Abstract
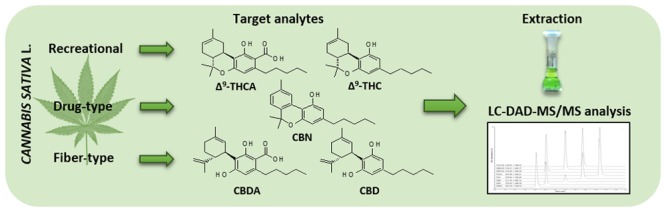
Cannabis sativa L. represents one of the most widely used source of drugs and drugs of abuse worldwide. Its biologically active compounds are mainly cannabinoids, including Δ9-tetrahydrocannabinol (THC), which is responsible for the psychoactive effects, tetrahydrocannabinolic acid (THCA), cannabinol (CBN), cannabidiol (CBD), and cannabidiolic acid (CBDA). Together with recreational and drug-type (or medicinal) Cannabis, some new products have been recently released into the market as fiber-type Cannabis variants (also known as hemp or industrial hemp) with low THC content and high content of nonpsychoactive CBD. In this research work, the aim was to characterize Cannabis recreational and drug-type samples by quantifying their active principles, after the development and validation of a suitable analytical method. In addition to the Cannabis samples described above, fiber-type plant varieties were also analyzed to monitor their content of nonpsychoactive compounds for both pharmaceutical and nutraceutical purposes. To do this, a highly efficient HPLC–DAD–MS/MS method, with an electrospray ionization (ESI) source and a triple-quadrupole mass analyzer acquiring in the multiple reaction monitoring (MRM) mode also coupled to a diode array detector (DAD), was developed and applied. Satisfactory validation results were obtained in terms of precision (RSD < 6.0% for all the analytes) and accuracy (>92.1% for all the compounds). The proposed methodology represents a versatile and reliable tool to assess both psychoactive and nonpsychoactive cannabinoid levels in Cannabis samples for a more rational use in both medicinal chemistry and nutraceutics.
Keywords: Cannabis sativa L, hemp, cannabinoids, drug of abuse, HPLC, MS, method validation
Cannabis sativa L. represents by far the most widely produced and consumed drug of abuse (DoA) worldwide; in particular, the users represent the 3.9% of the global population aged 15–64 years.1
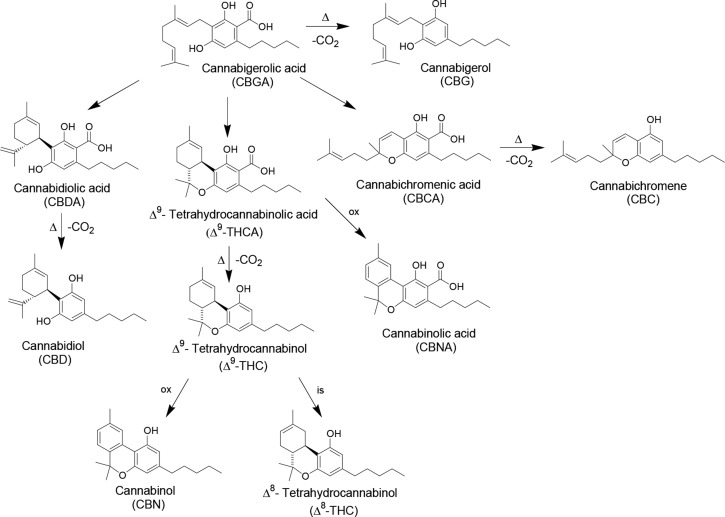
The most representative class of Cannabis bioactive compounds is composed of cannabinoids, which are terpenophenolics.2,3 Among them (Figure 1), Δ9-tetrahydrocannabinol (THC) is responsible for Cannabis psychoactive effects.4−7 Other main cannabinoids include tetrahydrocannabinolic acid (THCA), cannabinol (CBN), cannabidiol (CBD), and cannabidiolic acid (CBDA) (Figure 1). As shown in Figure 1, cannabigerolic acid (CBGA) is the biosynthetic precursor of both THCA and CBDA. Cannabinoids are biosynthesized in the acid form in plant tissues; then, they can generate their decarboxylated counterparts under the action of heat and light, by means of a spontaneous decarboxylation.3,4,8−10 CBN is the oxidative degradation product of Δ9-THC present in aged Cannabis.3,4,8,9
Figure 1.
