Abstract
Background
Few studies have determined the very long-term mortality risks in adult and childhood-diagnosed coeliac disease.
Objective
We quantified mortality risks in coeliac disease and determined whether age at diagnosis, or time following diagnosis, modified these risks.
Methods
Standardised mortality ratios were determined using data from a cohort of 602 coeliac patients assembled between 1979–1983 from Lothian, Scotland, and followed up from 1970–2016.
Results
All-cause mortality was 43% higher than in the general population. Excess deaths were primarily from haematological malignancies (standardised mortality ratio, 4.77) and external causes (standardised mortality ratio, 2.62) in adult and childhood-diagnosed cases respectively. Mortality risks declined steadily with time in adult-diagnosed cases (standardised mortality ratio, 4.85 in first year compared to 0.97, 25 years post-diagnosis). Beyond 15 years, this group had a significantly reduced risk of any malignancy (standardised mortality ratio, 0.57 (95% confidence interval: 0.33–0.92)). In contrast, for childhood-diagnosed cases an increased risk existed beyond 25 years (standardised mortality ratio, 2.24).
Conclusions
Adult-diagnosed coeliac patients have a temporarily increased mortality risk mainly from malignant lymphomas and a decreased risk of any malignancy beyond 15 years post-diagnosis. In contrast, childhood-diagnosed cases are at an increased risk of mortality mainly from external causes, and have long-term mortality risks that requires further investigation.
Keywords: Coeliac disease, mortality, cohort study, UK study, causes of death
Key summary
Established knowledge on subject
Coeliac disease is associated with increased risk of mortality mainly from specific malignancies.
Increased mortality risks in coeliac disease are greatest during the first few years of diagnosis.
Significant findings of current study
Adult-diagnosed coeliac disease patients have no significant excess risk of all-cause mortality beyond 25 years after diagnosis, with the confidence intervals around the standardised mortality ratio excluding a greater than 25% increase.
Adult-diagnosed coeliac disease patients have a reduced risk of death from any malignancy more than 15 years after diagnosis.
Childhood-diagnosed coeliac disease patients have a long-term mortality risk that merits further investigation.
Introduction
Coeliac disease (CD) patients are reported to have a higher mortality risk than the general population, although the reported magnitude of this increase in risk varies widely.1–8 There is consensus that these increased mortality risks are observed predominantly within a few years of diagnosis (1–4 years).1,4–6 Owing to limited follow-up, the majority of studies have not been able to assess whether these risks persist for long periods (>20 years) after diagnosis. Furthermore, studies have focused entirely on adults1,4 or included considerably more individuals diagnosed in adulthood (>85%) than in childhood,3,6,9 with many1,3,4 but not all9 reporting them to be at an increased risk of mortality primarily from lymphatic malignancies. Only a few studies3,5 have included children or assessed mortality risks in patients diagnosed in childhood, and these have been limited by short follow-up times5 and a focus on inpatients,3 who are more likely to have worse outcomes.
To our knowledge, the first study to have sufficient follow-up time to assess the difference in mortality risks between childhood and adulthood-diagnosed CD and the long-term trends in these risks was that carried out by Solaymani-Dodaran et al.10 on a cohort of CD patients followed up from 1970–2004 (Lothian cohort). Their finding of an increase in long-term mortality in childhood-diagnosed cases, including an excess mortality particularly from external causes has not been corroborated. Evidently, further research is needed to ascertain the magnitude of mortality risks associated with the disease and the very long-term (>20 years) changes in these risks. An additional 10 years of mortality data on the Lothian cohort enabled us to explore mortality risks further, and with greater statistical power; we aimed to quantify the overall and cause-specific mortality risks in adult and childhood-diagnosed CD, and determine whether these risks persist more than 25 years following diagnosis.
Methods
Study participants
Our cohort consisted of CD cases sourced from the Lothian region of Scotland between 1979–1983. The Lothian region comprises the administrative districts of West, East and Mid-Lothian and the City of Edinburgh. Its total population in 1979 was estimated at 764,688.11 Current population estimates indicate little variation in the population statistics, the most recent population estimate being 880,000.12
Participants were predominantly resident in the City of Edinburgh, and were identified through: diagnostic records of gastrointestinal units; existing histopathology records; 1961–1977 CD admissions recorded in the Scottish Hospital Inpatient Statistics; a postal survey of 440 general practitioners; and the region’s CD patient support group. Further details of the participants have been published elsewhere.11,13 We included all participants with at least one small bowel biopsy showing abnormalities typical of CD, some of which were confirmed by a second small bowel biopsy following a gluten withdrawal or gluten challenge (i.e. confirmed CD) and some of which were not (i.e. probable CD). Participants were further classified during enrolment into: childhood-diagnosed (patients diagnosed prior to their 15th birthday) and adulthood-diagnosed (patients diagnosed on or after their 15th birthday) CD cases.11
Study design
Follow-up commenced on the later of 1 January 1970 (date of first active tracking of cohort) or the date of clinical diagnosis until the earliest of loss to follow-up, death, or 20 October 2016. Given that participants were recruited into the study between 1979–1983, follow-up prior to 1979 was retrospective.
Cohort mortality data
Following an initial search of Scottish National Death Records, participants identified to be alive were flagged in the National Health Service Central Register. Upon death, copies of their death certificates were sent to the study team, who linked information on the underlying causes of death to the cohort participants. The underlying causes of death were coded using the 8th–10th revisions of the International Classification of Diseases (ICD).
