Abstract
Oxidative stress plays a major role in diabetic physiopathology; hence, the interest of using natural antioxidants as therapeutic tools exists. The aim of this study was the evaluation of in vitro antioxidant activity and inhibitory potential of organic extracts from Aristolochia longa roots against key enzymes linked to hyperglycemia. Antioxidant activity was performed using 2,2′-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radicals and ferric reducing/antioxidant power (FRAP) methods. The α-Glucosidase and β-Galactosidase inhibitory activities were investigated using an in vitro model. Moreover, phytochemical analysis of tested extracts was carried out. The aqueous fraction of this herb exhibited the highest antioxidant activity for both DPPH and ABTS methods, IC50=125.40±2.40 μg/mL and IC50=65.23±2.49 μg/mL, respectively. However, the ethyl acetate fraction possessed the strongest inhibitory effect towards α-Glucosidase (IC50=1.112±0.026 mg/mL). Furthermore, the result showed high levels of phenolic content. The results showed that this plant could be a significant source of medically important natural compounds.
1. Introduction
Medicinal plants are one of the main resources of therapeutic agents. Indeed, 80% of the world's population uses plants in health care [1]. Recently, the interest in the search for natural substances has considerably increased, because these substances are intended for use in foods or drugs to replace synthetic compounds, which are limited because of their side effects [2]. There is an increasing interest in using medicinal plants and their phytoconstituents as natural sources because of their well-known ability to scavenge free radicals. Effectively, plants are sources of natural antioxidants compounds that possess various pharmacological properties with little or no side effects and protect human health from many diseases [3–5]. The prevention of oxidative stress related disease by medicinal plant products is delaying the oxidation of lipids or other molecules by inhibiting the propagation of oxidative chain reactions [2].
Among Moroccan medicinal plants, Aristolochia longa (A. longa) is a medicinal plant belonging to Aristolochiaceae family, which is widely distributed in the tropical and temperate regions [6]. Aristolochia species contain secondary metabolites that have well-known beneficial effects [7]. A. longa locally known as “Barraztam” is a species commonly used in Moroccan traditional medicine. Many traditional healers also use a small amount of its rhizome powder with honey or salted butter for the treatment of abdominal pain and upper respiratory tract infections [8–10].
Likewise, diabetes mellitus is a major cause of mortality and the most common metabolic disorder characterized by hyperglycemia due to lack of insulin production by the pancreas or the inability of the insulin produced to control blood glucose [11, 12]. One interesting approach is to reduce postprandial hyperglycemia by retarding glucose uptake through the inhibition of carbohydrate-hydrolyzing enzymes, such as α-Glucosidase and β-Galactosidase [13, 14]. In this context, the aim of this study was to evaluate the in vitro antioxidant activity, α-Glucosidase and β-Galactosidase inhibitory potentials of A. longa root extracts.
2. Materials and Methods
2.1. Reagents
p-Nitrophenyl-α-D-glucopyranoside, 2-Nitrophenyl-β-D-galactopyranoside, α-Glucosidase from Saccharomyces cerevisiae, β-Galactosidase from Aspergillus oryzae, Acarbose, Folin-Ciocalteu reagent, rutin, catechin, 2,2′-diphenyl-1-picrylhydrazyl (DPPH), 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), and ascorbic acid were purchased from Sigma-Aldrich (France). All other reagents were of analytical grade.
2.2. Plant Material
2.2.1. Plant Collection
Roots of A. longa were collected in April 2016, in the province of Al Haouz in Morocco. The collected plant materials were authenticated at the Herbarium of Botany Department of the Scientific Institute of Rabat, Morocco. The roots of the plant were washed, dried at room temperature from 48 to 92 h, grounded into powder, and then stored in glass bottles preserved from light and moisture until use.
2.2.2. Preparation of Plant Extracts
To prepare the extracts, the technique of continuous hot extraction by a Soxhlet extractor was carried out using solvents of different polarities. Briefly, 10 g of root powder was extracted successively with 100 mL each of ethyl acetate, methanol, and water until the extracts were colorless in the siphon tube. The aqueous extract was prepared by adding 500 mL of distilled water to 50 g of A. longa dry roots powder. After 24 h of maceration under magnetic stirring at room temperature, the mixture was centrifuged. Then, all extracts already prepared were filtered through a filter paper (Whatman) and evaporated to dryness by a rotary evaporator at 50°C. The extracts obtained were kept at 4°C until further uses.
2.3. Phytochemical Analysis
2.3.1. Determination of Total Phenolic Content
Total phenolic content of A. longa extracts was assessed by Folin-Ciocalteu method [15] as described by Spanos and Wrolstad [16]. Gallic acid was used as standard for the calibration curve and the results are expressed as mg of gallic acid equivalent per g of extract dry weight (mg GAE/g edw).
2.3.2. Determination of Total Flavonoid Content
The total flavonoid content of A. longa extracts was determined according to the method described by Dewanto et al. [17] using Aluminium Chloride (AlCl3). Rutin was used to perform the standard curve, and the results were expressed as rutin equivalent per gram of extract dry weight (mg RE/g edw).
2.3.3. Determination of Proanthocyanidin Content
The proanthocyanidin content of A. longa extracts was determined as reported by Julkunen-Tiitto [18]. Catechin was used as a standard for constructing the calibration curve and the results are expressed as catechin equivalent per gram of extract dry weight (mg CE/g edw).
2.4. Antioxidant Activity
2.4.1. DPPH Radical Scavenging Activity Assay
Radical scavenging activity of the extracts was measured using the stable radical DPPH such as that determined by Sayah et al. [19] with some modifications. In tubes, 2.5 mL of different concentrations of each extract were introduced and 0.5 mL of methanol solution of DPPH (0.2 mM of DPPH, dissolved in methanol) freshly prepared was added. The mixture is vigorously vortexed and left in the dark at ambient temperature for 30 min. Then, the absorbance of the mixture was measured at 517 nm in a spectrophotometer. The antioxidant activity of our extracts is expressed as the percentage of DPPH radical inhibition and the IC50 was calculated for comparing the obtained results.