Chemical structures of main cannabinoids present in Cannabis sativa L. Abbreviation: Δ = heating; ox = oxidation; is = isomerization.
The vast majority of the psychoactive effects of THC are mediated by CB1 receptors, while nonpsychoactive cannabinoids, such as CBD, have low affinity for both CB1 and CB2 receptors.3 The interaction with CB1 receptors is responsible also for the analgesic effect of THC since their role in the transmission of the nociceptive information has been demonstrated.3 The immunomodulatory activity of THC can be explained by its binding to CB2 receptors, which are highly expressed in some cells of the immune system and are believed to have a role in the immune cell function.3 In addition, CB2 receptors are also considered to be involved in neuro-inflammation, atherosclerosis, and bone remodelling.3
Within nonpsychoactive cannabinoids, CBD has been found to possess a high antioxidant and anti-inflammatory activity,12 in addition to its antimicrobial, neuroprotective, anxiolytic, and anticonvulsant properties.3,12−17 The mechanism of action of CBD is believed to be multitarget.12,17
Recreational Cannabis, mainly marijuana, that it is usually sold illicitly in the black market or in the darknet, has high level of THC and low level of CBD. This type of Cannabis has been traditionally used in its smokeable preparations because this is the best way to achieve the wished psychoactive effects, but, more recently, it has been employed in unconventional ways, such as by means of vaporizers, edibles, beverages, or transdermal products.18 The severe effects of Cannabis consumption, mainly related to the presence of THC, include perceptive alterations, euphoria, hallucinations, slowed down reflexes, relaxation, and reduced coordination,1,19 which, for example, make driving under the influence of Cannabis extremely dangerous.
Drug-type or medicinal Cannabis has been proposed for the treatment of multiple sclerosis, neuropathic pain, chronic pain associated with rheumatoid arthritis and migraine, in oncological patients treated with chemotherapy, in mood disorders and in inflammatory diseases.20Cannabis for medicinal use has high/medium level of THC and low/medium level of CBD.
Recently, many countries have legalized also the use of fiber-type Cannabis, known as hemp or industrial hemp, which has to be characterized by a specific composition. In particular, THC has to be under a very low level (usually, 0.2–0.3% w/w) in order to obtain a product without psychoactive effects. The main compound of fiber-type plants is usually represented by CBD and its acidic precursor CBDA.3,4,8−11 Due to the biological activities previously described for CBD, extracts obtained from fiber-type Cannabis have a great potential for a possible use in pharmaceutical and nutraceutical products.
Cannabinoid profiling by means of advanced analytical techniques is of primary importance for a rational use of Cannabis for therapeutic purposes. The routine analysis of cannabinoids in both plant and biological matrices has been usually carried out by HPLC and GC methods.5,7,8,10,11 Nevertheless, the HPLC methods described so far have been mainly focused on a single class of cannabinoids (psychoactive or nonpsychoactive).21
In the light of all the above, the aim of this study was to develop a reliable and feasible analytical method based on HPLC–DAD–MS/MS to identify and quantify cannabinoids in Cannabis products. The innovative method developed in this study was successfully applied to the analysis of different kinds of Cannabis samples belonging to the categories mentioned above, i.e., recreational and drug-type. In particular, these samples were extracted and analyzed, and their active compounds were identified and quantified. In addition to these products, fiber-type plant varieties were also analyzed to monitor the content of nonpsychoactive compounds.
Results and Discussion
HPLC–DAD–MS/MS Conditions
At first, MS and MS/MS spectra of the analytes of interest and the ISs were acquired in the full-scan mode (m/z range 50–600) by direct infusion of reference standard solutions (1 μg/mL diluted using a mixture 50:50 of 0.1% v/v formic acid (FA) in acetonitrile (ACN) and 0.1% v/v FA in H2O) at 20 μL/min. In order to choose the most suitable conditions for each analyte and IS, all the spectra were acquired using both ESI+ and ESI–. A complete overview of the precursor and the product ions, together with the cone voltage, collision energy, and dwell time values for all the analytes and ISs are shown in Table 1. After a careful optimization of the MS/MS conditions, the most abundant transition for each analyte was used for quantitative analysis, while the second one for qualitative purposes (confirmatory daughter ions).