Population mortality data
Mortality rates were determined by applying population mortality estimates for the Lothian region obtained from the National Records of Scotland12,14 for the years 1974–2016. Population mortality estimates for the years 1970–1973 and cause-specific death estimates for 2016 were unavailable, and were extrapolated from the average mortality estimates for the periods of 1974–1976 and 2013–2015 respectively.
Statistical analysis
Overall and cause-specific mortality
We calculated overall standardised mortality ratios (SMRs) and their 95% confidence intervals (CIs) for the total person-years of follow-up using indirect standardisation, by estimating the number of deaths expected in this amount of person-time when age (five-year bands), gender and year specific mortality rates for the Lothian region were applied to our cohort. We then determined cause-specific SMRs for causes of death where we had population data (Appendix 1), using the same approach. Overall and cause-specific SMRs were calculated for the whole cohort and separately for adult and childhood-diagnosed cases.
Mortality risks by time since diagnosis
To determine whether mortality risks in the cohort were modified by the length of time following diagnosis, we calculated overall SMRs for the following times: first year of diagnosis, 0–4 years, 5–9 years, 10–14 years, 15–24 years and 25 years or more post-diagnosis and cause-specific SMRs for the following times: <15 years following diagnosis and ≥15 years following diagnosis.
Sensitivity analysis
To determine whether conclusions made were dependent on whether or not CD in the patient had been confirmed, we carried out a sensitivity analysis by repeating the analyses in participants with confirmed CD only.
All analyses were carried out using Stata v. 14.2.
Results
Cohort characteristics
Our cohort consisted of 602 participants diagnosed with CD between 1943–1983 (Table 1) (we excluded 23 participants from the original cohort of 625, who had died before our study start date). Of these, 73% had their diagnosis confirmed by one or more biopsies following gluten withdrawal or a gluten challenge. 318 (53%) participants were diagnosed as adults at a mean age of 45 years, and 284 (47%) were diagnosed as children at a median age of 1.58 years. Participants contributed to a total follow-up of 19,071 person-years, with childhood-diagnosed cases accounting for a greater percentage of the follow-up time (60%). Two hundred and five (64%) adult-diagnosed cases had died before the end of the study period compared with 32 (11%) childhood-diagnosed cases.
Table 1.
Cohort characteristics.
| Age at diagnosis |
|||
|---|---|---|---|
| Characteristic | <15 years (n = 284) | ≥15 years (n = 318) | Total (n = 602) |
| Number of participants, n (%) | |||
| Male | 129 (45.42) | 105 (33.02) | 234 (38.87) |
| Female | 155 (54.58) | 213 (66.98) | 368 (61.13) |
| Diagnosis, n (%) | |||
| Confirmed | 197 (69.37) | 242 (76.10) | 439 (72.92) |
| Probable | 87 (30.63) | 76 (23.90) | 163 (27.08) |
| Age group at diagnosis (years), n (%) | |||
| 0–4 | 223 (78.52) | – | 223 (37.04) |
| 5–9 | 46 (16.20) | – | 46 (7.64) |
| 9–14 | 15 (5.28) | – | 15 (2.49) |
| 15–24 | – | 35 (11.01) | 35 (5.81) |
| 25–34 | – | 63 (19.81) | 63 (10.47) |
| 35–44 | – | 60 (18.87) | 60 (9.97) |
| 45–54 | – | 58 (18.24) | 58 (9.63) |
| 55–64 | – | 71 (22.33) | 71 (11.79) |
| 65–74 | – | 27 (8.49) | 27 (4.49) |
| 75–84 | – | 4 (1.26) | 4 (0.66) |
| Age at diagnosis (years) | |||
| Mean ± SD | 2.97 ± 3.14 | 44.99 ± 15.49 | 25.17 ± 23.92 |
| Median (IQR) | 1.58 (0.91–4.21) | 45.46 (31.75–58.50) | 21.67 (1.67–46.82) |
| Year of diagnosis, n (%) | |||
| Prior to 1970 | 135 (47.54) | 93 (29.25) | 228 (37.87) |
| 1970–1983 | 149 (52.46) | 225 (70.75) | 374 (62.13) |
| Status at end of study,a n (%) | |||
| Dead | 32 (11.27) | 205 (64.47) | 237 (39.37) |
| Alive | 228 (80.28) | 97 (30.50) | 325 (53.99) |
| Censoredb | 24 (8.44) | 16 (5.03) | 40 (6.64) |
| Follow-up time (person-years) | |||
| Total | 11,500.79 | 7570.95 | 19,071.74 |
| Mean follow-up ± SD (per person) | 40.50 ± 9.87 | 23.81 ± 15.46 | 31.68 ± 15.54 |
IQR: interquartile range; SD: standard deviation.
End of study, 26 October, 2016.
Censored, lost to follow-up through emigration.
Overall and cause-specific mortality risks
The causes of deaths analysed and the average ages at which participants died from these causes are presented in Figures 1 and 2 respectively. We observed an excess of 70.8 deaths (237 observed deaths, 166.2 expected deaths) from all causes in the cohort during the entire study period (Table 2). This represents a relative excess mortality risk of 43% in the cohort compared to the general Lothian population (SMR = 1.43; 95% CI 1.25–1.62). Participants diagnosed in childhood had more than double the risk of dying (SMR = 2.11; 95% CI 1.44–2.97) compared with a 36% increase in risk in those diagnosed in adulthood (SMR = 1.36; 95% CI 1.18–1.56).
