Abstract
Background
About 5% of pancreatic ductal adenocarcinomas are inherited due to a deleterious germline mutation detected in 20% or fewer families. Pancreatic screening in high-risk individuals is proposed to allow early surgical treatment of (pre)malignant lesions. The outcomes of pancreatic surgery in high-risk individuals have never been correctly explored.
Objectives
To evaluate surgical appropriateness and search for associated factors in high-risk individuals.
Methods
A patient-level meta-analysis was performed including studies published since 1999. Individual classification distinguished the highest risk imaging abnormality into low-risk or high-risk abnormality, and the highest pathological degree of malignancy of lesions into no/low malignant potential or potentially/frankly malignant. Surgical appropriateness was considered when potentially/frankly malignant lesions were resected.
Results
Thirteen out of 24 studies were selected, which reported 90 high-risk individuals operated on. Low-risk/high-risk abnormalities were preoperatively detected in 46.7%/53.3% of operated high-risk individuals, respectively. Surgical appropriateness was consistent in 38 (42.2%) high-risk individuals, including 20 pancreatic ductal adenocarcinomas (22.2%). Identification of high-risk abnormalities was strongly associated with surgical appropriateness at multivariate analysis (P = 0.001). We proposed a score and nomogram predictive of surgical appropriateness, including high-risk abnormalities, age and existence of deleterious germline mutation.
Conclusion
Overall, 42.2% of high-risk individuals underwent appropriate surgery. The proposed score might help selecting the best candidates among high-risk individuals for pancreatic resection.
Keywords: Familial pancreatic carcinoma, pancreatic neoplasm, hereditary neoplastic syndromes, cancer screening, pancreatic surgery
Key summary
Summarise the established knowledge on this subject
Pancreatic screening in high-risk individuals is proposed to allow early surgical treatment of (pre)malignant lesions.
The outcomes of pancreatic surgery in individuals at high-risk with pancreatic lesions found at screening have never been properly explored.
What are the significant and/or new findings of this study?
In this patient-level meta-analysis, surgical appropriateness (resection of pre/malignant lesions) in high-risk individuals was consistent in 42% of cases.
We propose a score including imaging abnormalities, age and germline mutation, which might help in selecting the best candidates for pancreatic resection.
Introduction
The incidence of pancreatic ductal adenocarcinoma (PDAC) is increasing and is predicted to be the second leading cause of cancer-related death by 2030.1 Inherited forms of the disease represent 5% of cases.2,3 A putative causal germline mutation can be identified in less than 20% of families.4,5 In contrast, no deleterious germline mutation has been identified in most families with multiple PDACs (80–85%). Such familial aggregation of PDAC is called familial pancreatic cancer (FPC)2,5 and is defined as the occurrence of PDAC in two or more first-degree relatives (FDRs), or in three or more relatives whatever the degree.2,5,6
Currently, despite expert consensus have been proposed, there are no validated guidelines for screening programmes. Screening usually relies on yearly magnetic resonance imaging (MRI) and endoscopic ultrasonography (EUS) but its precise value has not been clearly assessed, and the role of computed tomography (CT) must still be evaluated.7
The aim of screening in high-risk individuals (HRIs) is to identify those who present morphological abnormalities suggesting the development of PDAC. Ideally, the goal is to propose early surgical treatment of premalignant lesions (such as pancreatic intraepithelial neoplasia (PanIN) or intraductal papillary mucinous neoplasms (IPMNs) with high-grade dysplasia)8 or even early stage invasive PDAC. Surgical resection of these (pre)malignant lesions could potentially cure up to 80% of patients.7,9,10
However, the optimal time to perform surgery in HRIs, i.e. neither too early (low grade lesions) nor too late (advanced PDAC), must still be determined and the rate of morbidity–mortality of pancreatic resection remains significant.10–13 Moreover, the outcomes of pancreatic surgery in HRIs have never been correctly explored, in particular due to the limited size of these studies. We aimed to perform a patient-level meta-analysis of all observational studies reporting on the surgical management of HRIs, with the objective to evaluate the appropriateness of prophylactic pancreatic resection in HRIs who were followed by screening and to develop a nomogram to predict surgical appropriateness.
Patients and methods
Selection of studies
We performed a patient-level meta-analysis of all series published between 1999 and 2017 on the screening of PDAC in HRIs and treatment of detected (pre)malignant lesions. Selected studies were identified by a search of the PubMed database with no language restriction. The keywords used were ‘familial pancreatic carcinoma’, ‘hereditary pancreatic cancer’, ‘high risk individuals’ and ‘screening’. Two independent searches were performed. Thirteen out of 24 identified studies were selected because they fulfilled the following criteria (Figure 1):
Studies including HRIs defined by the following strict criteria: (a) patients affected by a familial germline mutation of the LKB1/STK11 gene whatever the number of FDRs with PDAC or of another gene (i.e. BRCA1, BRCA2, PALB2, CDKN2A, TP53, PRSS1, ATM and mismatch repair genes) and one or more FDR with PDAC; or (b) patients with FPC defined by two or more FDRs with PDAC or three or more relatives whatever the degree.5,7
Studies reporting individual data on pancreatic surgery: indication according to morphological description of pancreatic abnormalities detected during screening, type of surgery and final pathological analysis of the resected specimen.