Table 1. Optimized MRM MS/MS Parameters.
| compound | MW | ion mode | precursor ion (m/z) | product iona (m/z) | collision energy (V) | cone voltage (V) | dwell time (s) |
|---|---|---|---|---|---|---|---|
| THC | 314.46 | + | 315.52 | 193.25 | 45 | 45 | 0.300 |
| 122.87 | 50 | 41 | |||||
| THCA | 358.47 | – | 357.46 | 245.21 | 41 | 40 | 0.300 |
| 191.33 | 45 | 36 | |||||
| CBD | 314.46 | + | 315.42 | 246.28 | 35 | 50 | 0.300 |
| 180.11 | 33 | 46 | |||||
| CBDA | 358.47 | – | 357.22 | 245.23 | 32 | 46 | 0.300 |
| 338.25 | 29 | 42 | |||||
| CBN | 310.43 | + | 311.19 | 280.14 | 55 | 51 | 0.300 |
| 172.08 | 52 | 48 | |||||
| THC-d3 | 317.48 | + | 318.45 | 196.22 | 43 | 40 | 0.300 |
| CBD-d3 | 317.48 | + | 318.39 | 249.30 | 40 | 36 | 0.300 |
| CBN-d3 | 313.45 | + | 314.21 | 283.17 | 53 | 48 | 0.300 |
In italic, confirmatory daughter ion.
Optimization of the Chromatographic Conditions
In order to achieve the simultaneous analysis of all the analytes considered in this study, an appropriate optimization of the chromatographic conditions was performed. During preliminary assays, several reversed phase columns based on C8 and C18 functionalized silica sorbents with different geometries were screened, with the aim to provide satisfactory peak shape and adequate retention time. A solid-core C18 column with medium particle size (2.7 μm) and narrow bore diameter (2.1 mm) was selected: Waters Cortecs C18+ column (100 mm × 2.1 mm i.d., 2.7 μm). Its geometry and solid-core technology increased peak resolution and symmetry, without the need of adding amine modifiers to the mobile phase. In order to obtain an acceptable total analysis time and avoid coelution with residual matrix components, a gradient elution starting at 50% of solvent A, linearly ramping up to 95% of the same solvent over 5 min, and flowing at a constant rate of 0.3 mL/min was used. The gradient was then restored to the starting conditions over 1 min and kept constant for 2 min to re-equilibrate the system. These conditions produced for each analyte symmetric, sharp, and baseline resolved peaks and guaranteed a total chromatographic run time of 13 min.
Optimization of the Extraction Conditions
Cannabis samples were subjected to an extraction procedure before being analyzed. The samples in the herbal form were finely cut by means of a commercial grinder, while hashish was chopped using a knife. Different sample-to-solvent ratios were tested in a range 1:1–1:10 (w/v) and finally the 1:5 (w/v) ratio was selected. Several solvents were considered for the extraction of the target analytes, such as pure MeOH, ethanol (EtOH), ACN, and their mixtures. The best results in terms of yield of cannabinoids were obtained by using 100% MeOH as the extraction solvent, and, for this reason, it was selected for the analytical method developed in this study. In addition, different extraction times were evaluated; in detail, the ultrasound-assisted extraction (UAE) was carried out up to 1 h, alternated to vortex mixing. The best combination between vortex mixing and UAE that led to satisfactory results was represented by 30 min UAE, followed by 1 min vortex mixing and a final 15 min UAE. The supernatant was then filtered and brought to dryness under vacuum by means of a rotary evaporator. The dried extract was then redissolved with 1 mL of a 50:50 (v/v) mixture of ACN and H2O containing 0.1% FA, suitably diluted and injected in the chromatographic system.
Validation Data
Complete validation data are shown in Table S2 in the Supporting Information.