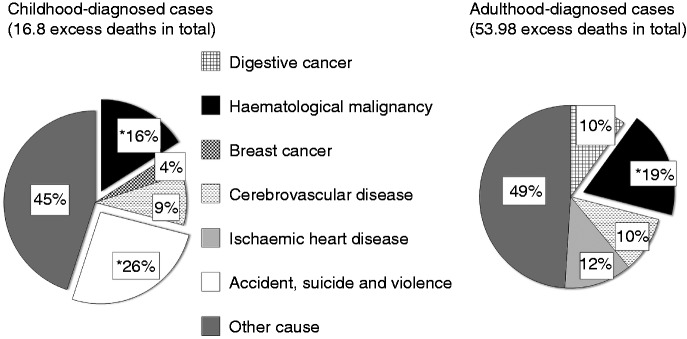
Figure 1.
Proportion of excess deaths from analysed causes.
Causes of death analysed in the cohort and the percentage of excess deaths from each of these causes, calculated as number of excess deaths from specific cause/total number of excess deaths × 100%. Definition of symbols used: *Significant number of excess deaths.
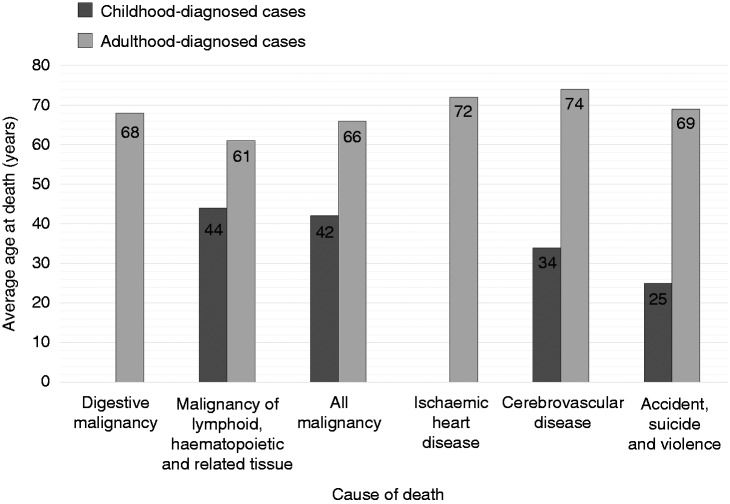
Figure 2.
Average ages at death from specific causes.
Average age of death among participants who died by cause of death.
Table 2.
Overall and cause-specific standardised mortality ratios (SMRs) by age at diagnosis.
| Age at diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <15 years
(n = 284) |
≥15 years
(n = 318) |
Overall (n = 602) |
|||||||
| Cause of death | Obs | Exp | SMR (95% CI) | Obs | Exp | SMR (95% CI) | Obs | Exp | SMR (95% CI) |
| All cause | 32 | 15.20 | 2.11 (1.44–2.97)a | 205 | 151.02 | 1.36 (1.18–1.56)a | 237 | 166.22 | 1.43 (1.25 – 1.62)a |
| All malignancy | 8 | 3.74 | 2.14 (0.92–4.21) | 50 | 41.57 | 1.20 (0.89–1.59) | 58 | 45.32 | 1.28 (0.97–1.65) |
| Digestive cancer | <5 | b | 0.00 (0.00–4.53) | 16 | 10.82 | 1.48 (0.85–2.40) | 16 | 11.63 | 1.38 (0.79–2.23) |
| Lymphatic and haematopoietic tissue malignancy | <5 | b | 8.03 (1.66–23)a | 13 | 2.73 | 4.77 (2.54–8.16)a | 16 | 3.1 | 5.16 (2.95–8.38)a |
| Breast cancer | <5 | b | 2.69 (0.07–15) | <5 | b | 0.00 (0.00–2.76) | <5 | b | 0.59 (0.01–3.26) |
| Cerebrovascular disease | <5 | b | 4.42 (0.53–16) | 25 | 19.43 | 1.29 (0.83–1.90) | 27 | 19.88 | 1.36 (0.90–1.98) |
| Ischaemic heart disease | <5 | b | 0.00 (0.00–2.86) | 42 | 35.63 | 1.18 (0.85–1.59) | 42 | 36.92 | 1.14 (0.82–1.54) |
| Accident, suicide and violence | 7 | 2.67 | 2.62 (1.05–5.40)a | <5 | b | 0.90 (0.24–2.30) | 11 | 7.13 | 1.54 (0.77–2.76) |
CI: confidence interval; Exp: expected deaths; Obs: observed deaths.
p < 0.05; bIn order to minimise the risk of disclosure of the identity of individual study participants, we have adhered to the rule of thumb suggested by the Office for National Statistics of suppressing all cell frequencies where the observed cell frequency is less than 5 (‘Review of the Dissemination of Health Statistics: Confidentiality Guidance: National Statistics; HMSO 2006’).
The overall excess mortality was accounted for at least in part by lymphatic and haematopoietic tissue (haematological) malignancies in adulthood-diagnosed cases, and by accidents, suicides and violence (external causes) in childhood-diagnosed cases (Figure 1). In adult cases there were 13 haematological malignancies (all of which were specifically lymphoma), compared with 2.7 expected, resulting in an SMR of 4.77 (95% CI 2.54–8.16). Childhood-diagnosed cases had an increase in risk of death from external causes (SMR = 2.62; 95% CI 1.05–5.40), haematological malignancies (SMR = 8.03; 95% CI 1.66–23) and cerebrovascular diseases (SMR = 4.42; 95% CI 0.53–16), although the last of these was not statistically significant.
