Abstract
Background
We previously showed that the endoscopic Kyoto classification for gastritis could predict Helicobacter pylori infection in individuals with a high negative titer of serum anti-H pylori antibodies. This study evaluated H pylori infection and the Kyoto classification score in patients with a low negative titer (<3 U/ml), high negative titer (3–9.9 U/ml), low positive titer (10–49.9 U/ml), and high positive titer (≥50 U/ml).
Methods
Serum antibody levels, Kyoto classification score and histology were investigated in 870 individuals with no history of H pylori-eradication therapy. Urea breath tests (UBTs) were additionally conducted for patients with a low negative titer and a Kyoto score ≥1 or an antibody titer ≥10 U/ml and a Kyoto score of 0 or 1. UBTs and/or histological studies were conducted for participants with a high negative titer.
Results
False diagnoses based on anti-H pylori antibody titers were observed in 0.3% of the low-negative-titer group, 11.7% of the high-negative-titer group, 18.9% of the low-positive-titer group and 2.2% of the high-positive-titer group. Surprisingly, false diagnoses based on antibody titers were noted in 63.2% of patients with a low positive titer and a Kyoto score of 0 and in 62.5% of patients with a high negative titer and a Kyoto score ≥2, respectively.
Conclusions
Endoscopic findings could predict false diagnoses determined using serum antibody titers.
Keywords: Antibody titer, H pylori, Kyoto classification score
Key summary
Summarize the established knowledge on this subject:
Serum anti-Helicobacter pylori antibody tests yield accuracies of 89% to 95%, sensitivities of 88% to 96%, and specificities of 86% to 96%.
The Kyoto classification is scored from 0 to 8 and is believed to provide an estimate of the risk of gastric cancer.
What are the new findings of this study?
A high rate of false anti-H pylori antibody test results was noted in patients with low positive titers as well as in those with high negative titers.
If the diagnoses according to an antibody assay and the Kyoto classification score are inconsistent, further examinations such as a urea breath test should be undertaken for a more accurate diagnosis.
Introduction
Helicobacter pylori infection is one of the most prevalent infectious diseases worldwide, with 40% to 50% of the global human population estimated to be infected.1 H pylori is associated with the development of atrophic gastritis and gastric cancer.2–6 Eradication of H pylori infection has been reported as an effective strategy for treating atrophic gastritis and peptic ulcer and preventing gastric cancer.7–11
The recently developed endoscopic Kyoto classification score is based on the summation of the following endoscopic findings: atrophy, intestinal metaplasia (IM), enlarged folds, nodularity and redness.12 The Kyoto classification is scored from 0 to 8 and is believed to provide an estimate of the risk of gastric cancer.13
Diagnostic methods of H pylori infection include 13C-urea breath tests (UBTs), measuring serum levels of anti-H pylori antibodies, stool antigen tests, rapid urease tests, culture and pathology.14,15 UBT is regarded as the gold standard because of its higher degree of accuracy. Serum anti-H pylori antibody tests are easy and inexpensive, with commercial test kits yielding accuracies of 89% to 95%, sensitivities of 88% to 96%, and specificities of 86% to 96%.16 In Japan, E-plate (Eiken Chemical, Tokyo, Japan) is the most commonly used commercial serology kit in daily clinical practice with an accuracy of 94.0%, a sensitivity of 95.2%, and a specificity of 92.6% based on the recommended cutoff point of 10 U/ml.17 We previously reported that 17% of individuals with a high negative titer (3–9.9 U/ml) were positive for H pylori infection.13 Furthermore, we determined that the Kyoto classification score could be a useful predictor of H pylori infection in patients with a high negative titer; the endoscopic Kyoto classification score could detect false-negative antibody test results. A combination of the antibody test and the Kyoto classification score might provide a more accurate diagnosis of H pylori infection. This study evaluated the effectiveness of combining the antibody test and the Kyoto classification score. Although our previous study focused on individuals with high negative titers (3–9.9 U/ml),13 this study included patients with low negative titers (<3 U/ml), low positive titers (10–49.9 U/ml) and high positive titers (≥50 U/ml).