Satisfactory linearity results were obtained over the 0.1–200 ng/mL concentration range for CBD with a linear regression correlation coefficient (r2) equal to 1.000. As to THCA and CBN, good linearity parameters were achieved, with r2 higher than 0.9995, over the 0.2–100 and 0.5–100 ng/mL concentration ranges, respectively. Satisfactory linearity was obtained for THC and CBDA over the 0.1–100 and 0.2–200 ng/mL concentration ranges, respectively (r2 > 0.9992). LOQ and LOD values were 0.1 and 0.03 ng/mL for CBD and THC, 0.2 and 0.06 ng/mL for THCA and CDBA, and 0.5 and 0.15 ng/mL for CBN, respectively.
Precision assays were carried out at three different levels (corresponding to the lower, the middle, and the higher value of each concentration range) to evaluate both the intra- and interday precision, obtaining good results, in terms of percentage relative standard deviation (%RSD): for all the target analytes, the %RSD values for intra- and interday data were found to be lower than 5.2 and 6.0%, respectively. The %RSD values for ISs were lower than 1.9 and 2.7% with regard to intra- and interday precision, respectively.
Accuracy was evaluated by means of the recovery assays: standard solutions of all the analytes at three different concentrations and IS at a constant concentration were added to Cannabis extracts with known analyte levels. Added concentrations corresponded to the lower limit, a middle point, and the higher value of each calibration curve, and percentage recovery of the standard addition was calculated. The high recovery values (>99.8% for THC, >98.6% for THCA, >99.4% for CBD, >98.5% for CBDA, and >92.1% for CBN) obtained for the cannabinoids and the ISs demonstrated that the developed analytical method is accurate.
Matrix effect (e.g., due to ion suppression or enhancement) was also investigated, and the data showed a negligible matrix effect for all the analytes since the sample/standard peak area ratios ranged between 86.7 and 99.0%.
Quantitative Results from Cannabis-Related Samples
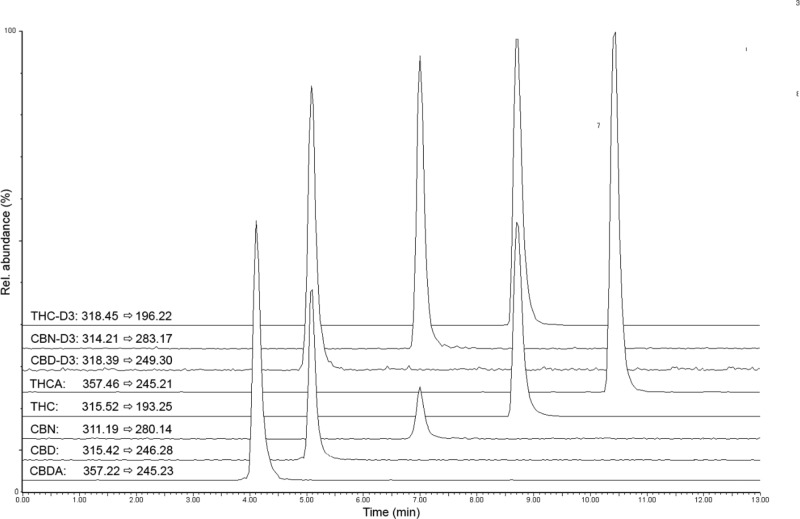
An overview of cannabinoid levels found in the analyzed samples is shown in Tables 2 and 3, while a representative LC–MS/MS chromatogram of a seized hashish sample is shown in Figure 2. With regard to recreational and drug-type Cannabis-related products, cannabinoid quantitative results are shown in Table 2. All the analytes of interest were effectively identified and quantified.
Table 2. Cannabinoid Levels in Recreational and Drug-Type Cannabis Samples.
| content
(mg/g ± SD) |
||||||
|---|---|---|---|---|---|---|
| sample type | sample no. | THC | THCA | CBD | CBDA | CBN |
| hashish | 1 | 15.2 ± 0.3 | 26.1 ± 0.6 | 8.6 ± 0.8 | 18.5 ± 0.4 | 1.3 ± 0.2 |
| 2 | 86.2 ± 0.7 | 90.3 ± 0.4 | 4.3 ± 0.3 | 17.4 ± 0.4 | 8.6 ± 0.3 | |
| marijuana | 3 | 4.2 ± 0.1 | 3.8 ± 0.2 | 2.7 ± 0.2 | 15.8 ± 0.5 | 2.2 ± 0.3 |
| 4 | 2.8 ± 0.3 | 4.6 ± 0.4 | 1.2 ± 0.1 | 37.3 ± 0.5 | 4.8 ± 0.4 | |
| drug-type Cannabis | 5 | 32.3 ± 0.4 | 27.1 ± 0.5 | 32.1 ± 0.4 | 47.3 ± 0.5 | 1.1 ± 0.4 |
Table 3. Cannabinoid Levels in Fiber-Type Cannabis Samples.
