Abstract
Background
Although acute kidney injury (AKI) often accompanies acute liver failure (ALF), its impact on long-term outcome is unknown.
Objective
This study examines the incidence, severity and outcomes of AKI in patients with ALF.
Methods
A total of 134 ALF patients treated at Hannover Medical School between 1995 and 2013 were retrospectively analyzed.
Results
Fifty-four ALF patients (40.3%) demonstrated AKI, as defined by the acute kidney injury network (AKIN) classification, on intensive care unit (ICU) admission, and 85 patients (63.4%) developed AKI prior to ALF recovery, emergency liver transplantation (ELT) or death. AKI severity was closely associated with other end-organ damage (p < 0.001). Follow-up creatinine levels in survivors were increased compared to baseline levels (76 versus 64 µmol/l, p = 0.003). One-hundred-and-three (76.9%) patients reached the combined endpoint of ELT or death, and 42 (31.3%) patients died within 28 days. AKIN stage 3 at ICU admission was the strongest independent predictor of 28-day overall mortality (hazard ratio 3.48, 95% confidence interval 1.75–6.93, p < 0.001) and ELT or death (hazard ratio 2.52, 95% confidence interval 1.60–3.96, p < 0.001).
Conclusions
AKI is a frequent complication in ALF that correlates with remote organ damage and long-term creatinine levels and independently predicts outcome.
Keywords: Liver transplantation, acute hepatitis, renal failure, renal replacement therapy, hemodialysis, hepatorenal syndrome
Key summary
Existing retrospective data, examining 1605 cases, has demonstrated that AKI complicated 70% of ALF, with implications for long-term rates of renal replacement therapy, ELT and survival. Some important information regarding baseline serum creatinine and urinary output was, however, lacking and no insight into the time course of AKIN stage deterioration as well as subsequent serum creatinine values was provided.
The results of our study in non-acetaminophen-induced ALF are: (a) in excess of 20% of patients exhibited elevated creatinine levels one week prior to ALF manifestation; (b) incorporation of urine output criteria revealed a comparable AKI incidence on ICU admission to that observed in the US ALF study group data (63.4 versus 70%); (c) a progression to peak AKIN stage (most frequently AKIN stage 3) occurred in 23% of patients within only 1 (0–4) day; and (d) compared to baseline levels, follow-up creatinine levels indicated a good renal recovery in survivors (76 versus 64 µmol/l, p = 0.003). Patients progressing to AKIN stage 3 on ICU demonstrated persistently elevated creatinine levels at follow-up (106 (69–123) versus 73 (59–84) µmol/l, p = 0.003).
Introduction
Acute kidney injury (AKI) in the context of acute liver failure (ALF) was first described some 60 years ago.1,2 Somewhat surprisingly, there have only been a few subsequent retrospective studies. These have focused on the development and implications of AKI in the setting of ALF, demonstrating an incidence of between 67% and 79%, depending on the etiologic distribution of ALF and the AKI definition used.3–6 The pathogenesis of AKI associated with ALF appears multifactorial. Acetaminophen (ACM) with its known associated renal toxicity, has dominated some of these studies.5,7 Reduced effective arterial blood volume, hepatorenal syndrome, endothelial dysfunction and sepsis are other causes of AKI development.5,8 Furthermore the high incidence of AKI in the acute versus the acute-on-chronic liver failure setting9,10 may result from rapidly declining liver function and associated hemodynamic disturbances.
The aim of this study was to evaluate the incidence and time course of AKI during early ALF, with particular emphasis on serum creatinine course and AKI network (AKIN) urine output criteria as well as its consequences for extra-hepatic organ dysfunction in patients with ALF of indeterminate etiology.
Patients and methods
Setting
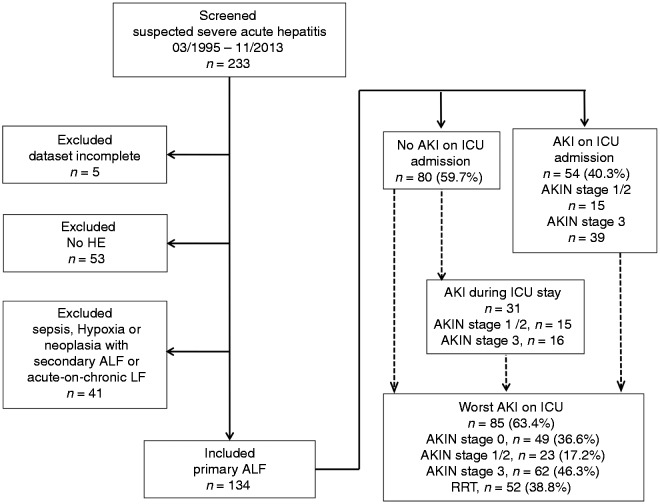
All patients admitted to our intensive care unit (ICU) between 1995 and 2013, who fulfilled the diagnostic criteria of ALF11 with documented hepatic encephalopathy (HE) and available renal function data were retrospectively screened. Patients with overt sepsis were excluded (Figure 1). All patients were managed with standard protocols.