2.4.2. ABTS Radical Scavenging Assay
The TEAC test (Trolox Equivalent Antioxidant Capacity) or discoloration test of ABTS∙+ was carried out according to the method described by Sayah et al. [19]. Briefly, the cationic radical (ABTS∙+) was prepared by the reaction between 10 mL of ABTS (2 mM) in H2O and 100 μL of potassium persulfate (K2S2O8) (70 mM). The mixture was incubated in the dark for 16 hours at room temperature. Then, the ABTS∙+ solution was diluted with methanol to obtain an absorbance of 0.70 at 734 nm. Then, 200 μL of each extract was mixed with 2 mL of the diluted ABTS∙+ solution and allowed to react for 1 minute. After, the absorbance of the ABTS∙+ radical is measured at 734 nm. All samples were made in triplicate. The results were represented as Trolox equivalent per gram of extract dry weight (mg TE/edw).
2.4.3. Ferric Reducing/Antioxidant Power (FRAP) Assay
The FRAP test is performed according to the method described by Sayah et al. [19]. Briefly, 1 mL of each extract (1 mg/mL) was mixed with 2.5 mL of the phosphate buffer solution (0.2 M, pH 6.6) and 2.5 mL of the 1% potassium ferricyanide aqueous solution. After incubation at 50°C for 20 min, 2.5 mL of 10% trichloroacetic acid were added to the mixture, and then the mixture was centrifuged at 3000 rpm for 10 min. At the end, 2.5 mL of the supernatant was mixed with 2.5 mL of distilled water and 0.5 mL of aqueous ferric chloride solution FeCl3 (0.1%, w/v). The absorbance was measured at 700 nm. The results are expressed as ascorbic acid equivalent per gram of extract dry weight (mg AAE/g edw).
2.5. In Vitro Antidiabetic Effects
2.5.1. α-Glucosidase Inhibitory Assay
The α-Glucosidase inhibitory activity was monitored using the substrate p-Nitrophenyl α-d-glucopyranoside (pNPG), which is hydrolyzed by α-Glucosidase to release p-Nitrophenyl (a colored agent which can be monitored at 405 nm), according to the method described by Marmouzi et al. [20, 21]. The results are expressed as percentage inhibition, while, for comparing results, the concentrations of inhibitor required to inhibit 50% of enzyme activity (IC50) were determined.
2.5.2. β-Galactosidase Inhibitory Assay
The β-Galactosidase inhibitory activity was assessed according to the method of Bouabid et al. [22] using 2-Nitrophenyl β-D-Galactopyranoside as substrate, which is hydrolyzed by β-Galactosidase to release 2-nitrophenyl (a colored agent; which can be monitored at 410 nm). Briefly, a mixture of 150 μL of the samples at different concentrations (0.5-5 mg/mL) and 100 μL of sodium phosphate buffer 0.1 M (pH=7.6) containing the enzyme β-Galactosidase solution (0.1U/mL) was incubated at 37°C for 10 min. After preincubation, 200 μL of gala solution 1 mM in sodium phosphate buffer 0.1 M (pH=7.6) was added. The reaction mixtures were incubated at 37°C for 30 min. After incubation, 1 mL of 0.1 M of Na2CO3 were added to stop the reaction and the absorbance was recorded at 405 nm using the spectrophotometer. The β-Galactosidase inhibitory activity was expressed as percentage inhibition and calculated using the same formula as the α-Glucosidase test, and the IC50 values were determined. Quercetin was used as positive control and the experiment was carried out in triplicate.
2.6. Statistical Analysis
Results are expressed as a mean ± standard error of the mean. Differences between the means were determined by one-way analysis of variance (one-way ANOVA). A difference in the mean values of p < 0.05 was considered to be statistically significant. All analyses were performed with GraphPad Prism 6. Also, IC50 values were determined using a nonlinear regression curve with the same program.
3. Results and Discussion
3.1. Total Phenolic, Flavonoid, and Proanthocyanidin Contents
Total phenolic, flavonoid, and proanthocyanidins contents are presented in Table 1. The phenolic contents in ethyl acetate fraction (EAF) of A. longa were found to be 32.55±0.43 mg GAE/g edw, which are significantly (p< 0.05) greater than methanolic fraction and aqueous extract (24.48±1.63 mg GAE/g edw and 13.13±0.48 mg GAE/g edw) respectively. There is no significant difference between EAF and aqueous fraction (AF) (28.90±1.36 mg GAE/g edw). Results of flavonoid and proanthocyanidin contents show that ethyl acetate fraction resulted also in significantly (p< 0.05) higher values of those compounds (116.86±4.12 mg RE/g edw and 77.64±1.93 mg CE/g edw, respectively) followed by the aqueous fractions that were 7.60±0.11 mg RE/g edw and 10.79±0.49 mg CE/g edw, respectively. The interesting phenolic contents of this herb indicate an important health promoting activity. Indeed, Djeridane et al. [23] worked on the A. longa roots and they estimated the total phenolics and flavonoids content as 1.47±0.02 mg GAE/g edw and 0.81±0.02 mg RE/g edw, respectively [23]. These compounds are secondary plant metabolites possessing a wide range of pharmacological activities such as anticancer, antiviral, anti-inflammatory activities and effects on capillary fragility [24–26]. Previous studies, which have been interested in other species of Aristolochia species such as A. bracteolata and A. indica, revealed that the methanol extract from each plant contains high amount of phenols and flavonoids [25, 26]. Phenolic compounds are an important and complex group of chemical constituents present in plants and are classic defense compounds to protect plants from herbivores, pathogenic, and parasite infections [27].
Table 1.
Total phenolic, flavonoid, and proanthocyanidin content of extracts.
| Extracts | Polyphenolic content (mg of GAE/g of extract) | Flavonoid content (mg of RE/g of extract) | Proanthocyanidins content (mg of CE/g of extract) |
|---|---|---|---|
| MF | 24.48±1.63b | 7.00±0.61b | 05.78±0.28a |
| EAF | 32.55±0.43c | 116.86±4.12c | 77.64±1.93c |
| AF | 28.90±1.36b,c | 07.60±0.11b | 10.79±0.49b |
| AE | 13.13±0.48a | 03.80±0.32a | 06.87±0.32a,b |
The values are the mean of three determinations ± standard error.
Values in the same column not sharing a common letter (a to c) differ significantly at p < 0.05.
MF: methanolic fraction; EAF: ethyl acetate fraction; AF: aqueous fraction; AE: aqueous extract; GAE: gallic acid equivalent; RE: rutin equivalent; CE: catechin equivalent.