| content
(mg/g ± SD) |
|||
|---|---|---|---|
| sample type | sample no. | CBD | CBDA |
| fiber-type Cannabis | 1 | 2.4 ± 0.1 | 15.5 ± 0.7 |
| 2 | 6.0 ± 0.3 | 2.2 ± 0.1 | |
| 3 | 3.3 ± 0.3 | 16.7 ± 1.2 | |
| 4 | 8.4 ± 0.1 | 17.2 ± 0.8 | |
| 5 | 9.8 ± 0.3 | 2.9 ± 0.3 | |
| 6 | 7.9 ± 0.5 | 14.5 ± 1.1 | |
| 7 | 3.3 ± 0.1 | 33.8 ± 0.3 | |
| Cannabis liquid extract | 1 | 251.9 ± 1.2a | 601.8 ± 1.6a |
| 2 | 261.1 ± 1.4a | 905.5 ± 1.8a | |
Content expressed as μg/mL.
Figure 2.
LC–MS/MS MRM chromatogram of a hashish sample.
With respect to recreational samples, the highest concentration of psychoactive cannabinoids was found in hashish samples, due to the fact that these nonherbal products represent a concentrate from raw Cannabis, illegally produced in order to obtain high-potency derivatives.21 With regard to the levels of psychoactive cannabinoid THC and its precursor THCA, they were found in such a concentration that the hashish samples and recreational-type herbal products considered for method application within this work resulted above the legal limit allowed for marketing and consumption. In particular, the two hashish samples have a considerably different potency in relation with their THC content: in fact, sample 2 showed a 5.7-fold higher level of THC and a 3.5-fold higher level of THCA with respect to sample 1 (Table 2). With regard to CBN levels in recreational-type Cannabis samples, they ranged from 1.1 to 8.6 mg/g in hashish and herbal samples (Table 2), without evident correlations with respect to the sample type and to other analyte levels. With CBN being the oxidative degradation product of THC, these differences could be due to different product aging and storage conditions before sample reception and analysis.
As for the drug-type Cannabis sample (Table 2), the contents found are in good agreement with those expected from a medium THC and CBD medical Cannabis strain.22
As for the content of cannabinoids in samples of fiber-type Cannabis, data are shown in Table 3. In general, the quantitative data obtained are in agreement with what has been previously described in the literature for this kind of Cannabis sample.10,11 It is known from the literature that the most abundant cannabinoid in this type of Cannabis is CBDA, followed by its neutral counterpart CBD.10,11 As a matter of fact, CBDA was found to be the most abundant cannabinoid in five samples out of the seven analyzed in this study, with a content from 2 to 10 times higher than that of CBD. These five samples of fiber-type Cannabis characterized by a remarkably high content of CBDA should be taken into consideration for the preparation of extracts to be used both in the pharmaceutical and the nutraceutical fields. In two fiber-type samples CBD had a content higher than CBDA, meaning that a decarboxylation process of the native compound in favor of CBD had occurred. With regard to THC, it was found to be under the legal limit of 0.2% in all the fiber-type samples analyzed in this work.
In the commercial liquid extracts derived from Cannabis, high amounts of CBD and CBDA were found (Table 3). This is probably due to the type of the starting raw plant material (fiber-type Cannabis) or to the technique used to obtain the extracts; anyway, without detailed information about these liquid products, it is not possible to hypothesize significant correlations.
Conclusions
The aim of this work was to characterize Cannabis recreational and drug-type samples by the analysis of both their psychoactive and nonpsychoactive cannabinoids. In addition, fiber-type plant varieties were also taken into account in this study to monitor their content of nonpsychoactive compounds.