Mortality risks by time since diagnosis
Overall, mortality risks for the entire cohort declined gradually over time (Table 3). This decreasing trend was driven by the results in adult-diagnosed cases. The highest mortality risk for this group was observed in the year following diagnosis (SMR = 4.85; 95% CI 2.42–8.68). Within 5–9 years post-diagnosis, the excess risks had decreased to 49% (SMR = 1.49; 95% CI 0.97–2.18). Beyond 25 years after diagnosis, adulthood-diagnosed CD patients had no significant excess risk, with the confidence intervals around the SMR excluding a greater than 25% increase (SMR = 0.97, 95% CI 0.74–1.24). Our analysis of childhood-diagnosed cases was somewhat hampered by a small number of expected deaths during the first 24 years following diagnosis. However, beyond 25 years after diagnosis, those diagnosed in childhood had more than double the mortality risk (SMR = 2.24; 95% CI 1.45–3.30).
Table 3.
Overall standardised mortality ratios (SMRs) by time since diagnosis.
| Age at diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <15 years
(n = 284) |
≥15 years
(n = 318) |
Total population
(n = 602) |
|||||||
| Time since diagnosis | Obs | Exp | SMR (95% CI) | Obs | Exp | SMR (95% CI) | Obs | Exp | SMR (95% CI) |
| First year | <5 | a | 0.00 (0.00–9.39) | 11 | 2.27 | 4.85 (2.42–8.68)b | 11 | 2.66 | 4.14 (2.06–7.40)b |
| 1–4 years | <5 | a | 0.00 (0.00–2.87) | 27 | 10.74 | 2.51 (1.66–3.66)b | 27 | 12.03 | 2.24 (1.48–3.27)b |
| 5–9 years | <5 | a | 11.22 (3.06–29.00)b | 26 | 17.5 | 1.49 (0.97–2.18) | 30 | 17.86 | 1.68 (1.13–2.40)b |
| 10–14 years | <5 | a | 2.18 (0.06–12.00) | 29 | 18.57 | 1.56 (1.05–2.24)b | 30 | 19.03 | 1.58 (1.06–2.25)b |
| 15–24 years | <5 | a | 1.31 (0.16–4.73) | 50 | 37.93 | 1.32 (0.98–1.74) | 52 | 39.45 | 1.32 (0.98–1.72) |
| 25+ years | 25 | 11.18 | 2.24 (1.45–3.30)b | 62 | 64.01 | 0.97 (0.74–1.24) | 87 | 75.19 | 1.16 (0.93–1.43) |
CI: confidence interval; Exp: expected deaths; Obs: observed deaths.
In order to minimise the risk of disclosure of the identity of individual study participants, we have adhered to the rule of thumb suggested by the Office for National Statistics of suppressing all cell frequencies where the observed cell frequency is less than 5 (‘Review of the Dissemination of Health Statistics: Confidentiality Guidance: National Statistics; HMSO 2006’): bp < 0.05.
When cause-specific deaths were analysed in the whole cohort, it appeared that most of the excess deaths from the different causes had been accrued during the first 15 years following diagnosis (Table 4). Among adult-diagnosed cases, there were significantly raised mortality risks in the first 15 years for any malignancy as well as for digestive cancers and haematological malignancies specifically. In contrast, beyond 15 years there was a modest but statistically significant decreased risk of mortality from any malignancy (SMR = 0.57; 95% CI 0.33–0.92). In childhood-diagnosed cases, the majority of deaths in the first 15 years were from external causes (SMR = 4.89; 95% CI 1.01–14). Beyond 15 years, the increased risks from these causes persisted, although lower than before (SMR = 1.95; 95% CI 0.53–4.98). For this group, increased mortality beyond 15 years of diagnosis was strongest for haematological malignancies (SMR = 9.44; 95% CI 1.95–28).
Table 4.
Cause-specific standardised mortality ratios (SMRs) by time since diagnosis.