3.2. Antioxidant Activity
Recently, many scientific studies have shown that free radicals play a major role in the development of cancer, heart disease, aging, cataracts, and immune system damage [28]. These unstable free radicals can be eliminated by antioxidants that inhibit the rate of oxidation and protect cells from damage [28]. Antioxidant drugs are used for the prevention and treatment of oxidative stress related disease such as diabetes, Alzheimer's disease, atherosclerosis, stroke, and cancer [29, 30]. However, its side effects and high prices force many people to take herbal medicines, which have fewer side effects [31]. Numerous methods for the analysis of in vitro and in vivo antioxidant activity have been developed, but only a few fast and reliable methods for a large number of plant extract samples exist [32–34]. Furthermore, to study the antioxidant activity of our plant extracts, the ability to scavenge the stable free radical DPPH and the cation ABTS and their ferric reducing antioxidant power (FRAP) was evaluated.
DPPH is one of the stable free radicals, commercially available, nitrogen centered, and largely used for evaluating scavenging activity of antioxidant standards and plant extracts with a characteristic absorbance at 517 nm [35], which decreases in the presence of free radical scavengers. By accepting hydrogen from a corresponding donor, the DPPH solution loses the characteristic dark purple color and becomes yellow diphenylpicryl hydrazine [36–39]. This scavenging activity has been widely used as a quick and reliable parameter to evaluate the general in vitro antioxidant activity of plant extracts [40, 41]. Recently, numerous studies reported the antioxidant properties of medicinal plant products using DPPH assay [42–44]. From these assay several molecules from medicinal plants were developed as antioxidant agents. Figure 1 illustrated the DPPH radical scavenging activity of different extracts of A. longa at various concentrations. All A. longa extracts showed scavenging effect, which increases with the concentration of samples. At 416 μg/mL concentration, aqueous and methanolic fraction of A. longa exhibited increased DPPH radical scavenging activity of 77.17% and 74.66%, respectively. Moreover, aqueous extract showed less activity at all concentrations. These results showed that A. longa roots contained high amount of radical scavenging compounds with proton-donating ability. Similar result was observed in one previous study of Benmehdi et al. [45], which showed a dose-dependent scavenging of DPPH radicals using A. clematitis roots.
Figure 1.
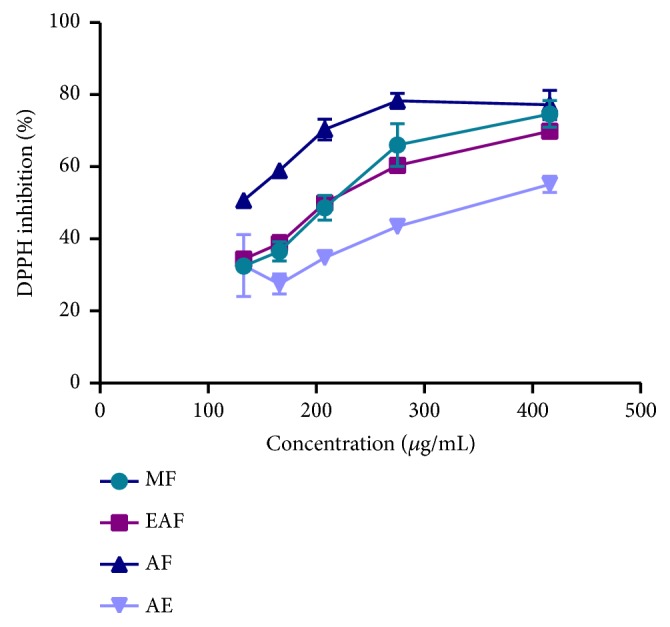
DPPH radical scavenging activity of extracts. The values are the mean of three determinations ± standard error.
The radical cation of 2,2-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) is stable in its free form. The concentration of this radical can be determined by measuring the absorbance at 734 nm. The addition of an antioxidant to a solution of this radical leads to its reduction and a decrease in absorbance. This decrease depends on the antioxidant activity of the test compound, but also on the time and the concentration [46]. The experiments were carried out using an improved ABTS decolorization assay [47]. The ability of the extracts to scavenge ABTS cation was expressed in Figure 2. The aqueous fraction exhibited potent ABTS radical cation scavenging activity in concentration dependent manner. At 181 μg/mL concentration, aqueous and methanolic fraction of A. longa possessed 90.89% and 82.58% scavenging activity on ABTS. At the concentrations investigated, this effect may indicate the capacity of the herb to minimize oxidative damage to certain vital tissues of the body [48]. These results are in agreement with the findings of Jegadeeswari et al. [49] using the same ABTS test on another Aristolochia species.
Figure 2.
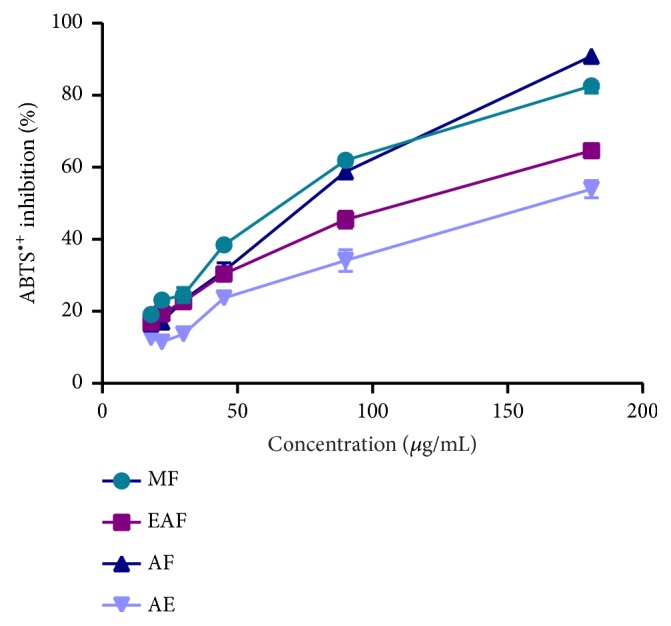
ABTS radical scavenging activity of extracts. The values are the mean of three determinations ± standard error.
In the FRAP method, antioxidants present in the sample reduced the Fe3+/ferricyanide complex to the blue ferrous form [50], which can serve as a measure of the antioxidant capacity interpreted as the reducing power [51]. The reducing power of the extracts is represented in Figure 3. A higher absorbance indicates a higher reducing power. At 2000 μg/mL concentration and among the solvents tested, aqueous fraction exhibited higher reducing activity 80.73%. The results obtained are consistent with the studies carried out on A. longa (aerial part) [52] and A. indica (aerial part) [53] and (roots) [54], which indicate that they have a reducing power.
Figure 3.

Ferric reducing antioxidant power of extracts. The values are the mean of three determinations ± standard error.