Methods
Patients
Consecutive patients who underwent an upper gastrointestinal endoscopy and a serum antibody test between September 2016 and August 2017 in the Toyoshima Endoscopy Clinic were retrospectively reviewed. We included patients who were evaluated for H pylori infection for the first time. We excluded patients with a history of eradication treatment, gastric cancer, or gastrectomy. Upper gastrointestinal endoscopy was used for screening, surveillance for upper gastrointestinal diseases, examination for abnormal findings of upper gastrointestinal radiography, or symptoms such as epigastric pain. Serum antibody levels were measured on the day of upper gastrointestinal endoscopy. This study was approved by the ethical review committee of Hattori Clinic on September 7, 2017. Written informed consent was obtained from all participants. All clinical investigations were conducted according to the ethical guidelines of the Declaration of Helsinki.
Serum anti-H pylori antibody
Antibody titers were measured using an enzyme immunoassay kit with antigens derived from H pylori isolated from Japanese individuals (E-plate Eiken H pylori antibody II; Eiken Chemical, Tokyo, Japan). A cutoff value of 10 U/ml was determined to indicate H pylori positivity.18,19 According to previous studies, we divided participants into four groups according to their serum antibody titers: < 3 U/ml (low negative), 3–9.9 U/ml (high negative), 10–49.9 U/ml (low positive) and ≥50 U/ml (high positive).13,20
Esophagogastroduodenoscopy, pathology and UBT
Upper gastrointestinal endoscopy was performed by expert physicians using the Olympus Evis Lucera Elite system with the GIF-HQ290 or GIF-H290Z endoscope (Olympus Corporation, Tokyo, Japan). Physicians met and discussed all endoscope images before this study. Furthermore, upper gastrointestinal endoscopy images were retrospectively reviewed by other expert physicians. Discrepancies in diagnosis between the two sets of physicians were resolved through discussion.
The Kyoto classification of gastritis is based on the sum of scores of the following five endoscopic findings, which are scored from 0 to 8: atrophy, IM, enlarged folds, nodularity and redness. A high score indicates an increased risk of gastric cancer.21,22 Gastric atrophy was classified according to the extent of mucosal atrophy as described by Kimura and Takemoto.23 C-II and C-III of the Kimura-Takemoto classification were scored as 1, and O-I to O-III as 2. IM is observed as grayish-whitish and slightly opalescent patches. IM within the antrum was scored as 1, and IM extending into the corpus as 2. The presence of folds enlarged over 5 mm or more was scored as 1. Nodularity is characterized by the appearance of multiple whitish, elevated lesions mainly in the pyloric gland mucosa. The presence of nodularity was scored as 1. Diffuse redness refers to uniform redness involving the entire fundic gland mucosa. The presence of redness with regular arrangements of collecting venules (RACs) was scored as 1, and without RACs as 2.
Pathological evaluation was conducted for a Kyoto classification score of gastritis ≥1. Pathological findings were evaluated using the updated Sydney System score.24 Biopsy specimens were obtained from the greater curve of the corpus and antrum. Histological diagnosis was performed by an expert gastrointestinal pathologist (H.W.).
