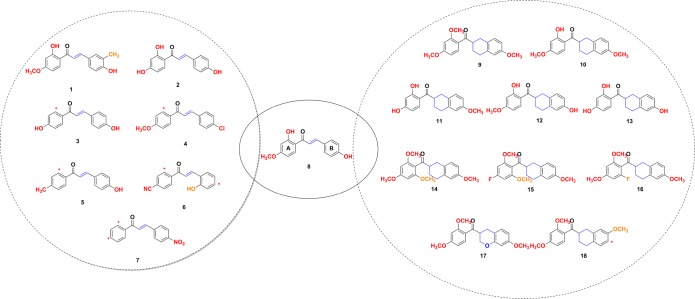
Abstract
Based on hit-likeness and chemical diversity, a number of chalcones and chalcone-mimetic compounds were selected as putative Notch inhibitors. The evaluation of the antiproliferative effect combined with the inhibition of Notch1 expression in KOPTK1 cell line identified compound 18, featuring a tetrahydronaphthalene-based scaffold, as a new promising Notch-blocking agent.
Keywords: Chalcone, tetrahydronaphthalene scaffold, Notch inhibitors, T-ALL, cancer
Notch signaling is an evolutionarily conserved cell communication pathway regulating many biological processes including stem cells self-renewal, cell differentiation, proliferation, and survival in different tissues during embryonic and adult life.1−3 The mammalian Notch family includes four trans-membrane receptors (namely, Notch1–4), and five trans-membrane ligands (namely, Jagged-1 and -2, Delta-like-1, -3, and -4). Notch signaling is a short-range intercellular communication system, wherein a membrane-tethered Notch ligand on the signal-sending cell interacts with a transmembrane Notch receptor on the juxtaposed signal-receiving cell.4 This activation induces a series of proteolytic events such as the S2 cleavage performed by the ADAM metalloproteases, and the S3 cleavage by the gamma secretase (GS) complex, which trigger the release of activated intracellular domain of Notch and its translocation to the nucleus.5 The subsequent interaction with the DNA binding protein CSL (CBF1/Suppressor of Hairless/LAG–1; also known as RBPJ) and Mastermind-like protein (MAML) promotes the transcription of Notch downstream target genes.5 Aberrant Notch activation due to either gene mutation or amplification or to post-translational modifications has been linked to the onset of different solid and hematological cancers, including T-cell acute lymphoblastic leukemia (T-ALL). Sixty percent of T-ALL cases are associated with activating NOTCH1 mutations and the majority of T-ALL patients shows increased expression and function of Notch3.6−8 Moreover, Notch signaling may contribute to chemotherapy resistance, thus suggesting Notch inhibition as a strategy in anticancer targeted therapy.9,10 Notwithstanding, small molecules (e.g., GS inhibitors (GSI) such as MK-0752, RO4929097 and PF-03084014) and large molecules (e.g., Notch1-specific antibody OMP-52M51) currently in clinical development are not effective in all patients and often cause severe side-effects that limit their widespread therapeutic use and raise the need for novel Notch-blocking agents.11,12
Recently, an in-house library of natural products and their derivatives was used as a source of potential inhibitors of the Notch signaling in T-ALL.13,14 Eight representative molecules of the library were selected through a cheminformatics approach and tested in vitro. The chalcone scaffold emerged as a promising tool to inhibit Notch signaling, and in particular, the synthesis of several chalcones combined with their biological evaluation highlighted the chalcone 8 as the most potent Notch blocking agent of the series, suggesting the synergistic activity of 2′- and 4-hydroxy groups.13,14 Herein, we designed and synthesized a number of chalcones and chalcone-mimetic compounds based on hit-likeness and chemical diversity, and we evaluated their antiproliferative activity in KOPTK1 cells. Small molecules able to reduce cell growth by more than 30% were further evaluated as inhibitors of Notch1 by means of Western blotting assay.