Figure 1.
Overview of the study cohort. Flow chart shows patients screened, excluded, AKIN stages at ICU admission and worst AKIN stages during ICU. AKI(N): acute kidney injury (network); (A)LF: (acute) liver failure; HE: hepatic encephalopathy; ICU: intensive care unit; RRT: renal replacement therapy.
Data acquisition and patient management
Clinical and laboratory data, including King’s College Criteria,12 Sequential Organ Failure Assessment (SOFA)13 and Simplified Acute Physiology Score (SAPS) III scores,14 were obtained by reviewing electronic charts. Prevalence and incidence of AKI from ICU admission to ICU discharge, death or emergency liver transplantation (ELT) were classified using the AKIN serum creatinine and urine output criteria.15 Baseline creatinine levels measured by referring hospitals along with follow-up creatinine levels were available in the majority of patients and were used to calculate the estimated glomerular filtration rate (eGFR) as suggested by the Chronic Kidney Disease Epidemiology Collaboration (CKD Epi)16 and the associated stages suggested by the Kidney Disease: Improving Global Outcomes (KDIGO) organization.17 AKI was not evaluated after ELT to exclude transplant-related confounders. However, long-term follow-up creatinine levels were analyzed in both patients with spontaneous recovery from ALF and patients undergoing ELT. HE was graded on a standard scale of I to IV as described previously.18 The bilirubin lactate and etiology (BiLE) score on admission was calculated as follows: BiLE score = baseline bilirubin (µmol/l)/100 + baseline lactate (mmol/l) + 4 (in case of indeterminate ALF, Budd-Chiari syndrome or phenprocoumon toxicity) – 2 (in case of ACM toxicity) + 0 (in case of any other ALF etiology).19 The decision to initiate renal replacement therapy (RRT) was made on an individual basis, taking into account the degree of metabolic acidosis, hyperkalemia, impending fluid overload and hyperammonemia.
Statistical analysis
Data are presented as numbers and percentages or medians with corresponding interquartile ranges (IQRs). Differences between groups were analyzed by the two-sided Mann–Whitney U test or Kruskal–Wallis test in the case of continuous variables, whereas Chi-squared test was used to compare categorical variables. The distribution of the time-to-event variables was estimated using the Kaplan–Meier method with log-rank testing. ELT-censored death and the composite endpoint of ELT or death from ICU admission to day 28 were the primary outcomes studied. Patients who survived to day 28 were censored at day 28. Parameters associated with outcome at day 28 were identified by univariate and multivariate Cox regression models. Variables found to be statistically significant at a 10% level in the univariate analysis were included in the multivariate model. Data analysis was performed using SPSS (SPSS Inc., Chicago, IL, USA). Figures were prepared using GraphPad Prism (GraphPad Prism Software Inc., San Diego, CA, USA).
Results
Study cohort characteristics at ICU admission
In total 134 ALF patients (76.1% female) with a median (IQR) age of 42.5 (31.5–51) years were included. Indeterminate cause (27.6%) and non-ACM drug-induced liver injury (DILI, 23.9%) were the leading ALF etiologies. ACM was associated with 9.7% of ALF presentations. Less frequently observed (“other”) ALF causes included: Budd-Chiari syndrome and veno-occlusive disease (n = 9), tumor infiltration and/or hemophagocytic syndrome (n = 4), Wilson’s disease (n = 3), autoimmune hepatitis (n = 2), ischemic hepatitis (n = 1) and heat stroke (n = 1). Median peak HE grade was 3 (2–4). Nineteen patients (14.2%) required catecholamine support on tertiary center admission. Characteristics of ALF patients according to AKIN stage at ICU admission are summarized in Table 1.
Table 1.