To do this, a new and highly efficient HPLC–DAD–MS/MS method was developed, coupled with a simple extraction procedure for the compounds of interest, based on UAE with MeOH. The overall analytical method was submitted to validation to show compliance with international requirements, and it was successfully applied to recreational, drug-type, and fiber-type Cannabis samples, providing a reliable cannabinoid profiling for a rational use of this plant, its extracts, and purified compounds in medicinal chemistry and other fields, including the nutraceutical one for fiber-type varieties.
Experimental Procedures
Chemicals, Solutions, and Equipment
Stock methanolic solutions (1.0 mg/mL) of (−)-Δ9-tetrahydrocannabinol (THC), cannabidiol (CBD), and cannabinol (CBN) and stock acetonitrile solutions (1.0 mg/mL) of tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA) were purchased from Cerilliant Corporation (Round Rock, TX, USA). THC-d3, CBD-d3, and CBN-d3 (used as the internal standards IS1, IS2, and IS3, respectively) stock methanolic solutions (100 μg/mL) were also purchased from Cerilliant Corporation. HPLC-grade methanol (MeOH), ACN (>99.9%) and 95% FA were purchased from Sigma-Aldrich (Saint Louis, MO, USA). Ultrapure water (H2O) (18.2 MΩ cm) was obtained by means of a Milli-Q apparatus by Millipore (Milford, MA, USA).
THC, CBD, CBN, and IS stock solutions were stable for 3 months when stored at −20 °C, while THCA and CBDA ones only if stored at −80 °C; working solutions were prepared fresh every day in mobile phase.
Cannabis Samples
Two types of seized recreational Cannabis-related products were analyzed to ascertain their cannabinoid levels, including two herbal products consisting of dried female inflorescences and two hashish samples. With regard to drug-type medical Cannabis, one sample of a variety with medium CBD and THC content was also analyzed. Recreational and drug-type herbal samples were treated and stored the same way as the fiber-type hemp, while hashish samples were stored at +4.0 °C until pretreatment and analysis.
Seven samples of fiber-type hemp female inflorescences were also analyzed in this study, including Antal, Carma, Carmagnola, China, Codimono, Fibrante, and Futura. These samples (about 100–500 g dry material each) were cultivated under the same agronomic conditions; they were kindly provided by Dr. Gianpaolo Grassi of the research center CREA-CIN (Rovigo, Italy), and they were certified for a content of THC below 0.2%. For each sample, hemp inflorescences were manually separated from twigs and seeds. After this procedure, the samples were stored at +4.0 °C until required for chemical analysis. Hemp inflorescences were ground in a mortar before the extraction.
The methodology developed in this work was also applied to two liquid hydroalcoholic herbal extracts obtained from fiber-type Cannabis to assess their cannabinoid content and their compliance with the legal limit for psychoactive compounds.
HPLC–DAD–MS/MS
The HPLC instrument was a Waters Alliance e2695 chromatographic pump system equipped with an autosampler. Chromatographic separation was carried out on a reversed phase Waters Cortecs C18+ column (100 mm × 2.1 mm i.d., 2.7 μm), maintained at room temperature, and equipped with a guard column. The mobile phase was a mixture of 0.1% FA in ACN (solvent A) and 0.1% FA in H2O (solvent B) at a constant flow rate of 0.3 mL/min. The mobile phase composition gradient program started with a 50/50 solvent A/solvent B ratio, linearly ramping up to 95% of solvent A over 5 min, and this ratio was maintained for 5 min. The gradient was then changed to the starting conditions over 1 min and kept constant for 2 min to re-equilibrate the system. The total run time was 13 min, and injection volume was 10 μL.
Mass spectrometry acquisition was performed on a Waters Micromass Quattro Micro triple quadrupole in multiple reaction monitoring (MRM) mode, and the analytes were acquired both in positive (ESI+) and negative (ESI−) ion mode with ionization polarity switching, under the following optimized settings: ion source voltage, 4.0 kV; ion source temperature, 1250 °C; desolvation temperature, 250 °C; desolvation gas (nitrogen) flow, 250 L/h; argon was used as the collision gas. The dwell times per channel were set at 300 ms for each analyte and IS. Precursor and product ions, with cone voltage and collision energy values for each analyte and IS, are reported in Table 1. Data were acquired in multiple reaction monitoring (MRM) mode and processed by using Waters MassLynx 4.1 software.