Results and Discussion
Synthesis of Chalcones and Chalcone-mimetic Derivatives
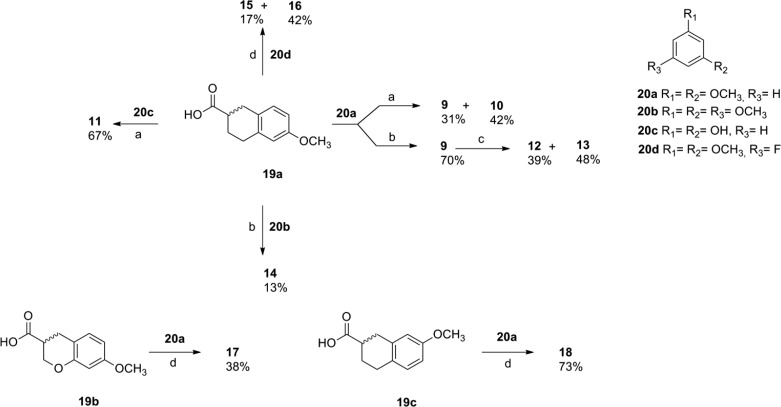
The general synthetic approach to efficiently yield chalcone scaffold bearing substituents at different positions (1–7) was based on the Claisen–Schmidt condensation as previously reported (Chart 1).13,15 For the synthesis of the chalcone mimetics (9–18) (Chart 1), the bicyclic acids (19a–c) were used to perform the Friedel–Crafts acylation of some arenes (20a–d) by using two different procedures (Scheme 1).
Chart 1. Chemical Structures of Chalcone-8 Derivatives (1–7) and Chalcone Mimetics (9–18).
Scheme 1. Synthesis of the Chalcone mimetics (9–18).
Reagents and conditions: (a) (1) SOCl2, toluene, reflux, 3 h; (2) BF3·OEt2, ClCH2CH2Cl anh., 60 °C, 16 h; (b) (1) SOCl2, toluene, reflux, 3 h; (2) TfOH, ClCH2CH2Cl anh., 60 °C, 1 h; (c) BBr3, anh. CH2Cl2, 4 °C, 48 h; (d) TFAA, TfOH, ClCH2CH2Cl anh, 15 min, rt.
The ketone 9 was prepared by the reaction between the activated form of 1,2,3,4-tetrahydronaphthalene-2-carboxylic acid 19a with 1,3-dimethoxybenzene 20a in the presence of BF3·OEt2. Since the catalyst induced the partial demethylation of the methoxy group vicinal to the ketone, the reaction afforded a mixture of 9 and 10 with 31% and 42% yields, respectively. Otherwise, performing the reaction with triflic acid,16 the ketone 9 was obtained in 70% yield. When resorcinol (20c) was used as the arene, compound 11 was obtained in 67% yield, so that the heating conditions favor the formation of the C-acylated product.17 Removal of the methoxy groups in 9 by using BBr318 led to the formation of a mixture of 12 and 13 in 39% and 48% yields, respectively. The trimethoxy derivative 14 was obtained in low yield probably due to steric hindrance. However, the direct acylation reaction of the fluoroarene 20d with the acid 19a by adapting previous reports using TFAA/TfOH,19,20 allowed the formation of the regioisomers 15 and 16, as shown in Scheme 1. Similarly, reaction of the 7-methoxychromane-3-carboxylic acid 19b or 7-methoxy-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid 19c with 20a in the presence of TFAA/TfOH afforded the aryl derivatives 17 and 18 in 38% and 73% yield, respectively. In all cases, compounds 9–18 were obtained as racemates.
Conformation of Chalcone 8 and Chalcone Mimetics
E-Chalcones can adopt s-cis or s-trans conformations.21 Nonsubstituted chalcones at the α-position adopt, in general, a s-cis conformation, and this was confirmed for the chalcone 8 by 2D-NOESY experiments (Figure S1). The incorporation of a bicyclic ring in the structure of the chalcone mimetics (exemplified by compound 12 in Figure 1), where the double bond conjugated to the enone has been removed, is meant to restrict the conformational freedom of these analogues. The energy minimized conformers (see Computational Methods S20) of the 12-R and 12-S enantiomers present in the racemic mixture were constructed and superimposed with the X-ray crystal structure of a model s-cis chalcone (that is, benzylidene acetophenone, CCDC 1185311).22 As shown in Figure 1, both enantiomers are able to locate the OH-substituent on ring B close to the 4-position of ring B in the s-cis chalcone. Thus, the compounds were assayed as racemic mixtures.
Figure 1.