Patient characteristics at intensive care unit admission.
| Variable | Total | No AKI | AKI |
p value | |
|---|---|---|---|---|---|
| 1–2 | 3 | ||||
| Demographics | |||||
| Number of patients (n, %) | 134 (100) | 80 (59.7) | 15 (11.1) | 39 (29.1) | |
| Age (years, median (IQR)) | 42.5 (31.5–51) | 39 (28–49) | 43.5 (36.3–52) | 48 (36–53.5) | 0.084 |
| Female sex (n, %) | 102 (76.1) | 64 (80) | 12 (80) | 26 (66.7) | 0.440 |
| Clinical data | |||||
| Body weight (kg, median (IQR)) | 71 (62.5–80) | 70 (62.5–80) | 76 (64.5–81) | 70 (61.5–82.5) | 0.87 |
| Temperature (℃, median (IQR)) | 36.4 (36–36.8) | 36.4 (36.1–36.8) | 36.4 (36–36.5) | 36.3 (35.6–36.9) | 0.27 |
| Heart rate (bpm, median (IQR)) | 93 (80.3–110) | 90 (80–110) | 95 (80.5–102) | 110 (90–117.5) | 0.006 |
| MAP (mmHg, median (IQR)) | 86.7 (76.7–96.7) | 86.7 (80.9–100) | 90 (70–95) | 80 (75.3–95) | 0.029 |
| pO2/FiO2 (median (IQR)) | 343 (293.8–428.6) | 353.6 (333.3–428.6) | 381 (311.7–428.6) | 300 (233.3–432.8) | 0.88 |
| Vasopressor requirement (n, %) | 19 (14.2) | 4 (5) | 2 (13.3) | 13 (33.4) | 0.001 |
| GCS (score, median (IQR)) | 14 (12–15) | 14 (13–15) | 14 (11.5–15) | 14 (10–15) | 0.046 |
| Maximum hepatic coma grade (score, median (IQR)) | 3 (2–4) | 3 (2–4) | 2 (1–3.5) | 3 (2–4) | 0.2 |
| SOFA score (score, median (IQR)) | 7 (5–9) | 5 (4–7) | 7 (6–10) | 10 (8–12.5) | <0.001 |
| SAPS III admission (score, median (IQR)) | 55 (49.3–63.8) | 51 (46–56) | 58 (55–63) | 65 (56–73) | <0.001 |
| BiLE score (score, median (IQR)) | 7.5 (5–10.4) | 6.7 (4.9–8.1) | 6.4 (3.6–10) | 11 (7.5–14.2) | <0.001 |
| King’s College Criteria fulfilled (n, %) | 71 (53) | 42 (52.5) | 5 (33.3) | 24 (61.5) | 0.132 |
| ALF etiology (n, %) | |||||
| ACM DILI | 13 (9.7) | 8 (10) | 2 (13.3) | 3 (7.7) | 0.851 |
| Non-ACM DILI | 32 (23.9) | 21 (26.3) | 3 (20) | 8 (20.5) | 0.893 |
| Viral | 31 (23.1) | 22 (27.5) | 2 (13.3) | 7 (17.9) | 0.391 |
| Other | 21 (15.7) | 9 (11.3) | 4 (26.7) | 8 (20.5) | 0.321 |
| Indeterminate | 37 (27.6) | 21 (26.3) | 4 (26.7) | 13 (33.3) | 0.663 |
| Laboratory data (median (IQR)) | |||||
| Platelets (106/µl) | 138.5 (94–208.5) | 159 (115–222.5) | 130 (104.5–222.5) | 100 (64.5–163) | 0.001 |
| Leukocytes (103/µl) | 10.95 (7.95–15.53) | 9.5 (7–12.1) | 13.3 (11.8–16.7) | 13.7 (10.43–17.1) | 0.014 |
| AST (U/l) | 1307 (317–3478) | 1208.5 (468.8–3503.5) | 893 (231.5–1633) | 1690 (224–4806) | 0.032 |
| ALT (U/l) | 1637 (543.8–3979.5) | 1716 (786.8–4201.3) | 1167 (202.5–2200) | 1617 (273–4093.5) | 0.32 |
| Bilirubin (µmol/l) | 240.5 (88–426) | 252 (120.8–402.3) | 108 (53–477) | 263 (73–471.5) | 0.77 |
| INR | 3.4 (2.5–4.7) | 3.5 (2.5–5.3) | 2.8 (2.2–4.5) | 3.5 (2.3–5) | 0.81 |
| pH | 7.43 (7.36–7.47) | 7.45 (7.41–7.49) | 7.42 (7.4–7.46) | 7.36 (7.25–7.42) | <0.001 |
| Lactate (mmol/l) | 3.8 (2.51–6.26) | 3.1 (2.3–4.5) | 3.5 (2.9–5) | 6.5 (4.4–11.8) | <0.001 |
| Kidney specific data | |||||
| Creatinine (µmol/l, median (IQR)) | 80 (57–168.3) | 61 (46.8–77.8) | 157 (136–201.5) | 226 (143–309) | <0.001 |
| Urine output (ml/24 h, median (IQR)) | 1730 (565–2935) | 2600 (1695–3740) | 1580 (985–2375) | 120 (0–470) | <0.001 |
| Baseline creatinine (µmol/l, median (IQR))a | 63.5 (53–79.3) | 63 (53–79.3) | 77 (62–81) | 70 (54.5–79) | 0.58 |
| Incidence of AKI in patients presenting with normal renal function at ICU admission (n, %) | 80 (100) | 49 (61.3) | 15 (18.8) | 16 (20) | |
| Worst creatinine (µmol/l, median (IQR))b | 137.5 (68–245.3) | 73 (57–144) | 194 (136.5–244) | 277 (203.5–351) | <0.001 |
| Worst urine output (ml/24 h, median (IQR))b | 970 (120–2450) | 1850 (920–2800) | 900 (105–2190) | 0 (0–225) | <0.001 |
| Time to maximum AKIN stage (days, median (IQR)) | 1 (0–4) | 2 (0–6) | 3 (0–7) | 1 (0–1) | 0.14 |
p values in bold denote statistical significance.