To compare the results, the IC50 are calculated as shown in Table 2. A lower value of IC50 indicates greater antioxidant activity. Indeed, the aqueous fraction showed the highest DPPH radical inhibition value (IC50=125.40±2.40 μg/mL), while the methanolic fraction was the most active against the ABTS radical cation (IC50=61.58±2.15 μg/mL), and the Trolox value for both tests was 1.79±0.35 μg/mL and 0.70±0.01 μg/mL, respectively. Our results are in analogy to those of Ramalalingam et al. [53] using the DPPH assay on the aqueous extract of A. indica, who showed IC50 value 182.31±0.31 μg/mL, while standard showed the value of 30.12±0.11 μg/mL [53]. In another study also using the DPPH assay, A. longa roots had the weakest activity compared to Trolox, among all the plants tested [23]. Furthermore, our study showed similar findings with this work. In ABTS method, we share the same results from a study of five extracts of A. bracteolate who found that the methanolic extract had potent antioxidant activity [25]. However, there is variability in the recorded activity of the different extracts; this is certainly related to the difference in the chemical composition of the extracts, especially polyphenols and flavonoids detected by phytochemical analysis in our study. These constituents have different degree of antioxidant activity against different free radicals [55]. The effectiveness of antioxidants may be due to the high amount of the main constituents and also to the presence of other constituents in small quantities or to the synergy between them. Indeed, previous studies reported the presence of chemical compounds such as Aristolactam la (ALIa), Limonen-6-ol, and Maaliol [56–58]. Phenolics are antioxidants with redox properties; the hydroxyl group helps them to work as reducing agents, hydrogen donors and singlet oxygen quenchers [59, 60]. Plant extracts that have high amounts of phenolic compounds do not always translate into high antioxidant capacity; it may be due to the presence of different active compounds, the synergistic effects of these compounds, and also to the position and extent of hydroxylation and conjugation [61]. Moreover, our activity was significantly weak compared to Trolox (p <0.05); this result can be interpreted by the fact that the extracts have several compounds, while the concentrations of those who could perform this activity will be really low. It is also noted that the antioxidant effects change depending on the test used. In fact, the antioxidant activity depends on the interactions in the reaction media between the substrate(s) (radicals) and the active molecule(s) that trap them. Other studies have shown that the roots of A. longa possess antioxidant activity [23]. Moreover, there is another study that was interested in the aerial part of this plant [52], and we found almost the same results for the DPPH method; IC50 values of fruit aqueous extract and fruit methanol extract were found to be 145.15±0.78 μg/mL and 186.21±6.24 μg/mL, respectively. The difference between the results is due, as already mentioned, to the difference of the phytoconstituents, also to the method used, the harvest period, and the studied part (root, leaf, stem…)
Table 2.
IC50 values (μg/mL) of extracts and Trolox on DPPH and ABTS inhibition activity.
| Products | DPPH (IC50 μg/mL) | ABTS (IC50 μg/mL) |
|---|---|---|
| MF | 199.35±1.25b | 61.58±2.15b |
| EAF | 220.80±2.40b | 103.62±8.62b,c |
| AF | 125.40±2.40b | 65.23±2.49b |
| AE | 354.60±5.20c | 144.40±2.07c |
| Trolox | 1.79±0.35a | 0.70±0.0a |
The values are the mean of three determinations ± standard error.
Values in the same column not sharing a common letter (a to c) differ significantly at p< 0.05.
3.3. α-Glucosidase and β-Galactosidase Inhibitory Activities
Diabetes is characterized by high blood sugar levels, which can lead to serious complications, so the goal of treating patients with diabetes is to maintain near-normal levels of glycemia control. In modern medicine, there is no treatment or medication to treat diabetes without side effects, which are related to the use of insulin and oral hypoglycemic agents [62]. Medicinal plants with antidiabetic properties may be a useful source for finding safer, cost-effective antidiabetic drugs. In the present research, different extracts of A. longa are evaluated for their antidiabetic activity. Two different in vitro assays were used to evaluate this activity, α-Glucosidase and β-Galactosidase uptake assay.
α-Glucosidase catalyzes the final step in the digestion of carbohydrates and is located in the brush-border surface membrane of intestinal cells [63]. Its inhibitors can retard the uptake of dietary carbohydrates in the small intestine and reduce postprandial hyperglycemia, which may be a useful mechanism in the preparation of antidiabetic drugs [64]. This is largely used as an effective pharmacological strategy for managing hyperglycemia related to the early stages of type 2 diabetes [65].
On the other hand, β-Galactosidase catalyzes the hydrolysis of β-Galactosides subtracts to simple carbohydrates in the intestine. Subsequently, the inhibition of this enzyme can lead to the intestinal hydrocarbon reduction and eventually decreases the glucose level. A. longa extracts showed inhibitory effects on both enzymes tested as presented in Table 3. The result revealed that the tested extracts inhibited α-Glucosidase and β-Galactosidase activity concentration dependently (0.5–5 mg/mL). Indeed, at the concentration of 1.5 mg/mL, the ethyl acetate fraction has the highest inhibitory activity against α-Glucosidase (76.56±2.54%) and β-Galactosidase (12.70±1.27%). The methanolic fraction showed a moderate inhibition of α-Glucosidase (21.94±1.34%) and β-Galactosidase (2.05±1.22%). However, the aqueous extract inhibited only the enzymatic activity of β-Galactosidase (2.20%). These results are consistent with those of Janani and Revathi, [66] who worked on another species of Aristolochiaceae (A. indica). Their study revealed that the methanolic extract of whole plant showed α-Glucosidase inhibitory activity, which increased with the increasing concentration.
Table 3.
α-Glucosidase and β-Galactosidase enzyme inhibition test results.
| Extract/ Standard |
α-Glucosidase inhibition | β-Galactosidase inhibition | ||
|---|---|---|---|---|
| % of inhibition at 1.5 mg/mL | IC50 (mg/mL) | % of inhibition at 1.5 mg/mL | IC50 (mg/mL) | |
| Ethyl acetate fraction | 76.56±2.54b | 1.112±0.026b | 12.70±1.27b | >5 |
| Methanolic fraction | 21.94±1.34a | 2.378±0.037b | 2.05±1.22a | >5 |
| Aqueous fraction | na | >5 | na | >5 |
| Aqueous extract | na | >5 | 2.20±0.13a | >5 |
| Acarbose | 96.78±0.03c | 0.199±0.014a | ---- | ---- |
| Quercetin | ---- | ---- | 92.62±0.14c | 0.247±0.006 |
na: nonactive; mean ± SD (n=3).