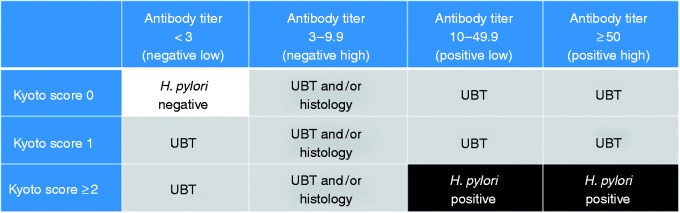
UBTs were additionally conducted for individuals with a low negative titer (<3 U/ml) and a Kyoto score ≥1 and those with an antibody titer ≥10 U/ml and a Kyoto score of 0 or 1. The cutoff value for UBT was 3.0%. If proton pump inhibitors (PPIs) were used, UBT was performed two weeks after discontinuation of the PPI treatment. If a diagnosis according to serum antibody levels and UBT was inconsistent, the UBT was adopted as the gold standard. For patients with a high negative titer (3–9.9 U/ml), UBTs and/or histological examinations for H pylori were conducted. If the results of at least one test were positive in participants with a high negative titer, patients were considered positive for H pylori. Individuals with an antibody titer <3 U/ml and a Kyoto score of 0 were considered negative for H pylori without confirmation using the UBT. Participants with an antibody titer ≥10 U/ml and a Kyoto score ≥2 were considered positive for H pylori without confirmation using the UBT (Figure 1).
Figure 1.
Diagnosis algorithm for Helicobacter pylori infection.
The white area was diagnosed as negative Helicobacter pylori without performing a urea breath test (UBT). The black area was diagnosed as positive H pylori without performing a UBT. The gray area was further investigated by UBT and/or histology.
Results
A total of 919 patients were enrolled. We investigated 870 patients, following the exclusion of 41 patients with a history of eradication therapy, four patients with past gastric cancer, and four patients with past gastrectomy. Among the 870 patients, 612, 139, 74 and 45 patients had antibody titers <3 U/ml (low negative), 3–9.9 U/ml (high negative), 10–49.9 U/ml (low positive) and ≥50 U/ml (high positive), respectively (Table 1).
Table 1.
Demographic data of patients undergoing upper gastrointestinal endoscopy and Helicobacter pylori antibody test.
| Antibody titer (U/ml) | < 3 (negative-low) | 3–9.9 (negative-high) | 10–49.9 (positive-low) | ≥ 50 (positive-high) |
|---|---|---|---|---|
| Patient number (%) | 612 (70.3%) | 139 (16.0%) | 74 (8.5%) | 45 (5.2%) |
| Male:Female (male %) | 241:371 (39.4%) | 47:92 (33.8%) | 32:42 (43.2%) | 14:31 (31.1%) |
| Age (mean ± SD) | 47.8 ± 13.1 | 47.0 ± 14.8 | 52.5 ± 15.8 | 50.9 ± 14.3 |
| Purposes for endoscopy | ||||
| Screening | 165 | 39 | 14 | 9 |
| Surveillance for upper GI diseases | 92 | 23 | 14 | 10 |
| Examination for upper GI radiography | 60 | 14 | 12 | 7 |
| Examination for symptoms | 295 | 63 | 34 | 19 |
GI: gastrointestinal.
In the low-negative-titer group, 5.7% (35/612) had a Kyoto score ≥1. Among them, 5.9% (2/34) were diagnosed as H pylori positive following a UBT, after excluding one patient who did not undergo a UBT. In the low-negative-titer group, 0.3% (2/612) were diagnosed as H pylori positive according to the UBT (Table 2). In these two cases, the histological findings also showed H pylori and neutrophil activity. One case (76 year-old female) involved O-III gastric atrophy and IM extending into the corpus (Kyoto score 4). The other case (58 year-old male) involved O-II gastric atrophy, IM within the antrum and diffuse redness without RACs (Kyoto score 5). False diagnoses based on antibody titers were reported in 13.3% (2/15) of patients with a low negative titer and Kyoto score ≥2 (Figure 2).
Table 2.