Figure 1.
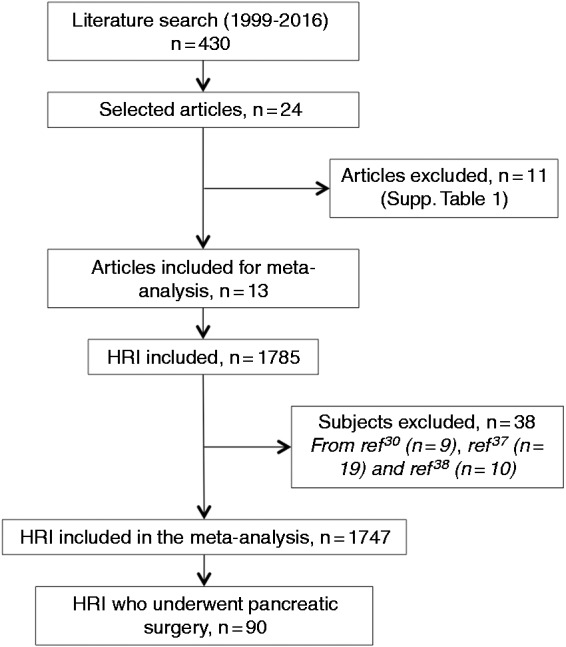
PRISMA flow chart of studies included in the meta-analysis and selection of high-risk individuals.
The 11 studies that did not fulfill these criteria are listed in Supplementary Table 1. The studies that included duplicate data from the same patients published elsewhere were excluded. In this case, only the most recent study was retained.14
Data collection
We excluded individuals who did not fulfill the above-mentioned criteria, such as those with only one FDR affected with PDAC.6,15,16 The following data were obtained for all included subjects: gender, known germline mutation or FPC, age at surgery, abnormalities identified at screening, type of resection, pathological analysis of surgical specimen, duration of postoperative follow-up and survival status of patients when available.
Pancreatic abnormalities identified during follow-up were reviewed at the individual level and were classified as:
Low-risk abnormalities (LRAs): cyst less than 30 mm, main pancreatic duct size less than 5 mm and no mural nodule.
High-risk abnormalities (HRAs): presence of ‘worrisome features’ (i.e. cyst ≥30 mm, enhancing mural nodule <5 mm, thickened/enhancing cyst walls, main pancreatic duct diameter 5–9 mm, abrupt change in the caliber of the pancreatic duct, cyst growth rate ≥5 mm/2 years and/or lymph nodes) or presence of ‘high-risk stigmata of malignancy’ (biliary obstruction, enhancing mural nodules ≥5 mm and/or main pancreatic duct diameter ≥10 mm) by extrapolation of international consensus for IPMN17,18 and the presence of a solid mass whatever the size and/or sample (cytology or biopsy) suspicious or positive for malignancy.
If a patient had multiple abnormalities, only that with the highest risk of malignancy was considered.
Individual pathological data of resected specimens were reviewed and classified into two categories depending on the lesion with highest malignant potential:
Lesions with no/low malignant potential: serous cystadenoma, branch duct IPMN with low-grade dysplasia or PanIN with low-grade dysplasia (previously called PanIN-1 or PanIN-2), or benign pancreatic neuroendocrine tumour (PNET).
Potentially/frankly malignant lesions: PanIN with high-grade dysplasia (previously called PanIN-3), branch duct IPMN with high-grade dysplasia, main duct IPMN with low or high-grade dysplasia, PDAC or malignant PNET.
Criteria used were based on the current international consensus guidelines.8 PDAC was classified following the 8th American Joint Commission on Cancer classification.19
Surgical appropriateness was defined as the presence of potentially/frankly malignant lesions on the final pathological examination of resected specimens. Conversely, surgical non-appropriateness was defined as the presence of only non-malignant lesions.
Statistical analysis
The primary judgement criterion was the rate of surgical appropriateness in HRIs who underwent pancreatic surgical resection for abnormal imaging findings diagnosed during screening.
Qualitative variables were described as frequencies (percentages) and compared with the chi-squared test. Quantitative variables were described as medians (25–75 interquartile) and compared with the Mann–Whitney test. The performances (sensitivity, specificity, positive and negative predictive values) of morphological criteria for the diagnosis of surgical appropriateness were calculated.
To explore the factors associated with surgical appropriateness, we performed univariate logistic regression analysis of all relevant variables. Then all clinically relevant variables with P ≤ 0.20 on univariate analysis were entered into a multivariate logistic regression model. The variables from multivariate analysis were used to create a nomogram to estimate the probability of surgical appropriateness. A predictive model was constructed and weighted with β-coefficient estimations from the multivariate model. An individual-level predictive score was built with nomogram total points.
Overall survival was defined as the time between the date of pancreatic surgery and death from any cause, or the date of the last news. Living patients were censored at the date of the last follow-up. Median overall survival was calculated using the Kaplan–Meier method. Survival curves were compared using the log rank test. All tests were performed with SPSS (version 20.0, IBM) and Prism (version 6, Graphpad) softwares. A P value of less than 0.05 was considered to be statistically significant. The PRISMA checklist is given in Supplementary Table 2.