| Age at diagnosis |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| <15 years
(n = 284) |
≥15 years
(n = 318) |
Total population |
|||||||
| Period after diagnosis | Obs | Exp | SMR (95% CI) | Obs | Exp | SMR (95% CI) | Obs | Exp | SMR (95% CI) |
| All malignancy | |||||||||
| <15 years | <5 | a | 0.00 (0.00–23) | 34 | 13.47 | 2.53 (1.75–3.53)b | 34 | 13.62 | 2.50 (1.73–3.49)b |
| ≥15 years | 8 | 3.58 | 2.23 (0.96–4.40) | 16 | 28.11 | 0.57 (0.33–0.92)b | 24 | 31.69 | 0.76 (0.49–1.13) |
| Digestive cancer | |||||||||
| <15 years | <5 | a | 0.00 (0.00–780) | 12 | 3.62 | 3.31 (1.71–5.79)b | 12 | 3.63 | 3.31 (1.71–5.78)b |
| ≥15 years | <5 | a | 0.00 (0.00–4.56) | <5 | a | 0.56 (0.15–1.42) | <5 | a | 0.50 (0.14–1.28) |
| Lymphatic and haematopoietic tissue malignancy | |||||||||
| <15 years | <5 | a | 0.00 (0.00–66) | 10 | 0.77 | 13.04 (6.25–24)b | 10 | 0.82 | 12.15 (5.83–22)b |
| ≥15 years | <5 | a | 9.44 (1.95–28)b | <5 | a | 1.53 (0.32–4.48) | 6 | 2.28 | 2.64 (0.97–5.74) |
| Cerebrovascular disease | |||||||||
| <15 years | <5 | a | 0.00 (0.00–260) | 8 | 6.20 | 1.29 (0.56–2.54) | 8 | 6.21 | 1.29 (0.56–2.54) |
| ≥15 years | <5 | a | 4.56 (0.55–16) | 17 | 13.23 | 1.29 (0.75–2.06) | 19 | 13.67 | 1.39 (0.84–2.17) |
| Ischemic heart disease | |||||||||
| <15 years | <5 | a | 0.00 (0.00–3200) | 13 | 14.18 | 0.92 (0.49–1.57) | 13 | 14.18 | 0.92 (0.49–1.57) |
| ≥15 years | <5 | a | 0.00 (0.00–2.86) | 29 | 21.45 | 1.25 (0.91–1.94) | 29 | 22.74 | 1.28 (0.85–1.83) |
| Accident, suicide and violence | |||||||||
| <15 years | <5 | a | 4.89 (1.01–14)b | <5 | a | 0.58 (0.01–3.22) | <5 | a | 1.71 (0.47–4.37) |
| ≥15 years | <5 | a | 1.95 (0.53–4.98) | <5 | a | 1.10 (0.23–3.21) | 7 | 4.79 | 1.46 (0.59–3.01) |
CI: confidence interval; Exp: expected deaths; Obs: observed deaths.
In order to minimise the risk of disclosure of the identity of individual study participants, we have adhered to the rule of thumb suggested by the Office for National Statistics of suppressing all cell frequencies where the observed cell frequency is less than 5 (‘Review of the Dissemination of Health Statistics: Confidentiality Guidance: National Statistics; HMSO 2006’): bp<0.05.
Sensitivity analysis
Restricting the analyses to confirmed cases did not produce any substantial differences in the estimates obtained (data not shown). We observed a similar pattern of higher mortality risks in childhood-diagnosed cases, as well as a decreasing trend in mortality risks with increasing time following diagnosis.
Discussion
Key findings
People diagnosed with CD were at a 43% increased risk of mortality compared to the general population. The largest relative risks of death were from haematological malignancies (>90% of which were specifically lymphoma) in both adult and childhood-diagnosed cases. Mortality risks declined steadily with time since diagnosis, particularly in the adulthood-diagnosed cases. For these, we observed a lower than expected risk of mortality from all malignancies after the first 15 years of diagnosis. We did not have sufficient follow-up at ages where mortality is expected to be high in the childhood-diagnosed cases to be able to make robust conclusions. We are nevertheless confident that beyond 25 years post-diagnosis, CD patients diagnosed as adults are no longer at a higher risk of mortality compared to the general population.
Strengths and limitations
Our study can boast a number of strengths, one of them being that it was population-based and therefore not prone to selection bias. Another strength is the use of the generally accepted gold standard15 of biopsies as a means of confirmation of CD. By using death certificates to ascertain the underlying causes of death, we have ensured a uniform quality of recording of causes of death in the cohort and the general population to which it was compared. We also have long follow-up, allowing us to assess longer-term risks, something impossible for earlier studies.
Supposing that CD truly raises mortality risks, then our estimates may have been underestimated by the inclusion of probable cases, if many of these did not indeed have CD (non-differential misclassification). In this instance, restricting the analyses to confirmed cases would have resulted in higher mortality estimates, at the cost of wider confidence intervals. However, when we restricted the analyses to confirmed cases, we observed slightly lower mortality risks and similar trends in all-cause and cause-specific mortality, implying that our probable cases were likely to be true CD cases. Given that over 70% of our cases were confirmed, we are quite confident that the inclusion of probable cases did not affect our results substantially.
There were other potential limitations. First, around 23% of the study participants lived outside the Lothian region (Fife or the Scottish borders) when diagnosed with CD.11 However, the demographic characteristics and mortality pattern of people living in Fife and the Scottish borders are generally similar to that of people in the Lothian region.16 Hence, we do not believe there are significant differences in the characteristics of the participants living within and outside the Lothian region to have rendered the population data used inappropriate. Second, we were unable to adjust for the effect of certain factors such as socio-economic status, smoking, compliance with gluten-free diets (GFDs), body mass index (BMI), existence of co-morbidities, etc., which may be associated with high mortality risks. Previous studies, however, have found that adjusting for BMI, smoking6 and education5 did not remove the effect. Third, approximately half of the deaths in the cohort were from causes that we did not have population data on. We are therefore unable to speculate as to whether people with CD have different mortality risks from causes of death that we have not analysed.
Other studies
Overall and cause-specific mortality
The 43% raised mortality risk observed is much lower than most of the earlier reports1,4 and more in agreement with recent studies,5,6 which have reported modest increases (30–40%) in mortality risks in CD patients. This lends support to the findings of decreased mortality risks with increasing length of time following diagnosis,4–6 as our current analysis was based on follow-up data more than 15 years following diagnoses in most cases.