Sample Preparation
Cannabis products were extracted and then analyzed by means of the developed method. The sample preparation from herbal products consisted of an extraction of 50 mg of the finely cut herb with 10 mL of MeOH (containing IS) by means of an ultrasonic bath for 30 min. The solution was agitated by vortex for one min and then the ultrasonic bath was used for other 15 min. The supernatant was filtered through an Agilent (Palo Alto, USA) membrane filter (13 mm membrane, 0.20 μm, nylon) and then evaporated through a rotary evaporator. The dry extract was dissolved in 1 mL of a 50:50 (v/v) mixture of ACN and H2OThe liquid Cannabis products were filtered through a 0.20 μm porosity nylon membrane filter and properly diluted with a 50:50 (v/v) mixture of ACN and H2O containing 0.1% FA and ISs before analysis.
Method Validation
Method validation was carried out according to United States Pharmacopeia (USP) and International Conference on Harmonization (ICH) guidelines. More details on the test performed for the method validation are included in the Supporting Information.
Acknowledgments
The authors are very grateful to Dr. Gianpaolo Grassi from CREA-CIN centre (Rovigo, Italy) for providing the fiber-type hemp samples.
Glossary
ABBREVIATIONS
- ACN
acetonitrile
- CB1
cannabinoids receptor 1
- CB2
cannabinoids receptor 2
- CBD
cannabidiol
- CBDA
cannabidiolic acid
- CBD-d3
deuterated cannabidiol
- CBN
cannabinol
- CBN-d3
deuterated cannabinol
- DAD
diode-array detector
- DoA
drug of abuse
- DUI
driving under influence
- ESI
electrospray ionization
- EtOH
ethanol
- FA
formic acid
- HPLC
high-performance liquid chromatography
- ICH
International Conference on Harmonization
- IS
internal standard
- MeOH
methanol
- MRM
multiple reaction monitoring
- MS/MS
tandem mass spectrometry
- THC
Δ9-tetrahydrocannabinol
- THCA
Δ9-tetrahydrocannabinolic acid
- THC-d3
deuterated Δ9-tetrahydrocannabinol
- UAE
ultrasound-assisted extraction
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00571.
Experimental procedures for method validation and validation parameters (PDF)
Author Contributions
All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Hall W.; Degenhardt L. Adverse health effects of non-medical Cannabis use. Lancet 2009, 374, 1383–1391. 10.1016/S0140-6736(09)61037-0. [DOI] [PubMed] [Google Scholar]
- Andre C. M.; Hausman J. F.; Guerriero G. Cannabis sativa: the plant of the thousand and one molecule. Front. Plant Sci. 2016, 7, 1–17. 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appendino G.; Chianese G.; Taglialatela-Scafati O. Cannabinoids: occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. 10.2174/092986711794940888. [DOI] [PubMed] [Google Scholar]
- Elsohly M.; Slade D. Chemical constituents of marijuana: the complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. 10.1016/j.lfs.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Mercolini L.; Musenga A.; Comin I.; Baccini C.; Conti M.; Raggi M. A. Determination of plasma and urine levels of Δ9-tetrahydrocannabinol and its main metabolite by liquid chromatography after solid-phase extraction. J. Pharm. Biomed. Anal. 2008, 47, 156–163. 10.1016/j.jpba.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Rubino T.; Zamberletti E.; Parolaro D. Adolescent exposure to Cannabis as a risk factor for psychiatric disorders. J. Psychopharmacol. 2012, 26, 177–188. 10.1177/0269881111405362. [DOI] [PubMed] [Google Scholar]
- Mercolini L.; Mandrioli R.; Protti M.; Conti M.; Serpelloni G.; Raggi M. A. Monitoring of chronic Cannabis abuse: an LC–MS/MS method for hair analysis. J. Pharm. Biomed. Anal. 2013, 76, 119–125. 10.1016/j.jpba.2012.12.015. [DOI] [PubMed] [Google Scholar]