Results
Study characteristics
All 13 studies included were prospective and six were multicentre 6,14–16,20–28 (Table 1). The 13 studies included 1747 screened HRIs. The proportion of screened HRIs with morphological abnormalities was only reported in eight studies (511/1150, 44.4%). The median number per study of screened HRIs was 95 (44–216) and of operated HRIs was five (three to seven).
Table 1.
Main characteristics of the studies that have included only high-risk individuals for pancreatic ductal adenocarcinoma, as defined in the Methods section.
| First author/year | Setting | Main inclusion criteria | Patients included | Patients operated on | Modalities of initial screening | Modalities of follow-up and minimal intervala |
|---|---|---|---|---|---|---|
| Canto et al., 200420 | Prospective, monocentric | FPC, LKB1 | 38 | 7 | EUS, ±ERCP, CT, FNA | EUS yearly |
| Canto et al., 200621 | Prospective, monocentric | FPC, LKB1 | 78 | 7 | EUS, CT ± ERCP, FNA | EUS + CT yearly |
| Poley et al., 200922 | Prospective, multicentric | FPC, BRCA1/2, CDKN2A, LKB1, TP53, PRSS1 | 44 | 3 | EUS ± CT, MRI | NR |
| Verna et al., 201023 | Prospective, monocentric | FPC, BRCA1/2, CDKN2A, MMR | 51 | 5 | EUS or MRI ± ERCP, FNA | EUS + MRI yearly |
| Ludwig et al., 201115 | Prospective, monocentric | FPC, BRCA1/2 | 100 | 6 | MRI or CT ± EUS, FNA | MRI or CT yearly |
| Canto et al., 201224 | Prospective, multicentric | LKB1, BRCA1/2 | 216 | 5 | CT, EUS, MRI | CT and/or EUS and/or MRI yearly |
| Al-Sukhni et al., 201216 | Prospective, monocentric | FPC, BRCA1/2, CDKN2A, LKB1 | 252 | 4 | MRI ± CT, EUS, ERCP | MRI ± EUS yearly |
| Sud et al., 201425 | Prospective, monocentric | FPC, BRCA1/2, MMR, CDKN2A, LKB1, PRSS1 | 30 | 3 | EUS ± FNA | EUS yearly |
| Mocci et al., 201526 | Prospective, multicentric | FPC, CDKN2A, MMR, PRSS1 | 41 | 1 | EUS, CT ± MRI, FNA | EUS + CT yearly |
| Danset et al., 201627 | Prospective, monocentric | FPC, BRCA1/2, CDKN2A, PALB2, MMR, LKB1, PRSS1 | 95 | 16 | EUS, MRI ± CT, FNA | EUS + MRI yearly |
| Vasen et al., 201614 | Prospective, multicentric | FPC, BRCA1/2, PALB2, CDKN2A | 411 | 30 | MRI ± EUS, CT, FNA | MRI ± EUS yearly |
| Bartsch et al., 20166 | Prospective, multicentric | FPC, BRCA1/2, PALB2 | 234 | 2 | MRI, EUS ± CT, FNA | MRI ± EUS yearly |
| Harinck et al., 201628 | Prospective, multicentric | FPC, BRCA1/2, CDKN2A, LKB1, TP53, MMR | 139 | 1 | MRI ± EUS | MRI ± EUS yearly |
FPC: familial pancreatic cancer; EUS: endoscopic ultrasonography; ERCP: endoscopic retrograde cholangio-pancreatography; FNA: fine-needle aspiration; CT: computed tomography; MRCP: magnetic resonance cholangio-pancreato; MRI: magnetic resonance imaging; NR: not reported;
Interval could be shortened in case of morphological abnormalities.
Screening was performed by different imaging modalities, but mainly EUS and MRI. MRI was first reported for screening in 2009 and became routine after 2012. Four series did not describe the MRI protocol,20–22,26 one provided a minimum protocol (T2-weighted only),15 and one reported MRI with secretin injection,24 although the usefulness of the latter was not assessed. Finally, a few series used an exhaustive MRI protocol with gadolinium injection,14,23,24 and only one included additional diffusion-weighted sequences.28 The usual interval between imaging examinations was one year. In the case of imaging abnormalities, a CT scan and EUS were usually performed in addition to the initial MRI, and then repeated with shorter (3–6 months) intervals.
Patient characteristics
The 13 included studies evaluated 90 HRIs who were operated on due to abnormal pancreatic imaging findings during screening, with lesions considered to be at risk of (pre)malignancy. There were too few reports on the delay between the beginning of screening and surgery to be analysed. The characteristics of operated HRIs are presented in Table 2. The median age was 58 (51–66) years and 20 patients (22.2%) were less than 50 years old. The two most frequent morphological abnormalities leading to surgery were cystic lesions with benign features (46.7%) and solid masses (28.9%). Overall, LRAs or HRAs were identified during screening in 46.7% and 53.3% of operated patients, respectively. In patients with HRAs, the detected lesion with the highest theoretical risk was a cystic lesion(s) with worrisome features in 13.3%, high-risk stigmata in 1.1%, or a solid mass in 28.9%. Finally, 10% of patients had positive cytology/biopsy (Table 2).
Table 2.