We have just a few studies with which to compare our findings of higher risks in childhood than adulthood-diagnosed cases. While two studies3,5 have similarly reported high risks in children, they either included young adults5 or had a shorter follow-up time.3
Our finding of excess risks of death from malignant lymphomas in adult and childhood-diagnosed CD is consistent with other studies1,3,4,7 and our previous observation from this cohort that malignancy itself was raised in CD patients,17 a finding not supported by all previous research.18 Previous research has highlighted a reduced risk of breast cancer in CD patients,6,9 however, with only small numbers of deaths (<5) we were unable to say whether this extends to mortality from breast cancer.
The observed excess deaths in childhood-diagnosed cases from external causes is consistent with prior publications from this cohort10,11 and reports of causes of death in children in the UK and worldwide.19 However, it is unclear why childhood-diagnosed CD patients would have significantly more of such deaths than the general population. According to Cinquetti et al.20 the acceptance of GFDs in children and adolescents can be associated with adverse psychological effects such as depression and anxiety. In addition, there is evidence that hypervigilance to GFDs may have a negative impact on quality of life.21 This may therefore make such individuals more inclined to intentional self-harm; however, this reasoning is speculative and further research is needed.
Mortality risks with time following diagnosis
Similar to most studies,1,4,5,11 we have confirmed that the mortality risks in CD patients are greatest during the first few years after diagnosis. It is plausible that at the time of diagnosis, patients in our cohort (all of whom were diagnosed prior to 1984) were more symptomatic and presented with more severe forms of the disease, as has been hypothesised to be the case with patients diagnosed in the pre-serological era.8,22 This coincides with the recognition that diagnosis at this time was more difficult than what it has become with serology and endoscopic biopsy. This is evidenced from co-existing symptoms at the time of diagnosis among the present cohort.23 Another possible explanation is that the cases may have presented with illnesses other than CD, leading to an ascertainment bias with respect to CD diagnosis.
Alternatively, it could be argued that CD patients adopt healthier lifestyles after their diagnoses and are therefore less likely to have higher mortality risks than before. As reported in some studies, CD patients are generally less likely than the general population to smoke or be obese6 and, also, they have lower fat intake and better cholesterol profiles,24,25 all of which are associated with lower mortality risks.
The reduction in all malignancies in adult cases 15 years following diagnosis is an interesting finding which needs further exploration through research. Few studies9,17,26 have reported a decrease in the incidence of malignancies in CD 10 years or more following diagnoses, but we are not aware of any study, that corroborates our finding of decreased mortality risk from these causes 15 years after diagnosis. The results may have been driven by a combination of breast cancer and other cancers sharing similar biological properties with breast and lung cancers, which some studies have shown CD patients to be protected from.6,9 Understanding the mechanism of protection from these cancers could provide valuable information on the treatment of cancers in the general population.
The persistent increase in mortality risks in childhood-diagnosed cases more than 25 years after diagnosis is consistent with the earlier report on the cohort,10 and requires further investigation. A longer follow-up of these cases will enable a better assessment of the risks from diseases that occur in later years in life but this will necessitate several additional decades of follow-up of this cohort for us to be able to estimate these risks with sufficient precision. Therefore, retrospectively collected data available from people with childhood-diagnosed CD in the distant past is likely to offer our best hope of realising this.
Mortality risks in the Lothian cohort over time
A summary of key findings from all mortality follow-up of the Lothian cohort is provided in Table 5.
Table 5.
Summary of key findings from all follow-up of the Lothian cohort.
| Study | No. patients | Follow-up time | Statistical measure | Key findings and comments |
|---|---|---|---|---|
| Logan et al.11 | 653 | Date of clinical diagnosis of CD to 30 June 1986 (8823 person years at risk, 115 deaths) | Overall and cause-specific SMRs | • Mortality overall was 1.9-fold more for CD patients than that of the general population. The SMR was similar in men and women. • 10 deaths were directly attributable to CD, compared to 44 from malignancies (corresponding SMR of 3.0 fold for malignancies). • Only four deaths occurred in cases diagnosed in childhood (aged<15 years, total sample size of 292). All these deaths were from external causes. • Overall, there was a gradual decline in SMR with increased time from diagnosis. For 15+ years, there was an SMR of 1.2; however, this was based on just 11.64 expected deaths and was not statistically significant. |
| Solaymani-Dodaran et al.10 | 602a | Date of clinical diagnosis of CD or 1 January 1970 (whichever was later) to 31 December 2004 (14,926 person-years, 195 deaths)b | Overall and cause-specific SMRs | • All analyses in this paper were presented separately for child (0–14 years) and adult (15+ years) diagnosed cases. The overall raised mortality risk was higher in childhood-diagnosed cases (SMR = 2.6) than for adult-diagnosed cases (SMR = 1.6) • Among childhood-diagnosed cases there were 21 deaths, of which seven were due to external causes (SMR = 2.9) and five due to neoplasms (SMR = 3.6). Both these increases were statistically significant. • Due to the increased follow-up, the expected number of deaths 15 + years following diagnosis had increased to 68.71. In childhood-diagnosed cases there was a raised risk 25+ years after diagnosis (SMR = 3.5). For adult-diagnosed cases, no corresponding risk was observed but the confidence interval was wide (SMR = 1.26; 95% CI 0.86–1.77). |
| Current study | 602 | Date of clinical diagnosis of CD or 1 January 1970 (whichever was later) to 20 October 2016 (19,071 person years, 237 deaths)b | Overall and cause-specific SMRs | • In the current study, the overall increase in mortality was 2.1-fold in childhood-diagnosed cases and 1.4-fold in adult-diagnosed cases. The decrease in overall SMRs compared with the earlier reports reflects the decline in risk following increased time from diagnosis. • There were 32 deaths among childhood-diagnosed cases, of which the number of deaths from malignancy increased to eight and the number from external causes remained at seven. However, the expected number of deaths from external causes only increased slightly compared with the previous report (2.67 vs 2.39) as childhood-diagnosed participants are now at an age where deaths attributed to this cause are less common. • The expected number of deaths 15+ years following diagnosis has increased to 114.64. Among adult-diagnosed cases, the absence of an increased risk beyond 25 years reported previously remains but the confidence interval around this value has tightened considerably (SMR = 0.97; (95% CI 0.74–1.24). The equivalent SMR for childhood-diagnosed cases was reduced in this updated follow-up (SMR = 2.2) as the impact of deaths from external causes on this overall figure has been weakened. • There was a reduced risk of death from any malignancy more than 15 years after diagnosis in adult-diagnosed CD patients. Previously Grainge et al.17 reported no change in malignancy incidence in this group compared with the general population 15 years after diagnosis. In the previous mortality report, cause-specific results only stratified time since diagnosis into <5 years and 5+ years. |
CD: coeliac disease; CI: confidence interval; SMR: standardised mortality ratio.