Evaluation of Helicobacter pylori antibody.
| Antibody titer (U/ml) | < 3 (negative-low) | 3–9.9 (negative-high) | 10–49.9 (positive-low) | ≥ 50 (positive-high) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient number | 612 | 137 | 74 | 45 | |||||||
| Kyoto score | 0 | ≥1 | ≤1 | ≥2 | ≤1 | ≥2 | |||||
| Urea breath test | (–) | (+) | (–) | (+) | (–) | (+) | (–) | (+) | |||
| H pylori diagnosis | (–) | (–) | (+) | (–) | (+) | (–) | (+) | (+) | (–) | (+) | (+) |
| Patient number | 577 | 32 | 2 | 121 | 16 | 14 | 18 | 42 | 1 | 12 | 32 |
| Rate of false diagnosis | 0.3% | 11.7% | 18.9% | 2.2% | |||||||
| based on antibody | (2/612) | (16/137) | (14/74) | (1/45) | |||||||
Figure 2.
Helicobacter pylori-positive rates in subgroups based on antibody titer and Kyoto score.
In the high-negative-titer group, two patients did not undergo a UBT or histological assessment for H pylori. After excluding these two patients, 137 patients in the high-negative-titer group were analyzed for the accuracy of the anti-H pylori antibody results. In the high-negative-titer group, 11.7% (16/137) were diagnosed as H pylori positive (Table 2). False diagnoses based on antibody titers were found in 35.7% (5/14) of patients with a high negative titer and a Kyoto score of 1 and in 62.5% (10/16) of patients with a high negative titer and a Kyoto score ≥2 (Figure 2).
In the low-positive-titer group, 18.9% (14/74) were diagnosed as H pylori negative according to the UBT (Table 2), and 25.7% (19/74) and 17.6% (13/74) were determined to have a Kyoto score of 0 and 1, respectively. According to the UBT results, 63.2% (12/19) of the low-positive-titer group with a Kyoto score of 0 and 15.4% (2/13) of the low-positive-titer group with a Kyoto score of 1 were diagnosed as H pylori negative (Figure 2). The two patients with a Kyoto score of 1 were given that score according to their Kimura-Takemoto C-II or C-III classification.
In the high-positive-titer group, 2.2% (1/45) were diagnosed as H pylori negative according to the UBT (Table 2), and 8.9% (4/45) and 20% (9/45) were determined to have a Kyoto score of 0 and 1, respectively. Furthermore, 25% (1/4) of the high-positive-titer group with a Kyoto score of 0 were diagnosed as H pylori negative according to the UBT (Figure 2). This case involved a 47-year-old woman who showed a titer of 88.9 U/ml.
In this study, anti-H pylori antibody titers had an accuracy of 96.2%, a sensitivity of 85.2%, a specificity of 98.0%, a positive predictive value of 87.4% and a negative predictive value of 97.6% for the diagnosis of H pylori infection.
Discussion
False diagnoses based on anti-H pylori antibody titers were reported in 0.3% of the low-negative-titer group, 11.7% of the high-negative-titer group, 18.9% of the low-positive-titer group and 2.2% of the high-positive-titer group. The rate of false anti-H pylori antibody test results was relatively high in the high-negative-titer group and even higher in the low-positive-titer group. Therefore, it appears that it is not only high negative titers but also low positive titers that fall into the gray diagnostic zone. The results of our study therefore highlight to physicians the importance of not relying solely on anti-H pylori antibody titers for diagnosing H pylori infection.
We previously showed that a Kyoto classification score of 2 or more could predict H pylori infection in high-negative-titer patients.13 On the other hand, a Kyoto classification score of 1 or 0 indicates that a patient is negative for H pylori infection. If the diagnoses according to an antibody assay and the Kyoto classification score are inconsistent, further examinations such as a UBT should be undertaken for a more accurate diagnosis.
The Kyoto classification score is believed to provide an estimate of the risk of gastric cancer. Sugimoto et al. showed that the Kyoto classification score in patients with gastric cancer was significantly higher than that in patients with gastritis alone.25 Our study showed that the Kyoto classification score might be useful not only for estimating the risk of gastric cancer but also for predicting false diagnoses following anti-H pylori antibody test results.
In the low-negative-titer group, 0.3% (2/612) were diagnosed as H pylori positive according to the UBT. These two patients had severe atrophic gastritis with IM. Both patients were in their seventies. These two patients were similar to Group D according to the ABC method.26 Severe atrophic gastritis with IM could induce a significant decrease in the H pylori count, resulting in a decreased antibody titer.