Pooled characteristics of the 90 high-risk individuals who underwent pancreatic surgery during pancreatic screening.
| Characteristics | All patients | Surgical appropriateness | No surgical appropriateness |
|---|---|---|---|
| Number of patients, n (%) | 90 | 38 | 52 |
| Male gender, n (%)* | 28/79 (35.4) | 11/33 (33.3) | 17/46 (37) |
| Age, median (IQR)** | 58 (51–66) | 60 (52–67) | 58 (48–64) |
| Indication of screening, n (%) | |||
| FPC (no germline mutation) | 63 (70) | 21 (55.3) | 42 (80.8) |
| LKB1/STK11 mutation carriers | 3 (3.3) | 1 (2.6) | 2 (3.8) |
| BRCA2 mutation carriers | 10 (11.1) | 5 (13.2) | 5 (9.6) |
| CDKN2A mutation carriers | 13 (14.4) | 11 (28.9) | 2 (3.8) |
| PALB2 mutation carriers | 1 (1.1) | 0 (0) | 1 (1.9) |
| Abnormality of highest risk per patient, n (%) | |||
| Low-risk abnormality | 42 (46.7) | 7 (18.4) | 35 (67.3) |
| Cystic lesion(s) with benign features | 42 (46.7) | 7 (18.4) | 35 (67.3) |
| High-risk abnormality | 48 (53.3) | 31 (81.6) | 17 (32.7) |
| Cystic lesion(s) with worrisome features | 12 (13.3) | 6 (15.8) | 6 (11.5) |
| Cystic lesion(s) with high-risk stigmata of malignancy | 1 (1.1) | 0 | 1 (1.9) |
| Solid mass | 26 (28.9) | 19 (50) | 7 (13.5) |
| Positive cytology/biopsy (suspicious or positive for IPMN with HGD, PanIN3 or PDAC) | 9 (10) | 6 (15.8) | 3 (5.8) |
| Type of surgical procedure, n (%) | |||
| Pancreatico-duodenectomy | 23 (25.6) | 11 (28.9) | 12 (23.1) |
| Total pancreatectomy | 12 (13.3) | 8 (21.1) | 4 (7.7) |
| Left pancreatectomy | 49 (54.4) | 16 (42.1) | 33 (63.5) |
| Limited pancreatectomy | 6 (6.7) | 3 (7.9) | 3 (5.8) |
| Highest-malignancy pathological lesion | |||
| Lesions of no/low potential of malignancy | 52 (57.8%) | ||
| Serous cystadenoma | 6 (6.7) | 0 | 6 (6.7) |
| BD-IPMN with low-grade dysplasia | 31 (34.4) | 0 | 31 (34.4) |
| PanIN with low-grade dysplasia | 15 (16.7) | 0 | 15 (16.7) |
| Potentially/frankly malignant lesions | 38 (42.2%) | ||
| MD-IPMN with low-grade dysplasia | 2 (2.2) | 2 (2.2) | 0 |
| MD-IPMN with high-grade dysplasia | 5 (5.6) | 5 (5.6) | 0 |
| BD-IPMN with high-grade dysplasia | 1 (1.1) | 1 (1.1) | 0 |
| PanIN with high-grade dysplasia | 9 (10) | 9 (10) | 0 |
| Invasive adenocarcinoma | 20 (22.2) | 20 (52.6) | 0 |
| Malignant neuroendocrine tumor | 1 (1.1) | 1 (2.6) | 0 |
Missing data: *11, **4.
BD: branch duct; FPC: familial pancreatic cancer; HGD: high-grade dysplasia; IPMN: intraductal papillary mucinous neoplasms; LGD: low-grade dysplasia; MD: main duct; PanIN: pancreatic intraepithelial neoplasia; PDAC: pancreatic ductal adenocarcinoma.
The two most frequent surgical procedures were left pancreatectomy (54.4%) and pancreatico-duodenectomy (25.6%). Twelve patients (13.3%) underwent a total pancreatectomy. No surgery-related deaths were reported. Left pancreatectomy was more frequently performed for LRAs than pancreatico-duodenectomy or total pancreatectomy (57% vs. 31%, P = 0.02). Pathological examination of the resected specimen showed non-malignant lesions in 57.8%, mostly branch duct IPMN or PanIN with low-grade dysplasia. Potentially/frankly malignant lesions were identified in the remaining 42.2%, including 20 (52.6%) with invasive PDAC. Among those 20 PDAC, eight were classified stage I (six stage IA and two stage IB), seven were classified stage II (two stage IIA and five stage IIB) and five were classified stage III. It was generally not determined whether PDAC was identified at the first screening or during follow-up.
Proposal of the Beaujon score and nomogram
Overall, based on the definition proposed above, surgery was found to be appropriate in 38 HRIs or 42.2% of all operated HRIs. The sensitivity, specificity, positive and negative predictive values of the proposed morphological criteria (HRAs vs. LRAs) was 81.6%, 67.3%, 64.6% and 83.3%, respectively.
The factors associated with surgical appropriateness on univariate analysis (Table 3) were the presence of a germline mutation in screened HRIs (vs. FPC, P = 0.011) and HRAs identified during screening (vs. LRAs, P < 0.001). Age greater than 50 years (P = 0.20) tended to be associated with surgical appropriateness. At multivariate analysis, the only factor independently and significantly associated with surgical appropriateness was surgery performed for HRAs (vs. LRAs, hazard ratio (HR) 11.69, 95% confidence interval (CI) 3.71–36.81, P = 0.001).