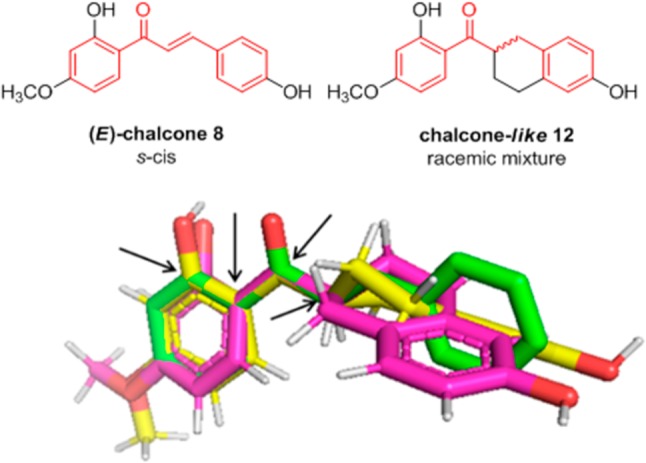
Superposition of the crystal structure of benzylidene acetophenone (green) with energy-minimized conformers of 12-S (magenta) and 12-R (yellow). Atom pairs used for the superimposition are indicated with black arrows.
Biological Results and Discussion
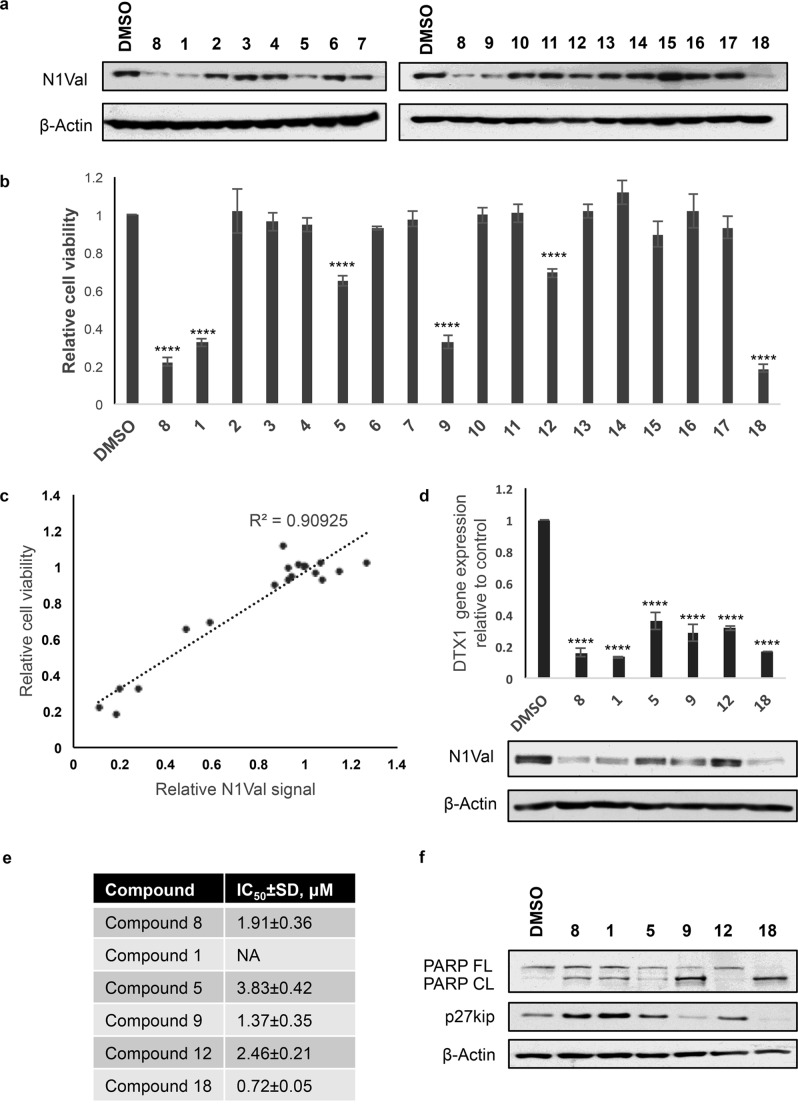
To evaluate the effectiveness of chalcones 1–7 and chalcone mimetics 9–18, we investigated their growth-inhibitory potency in KOPTK1 T-ALL cell line. These cells are sensitive to Notch inhibition by GSI and harbor activating Notch1 gene mutations.7 As we demonstrated previously, in this cell line the reference compound 8 inhibited Notch1 signaling and cell growth in the concentration range between 1 and 2.5 μM.13 Therefore, we compared the effects of compounds 1–7 and 9–18 in KOPTK1 cells on the endogenous levels of the activated domain of Notch1 (N1Val) by Western blotting and on cell growth by MTS assay, treating the cells with 2.5 μM of each compound for 36 h and taking 30% decrease of cell viability as a threshold value for further screening (Figures 2a,b and S2). Notably, the comparison of the viability data and the N1Val levels after exposure to all chalcone derivatives confirmed a remarkably positive correlation between N1Val protein levels and the sensitivity of KOPTK1 cells to the bioactive compounds 1, 5, 9, 12, and 18 indicating that their antiproliferative effects were tightly associated with Notch signaling inhibition (Figure 2c). Further proving Notch inhibition, selected compounds decreased the N1Val protein expression and the endogenous mRNA levels of the Notch target gene DELTEX1 (DTX1). Interestingly, among them, only compounds 1 and 18 showed Notch1-inhibitory effects comparable to that of compound 8, whereas the Notch inhibition exerted by 5, 9, and 12 was weaker than that of compound 8 (Figures 2d and S3). In addition, we determined the half-maximal growth-inhibitory concentration (IC50) in a dose–response proliferation assay with increasing concentrations (namely, 0, 0.5, 1, 2.5, 5, 10, 20, and 50 μM) of selected compounds 1, 5, 9, 12, and 18. These molecules inhibited leukemic cell growth to a similar extent each other, with IC50 values comparable to that of the reference compound 8 and ranging from 0.7 to 3.8 μM (Figure 2e). Notably, statistical analysis of single-point comparative treatment showed no significant differences in the inhibition of cell growth between compounds 8, 1, 9, and 18, which provided a comparable effect at 2.5 μM concentration. The slightly higher IC50 values of 5 and 12 are in line with their weaker