Values in the same column not sharing a common letter (a to c) differ significantly at p< 0.05.
To measure the inhibitory effectiveness of each extract, we used the IC50 which represents the concentration of an inhibitor that is required for 50% inhibition of its targeted enzyme. The fractions of ethyl acetate and methanol showed a strong inhibitory capacity against α-Glucosidase with IC50 values of 1.112±0.026 and 2.378±0.037 mg/mL, respectively. These inhibition values are greater than that obtained by Acarbose (0.199±0.014 mg/mL), used as standard antidiabetic. Similar effects were observed on A. indica [67]. Likewise, the α-Glucosidase inhibition values by the aqueous extract and aqueous fraction are above 5 mg/mL. For β-Galactosidase inhibitory capacity, all extracts showed an IC50 value greater than 5 mg/mL. The fact that α-Glucosidase and β-Galactosidase showed difference is due to structural differences related to the origins of enzymes [68].
The inhibitory effects of A. longa extracts against the enzyme α-Glucosidase demonstrate their potential abilities to reduce the postprandial increase of blood glucose levels in diabetic patients and their capacities to prevent type 2 diabetes. Hence, it is suggested that the mechanism of antihyperglycemic may be due to the antioxidant activity of this herb. Our finding is in accordance with earlier reports that showed that, in animal models, two of its species, aerial parts of A. indica [69] and the A. ringens roots extracts [70], showed a reduction in elevated blood glucose level. Moreover, the results are in line with a study performed on 71 herb plants to test their antidiabetic effects that showed that 36 herbs had α-Glucosidase inhibition including a species of Aristolochiaceae (Asarum heterotropoides) [71]. The differences observed for the inhibitory activity of the enzyme could be explained by the changes in the percentage of inhibition relative to the phytochemical composition of the plant species and also by the sensitivity of enzymes. Consequently, as mentioned above, phytochemical studies on A. longa demonstrated its abilities to produce a high amount of phenolic compounds and several flavonoids, including alkaloids [57], saponins, and tannins [72]. The phenolic compounds are known by their capacity to inhibit the activities of carbohydrate-hydrolyzing enzymes because of their ability to bind to proteins [73]. Moreover, the presence of flavonoids, especially in ethyl acetate fraction, may account for the inhibitory activity observed. Indeed, flavonoids have been known to possess high inhibitory potential towards α-Glucosidase in both in vitro and in vivo studies [74] and may prevent the malfunction of pancreatic beta cell due to oxidative stress and can thus reduce the onset of type 2 diabetes [75]. Importantly, some researchers have indicated that there is a positive relationship between total flavonoid and polyphenol content and the ability to inhibit α-Glucosidase [76]. The inhibitory effect observed for methanolic fraction of A. longa may be associated with the presence of other phytoconstituents like alkaloids, tannins, and saponins [76, 77]. These last have been responsible for suppressing the absorption of liquid and glucose at the brush borders [78]. These compounds, which can also inhibit α-Glucosidase, have fewer side effects and are less expensive compared to synthetic pharmacotherapeutics like Acarbose [79], and they perform several other biological activities such as antibacterial, antioxidant, and anticancer [80]. Generally, herbal medicine is based on the therapeutic action of a mixture of different compounds acting often in synergy to exert all their beneficial effects. This suggests that the biologically active compounds present in the extracts studied may act in a synergistic way to exercise their carbohydrate-hydrolyzing enzymes inhibition activities and antioxidant effects. The observed variations in chemical composition of Aristolochia sp from different parts of the world are not only due to the type of specie but also different agroclimatic conditions, extraction method, harvest period, and characterization techniques [81, 82] and also to the selected part of plant and polarity of extraction solvents. Concerning plant extracts, other studies have shown that some of them can increase insulin secretion and insulin signaling in adipose and skeletal muscles [83]. In the present study, we have investigated the antidiabetic potential of A. longa, which is used in traditional medicine for the treatment of several diseases. This herb was not previously investigated for its in vitro antidiabetic activity. The results from this work should be relevant to the human body.
4. Conclusion
The aqueous fraction of the A. longa roots had the best antioxidant effects against the DPPH and ABTS radicals and a strong ferric reducing power. This suggests that A. longa can be used to prevent and control the oxidative stress induced by free radicals. The antidiabetic activity was also investigated, focusing on the inhibitory effects on α-Glucosidase and β-Galactosidase. Our study is the first to report a potential mode of action of A. longa and suggests that the effect of this plant is due to the inhibition of digestive enzymes. On the other hand, the presence of flavonoids and phenols concludes that this herb has multiple biological properties. Other studies must be conducted to isolate the active ingredients of this plant, identify them, and study their bioactivity.