Before hospitalization.
During hospitalization.
ACM: acetaminophen; AKI: acute kidney injury; AKIN: acute kidney injury network; ALF: acute liver failure; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BiLE: bilirubin lactate and etiology; DILI: drug-induced liver injury; GCS: Glasgow coma scale; INR: international normalized ratio; IQR: interquartile range; MAP: mean arterial pressure; pO2/FiO2: partial pressure of oxygen in arterial blood divided by the fraction of inspired oxygen; SAPS: Simplified Acute Physiology Score; SOFA: Sequential Organ Failure Assessment.
Incidence and progression of AKI in ALF
Baseline creatinine levels were available for 80 patients (59.7%) and did not differ between those presenting with unaffected renal function and those with AKIN stage 1/2 or 3 renal dysfunction (63 versus 77 versus 70 µmol/l, p = 0.58). Fifty-four ALF patients (40.3%) demonstrated AKI at ICU admission (AKIN stage 1/2, n = 15 (11.1%), AKIN stage 3, n = 39 (29.1%)). By definition, admission creatinine levels increased and urine output steadily decreased within the first 24 hours across all AKIN stages (p < 0.001) (Table 1). Eighty-five patients (63.4%) developed AKI at any time prior to ALF recovery, ELT or death. Median progression to peak AKIN stage (most frequently AKIN stage 3 (n = 62, 46.3%)) occurred within 1 (0–4) day (Figures 1 and 2). Fifty-two patients (38.8%) required RRT before recovery, ELT or death. AKI at ICU admission was associated with subsequent RRT requirement (p < 0.001). Over 87% patients with AKIN stage 3 at ICU admission required RRT within the first 24 hours. Patients without renal dysfunction at admission had a lower need for RRT (15%), which was initiated after 4 (2–5) days. AKI incidence and severity were similar between different ALF etiologic entities for AKI on ICU admission (Table 1, Supplementary Table 1).
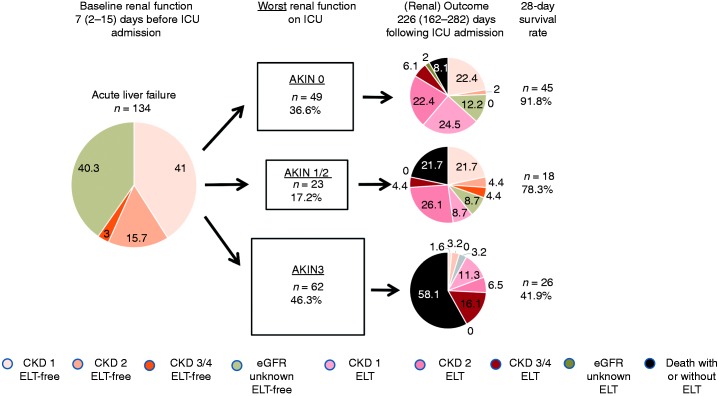
Figure 2.
Overview on baseline renal function, long-term renal and overall outcome (spontaneous survival, ELT and/or death) in relation to worst AKIN stages on ICU. Numbers within each pie chart represent percent values. AKIN: acute kidney injury network; CKD: chronic kidney disease; eGFR: estimated glomerular filtration rate; ELT: emergency liver transplantation; ICU: intensive care unit.