effect on Notch1 inhibition (Figures 2a,d and S3). Determination of the IC50 value for compound 1 was not possible due to the irregular shape of its growth inhibition curve (Figure S4). Moreover, consistent with the effect induced by chalcone 8 in G1-phase cell cycle arrest and apoptosis,13 we found that growth inhibition by 1 was accompanied by both increased levels of the antiproliferative factor p27kip and of the apoptotic marker cleaved poly ADP-ribose polymerase (CL PARP). On the contrary, the most promising chalcone mimetics 9 and 18 failed to upregulate p27kip and promoted the highest levels of PARP cleavage among tested compounds (Figure 2f), whereas the weakest Notch inhibitors 5 and 12 induced p27kip without affecting PARP processing (Figure 2f). Finally, chalcone mimetics 9 and 18 affected cell proliferation also in GSI-resistant JURKAT cell line23 and in immortalized nontumorigenic human keratinocyte HaCat cells in which Notch signaling acts as an onco-suppressor.24 These latter observations suggest different mechanisms of action and/or off-target effects by chalcones and chalcone mimetics (Figure S5).
Figure 2.
Antiproliferative and molecular effects of chalcones and chalcone-like derivatives in KOPTK1 T-ALL cells. (a) Protein expression levels of activated domain of Notch1 (N1Val) in KOPTK1 cells treated for 36 h with 2.5 μM of compounds 1–18 or vehicle alone (DMSO). β-Actin is used as loading control. (b) Histogram shows the mean of relative cell viability of KOPTK1 cells treated with 2.5 μM of compounds 1–18 or DMSO for 36 h measured with MTS assay ± SEM obtained from two independent experiments performed in triplicate. ****P < 0.0001, comparing with DMSO. (c) Representative graph showing the results of Pearson’s correlation test between the average values of relative N1Val expression obtained with optical densitometry and cell viability in KOPTK1 treated with compounds 1–18 vs DMSO. R = 0.9535, R2 = 0.90925, p < 0.0001. (d) N1Val and β-actin protein expression levels (lower panel) and DELTEX1 (DTX1) relative gene expression levels (upper panel) in KOPTK1 cells treated with 2.5 μM of compounds 8, 1, 5, 9, 12, and 18 or vehicle alone (DMSO) for 36 h. Data represent mean values for three independent experiments performed in triplicate ± SEM. (e) IC50 values of compounds 8, 1, 5, 9, 12, and 18 determined in KOPTK1 after 36 h of incubation. Data represent mean values for three independent experiments performed in triplicate ± SD. (f) Protein expression of p27kip and of the nonprocessed PARP (FL PARP) and its cleaved form (CL PARP). β-Actin is used as loading control.