Data Availability
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Oliveira S. G. D., de Moura F. R. R., Demarco F. F., Nascente P. D. S., Pino F. A. B. D., Lund R. G. An ethnomedicinal survey on phytotherapy with professionals and patients from basic care units in the brazilian unified health system. Journal of Ethnopharmacology. 2012;140(2):428–437. doi: 10.1016/j.jep.2012.01.054. [DOI] [PubMed] [Google Scholar]
- 2.Velioglu Y. S., Mazza G., Gao L., Oomah B. D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. Journal of Agricultural and Food Chemistry. 1998;46(10):4113–4117. doi: 10.1021/jf9801973. [DOI] [Google Scholar]
- 3.Piao M. J., Kang K. A., Zhang R., et al. Antioxidant properties of 1,2,3,4,6-penta-O-galloyl-β-d-glucose from Elaeocarpus sylvestris var. ellipticus. Food Chemistry. 2009;115(2):412–418. doi: 10.1016/j.foodchem.2008.12.020. [DOI] [Google Scholar]
- 4.Di Matteo V., Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimers disease, Parkinsons disease, and amyotrophic lateral sclerosis. Current Drug Targets-CNS & Neurological Disorders. 2003;2(2):95–107. doi: 10.2174/1568007033482959. [DOI] [PubMed] [Google Scholar]
- 5.Gerber M., Boutron-Ruault M.-C., Hercberg S., Riboli E., Scalbert A., Siess M.-H. Food and cancer: state of the art about the protective effect of fruits and vegetables. Bulletin du Cancer. 2002;89(3):293–312. [PubMed] [Google Scholar]
- 6.Shirwaikar A., Somashekar A. P., Udupa A. L., Udupa S. L., Somashekar S. Wound healing studies of Aristolochia bracteolata Lam. with supportive action of antioxidant enzymes. Phytomedicine. 2003;10(6-7):558–562. doi: 10.1078/094471103322331548. [DOI] [PubMed] [Google Scholar]
- 7.Pacheco A. G., De Oliveira P. M., Piló-Veloso D., De Carvalho Alcântara A. F. C-NMR data of diterpenes isolated from Aristolochia species. Molecules. 2009;14(3):1245–1262. doi: 10.3390/molecules14031245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellakhdar J., Claisse R., Fleurentin J., Younos C. Repertory of standard herbal drugs in the Moroccan pharmacopoea. Journal of Ethnopharmacology. 1991;35(2):123–143. doi: 10.1016/0378-8741(91)90064-K. [DOI] [PubMed] [Google Scholar]
- 9.Benchaabaneand A., Abbad A. Trace of the Present. Marrakech. 1997. Medicinal Plants marked in Marrakech. [Google Scholar]
- 10.Bellakhdar J. The Traditional Moroccan Pharmacopoeia. Ibis Press; 1997. [Google Scholar]
- 11.Bailes B. K. Diabetes mellitus and its chronic complications. AORN Journal. 2002;76(2):265–282. doi: 10.1016/S0001-2092(06)61065-X. [DOI] [PubMed] [Google Scholar]
- 12.Gardner D. G., Shoback D. M. Greenspan's Basic & Clinical Endocrinology. 9th. New York, NY, USA: McGraw-Hill Medical; 2011. [Google Scholar]
- 13.Bhandari M. R., Jong-Anurakkun N., Hong G., Kawabata J. α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Journal of Food Chemistry and Nutrition. 2008;106(1):247–252. doi: 10.1016/j.foodchem.2007.05.077. [DOI] [Google Scholar]
- 14.Puls W., Keup U., Krause H. P., Thomas G., Hoffmeister F. Glucosidase inhibition. A new approach to the treatment of diabetes, obesity, and hyperlipoproteinaemia. Naturwissenschaften. 1977;64(10):536–537. doi: 10.1007/bf00483562. [DOI] [PubMed] [Google Scholar]
- 15.Singleton V. L., Orthofer R., Lamuela-Raventós R. M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods in Enzymology. 1999;299:152–178. doi: 10.1016/S0076-6879(99)99017-1. [DOI] [Google Scholar]
- 16.Spanos G. A., Wrolstad R. E. Influence of processing and storage on the phenolic composition of thompson seedless grape juice. Journal of Agricultural and Food Chemistry. 1990;38(7):1565–1571. doi: 10.1021/jf00097a030. [DOI] [Google Scholar]
- 17.Dewanto V., Wu X., Liu R. H. Processed sweet corn has higher antioxidant activity. Journal of Agricultural and Food Chemistry. 2002;50(17):4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- 18.Julkunen-Tiitto R. Phenolic constituents in the leaves of Northern willows: methods for the analysis of certain phenolics. Journal of Agricultural and Food Chemistry. 1985;33(2):213–217. doi: 10.1021/jf00062a013. [DOI] [Google Scholar]
- 19.Sayah K., Marmouzi I., Naceiri Mrabti H., Cherrah Y., Faouzi M. E. A. Antioxidant activity and inhibitory potential of cistus salviifolius (L.) and cistus monspeliensis (L.) aerial parts extracts against key enzymes linked to hyperglycemia. BioMed Research International. 2017;2017:7. doi: 10.1155/2017/2789482.2789482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marmouzi I., Karym E. M., Saidi N., et al. In vitro and in vivo antioxidant and anti-hyperglycemic activities of moroccan oat cultivars. Antioxidants. 2017;6(4) doi: 10.3390/antiox6040102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berrani A., Marmouzi I., Kharbach M., et al. Anabasis aretioides Coss. & Moq. phenolic compounds exhibit in vitro hypoglycemic, antioxidant and antipathogenic properties. Journal of Basic and Clinical Physiology and Pharmacology. 2018 doi: 10.1515/jbcpp-2018-0154. [DOI] [PubMed] [Google Scholar]
- 22.Bouabid K., Lamchouri F., Toufik H., Sayah K., Cherrah Y., Faouzi M. E. Phytochemical screening and in vitro evaluation of alpha amylase, alpha glucosidase and beta galactosidase inhibition by aqueous and organic Atractylis gummifera L. extracts. Plant Science Today. 2018;5(3):103–112. doi: 10.14719/pst.2018.5.3.393. [DOI] [Google Scholar]
- 23.Djeridane A., Yousfi M., Nadjemi B., Maamri S., Djireb F., Stocker P. Phenolic extracts from various Algerian plants as strong inhibitors of porcine liver carboxylesterase. Journal of Enzyme Inhibition and Medicinal Chemistry. 2006;21(6):719–726. doi: 10.1080/14756360600810399. [DOI] [PubMed] [Google Scholar]
- 24.Abdel-Hameed E. S. S., Bazaid S. A., Shohayeb M. M. Phytochemical Studies and Evaluation of Antioxidant, Anticancer and Antimicrobial Properties of Conocarpus erectus L. Growing in Taif, Saudi Arabia. European Journal of Medicinal Plants. 2012;2(2):93–112. doi: 10.9734/EJMP/2012/1040. [DOI] [Google Scholar]
- 25.Badami S., Jose C. K., Kumar C. R. K., et al. In vitro antioxidant activity of various extracts of Aristolochia bracteolata leaves. Oriental Pharmacy and Experimental Medicine. 2005;5(4):316–321. doi: 10.3742/OPEM.2005.5.4.316. [DOI] [Google Scholar]