Individuals with positive anti-H pylori antibody levels and negative UBT results could include those in whom the infection resolved spontaneously. Helicobacter infections other than H pylori could also result in positive serum antibody levels.27 These cases would not require H pylori eradication treatment. Our study showed that a combination of serum anti-H pylori antibody titer and a Kyoto classification score could avoid unnecessary eradication treatment. This study could serve as a warning against unnecessary eradication treatment based on just the anti-H pylori antibody test.
There are some limitations to this study. First, we used the UBT as the gold standard for diagnosing H pylori infection; however, its accuracy is not 100%. Second, we did not perform a UBT in individuals with an antibody titer <3 U/ml and a Kyoto score of 0 or in participants with an antibody titer ≥10 U/ml and a Kyoto score ≥2. Kyoto score ≥ 2 has been reported to predict H pylori infection in high-negative-titer patients.13 Therefore we judged that individuals with an antibody titer ≥10 U/ml and a Kyoto score ≥2 had H pylori infection. Furthermore, we considered that conducting UBTs for these cases would be excessive in daily clinical practice. Further study should be conducted to analyze the association between H pylori infection and the Kyoto classification score.
In conclusion, a high rate of false anti-H pylori antibody test results was noted in patients with low positive titers as well as in those with high negative titers. If the diagnoses according to the antibody assay and endoscopic findings are inconsistent, further examination such as a UBT should be added.
Acknowledgments
We would like to thank Editage for English language editing. Author contributions include the following: T.N. analyzed data and wrote the manuscript; K.S. critically revised the manuscript; H.S. was responsible for interpretation of data; T.Y and Y.T. collected data; N.Y., Y.S. and K.K. supervised the study; H.W. was responsible for pathological analysis; and O.T. developed the study concept and design.
Declaration of conflicting interests
None declared.
Ethics approval
This study was approved by the ethical review committee of Hattori Clinic on September 7, 2017. All clinical investigations were conducted according to the ethical guidelines of the Declaration of Helsinki.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Informed consent
Written informed consent was obtained from all participants.
References
- 1.Suzuki H, Nishizawa T, Hibi T. Helicobacter pylori eradication therapy. Future Microbiol 2010; 5: 639–648. [DOI] [PubMed] [Google Scholar]
- 2.Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med 2001; 345: 784–789. [DOI] [PubMed] [Google Scholar]
- 3.Nishizawa T, Suzuki H, Sakitani K, et al. Family history is an independent risk factor for the progression of gastric atrophy among patients with Helicobacter pylori infection. United European Gastroenterol J 2017; 5: 32–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Toyoshima O, Tanikawa C, Yamamoto R, et al. Decrease in PSCA expression caused by Helicobacter pylori infection may promote progression to severe gastritis. Oncotarget 2018; 9: 3936–3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suzuki H, Nishizawa T, Hibi T. Can Helicobacter pylori-associated dyspepsia be categorized as functional dyspepsia?. J Gastroenterol Hepatol 2011; 26(Suppl 3): 42–45. [DOI] [PubMed] [Google Scholar]
- 6.Nishizawa T, Suzuki H, Nakagawa I, et al. Early Helicobacter pylori eradication restores sonic hedgehog expression in the gastric mucosa of Mongolian gerbils. Digestion 2009; 79: 99–108. [DOI] [PubMed] [Google Scholar]
- 7.Lee YC, Chiang TH, Chou CK, et al. Association between Helicobacter pylori eradication and gastric cancer incidence: A systematic review and meta-analysis. Gastroenterology 2016; 150: 1113–1124.e5. [DOI] [PubMed] [Google Scholar]