- De Backer B.; Debrus B.; Lebrun P.; Theunis L.; Dubois N.; Decock L.; Verstraete A.; Hubert P.; Charlier C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in Cannabis plant material. J. Chromatogr. B: Anal. Technol. Biomed. Life Sci. 2009, 877, 4115–4124. 10.1016/j.jchromb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Hanuš L. O.; Meyer S. M.; Muñoz E.; Taglialatela-Scafati O.; Appendino G. Phytocannabinoids: a unified critical inventory. Nat. Prod. Rep. 2016, 33, 1357–1392. 10.1039/C6NP00074F. [DOI] [PubMed] [Google Scholar]
- Brighenti V.; Pellati F.; Steinbach M.; Maran D.; Benvenuti S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. 10.1016/j.jpba.2017.05.049. [DOI] [PubMed] [Google Scholar]
- Pellati F.; Brighenti V.; Sperlea J.; Marchetti L.; Bertelli D.; Benvenuti S. New methods for the comprehensive analysis of bioactive compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. 10.3390/molecules23102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellati F.; Borgonetti V.; Brighenti V.; Biagi M.; Benvenuti S.; Corsi L. Cannabis sativa L. and nonpsychoactive cannabinoids: their chemistry and role against oxidative stress, inflammation and cancer. BioMed Res. Int. 2018, 2018, 1. 10.1155/2018/1691428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernàndez-Ruiz J.; Moreno-Martet M.; Rodrìguez-Cueto C.; Palomo-Garo C.; Gòmez-Caña M.; Valdeolivas S.; Guaza C.; Romero J.; Guzmàn M.; Mechoulam R.; Ramos J. A. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 2011, 163, 1365–378. 10.1111/j.1476-5381.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander S. P. H. Therapeutic potential of Cannabis-related drugs. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 157–166. 10.1016/j.pnpbp.2015.07.001. [DOI] [PubMed] [Google Scholar]
- Campos A. C.; Fogaça M. V.; Sonego A. B.; Guimarães F. S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. 10.1016/j.phrs.2016.01.033. [DOI] [PubMed] [Google Scholar]
- Corsi L.; Pellati F.; Brighenti V.; Plessi N.; Benvenuti S. Chemical composition and in vitro neuroprotective activity of fibre-type Cannabis sativa L. (Hemp). Curr. Bioact. Compd. 2018, 10.2174/1573407214666180809124952. [DOI] [Google Scholar]
- Pisanti S.; Malfitano A. M.; Ciaglia E.; Lamberti A.; Ranieri R.; Cuomo G.; Abate M.; Faggiana G.; Proto M. C.; Fiore D.; Laezza C.; Bifulco M. Cannabidiol: state of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- Schlienz N. J.; Cone E. J.; Herrmann E. S.; Lembeck N. A.; Mitchell J. M.; Bigelow G. E.; Flegel R.; LoDico C. P.; Hayes E. D.; Vandrey R. Pharmacokinetic characterization of 11-nor-9-carboxy-Δ9-tetrahydrocannabinol in urine following acute oral Cannabis ingestion in healthy adults. J. Anal. Toxicol. 2018, 42, 232–247. 10.1093/jat/bkx102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercolini L.; Mandrioli R.; Sorella V.; Somaini L.; Giocondi D.; Serpelloni G.; Raggi M. A. Dried blood spots: liquid chromatography–mass spectrometry analysis of Δ9-tetrahydrocannabinol and its main metabolites. J. Chromatogr. A 2013, 1271, 33–40. 10.1016/j.chroma.2012.11.030. [DOI] [PubMed] [Google Scholar]
- Pertwee R. G. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol. 2009, 156, 397–411. 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leghissa A.; Hildenbrand Z. L.; Schug K. A. A review of methods for the chemical characterization of Cannabis natural products. J. Sep. Sci. 2018, 41, 398–415. 10.1002/jssc.201701003. [DOI] [PubMed] [Google Scholar]
- Lewis M. M.; Yang Y.; Wasilewski E.; Clarke H. A.; Kotra L. P. Chemical profiling of Medical Cannabis Extracts. ACS Omega 2017, 2, 6091–6103. 10.1021/acsomega.7b00996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.