- 26.Sivaraj D., Shanmugam S., Rajan M., et al. Evaluation of Aristolochia indica L. and Piper nigrum L. methanol extract against centipede Scolopendra moristans L. using Wistar albino rats and screening of bioactive compounds by high pressure liquid chromatography: a polyherbal formulation. Biomedicine & Pharmacotherapy. 2018;97:1603–1612. doi: 10.1016/j.biopha.2017.11.114. [DOI] [PubMed] [Google Scholar]
- 27.Walton N. J., Mayer M. J., Narbad A. Molecules of interest: vanillin. Phytochemistry. 2003;63(5):505–515. doi: 10.1016/S0031-9422(03)00149-3. [DOI] [PubMed] [Google Scholar]
- 28.Asimi O. A., Sahu N. P., Pal A. K. Antioxidant activity and antimicrobial property of some Indian spices. International Journal Scientific Research Publications. 2013;3(3):1–8. [Google Scholar]
- 29.Devasagayam T. P. A., Tilak J. C., Boloor K. K., et al. Free radicals and antioxidants in human health: current status and future prospects. The Journal of the Association of Physicians of India. 2004;52(794804):4. [PubMed] [Google Scholar]
- 30.Howlader S. I. M., Rahman M. M., Khalipha A. B. R., Rahman M. M., Ahmed F. Antioxidant and antidiarrhoeal potentiality of Diospyros blancoi. International Journal of Pharmacology. 2012;8(5):403–409. doi: 10.3923/ijp.2012.403.409. [DOI] [Google Scholar]
- 31.Kala C. P. Current status of medicinal plants used by traditional Vaidyas in Uttaranchal state of India. Ethnobotany Research & Applications. 2005;3:267–278. [Google Scholar]
- 32.Miller N. J., Rice-Evans C., Davies M. J., Gopinathan V., Milner A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clinical Science. 1993;84(4):407–412. doi: 10.1042/cs0840407. [DOI] [PubMed] [Google Scholar]
- 33.Brand-Williams W., Cuvelier M. E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Science and Technology. 1995;28(1):25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 34.Aruoma O. I., Cuppett S. L. Antioxidant Methodology: In Vivo and In Vitro Concepts. Champaign, IL, USA: The American Oil Chemists Society Press; 1997. [Google Scholar]
- 35.Helfand S. L., Rogina B. Genetics of aging in the fruit fly, Drosophila melanogaster. Annual Review of Genetics. 2003;37(1):329–348. doi: 10.1146/annurev.genet.37.040103.095211. [DOI] [PubMed] [Google Scholar]
- 36.Huang D., Ou B., Prior R. L. The chemistry behind antioxidant capacity assays. Journal of Agricultural and Food Chemistry. 2005;53(6):1841–1856. doi: 10.1021/jf030723c. [DOI] [PubMed] [Google Scholar]
- 37.Conforti F., Sosa S., Marrelli M., et al. In vivo anti-inflammatory and in vitro antioxidant activities of Mediterranean dietary plants. Journal of Ethnopharmacology. 2008;116(1):144–151. doi: 10.1016/j.jep.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 38.Tirzitis G., Bartosz G. Determination of antiradical and antioxidant activity: basic principles and new insights. Acta Biochimica Polonica. 2010;57(1):139–142. [PubMed] [Google Scholar]
- 39.Prashith K., Manasa M., Poornima G., et al. Antibacterial, cytotoxic and antioxidant potential of Vitex negundo var. negundoand Vitex negundo var. purpurascens- A comparative study. Science, Technology and Arts Research Journal. 2013;2(3):59–68. [Google Scholar]
- 40.Bonina F., Puglia C., Tomaino A., et al. In-vitro antioxidant and in-vivo photoprotective effect of three lyophilized extracts of Sedum telephium L. leaves. Journal of Pharmacy and Pharmacology. 2000;52(10):1279–1285. doi: 10.1211/0022357001777261. [DOI] [PubMed] [Google Scholar]
- 41.Soares J. R., Dinis T. C. P., Cunha A. P., Almeida L. M. Antioxidant activities of some extracts of Thymus zygis. Free Radical Research. 1997;26(5):469–478. doi: 10.3109/10715769709084484. [DOI] [PubMed] [Google Scholar]
- 42.Sarr S., Fall A., Gueye R., et al. Etude de l’activité antioxydante des extraits des feuilles de Vitex doniana (Verbenacea) International Journal of Biological and Chemical Sciences. 2015;9(3):1263–1269. [Google Scholar]
- 43.Amezouar F., Badri W., Hsaine M., Bourhim N., Fougrach H. Antioxidant and anti-inflammatory activities of Moroccan Erica arborea L. Pathologie Biologie. 2013;61(6):254–258. doi: 10.1016/j.patbio.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 44.Ghedadba N., Bousselsela H., Hambaba L., Benbia S., Mouloud Y. Évaluation de l’activité antioxydante et antimicrobienne des feuilles et des sommités fleuries de Marrubium vulgare L.Evaluation of the antioxidant and antimicrobial activities of the leaves and flowered tops of Marrubium vulgare L. Phytothérapie. 2014;12(1):15–24. doi: 10.1007/s10298-014-0832-z. [DOI] [Google Scholar]
- 45.Benmehdi H., Behilil A., Memmou F., Amrouche A. Free radical scavenging activity, kinetic behaviour and phytochemical constituents of Aristolochia clematitis L. roots. Arabian Journal of Chemistry. 2017;10:S1402–S1408. doi: 10.1016/j.arabjc.2013.04.015. [DOI] [Google Scholar]
- 46.Cano A., Acosta M., Arnao M. B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Report. 2000;5(6):365–370. doi: 10.1179/135100000101535933. [DOI] [PubMed] [Google Scholar]
- 47.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biology & Medicine. 1999;26(9-10):1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 48.Atawodi S. E. Antioxidant potential of African medicinal plants. African Journal of Biotechnology. 2005;4(2):128–133. [Google Scholar]
- 49.Jegadeeswari P., Nishanthini A., Muthukumarasamy S., Mohan V. R. Evaluation of antioxidant activity of Aristolochia Krysagathra (Aristolochiaceae)- An important medicinal herb. International Journal of Pharmacy. 2014;4(1):410–416. [Google Scholar]
- 50.Gülçin I., Oktay M., Kireçci E., Küfrevıoğlu Ö. I. Screening of antioxidant and antimicrobial activities of anise (Pimpinella anisum L.) seed extracts. Food Chemistry. 2003;83(3):371–382. doi: 10.1016/S0308-8146(03)00098-0. [DOI] [Google Scholar]
- 51.Samaradivakara S. P., Samarasekera R., Handunnetti S. M., Weerasena O. V. D. S. J. Cholinesterase, protease inhibitory and antioxidant capacities of Sri Lankan medicinal plants. Industrial Crops and Products. 2016;83:227–234. doi: 10.1016/j.indcrop.2015.12.047. [DOI] [Google Scholar]