SAR of Chalcones and Chalcone-mimetic Derivatives as Notch Inhibitors
Structure–activity relationships (SAR) of a previous series of chalcone derivatives revealed how the 2′- and 4-hydroxy groups are essential for both cell proliferation and Notch inhibition.13 The biological results obtained with the chalcones described in this work confirmed the previous SAR.
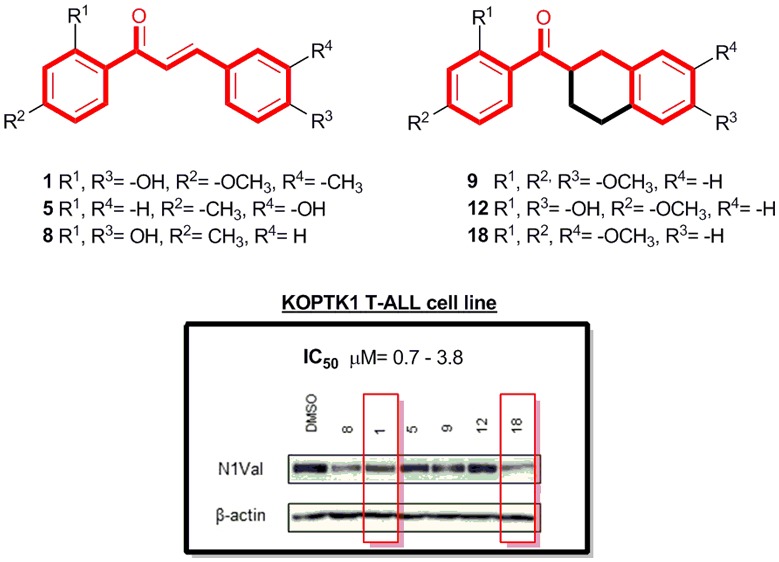
Accordingly, the effectiveness of the chalcones 1 and 5 on growth inhibition of KOPTK1 T-ALL cell line highlighted the pivotal role of 4-OH substitution to ring B (Figure 2b). However, the Western blotting assay on N1Val expression using chalcone 8 as the reference compound showed a comparable inhibitory effect just for 1 (Figure 2a). These results reinforced the previous evidence also for the ring A showing how the lack of 4-OH substitution led to a weaker inhibitory activity toward activated Notch1. The introduction of 3-CH3 group at ring B did not influence the inhibition of endogenous Notch signaling activity and cell growth.
Chalcone mimetics 9, 12, and 18 proved to decrease the cell viability of KOPTK1 T-ALL cells with IC50 values in the low- up to submicromolar range (Figure 2e). While compound 12, with a substitution pattern at rings A and B similar to that of the reference chalcone 8, showed some Notch-inhibitory effects, the best inhibition of Notch1 intracellular domain in this series was obtained by compound 18 (Figure 2d). Thus, it is interesting to note that compounds incorporating a tetrahydronaphthalene scaffold are also able to exert inhibition of the Notch pathway, although with some differences compared to chalcones.
Conclusion
Based on previous evidence and molecular modeling studies, several chalcones 1–7 and chalcone mimetics 9–18 were selected, synthesized, and tested toward antiproliferative activity and Notch inhibition in KOPTK1 T-ALL cells. Compounds 1 and 18 exhibited cell growth reduction combined with inhibition of Notch1 intracellular domain, mostly comparable to the reference compound 8. SAR analysis of the chalcone series confirmed the key role played by the 2′- and 4-OH groups on both antiproliferative and Notch1 inhibitory activity. However, the introduction of bicyclic ring in the structure of the chalcone mimetics promotes Notch inhibition through different biological responses, thus suggesting that more in-depth investigations are needed to further develop tetrahydronaphthalene derivatives as Notch blocking agents.
Acknowledgments
The authors acknowledge networking contribution by the COST Action CM1407 “Challenging organic syntheses inspired by nature - from natural products chemistry to drug discovery”. V.C. thanks the Universidad del Valle (CIAM-2017) and the Science, Technology and Innovation Fund-General Royalties System (FCTeI-SGR) under contract BPIN 2013000100007 for a predoctoral fellowship. This work has been supported by Italian Ministry of Education, University and Research - Dipartimenti di Eccellenza - L. 232/2016. This work has been partially supported by a grant from MINECO/FEDER SAF2015-64629-C2-1-R (to M-J.P.-P and E-M.P), MIUR PNR 2015–2020 (ARS01_00432) (to I.S.), and Sapienza University 2016 Project number RG116154E2C7A6FB (to I.S.).