Table 3.
Univariate and multivariate logistic regression analyses of factors associated with appropriateness from pancreatic surgery in high-risk individuals undergoing screening.
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |
| Gender | ||||||
| Female | 1 | |||||
| Male | 0.85 | 0.33–2.18 | 0.74 | |||
| Age | ||||||
| <50 | 1 | 1 | ||||
| ≥50 | 1.94 | 0.66–5.68 | 0.20 | 3.11 | 0.87–11.14 | 0.081 |
| Deleterious germline mutation identified | ||||||
| No | 1 | 1 | ||||
| Yes | 3.4 | 1.33–8.71 | 0.011 | 2.25 | 0.72–7.05 | 0.16 |
| Abnormality of highest risk at screening | ||||||
| Low-risk abnormality | 1 | 1 | ||||
| High-risk abnormality | 9.12 | 3.34–24.89 | <0.001 | 11.69 | 3.71–36.81 | 0.001 |
HR: hazard ratio; CI: confidence interval.
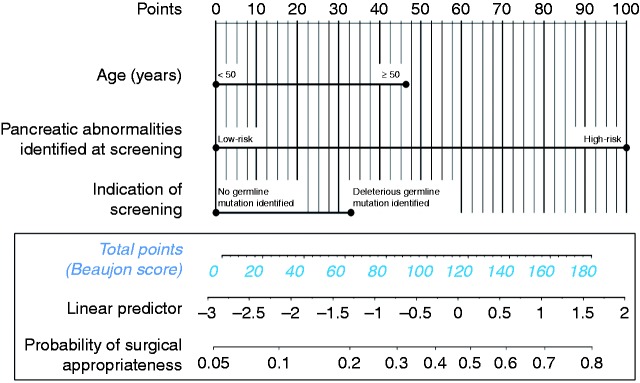
A nomogram was built that included the three variables entered into multivariate analysis. The Beaujon score was then developed to predict the probability of surgical appropriateness of pancreatic resection in HRIs (Figure 2). This score was based on the total number of points obtained from the nomogram, with a minimum of 0 (all three variables negative) corresponding to a probability of surgical appropriateness of approximately 5%, and a maximum of 180 (all three variables positive) corresponding to a probability of surgical appropriateness of 80%. The area under the receiver operating characteristic (ROC) curve of the predictive model was 0.81 (compared with 0.77 when non-weighted variables were used).
Figure 2.
Beaujon score and nomogram to predict the probability of the appropriateness of prophylactic pancreatic resection in high-risk individuals following screening. First, the points associated with each of the three predictive factors are obtained by vertical translation from the patient’s value to the ‘points’ line. All variables are binary. Next, the points are summed and the corresponding total number is reported on the ‘total points’ line. A vertical line is then drawn downward from the total points line to obtain the probability of surgical appropriateness.
Survival analyses
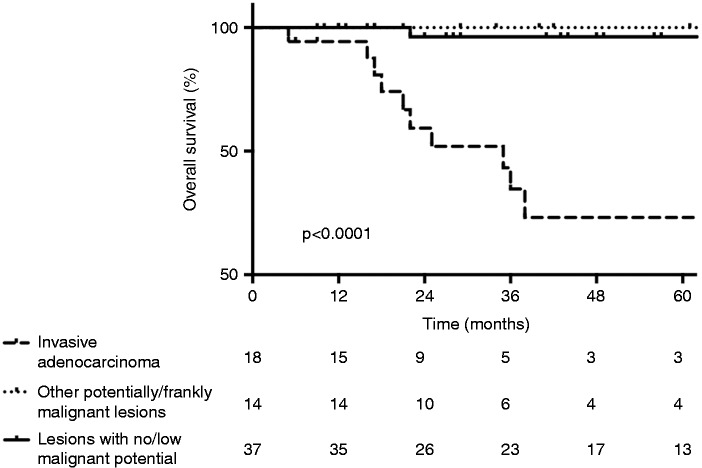
The duration of follow-up and survival in non-operated HRIs was not reported. Reports of recurrence in operated HRIs were too rare for analysis and classification into recurrence or new lesions. Survival was reported in 69 operated HRIs. Median postoperative follow-up was 29 months (17–57). Overall, 11/69 patients (15.9%) died. Ten out of 32 patients (31.3%) in the surgical appropriateness group died of recurrent PDAC. All had been found to have invasive PDAC on the surgical specimen. One out of 38 patients (2.6%) in the non-surgical appropriateness group died of intrahepatic cholangiocarcinoma 16 months after pancreatic surgery. Patients with potentially/frankly malignant lesions had a significantly higher risk of death (HR 15.1, 95% CI 2.58–29.44, P = 0.0011). In particular, survival in HRIs with invasive PDAC was significantly lower than in those with other potentially/frankly malignant lesions or with no/low potential of malignancy (Figure 3). One, 2 and 3-year overall survival rates were 94.4%, 59.4% and 34.6%, respectively, in HRIs with invasive PDAC (median overall survival 35 months), 100%, 100% and 100%, respectively, in those with other types of potentially/frankly malignant lesions, and 100%, 96.3% and 96.3%, respectively, in those with lesions of no/low potential of malignancy, respectively.