The sample size reported in the paper was 625, however, the authors excluded 23 deaths (out of 218 deaths that had occurred in the 625 CD cases) that occurred before 1 January 1970 from the main analysis reported in the paper.
The survival analysis commenced in 1970 in the analysis presented in this article, to circumvent the problem of survival bias and because population mortality estimates by cause were more reliable from this date onwards.
Conclusion
Our study indicates that individuals with CD are at a modestly increased risk of mortality compared to the general population. The increased risk, primarily from malignant lymphomas, is only temporary in adults and generally declines in the long-term. The increased risk of death from external causes in childhood-diagnosed cases which we highlighted in our previous report persisted with this longer-term follow-up and should form the focus of further research.
Supplemental Material
Supplemental Material for Mortality in people with coeliac disease: Long-term follow-up from a Scottish cohort by Wilhemina Quarpong, Timothy R Card, Joe West, Masoud Solaymani-Dodaran, Richard FA Logan and Matthew J Grainge in United European Gastroenterology Journal
Acknowledgements
The authors wish to thank the late Anne Ferguson and Edith Rifkin who obtained funding for and helped set up this study. National Records of Scotland supplied all follow-up data on our study participants.
Appendix 1. Causes of death analysed and their corresponding International Classification of Diseases (ICD) codes
| ICD code |
|||
|---|---|---|---|
| Disease group | ICD 8 | ICD 9 | ICD 10 |
| Neoplasms | |||
| All malignant neoplasms | 140–209 | 140–209 | C00–C97 |
| Malignant neoplasm of lymphoid, haematopoietic and related tissue | 200–209 | 200–209 | C81–C96 |
| Malignant neoplasm of breast | 174 | 174–175 | C50 |
| Malignant neoplasm of digestive organsa | 150–151, 153–155, 157–158 | 150–151, 153–155, 157–159 | C15–C16, C18, C19–C22, C25 |
| Diseases of the circulatory system | |||
| Ischaemic heart disease | 410–414 | 410–414 | I20–I25 |
| Cerebrovascular disease | 430–438 | 430–438 | I60–I69 |
| External causes of morbidity and mortality | |||
| Accidentb | E800–E929, E940–E946 | E800–E929 | V01–X59, Y85, Y86 |
| Intentional self-harm (suicide) | E950–E959 | E950–E959 | X60–X84, Y87.0 |
| Assault (violence) | E960–E969 | E960–E969 | X85–Y09, Y87.1 |
| Event of undetermined intent | E980–E989 | E980–E989 | Y10–Y34, Y87.2 |
Excludes malignant neoplasms of small intestine, gall bladder, other and ill-defined digestive organs.
Includes transport accidents, falls and poisoning.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Ethics approval
Initial approval was obtained in 1978/1979, with permission from consultants in the Lothian region for the accessing of patient records and review of biopsies. In terms of the long-term follow-up of the study participants, members of the study team who had access to death certificate and other personally identifiable information supplied by National Records of Scotland signed a confidentiality agreement with National Records of Scotland. Other members of the study team only had access to anonymised patient data. The current study was approved by the Division of Epidemiology and Public Health Ethics Committee, University of Nottingham (9/Mar/2017).
Funding
The work was originally funded by a grant from the Scottish Home and Health Department.
Informed consent
Not required.