Glossary
ABBREVIATIONS
- ADAM
a disintegrin and metalloproteinase
- GS
gamma secretase
- GSI
gamma secretases inhibitor
- CSL
CBF-1/SuH/Lag-1
- MAML
mastermind-like
- T-ALL
T-cell acute lymphoblastic leukemia
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00608.
Experimental details for synthetic procedures, associated chemical data for compounds 1–18, computational methods, and biological assays (PDF)
Author Present Address
◆ (V.C.) Grupo de Investigación de Compuestos Heterocíclicos, Departamento de Química, Universidad del Valle, A.A. 25360 Cali, Colombia. E-mail: viviana.cuartas@correounivalle.edu.co.
Author Present Address
■ (N.Z.) Department of Physics, University of Rome “Sapienza”, Rome, Italy.
Author Contributions
○ These authors contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Liu J.; Sato C.; Cerletti M.; Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010, 92, 367–409. 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- Siebel C.; Lendahl U. Physiol. Rev. 2017, 97, 1235–1294. 10.1152/physrev.00005.2017. [DOI] [PubMed] [Google Scholar]
- Koch U.; Lehal R.; Radtke F. Stem cells living with a Notch. Development 2013, 140 (4), 689–704. 10.1242/dev.080614. [DOI] [PubMed] [Google Scholar]
- Kopan R.; Ilagan M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 2009, 137 (2), 216–33. 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo R.; Checquolo S.; Bellavia D.; Talora C.; Screpanti I. The molecular basis of notch signaling regulation: a complex simplicity. Curr. Mol. Med. 2014, 14 (1), 34–44. 10.2174/1566524013666131118105216. [DOI] [PubMed] [Google Scholar]
- Bellavia D.; Palermo R.; Felli M. P.; Screpanti I.; Checquolo S. Notch signaling as a therapeutic target for Acute Lymphoblastic Leukemia. Expert Opin. Ther. Targets 2018, 22 (4), 331–342. 10.1080/14728222.2018.1451840. [DOI] [PubMed] [Google Scholar]
- Weng A. P.; Ferrando A. A.; Lee W.; Morris J. P. 4th; Silverman L. B.; Sanchez-Irizarry C.; Blacklow S. C.; Look A. T.; Aster J. C. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004, 306 (5694), 269–271. 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- Bellavia D.; Campese A. F.; Checquolo S.; Balestri A.; Biondi A.; Cazzaniga G.; Lendahl U.; Fehling H. J.; Hayday A. C.; Frati L.; von Boehmer H.; Gulino A.; Screpanti I. Combined expression of pTalpha and Notch3 in T cell leukemia identifies the requirement of preTCR for leukemogenesis. Proc. Natl. Acad. Sci. U. S. A. 2002, 99 (6), 3788–3793. 10.1073/pnas.062050599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aster J. C.; Pear W. S.; Blacklow S. C. The Varied Roles of Notch in Cancer. Annu. Rev. Pathol.: Mech. Dis. 2017, 12, 245–275. 10.1146/annurev-pathol-052016-100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palermo R.; Ghirga F.; Piccioni M. G.; Bernardi F.; Zhdanovskaya N.; Infante P.; Mori M. Natural products inspired modulators of cancer stem cells-specific signaling pathways Notch and Hedgehog. Curr. Pharm. Des. 2019, 25, 1. 10.2174/1381612825666190111124822. [DOI] [PubMed] [Google Scholar]
- Andersson E. R.; Lendahl U. Therapeutic modulation of Notch signalling-are we there yet?. Nat. Rev. Drug Discovery 2014, 13 (5), 357–78. 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- Takebe N.; Nguyen D.; Yang S. X. Targeting notch signaling pathway in cancer: clinical development advances and challenges. Pharmacol. Ther. 2014, 141 (2), 140–9. 10.1016/j.pharmthera.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M.; Tottone L.; Quaglio D.; Zhdanovskaya N.; Ingallina C.; Fusto M.; Ghirga F.; Peruzzi G.; Crestoni M. E.; Simeoni F.; Giulimondi F.; Talora C.; Botta B.; Screpanti I.; Palermo R. Identification of a novel chalcone derivative that inhibits Notch signaling in T-cell acute lymphoblastic leukemia. Sci. Rep. 2017, 7 (1), 2213. 10.1038/s41598-017-02316-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botta B.; Screpanti I.; Tottone L.; Zhdanovskaya N.; Ingallina C.; Giulimondi F.; Quaglio D.; Palermo R.; Mori M.; Ghirga F.. Inibitori di Notch per uso nel trattamento della leucemia linfoblastica acuta a cellule T. Domanda internazionale nr. PCT/IB2017/0582042017. Deposito 20-12-2017.