Figure 3.
Overall survival following pancreatic surgical resection of high-risk individuals undergoing pancreatic ductal adenocarcinoma (PDAC) screening, depending on the presence of invasive adenocarcinoma, other potentially/frankly malignant lesions (i.e. pancreatic intraepithelial neoplasia (PanIN)-3, branch duct intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia, main duct IPMN or malignant neuroendocrine tumour) or non-malignant lesions (i.e. serous cystadenoma, PanIN-1, PanIN-2 or branch duct IPMN with low-grade dysplasia) at pathological examination of the resected specimen.
Discussion
To our knowledge, this is the first study to propose a patient-level meta-analysis to evaluate the controversial question of the ‘appropriateness’ of pancreatic surgery in HRIs who receive pancreatic screening. All the previously published studies limited populations and were mainly descriptive. The present meta-analysis was performed to obtain sufficient statistical power for inferential analysis. Our main result is that 42.2% of HRI surgeries were appropriate while 57.8% of the remaining patients had lesions of no/low malignant potential. In addition, surgery was beneficial in 20% of operated HRIs who underwent resection of potentially/frankly malignant lesions before the development of invasive adenocarcinoma and who did not die from PDAC.
These results suggest that selection of HRIs for pancreatic surgery should be optimised. Performing surgical resection before the disease becomes invasive (high-grade dysplasia), or achieving R0 resection at the earliest stage possible in patients with invasive tumours would provide the highest chance of cure. Hence, only HRIs with potentially/frankly malignant lesions should be ideally selected for surgery, while follow-up should be continued in those without. Our study showed that proposing surgery in cases of high-risk dysplasia could increase survival (2-year rate of 100% vs. 55.8% in the case of invasive PDAC). Interestingly, the outcome in this subgroup of patients was highly favourable and similar to that in patients operated for lesions with no/low potential malignancy, with a high chance of cure. Conversely, the survival of HRIs operated with invasive PDAC (median, 35 months) was similar, or slightly better, to that of patients operated on for sporadic PDAC not undergoing any previous screening (25–28 months in one recently published trial).29 Hence, surgery might have been performed too late in those patients despite screening.
This study was not designed to evaluate the value or benefit of pancreatic screening itself, but to assess surgical appropriateness. The number of HRIs who underwent surgery cannot be compared with those who were screened. First, the delay between the beginning of screening and surgery could not be analysed and postsurgical follow-up was only 29 months, thus the possibility of developing late recurrence or second pancreatic premalignant lesions could not be excluded. Second, the rate of patients with PDAC who did not undergo surgery and the duration of screening in non-operated HRIs were not reported. Thus this study only provides an image at the time of surgery and does not give information on the natural history of pancreatic (pre)malignant lesions in HRIs.
Our analyses showed that the identification of HRAs at screening was significantly associated with potentially/frankly malignant lesions in the surgical specimen, which is consistent with recent published data.30 There is a consensus on the morphological description of sporadic IPMN-associated worrisome features and high-risk stigmata of malignancies.18 While hereditary PDAC develops from both IPMN and PanIN, the imaging features of the latter have been poorly described, and data on the morphological distinction between low-grade and high-grade PanIN are even more limited.31 Hence the morphological criteria used to define LRAs and HRAs in our study were voluntarily inspired from criteria for sporadic IPMN, with a relevant prognostic impact on our model.
Although both the sensitivity (81.6%) and negative predictive value (83.3%) of the morphological features of pancreatic lesions were acceptable, the specificity (67.3%) and positive predictive value (64.6%) were insufficient. Thus, it seems inadequate as a single criterion for patient selection because this would result in a high rate of surgery for benign lesions. Other original criteria were identified in our analysis, including the presence of a deleterious germline mutation (vs. FPC setting) and age greater than 50 years, although they did not reach statistical significance probably due to low statistical power. Bartsch et al.6 reported that pancreatic screening rarely revealed significant and potentially relevant lesions before the age of 50 years, although almost all screening programmes propose to begin screening at age 40–45 years or 10 years less than the youngest age of PDAC in the family. We proposed a nomogram to predict surgical appropriateness at the individual scale. Even if some variables were not independently associated with surgical appropriateness, some of them interacted significantly in the model, justifying the use of three variables in the Beaujon score. The nomogram was accurate, with an area under the ROC curve of 0.81. Finally, while this score could help improve the case-by-case management of patients, it was developed based on data obtained from a meta-analysis and it must be prospectively validated in an independent cohort of HRIs.