- 8.Sakitani K, Nishizawa T, Arita M, et al. Early detection of gastric cancer after Helicobacter pylori eradication due to endoscopic surveillance. Helicobacter 2018; 23: e12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nishizawa T, Suzuki H, Nakagawa I, et al. Gatifloxacin-based triple therapy as a third-line regimen for Helicobacter pylori eradication. J Gastroenterol Hepatol 2008; 23(Suppl 2): S167–S170. [DOI] [PubMed] [Google Scholar]
- 10.Nishizawa T, Suzuki H, Takahashi M, et al. Delay of second-line eradication therapy for Helicobacter pylori can increase eradication failure. J Gastroenterol Hepatol 2013; 28: 1608–1610. [DOI] [PubMed] [Google Scholar]
- 11.Toyoshima O, Yamaji Y, Yoshida S, et al. Endoscopic gastric atrophy is strongly associated with gastric cancer development after Helicobacter pylori eradication. Surg Endosc 2017; 31: 2140–2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato M. Endoscopic findings of H. pylori infection. In: Suzuki H, Warren R, Marshall B. (eds). Helicobacter pylori, Tokyo: Springer Japan, 2016, pp. 157–167. [Google Scholar]
- 13.Toyoshima O, Nishizawa T, Arita M, et al. Helicobacter pylori infection in subjects negative for high titer serum antibody. World J Gastroenterol 2018; 24: 1419–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki H, Nishizawa T, Tsugawa H, et al. Molecular approaches and modern clinical strategies for the management of Helicobacter pylori infection in Japan. Keio J Med 2012; 61: 109–119. [DOI] [PubMed] [Google Scholar]
- 15.Nishizawa T, Suzuki H. Mechanisms of Helicobacter pylori antibiotic resistance and molecular testing. Front Mol Biosci 2014; 1: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talley NJ, Kost L, Haddad A, et al. Comparison of commercial serological tests for detection of Helicobacter pylori antibodies. J Clin Microbiol 1992; 30: 3146–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toyoshima O, Nishizawa T, Sakitani K, et al. Serum anti-Helicobacter pylori antibody titer and its association with gastric nodularity, atrophy, and age: A cross-sectional study. World J Gastroenterol 2018; 24: 4061–4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nishizawa T, Suzuki H, Arano T, et al. Characteristics of gastric cancer detected within 1 year after successful eradication of Helicobacter pylori. J Clin Biochem Nutr 2016; 59: 226–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishizawa T, Suzuki H, Fujimoto A, et al. Effects of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr 2017; 60: 208–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kishikawa H, Kimura K, Takarabe S, et al. Helicobacter pylori antibody titer and gastric cancer screening. Dis Markers 2015; 2015: 156719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kiso M, Yoshihara M, Ito M, et al. Characteristics of gastric cancer in negative test of serum anti-Helicobacter pylori antibody and pepsinogen test: A multicenter study. Gastric Cancer 2017; 20: 764–771. [DOI] [PubMed] [Google Scholar]
- 22.Shichijo S, Hirata Y, Niikura R, et al. Association between gastric cancer and the Kyoto classification of gastritis. J Gastroenterol Hepatol 2017; 32: 1581–1586. [DOI] [PubMed] [Google Scholar]
- 23.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy 1969; 3: 87–97. [Google Scholar]
- 24.Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol 1996; 20: 1161–1181. [DOI] [PubMed] [Google Scholar]
- 25.Sugimoto M, Ban H, Ichikawa H, et al. Efficacy of the Kyoto classification of gastritis in identifying patients at high risk for gastric cancer. Intern Med 2017; 56: 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miki K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels—“ABC method”. Proc Jpn Acad Ser B Phys Biol Sci 2011; 87: 405–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakamura M, Matsui H, Takahashi T, et al. Suppression of lymphangiogenesis induced by Flt-4 antibody in gastric low-grade mucosa-associated lymphoid tissue lymphoma by Helicobacter heilmannii infection. J Gastroenterol Hepatol 2010; 25: 1–6. [DOI] [PubMed] [Google Scholar]