Association of AKI severity with organ dysfunction
AKI severity at ICU admission correlated with various baseline clinical parameters including heart rate (p = 0.006), mean arterial pressure (p = 0.029), vasopressor requirement (p = 0.001), platelet count (p = 0.001), leukocyte count (p = 0.014) and lactate levels (p < 0.001). Similarly, severity of non-hepatorenal end-organ damage as scored by SAPS III and SOFA increased in parallel with the severity of AKI (p < 0.001).The association between the extent of liver injury and dysfunction and AKI severity was less apparent. Aspartate aminotransferase levels increased significantly across AKIN stages (p = 0.032), whereas alanine aminotransferase (p = 0.32), prothrombin time (p = 0.72) and serum bilirubin (p = 0.77) did not differ between non-AKI and AKI patients. BiLE score, a predictive instrument in ALF, which was associated with the incidence and severity of AKI (p < 0.001), probably exerted its prognostic value by means of the lactate component (Table 1).
Renal outcomes
Follow-up creatinine levels were available for 78/89 surviving patients (87.6%) at a median of 226 (162–282) days following ICU admission. These demonstrated a small but significant increase, compared to baseline creatinine (76 versus 64 µmol/l, p = 0.003) (Tables 1 and 2, Supplementary Figure 1). Patients with AKIN stage 3 on admission or progression to AKIN stage 3 during ICU stay (follow-up creatinine available in 60 of 62 patients (96.7%)) exhibited higher follow-up creatinine levels (113 (68–133) versus 74 (62–93) µmol/l, p = 0.01, and 106 (69–123) versus 73 (59–84) µmol/l, p = 0.003, respectively). Although 25% of patients with spontaneous recovery and without renal dysfunction on ICU preserved normal creatinine levels until follow-up, only 3.2% of patients did so if they had developed AKIN stage 3 at any time following ALF. Accordingly, there was a (non-significant) trend towards higher eGFR-based KDIGO-CKD stages (p = 0.070). ELT was associated with higher follow-up creatinine levels compared to spontaneous survival (82 (67–111) versus 62 (57–74) µmol/l, p = 0.001, Figure 2).
Table 2.
Outcome depending on admission acute kidney injury network stage.
| Variable | Total | No AKI | AKI |
p value | |
|---|---|---|---|---|---|
| 1–2 | 3 | ||||
| Number of patients (n, %) | 134 (100) | 80 (59.7) | 15 (11.1) | 39 (29.1) | |
| Renal outcome | |||||
| Need of RRT (n, %)a | 52 (38.8) | 12 (15) | 6 (40) | 34 (87.2) | <0.001 |
| Time to first RRT (days, median (IQR)) | 1 (0–2) | 4 (2–5) | 1.5 (1–5.8) | 0 (0–1) | <0.001 |
| Follow-up creatinine (µmol/l, median (IQR))b | 75.5 (63–101.3) | 72.5 (59.3–93) | 78 (70–99.3) | 112.5 (67.8–132.8) | 0.01 |
| Interval for follow-up creatinine (days, median (IQR)) | 226 (162–282) | 210.5 (126.3–282.3) | 225 (129.3–487) | 230 (164–302.5) | 0.9 |
| Delta creatinine (µmol/l, median (IQR))c | 15 (–7–28) | 14.5 (–7.3–26.3) | 6.5 (–18–25.5) | 50 (–3–91) | 0.006 |
| 28-day survival and ELT | |||||
| Overall survival (n, %) | 89 (66.4) | 60 (75) | 13 (86.6) | 16 (41) | 0.001 |
| ELT-free survival (n, %) | 31 (23.1) | 22 (27.5) | 5 (33.3) | 4 (10.2) | 0.068 |
| ELT (n, %) | 63 (47) | 41 (51.2) | 8 (53.3) | 14 (35.9) | 0.253 |
| Death or ELT (n, %) | 103 (76.9) | 58 (72.5) | 10 (66.7) | 35 (89.7) | 0.068 |
| ELT-censored mortality (n, %) | 42 (31.3) | 17 (40.5) | 2 (4.7) | 23 (54.8) | <0.001 |
p values in bold denote statistical significance.
During hospitalization.
Of surviving patients with and without ELT.
Difference between follow-up creatinine and baseline creatinine.
AKI: acute kidney injury; ELT: emergency liver transplantation; IQR: interquartile range; RRT: renal replacement therapy.