- Schiano Moriello A.; Luongo L.; Guida F.; Christodoulou M. S.; Perdicchia D.; Maione S.; Passarella D.; Marzo V. D.; De Petrocellis L. Chalcone Derivatives Activate and Desensitize the Transient Receptor Potential Ankyrin 1 Cation Channel, Subfamily A, Member 1 TRPA1 Ion Channel: Structure-Activity Relationships in vitro and Anti-Nociceptive and Anti-inflammatory Activity in vivo. CNS Neurol. Disord.: Drug Targets 2016, 15, 987–994. 10.2174/1871527315666160413123621. [DOI] [PubMed] [Google Scholar]
- Bueno O.; Tobajas G.; Quesada E.; Estévez-Gallego J.; Noppen S.; Camarasa M.-J.; Díaz J.-F.; Liekens S.; Priego E.-M.; Pérez-Pérez M.-J. Conformational mimetics of the α-methyl chalcone TUB091 binding tubulin: Design, synthesis and antiproliferative activity. Eur. J. Med. Chem. 2018, 148, 337–348. 10.1016/j.ejmech.2018.02.019. [DOI] [PubMed] [Google Scholar]
- Adogla E. A.; Janser R. F. J.; Fairbanks S. S.; Vortolomei C. M.; Meka R. K.; Janser I. Selective methoxy ether cleavage of 2,6-dimethoxyphenol followed by a selective acylation. Tetrahedron Lett. 2012, 53, 11–14. 10.1016/j.tetlet.2011.10.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McOmie J. F. W.; Watts M. L.; West D. E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron 1968, 24, 2289–2292. 10.1016/0040-4020(68)88130-X. [DOI] [Google Scholar]
- Tachrim Z.; Wang L.; Murai Y.; Yoshida T.; Kurokawa N.; Ohashi F.; Hashidoko Y.; Hashimoto M. Trifluoromethanesulfonic Acid as Acylation Catalyst: Special Feature for C- and/or O-Acylation Reactions. Catalysts 2017, 7, 40. 10.3390/catal7020040. [DOI] [Google Scholar]
- Plażuk D.; Zakrzewski J. Friedel–Crafts acylation of ferrocene with alkynoic acids. J. Organomet. Chem. 2009, 694, 1802–1806. 10.1016/j.jorganchem.2009.01.007. [DOI] [Google Scholar]
- Padhye S.; Ahmad A.; Oswal N.; Sarkar F. H. J. Hematol. Oncol. 2009, 2 (38), 38. 10.1186/1756-8722-2-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovich D. Topocehmistry. Part XXX. Crystal and molecular structures of chalcone. J. Chem. Soc. B 1970, 0, 11–16. 10.1039/j29700000011. [DOI] [Google Scholar]
- Rao S. S.; O’Neil J.; Liberator C. D.; Hardwick J. S.; Dai X.; Zhang T.; Tyminski E.; Yuan J.; Kohl N. E.; Richon V. M.; Van der Ploeg L. H.; Carroll P. M.; Draetta G. F.; Look A. T.; Strack P. R.; Winter C. G. Inhibition of NOTCH signaling by gamma secretase inhibitor engages the RB pathway and elicits cell cycle exit in T-cell acute lymphoblastic leukemia cells. Cancer Res. 2009, 69 (7), 3060–8. 10.1158/0008-5472.CAN-08-4295. [DOI] [PubMed] [Google Scholar]
- Niimi H.; Pardali K.; Vanlandewijck M.; Heldin C. H.; Moustakas A. Notch signaling is necessary for epithelial growth arrest by TGF-beta. J. Cell Biol. 2007, 176, 695–707. 10.1083/jcb.200612129. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.