The relevance of surgical decision-making in HRIs involves the detection methods used during initial screening and subsequent follow-up. The present meta-analysis reveals wide heterogeneity between studies.7 For instance, MRI has progressively replaced CT scan and became routine after 2012. EUS and MRI are complementary and their contribution to pancreatic screening is essential as they have increased the detection of abnormal findings in HRIs.28 Systematic sampling is an invasive tool, not always feasible due to the limited size of pancreatic abnormalities. In our meta-analysis one third of patients (three out of nine) with positive cytology or biopsy did not have potentially/frankly malignant lesions on the resected specimen. In all three patients sampling was performed by fine-needle aspiration. This rate of false positive is unusually high, in comparison with the 2% rate reported in the literature.32 Explanations are unknown; this might be due to interpretation errors, or subjectivity bias, i.e. overestimation of the grade of dysplasia due to the acknowledgement of the setting of HRI screening. Besides, the sensitivity of malignant cytology was lower in our analysis (66%) in comparison with the usual sensitivity (85%).32 This might be explained by the fact the patients included in our meta-analysis may have been treated a longer time ago, using techniques of endoscopic sampling and pathological analysis with lower performances. Still, in the case of sample indicating potentially/frankly malignant lesions, it is currently mandatory to propose surgical resection, while explaining to the patient that cytology may have been falsely positive. The future probably lies in molecular biomarkers of pancreatic juices,33–35 but detecting biological stigmata of HRAs before the occurrence of invasive carcinoma will be an even more difficult challenge. The sensitivity of circulating tumour cells/DNA plasma detection is still low and has not been assessed for the screening of HRIs.
This study has the limitations associated with its retrospective design. To limit selection bias, we performed a strict selection of articles and a thorough analysis of all individual data, to avoid multiple inclusions of redundant patients already included in previous studies from the same team. Although there was heterogeneity among studies, all data (including morphological, surgical and pathological) were re-analysed using standardised and consensual grading systems to enable pooled analysis. Finally, the number of included HRIs in each study was relatively low, which justified that meta-analysis; but still, it may have induced a lack of statistical power.
In conclusion, this patient-level meta-analysis suggests that surgery was appropriate in 42.2% of HRIs who underwent pancreatic surgery in the literature. Morphological characterisation of lesions was strongly associated with surgical appropriateness, but age (≥50 years) and the presence of a deleterious germline mutation could also improve patient selection for surgery. The score and the nomogram we proposed to help in surgical decision require prospective validation in large cohorts with adequate longer-term follow-up.
Supplemental Material
Supplemental Material for Appropriateness of pancreatic resection in high-risk individuals for familial pancreatic ductal adenocarcinoma: a patient-level meta-analysis and proposition of the Beaujon score by Louis de Mestier, Marie Muller, Jérôme Cros, Marie-Pierre Vullierme, Dewi Vernerey, Frédérique Maire, Safi Dokmak, Vinciane Rebours, Alain Sauvanet, Philippe Lévy and Pascal Hammel in United European Gastroenterology Journal
Acknowledgement
The authors would like to thank Mrs Dale Roche-Lebrec for technical assistance in the preparation of the manuscript.
Footnotes
The first two authors contributed equally and share first authorship.
Author contribution
Conception of the study: LdM, MM and PH. Data collection and analysis: LdM, MM, JC, FM, MPV, DW, SD, AS, VR, PL and PH. Statistical analysis: LdM and DW. Manuscript writing: LdM, MM and PH. Review and approval of the manuscript: all the authors.
Declaration of conflicting interests
The authors declared no conflict of interest.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Ethics approval
This type of study is exempt from ethics approval.
Informed consent
Not applicable.
References
- 1.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014; 74: 2913–2921. [DOI] [PubMed] [Google Scholar]
- 2.Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer – current knowledge. Nat Rev Gastroenterol Hepatol 2012; 9: 445–453. [DOI] [PubMed] [Google Scholar]
- 3.de Mestier L, Danset J-B, Neuzillet C, et al. Pancreatic ductal adenocarcinoma in BRCA2 mutation carriers. Endocr Relat Cancer 2016; 23: T57–67. [DOI] [PubMed]
- 4.Shindo K, Yu J, Suenaga M, et al. Deleterious germline mutations in patients with apparently sporadic pancreatic adenocarcinoma. J Clin Oncol 2017; 35: 3382–3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brentnall TA. Cancer surveillance of patients from familial pancreatic cancer kindreds. Med Clin North Am 2000; 84: 707–718. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch DK, Slater EP, Carrato A, et al. Refinement of screening for familial pancreatic cancer. Gut 2016; 65: 1314–1321. [DOI] [PubMed] [Google Scholar]