Patient outcome and predictors of survival
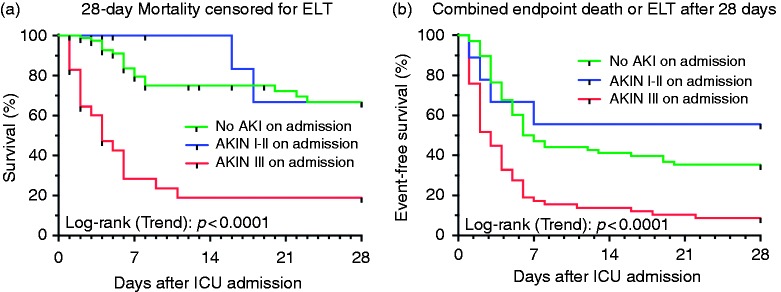
In the entire ALF cohort, 31 (23.1%) patients recovered spontaneously and 63 (47%) patients underwent ELT. One-hundred-and-three (76.9%) patients reached the combined endpoint of ELT and/or death, of whom 42 (31.3%) non-transplanted patients died within 28 days of admission. AKIN stage 3 at admission increased ELT-censored 28-day mortality (54.8 versus 40.5%, p < 0.001), conferring low ELT-free survival (10.3 versus 28.4%, p = 0.024). The adjusted Cox proportional hazards regression analysis confirmed AKIN stage 3 at admission as an independent predictor of overall 28-day mortality (hazard ratio (HR) 3.48 (95% confidence interval (CI) 1.75–6.93), p < 0.001) and 28-day combined endpoint of ELT and/or death (HR 2.52 (95% CI 1.60–3.96), p < 0.001) (Table 3, Figure 3). Comparing peak AKIN stage during clinical course rather than admission AKIN stage, differences in 28-day mortality were even more apparent: 8.2 (no renal dysfunction) versus 21.7 (worst AKIN stage 1/2) versus 58.1% (worst AKIN stage 3). Development of AKIN stage 3 reduced the likelihood of ELT-free survival with a normal follow-up creatinine to 3.2%, compared to 24.5% in the absence of renal dysfunction (Figure 2). AKIN stage 3 and BiLE score > 6.9 on ICU admission served as independent predictors of mortality and/or the combined endpoint of ELT/death at day 28 (Table 3).
Table 3.
Prediction of 28-day overall mortality and the combined endpoint of emergency liver transplantation or mortality within 28 days in relation to selected variables on admission.
| Variable | 28-day overall mortality HR (95% CI) | p value | ELT and/or death within 28 days HR (95% CI) | p value |
|---|---|---|---|---|
| Indeterminate etiology | NS | NS | ||
| King’s College Criteria | NS | NS | ||
| BiLE score > 6.9 | NS | 1.95 (1.22–3.12) | 0.005 | |
| AKIN stage 3 | 3.48 (1.75–6.93) | <0.001 | 2.52 (1.60–3.96) | <0.001 |
Estimated hazard ratio (HR), 95% confidence interval (CI) and p value were calculated using adjusted logistic regression models.
AKIN: acute kidney injury network; BiLE: bilirubin lactate and etiology score; ELT: emergency liver transplantation; NS: not significant.
Figure 3.
Outcome of acute liver failure patients stratified by admission acute kidney injury network stages. Kaplan–Meier curves show outcome of patients with and without AKI on admission to ICU. Admission AKIN stage 3 is significantly associated with a high 28-day ELT-censored mortality of the patients (a) as well as a low ELT-free survival (b). ELT: emergency liver transplantation; AKI(N): acute kidney injury (network); ICU: intensive care unit.
Discussion
Within this predominantly non-ACM-induced ALF cohort it could be shown that 40% presented with AKI at ICU admission, and a further 20% developed AKI prior to recovery, ELT or death. Furthermore, it was observed that AKI severity was closely associated with non-hepatorenal end-organ damage and that AKIN stage 3 at admission was an independent predictor of 28-day survival, with the majority of survivors exhibiting acceptable renal recovery.
In comparison to the US ALF study group,6 our patients exhibited similar age, were more likely to be female, possessed less favorable ALF etiologies 13 (9.7%) versus 738 (46%) ACM DILI), alongside greater hepatic dysfunction (international normalized ratio (INR) 3.4 versus 2.7). Unsurprisingly, rates of RRT were higher (38.8 versus 30.6%) and ELT-free survival lower (23.1 versus 48.4%) in our cohort (Supplementary Table 2). Extrapolation of the US ALF data is, however, difficult, given the inherent limitations of the missing AKIN relevant parameters and the high prevalence of ACM-induced ALF (46%) which differs from that in many other geographical regions.20
Our findings clearly contribute to clarifying some of the pertinent aspects. By including baseline creatinine levels, we could show that the majority of patients developed AKI as a consequence of ALF. It remains speculative, however, if inclusion of the AKIN criteria enhances the diagnostic sensitivity of AKI: 54 of 134 patients (40.3%) in our cohort versus 716 of 1605 (44.6%) in the US ALF cohort showed some degree of AKI at presentation; 85 of 134 patients (63.4%) developed AKI at some point in ALF during clinical course in our cohort. Equivalent data were not presented by Tujios et al.6 Kumar et al.21 recommended to repeatedly evaluate liver-specific prognostic parameters (ammonia, bilirubin, INR and HE) over the first 3 days. This “early dynamic” approach demonstrated excellent mortality discrimination in comparison to King’s College Criteria and Model of End-stage Liver Disease score.21 It appears rational to include renal dysfunction in such a dynamic model as well, particularly in patients with non-ACM DILI and indeterminate ALF. In addition, by analyzing follow-up creatinine levels, we could demonstrate that survival with acceptable CKD stage is achievable in the majority of surviving patients. However, the influence of ELT requires due consideration as this inevitably improved renal outcomes.
