lima JP, et al. Clinical impact of the immunochemical fecal occult blood test for colorectal cancer screening in Brazil. Ann Gastroenterol 2017; 30: 442–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arana-Arri E, Idigoras I, Uranga B, et al. Population-based colorectal cancer screening programmes using a faecal immunochemical test: Should faecal haemoglobin cut-offs differ by age and sex? BMC Cancer 2017; 17: 577–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corley DA, Jensen CD, Quinn VP, et al. Association between time to colonoscopy after a positive fecal test result and risk of colorectal cancer and cancer stage at diagnosis. JAMA 2017; 317: 1631–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grazzini G, Castiglione G, Isu A, et al. Colorectal cancer screening by fecal occult blood testing: Results of a population-based experience. Tumori 2000; 86: 384–388. [DOI] [PubMed] [Google Scholar]
- 39.Choi KS, Lee HY, Jun JK, et al. Adherence to follow-up after a positive fecal occult blood test in an organized colorectal cancer screening program in Korea, 2004–2008. J Gastroenterol Hepatol 2012; 27: 1070–1077. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari Bravo M, De Conca V, Devoto GL, et al. Colorectal cancer screening in LHU4 Chiavarese, Italy: Ethical, methodological and outcome evaluations at the end of the first round. J Prev Med Hyg 2012; 53: 37–43. [PubMed] [Google Scholar]
- 41.Parente F, Boemo C, Ardizzoia A, et al. Outcomes and cost evaluation of the first two rounds of a colorectal cancer screening program based on immunochemical fecal occult blood test in northern Italy. Endoscopy 2013; 45: 27–34. [DOI] [PubMed] [Google Scholar]
- 42.Wu PH, Lin YM, Liao CS, et al. Comparing the outcomes of two strategies for colorectal tumor detection: Policy-promoted screening program versus health promotion service. J Chin Med Assoc 2013; 76: 325–329. [DOI] [PubMed] [Google Scholar]
- 43.Abu Hassan MR, Leong TW, Othman Andu DF, et al. Evaluation of a colorectal carcinoma screening program in Kota Setar and Kuala Muda Districts, Malaysia. Asian Pac J Cancer Prev 2016; 17: 569–573. [DOI] [PubMed] [Google Scholar]
- 44.Portillo I, Arana-Arri E, Idigoras I, et al. Colorectal and interval cancers of the colorectal cancer screening program in the Basque Country (Spain). World J Gastroenterol 2017; 23: 2731–2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burón A, Auge JM, Sala M, et al. Association between socioeconomic deprivation and colorectal cancer screening outcomes: Low uptake rates among the most and least deprived people. PLoS One 2017; 12: e0179864–e0179864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Issaka RB, Singh MH, Oshima SM, et al. Inadequate utilization of diagnostic colonoscopy following abnormal FIT results in an integrated safety-net system. Am J Gastroenterol 2017; 112: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quintero E, Castells A, Bujanda L, et al. Colonoscopy versus fecal immunochemical testing in colorectal-cancer screening. N Engl J Med 2012; 366: 697–706. [DOI] [PubMed] [Google Scholar]
- 48.Yabroff KR, Washington KS, Leader A, et al. Is the promise of cancer-screening programs being compromised? Quality of follow-up care after abnormal screening results. Med Care Res Rev 2003; 60: 294–331. [DOI] [PubMed] [Google Scholar]
- 49.Meester RG, Zauber AG, Doubeni CA, et al. Consequences of increasing time to colonoscopy examination after positive result from fecal colorectal cancer screening test. Clin Gastroenterol Hepatol 2016; 14: 1445–1451.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bretthauer M, Kaminski MF, Løberg M, et al. Population-based colonoscopy screening for colorectal cancer: A randomized clinical trial. JAMA Intern Med 2016; 176: 894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for The compliance rate for the second diagnostic evaluation after a positive fecal occult blood test: A systematic review and meta-analysis by Rachel Gingold-Belfer, Haim Leibovitzh, Doron Boltin, Nidal Issa, Tsachi Tsadok Perets, Ram Dickman and Yaron Niv in United European Gastroenterology Journal