- 7.Canto MI, Harinck F, Hruban RH, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013; 62: 339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basturk O, Hong S-M, Wood LD, et al. A revised classification system and recommendations from the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol 2015; 39: 1730–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maire F, Hammel P, Terris B, et al. Prognosis of malignant intraductal papillary mucinous tumours of the pancreas after surgical resection. Comparison with pancreatic ductal adenocarcinoma. Gut 2002; 51: 717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poruk KE, Firpo MA, Adler DG, et al. Screening for pancreatic cancer: why, how, and who? Ann Surg 2013; 257: 17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lami G, Biagini MR, Galli A. Endoscopic ultrasonography for surveillance of individuals at high risk for pancreatic cancer. World J Gastrointest Endosc 2014; 6: 272–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Capurso G, Signoretti M, Valente R, et al. Methods and outcomes of screening for pancreatic adenocarcinoma in high-risk individuals. World J Gastrointest Endosc 2015; 7: 833–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu C. Screening for pancreatic cancer in familial high-risk individuals: a systematic review. World J Gastroenterol 2015; 21: 8678–8678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vasen H, Ibrahim I, Ponce CG, et al. Benefit of surveillance for pancreatic cancer in high-risk individuals: outcome of long-term prospective follow-up studies from three European expert centers. J Clin Oncol 2016; 34: 2010–2019. [DOI] [PubMed] [Google Scholar]
- 15.Ludwig E, Olson SH, Bayuga S, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. Am J Gastroenterol 2011; 106: 946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Sukhni W, Borgida A, Rothenmund H, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012; 16: 771–783. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology 2012; 12: 183–197. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka M, Fernández-del Castillo C, Kamisawa T, et al. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017; 17: 738–753. [DOI] [PubMed] [Google Scholar]
- 19.Kamarajah SK, Burns WR, Frankel TL, et al. Validation of the American Joint Commission on Cancer (AJCC) 8th Edition Staging System for Patients with Pancreatic Adenocarcinoma: a surveillance, epidemiology and end results (SEER) analysis. Ann Surg Oncol 2017; 24: 2023–2030. [DOI] [PubMed] [Google Scholar]
- 20.Canto MI, Goggins M, Yeo CJ, et al. Screening for pancreatic neoplasia in high-risk individuals: an EUS-based approach. Clin Gastroenterol Hepatol 2004; 2: 606–621. [DOI] [PubMed] [Google Scholar]
- 21.Canto MI, Goggins M, Hruban RH, et al. Screening for early pancreatic neoplasia in high-risk individuals: a prospective controlled study. Clin Gastroenterol Hepatol 2006; 4: 766–781; quiz: 665. [DOI] [PubMed] [Google Scholar]
- 22.Poley JW, Kluijt I, Gouma DJ, et al. The yield of first-time endoscopic ultrasonography in screening individuals at a high risk of developing pancreatic cancer. Am J Gastroenterol 2009; 104: 2175–2181. [DOI] [PubMed] [Google Scholar]
- 23.Verna EC, Hwang C, Stevens PD, et al. Pancreatic cancer screening in a prospective cohort of high-risk patients: a comprehensive strategy of imaging and genetics. Clin Cancer Res 2010; 16: 5028–5037. [DOI] [PubMed] [Google Scholar]
- 24.Canto MI, Hruban RH, Fishman EK, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142: 796–804; quiz: e14–e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sud A, Wham D, Catalano M, et al. Promising outcomes of screening for pancreatic cancer by genetic testing and endoscopic ultrasound. Pancreas 2014; 43: 458–461. [DOI] [PubMed] [Google Scholar]
- 26.Mocci E, Guillen-Ponce C, Earl J, et al. PanGen-Fam: Spanish registry of hereditary pancreatic cancer. Eur J Cancer 2015; 51: 1911–1917. [DOI] [PubMed] [Google Scholar]
- 27.Danset J-B, Cros J, Rebours V, et al. Rentabilité du dépistage des sujets à haut risque (SHR) de cancer du pancréas (CaPa): résultat d’une série française. Presented at the JFHOD Congress 2016, reference C0-45. www.snfge.org (accessed 5 January 2019).
- 28.Harinck F, Konings ICAW, Kluijt I, et al. A multicentre comparative prospective blinded analysis of EUS and MRI for screening of pancreatic cancer in high-risk individuals. Gut 2016; 65: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 29.Neoptolemos JP, Palmer DH, Ghaneh P, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 2017; 389: 1011–1024. [DOI] [PubMed] [Google Scholar]
- 30.Imbe K, Nagata N, Hisada Y, et al. Validation of the American Gastroenterological Association guidelines on management of intraductal papillary mucinous neoplasms: more than 5 years of follow-up. Eur Radiol 2018; 28: 170–178. [DOI] [PubMed] [Google Scholar]
- 31.Maire F, Couvelard A, Palazzo L, et al. Pancreatic intraepithelial neoplasia in patients with intraductal papillary mucinous neoplasms: the interest of endoscopic ultrasonography. Pancreas 2013; 42: 1262–1266. [DOI] [PubMed] [Google Scholar]
- 32.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc 2012; 75: 319–331. [DOI] [PubMed] [Google Scholar]
- 33.Eshleman JR, Norris AL, Sadakari Y, et al. KRAS and guanine nucleotide-binding protein mutations in pancreatic juice collected from the duodenum of patients at high risk for neoplasia undergoing endoscopic ultrasound. Clin Gastroenterol Hepatol 2015; 13: 963–969.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kanda M, Sadakari Y, Borges M, et al. Mutant TP53 in duodenal samples of pancreatic juice from patients with pancreatic cancer or high-grade dysplasia. Clin Gastroenterol Hepatol 2013; 11: 719–730.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jabbar KS, Arike L, Verbeke CS, et al. Highly accurate identification of cystic precursor lesions of pancreatic cancer through targeted mass spectrometry: a phase IIc diagnostic study. J Clin Oncol 2017; 36: 367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Appropriateness of pancreatic resection in high-risk individuals for familial pancreatic ductal adenocarcinoma: a patient-level meta-analysis and proposition of the Beaujon score by Louis de Mestier, Marie Muller, Jérôme Cros, Marie-Pierre Vullierme, Dewi Vernerey, Frédérique Maire, Safi Dokmak, Vinciane Rebours, Alain Sauvanet, Philippe Lévy and Pascal Hammel in United European Gastroenterology Journal