Our data correspond well to the data from the US ALF study group regarding the distribution of AKIN stages. Only 11.2% of ALF patients in our cohort (9.7% in the US ALF study group) presented with AKIN stage 1/2 at admission, whereas the majority of patients either lacked renal dysfunction or suffered from AKIN stage 3 renal failure, often requiring RRT within 24 hours. This “all or nothing” pattern of AKI in the setting of ALF may point towards a certain cut-off above which AKI develops. Our data suggest that the extent of liver dysfunction is, however, not the driving force of AKI. The association between aspartate aminotransferase elevation and AKIN stage may point towards hemodynamic compromise with reduced venous return as a common cause of renal dysfunction. However, many subacute ALFs develop AKI in the absence of overt right heart failure. We have recently shown that circulating angiopoietin-2 (Angpt-2), which is markedly upregulated in the injured liver, is a potential mediator of severe remote organ injury though not associated with the severity of liver dysfunction.8 It remains unclear as to what extent Angpt-2 contributes to AKI in ALF, but antagonizing Angpt-2 completely prevented AKI in a murine ischemia/reperfusion injury model.22 Another explanation of the AKI pattern observed might be given by the study setting. Most ALF patients were admitted to tertiary care several days after symptom onset, leading to the early phases of AKI being missed.
An association between ACM-induced ALF and a development to higher AKIN stages has been reported by several authors.4,6,20 Leithead et al.4 investigated AKI in 308 Scottish ALF patients, 207 (70.5%) of whom were caused by ACM. At the time of admission to hospital, 133 (43%) had AKI (defined as creatinine levels >2 times a presumed baseline level), and 208 patients (67%) developed AKI at some point of their illness. Variables independently associated with AKI were patient age, fulfilling King’s College Criteria, hypotension, systemic inflammatory response syndrome, superimposed infection and ACM ALF.4 Given these observations, the lack of association between ACM ALF and AKI in our cohort was surprising. Of note, Leithead et al.4 included only a small proportion of patients with seronegative hepatitis, that is cryptogenic ALF (12.7%), and Tujios et al.6 summarized all non-ACM, non-ischemic patients as “other” etiologies. The studies are therefore difficult to compare. Our results indicate, however, that cryptogenic and other less frequently encountered ALF entities may imply a high risk for AKI.
For ALF patients presenting with AKIN stage 3 renal dysfunction at ICU admission, the main implication is clearly prognosis, as this—in the absence of ELT—is a strong indicator for impending death. Patients with an unfavorable prognosis should be considered early and with a comparably low threshold for ELT.
Limitations of our study are the single-center design and the retrospective data acquisition which both limit the generalizability of our conclusions. In order to avoid any bias implicated by the therapeutic complexity of an ELT procedure, we limited our observation period to the early period of ALF until one of the predefined endpoints (spontaneous recovery, ELT or death) appeared. Although late, unexpected fatal outcomes are rare following ALF, we cannot exclude that our combined endpoint of ELT and/or death until hospital discharge may have missed some patients deteriorating afterwards.
Conclusion
AKI represents a major problem in non-ACM-induced ALF. Sixty percent of ALF patients develop AKI at some point in time during the acute phase of illness. The incidence of AKI is closely associated with non-hepatorenal end-organ damage and represents a strong independent predictor of 28-day mortality. The majority of ALF patients require ELT which enables renal recovery in almost all surviving patients.
Supplemental Material
Supplemental Material for Outcomes of renal dysfunction in patients with acute liver failure by Johannes Hadem, Jan T. Kielstein, Michael P. Manns, Philipp Kümpers and Alexander Lukasz in United European Gastroenterology Journal
Acknowledgment
We are grateful for scientific advice by Dr Matthias Bahr, Sana Kliniken Lübeck, Germany. We are much obliged for thorough revision of the manuscript by Dr Mark Greer, Department of Respiratory Medicine, Hannover Medical School, Germany.