References
- 1.Cottone M, Termini A, Oliva L, et al. Mortality and causes of death in celiac disease in a Mediterranean area. Dig Dis Sci 1999; 44: 2538–2541. [DOI] [PubMed] [Google Scholar]
- 2.Nielsen OH, Jacobsen O, Pedersen ER, et al. Malignant diseases and mortality rate. Scand J Gastroenterol 1985; 20: 13–18. [DOI] [PubMed] [Google Scholar]
- 3.Peters U, Askling J, Gridley G, et al. Causes of death in patients with celiac disease in a population-based Swedish cohort. Arch Intern Med 2003; 163: 1566–1572. [DOI] [PubMed] [Google Scholar]
- 4.Corrao G, Corazza GR, Bagnardi V, et al. Mortality in patients with coeliac disease and their relatives: A cohort study. Lancet 2001; 358: 356–361. [DOI] [PubMed] [Google Scholar]
- 5.Ludvigsson JF, Montgomery SM, Ekbom A, et al. Small-intestinal histopathology and mortality risk in celiac disease. JAMA 2009; 302: 1171–1178. [DOI] [PubMed] [Google Scholar]
- 6.West J, Logan RFA, Smith CJ, et al. Malignancy and mortality in people with coeliac disease: Population based cohort study. BMJ 2004; 329: 716–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viljamaa M, Kaukinen K, Pukkala E, et al. Malignancies and mortality in patients with coeliac disease and dermatitis herpetiformis: 30-Year population-based study. Dig Liver Dis 2006; 38: 374–380. [DOI] [PubMed] [Google Scholar]
- 8.Holmes GKT, Muirhead A. Mortality in coeliac disease: A population-based cohort study from a single centre in Southern Derbyshire, UK. BMJ Open Gastroenterology 2018; 5: e000201–e000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sultan AA, Crooks CJ, Card T, et al. Causes of death in people with coeliac disease in England compared with the general population: A competing risk analysis. Gut 2015; 64: 1220–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Solaymani-Dodaran M, West J, Logan RFA. Long-term mortality in people with celiac disease diagnosed in childhood compared with adulthood: A population-based cohort study. Am J Gastroenterol 2007; 102: 864–870. [DOI] [PubMed] [Google Scholar]
- 11.Logan RF, Rifkind EA, Turner ID, et al. Mortality in celiac disease. Gastroenterology 1989; 97: 265–271. [DOI] [PubMed] [Google Scholar]
- 12.National Records of Scotland. Population estimates time series data, www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/population/population-estimates/mid-year-population-estimates/population-estimates-time-series-data (2017, accessed 13 July 2017).
- 13.Logan RF, Rifkind EA, Busuttil A, et al. Prevalence and ‘incidence’ of celiac disease in Edinburgh and the Lothian region of Scotland. Gastroenterology 1986; 90: 334–342. [DOI] [PubMed] [Google Scholar]
- 14.National Records of Scotland. Deaths time series data, www.nrscotland.gov.uk/statistics-and-data/statistics/statistics-by-theme/vital-events/deaths/deaths-time-series-data (2017, accessed 13 June 2017).
- 15.Ludvigsson JF, Bai JC, Biagi F, et al. Diagnosis and management of adult coeliac disease: Guidelines from the British Society of Gastroenterology. Gut 2014; 63: 1210–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.National Records of Scotland. Council area profiles, www.nrscotland.gov.uk/statistics-and-data/statistics/stats-at-a-glance/council-area-profiles (2017, accessed 13 June 2017).
- 17.Grainge MJ, West J, Solaymani-Dodaran M, et al. The long-term risk of malignancy following a diagnosis of coeliac disease or dermatitis herpetiformis: A cohort study. Aliment Pharmacol Ther 2012; 35: 730–739. [DOI] [PubMed] [Google Scholar]
- 18.Card TR, West J, Holmes GK. Risk of malignancy in diagnosed coeliac disease: A 24-year prospective, population-based, cohort study. Aliment Pharmacol Ther 2004; 20: 769–775. [DOI] [PubMed] [Google Scholar]
- 19.Santos T, Ferreira M, Simões MC, et al. Chronic condition and risk behaviours in Portuguese adolescents. Glob J Health Sci 2014; 6: 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cinquetti M, Trabucchi C, Menegazzi N, et al. Psychological problems connected to the dietary restrictions in the adolescent with coeliac disease. Pediatr Med Chir 1999; 21: 279–283. [PubMed] [Google Scholar]
- 21.Wolf RL, Lebwohl B, Lee AR, et al. Hypervigilance to a gluten-free diet and decreased quality of life in teenagers and adults with celiac disease. Dig Dis Sci 2018; 63: 1438–1448. [DOI] [PubMed] [Google Scholar]
- 22.Grainge MJ, West J, Card TR, et al. Causes of death in people with celiac disease spanning the pre-and post-serology era: A population-based cohort study from Derby, UK. Am J Gastroenterol 2011; 106: 933–939. [DOI] [PubMed] [Google Scholar]
- 23.Logan RF, Tucker G, Rifkind EA, et al. Changes in clinical features of coeliac disease in adults in Edinburgh and the Lothians 1960–79. Br Med J (Clin Res Ed) 1983; 286: 95–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brar P, Kwon GY, Holleran S, et al. Change in lipid profile in celiac disease: Beneficial effect of gluten-free diet. Am J Med 2006; 119: 786–790. [DOI] [PubMed] [Google Scholar]
- 25.Lewis NR, Sanders DS, Logan RF, et al. Cholesterol profile in people with newly diagnosed coeliac disease: A comparison with the general population and changes following treatment. Br J Nutr 2009; 102: 509–513. [DOI] [PubMed] [Google Scholar]
- 26.Askling J, Linet M, Gridley G, et al. Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis. Gastroenterology 2002; 123: 1428–1435. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Mortality in people with coeliac disease: Long-term follow-up from a Scottish cohort by Wilhemina Quarpong, Timothy R Card, Joe West, Masoud Solaymani-Dodaran, Richard FA Logan and Matthew J Grainge in United European Gastroenterology Journal