Declaration of conflicting interests
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Author contributions
JH devised the study, included patients, collected the data and wrote the manuscript; JTK and MPM supervised the project and revised the manuscript; PK performed statistical analysis and revised the manuscript; AL performed statistical analysis and wrote and revised the manuscript.
Ethics approval
The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institutional ethics committee (28 Jan 2004, #3493).
Informed consent
As this retrospective study relied on clinical data acquired as part of routine care, the need for informed consent was waived by the institutional ethics committee.
References
- 1.Hecker R, Sherlock S. Electrolyte and circulatory changes in terminal liver failure. Lancet 1956; 271: 1121–1125. [DOI] [PubMed] [Google Scholar]
- 2.Ritt DJ, Whelan G, Werner DJ, et al. Acute hepatic necrosis with stupor or coma. Medicine (Baltimore) 1969; 48: 151–172. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson SP, Blendis LM, Williams R. Frequency and type of renal and electrolyte disorders in fulminant hepatic failure. Br Med J 1974; 1: 186–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leithead JA, Ferguson JW, Bates CM, et al. The systemic inflammatory response syndrome is predictive of renal dysfunction in patients with non-paracetamol-induced acute liver failure. Gut 2009; 58: 443–449. [DOI] [PubMed] [Google Scholar]
- 5.O’Riordan A, Brummell Z, Sizer E, et al. Acute kidney injury in patients admitted to a liver intensive therapy unit with paracetamol-induced hepatotoxicity. Nephrol Dial Transplant 2011; 26: 3501–3508. [DOI] [PubMed] [Google Scholar]
- 6.Tujios SR, Hynan LS, Vazquez MA, et al. Risk factors and outcomes of acute kidney injury in patients with acute liver failure. Clin Gastroenterol Hepatol 2015; 13: 352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blakely P, McDonald BR. Acute renal failure due to acetaminophen ingestion: a case report and review of the literature. J Am Soc Nephrol 1995; 6: 48–53. [DOI] [PubMed] [Google Scholar]
- 8.Hadem J, Bockmeyer CL, Lukasz A, et al. Angiopoietin-2 in acute liver failure. Crit Care Med 2012; 40: 1499–1505. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology 2008; 48: 2064–2077. [DOI] [PubMed] [Google Scholar]
- 10.Moreau R, Jalan R, Gines P, et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013; 144: 1426–1437. [DOI] [PubMed] [Google Scholar]
- 11.Polson J, Lee WM, American Association for the Study of Liver Disease AASLD position paper: the management of acute liver failure. Hepatology 2005; 41: 1179–1197. [DOI] [PubMed] [Google Scholar]
- 12.O’Grady JG, Alexander GJM, Hayllar KM, et al. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology 1989; 97: 439–455. [DOI] [PubMed] [Google Scholar]
- 13.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 1996; 22: 707–710. [DOI] [PubMed] [Google Scholar]
- 14.Moreno RP, Metnitz PGH, Almeida E, et al. SAPS 3—From evaluation of the patient to evaluation of the intensive care unit. Part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med 2005; 31: 1345–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31–R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens PE, Levin A, Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Int Med 2013; 158: 825–830. [DOI] [PubMed] [Google Scholar]
- 18.Lee WM. Acute liver failure. N Engl J Med 1993; 329: 1862–1872. [DOI] [PubMed] [Google Scholar]
- 19.Hadem J, Stiefel P, Bahr MJ, et al. Prognostic implications of lactate, bilirubin, and etiology in German patients with acute liver failure. Clin Gastroenterol Hepatol 2008; 6: 339–345. [DOI] [PubMed] [Google Scholar]
- 20.Bernal W, Wendon J. Acute liver failure. N Engl J Med 2013; 369: 2525–2534. [DOI] [PubMed] [Google Scholar]
- 21.Kumar R, Shalimar, Sharma H, et al. Prospective derivation and validation of early dynamic model for predicting outcome in patients with acute liver failure. Gut 2012; 61: 1068–1075. [DOI] [PubMed] [Google Scholar]
- 22.Rübig E, Stypmann J, van Slyke P, et al. The synthetic Tie2 agonist peptide vasculotide protects renal vascular barrier function in experimental acute kidney injury. Sci Rep 2016; 6: 22111–22111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Outcomes of renal dysfunction in patients with acute liver failure by Johannes Hadem, Jan T. Kielstein, Michael P. Manns, Philipp Kümpers and Alexander Lukasz in United European Gastroenterology Journal