- 52.Merouani N., Belhattab R., Sahli F. Evaluation of the biological activity of Aristolochia longa l. Extracts. International Journal of Pharmaceutical Sciences and Research. 2017;8(5):1978–1992. [Google Scholar]
- 53.Subramaniyan V., Saravanan R., Baskaran D., Ramalalingam S. In vitro free radical scavenging and anticancer potential of aristolochia indica L. Against MCF-7 cell line. International Journal of Pharmacy and Pharmaceutical Sciences. 2015;7(6):392–396. [Google Scholar]
- 54.Nile S. H., Nile A. S., Keum Y.-S. Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods. 3 Biotech. 2017;7(1):76. doi: 10.1007/s13205-017-0706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun J., Chu Y.-F., Wu X., Liu R. H. Antioxidant and antiproliferative activities of common fruits. Journal of Agricultural and Food Chemistry. 2002;50(25):7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 56.Hinou J., Demetzos C., Harvala C., Roussakis C. Cytotoxic and antimicrobial principles from the roots of aristolochia longa. International Journal of Crude Drug Research. 1990;28(2):149–151. doi: 10.3109/13880209009082801. [DOI] [Google Scholar]
- 57.Dhouioui M., Boulila A., Chaabane H., Zina M. S., Casabianca H. Seasonal changes in essential oil composition of Aristolochia longa L. ssp. paucinervis Batt. (Aristolochiaceae) roots and its antimicrobial activity. Industrial Crops and Products. 2016;83:301–306. doi: 10.1016/j.indcrop.2016.01.025. [DOI] [Google Scholar]
- 58.Aneb M., Talbaoui A., Bouyahya A., et al. In vitro cytotoxic effects and antibacterial activity of moroccan medicinal plants aristolochia longa and lavandula multifida. European Journal of Medicinal Plants. 2016;16(2):1–13. doi: 10.9734/EJMP/2016/28534. [DOI] [Google Scholar]
- 59.Chua M.-T., Tung Y.-T., Chang S.-T. Antioxidant activities of ethanolic extracts from the twigs of Cinnamomum osmophloeum. Bioresource Technology. 2008;99(6):1918–1925. doi: 10.1016/j.biortech.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 60.Mudoi T., Deka D. C., Devi R. In vitro antioxidant activity of Garcinia pedunculata, an indigenous fruit of North Eastern (NE) region of India. International Journal of PharmTech Research. 2012;4(1):334–342. [Google Scholar]
- 61.Pietta P. G. Flavonoids as antioxidants. Journal of Natural Products. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 62.Marles R. J., Farnsworth N. Plants as sources of antidiabetic agents. Economic and Medicinal Plant Research. 1994;6:149–187. [Google Scholar]
- 63.Caspary W. F. Sucrose malabsorption in man after ingestion of α-glucosidehydrolase inhibitor. The Lancet. 1978;311(8076):1231–1233. doi: 10.1016/s0140-6736(78)92466-2. [DOI] [PubMed] [Google Scholar]
- 64.Toeller M. α-glucosidase inhibitors in diabetes: efficacy in NIDDM subjects. European Journal of Clinical Investigation. 1994;24(Suppl. 3):31–35. doi: 10.1111/j.1365-2362.1994.tb02253.x. [DOI] [PubMed] [Google Scholar]
- 65.Hanhineva K., Törrönen R., Bondia-Pons I., et al. Impact of dietary polyphenols on carbohydrate metabolism. International Journal of Molecular Sciences. 2010;11(4):1365–1402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Janani N., Revathi K. In vitro evaluation of Aristolochia indica for its anti-inflammatory, antidiabetic and anticancer efficacy. International Journal of Current Research in Medical Sciences. 2018;4(6):23–30. [Google Scholar]
- 67.Nile S. H., Keum Y. S. Antioxidant, anti-inflammatory and enzyme inhibitory activities of 10 selected unani herbs. Bangladesh Journal of Pharmacology. 2017;12(2):162–164. [Google Scholar]
- 68.Chiba S. Molecular mechanism in α-glucosidase and glucoamylase. Bioscience, Biotechnology, and Biochemistry. 1997;61(8):1233–1239. doi: 10.1271/bbb.61.1233. [DOI] [PubMed] [Google Scholar]
- 69.Karan S. K., Mishra S. K., Pal D., Mondal A. Isolation of β-sitosterol and evaluation of antidiabetic activity of Aristolochia indica in alloxan-induced diabetic mice with a reference to in-vitro antioxidant activity. Journal of Medicinal Plants Research. 2012;6(7):1219–1223. doi: 10.5897/JMPR11.973. [DOI] [Google Scholar]
- 70.Sulyman A. O., Akolade J. O., Sabiu S. A., Aladodo R. A., Muritala H. F. Antidiabetic potentials of ethanolic extract of Aristolochia ringens (Vahl.) roots. Journal of Ethnopharmacology. 2016;182:122–128. doi: 10.1016/j.jep.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Shin S. M., Jeong Y. J., Park D. W., et al. Screening for anti-diabetic effects of prescribed korean traditional medicines. Korean Journal of Plant Resources. 2012;25(6):670–681. doi: 10.7732/kjpr.2012.25.6.670. [DOI] [Google Scholar]
- 72.Benarba B., Meddah B. Ethnobotanical study, antifungal activity, phytochemical screening and total phenolic content of Algerian Aristolochia longa. Journal of Intercultural Ethnopharmacology. 2014;3(4):p. 150. doi: 10.5455/jice.20140826030222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shobana S., Sreerama Y. N., Malleshi N. G. Composition and enzyme inhibitory properties of finger millet (Eleusine coracana L.) seed coat phenolics: mode of inhibition of α-glucosidase and pancreatic amylase. Food Chemistry. 2009;115(4):1268–1273. doi: 10.1016/j.foodchem.2009.01.042. [DOI] [Google Scholar]
- 74.Adefegha S. A., Oboh G. In vitro inhibition activity of polyphenol-rich extracts from Syzygium aromaticum (L.) Merr. & Perry (Clove) buds against carbohydrate hydrolyzing enzymes linked to type 2 diabetes and Fe2+-induced lipid peroxidation in rat pancreas. Asian Pacific Journal of Tropical Biomedicine. 2012;2(10):774–781. doi: 10.1016/S2221-1691(12)60228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Song Y., Manson J. E., Buring J. E., Sesso H. D., Liu S. Associations of dietary flavonoids with risk of type 2 diabetes, and markers of insulin resistance and systemic inflammation in women: a prospective study and cross-sectional analysis. Journal of the American College of Nutrition. 2005;24(5):376–384. doi: 10.1080/07315724.2005.10719488. [DOI] [PubMed] [Google Scholar]
- 76.Ramkumar K. M., Thayumanavan B., Palvannan T., Rajaguru P. Inhibitory effect of Gymnema montanum leaves on α-glucosidase activity and α-amylase activity and their relationship withpolyphenolic content. Medicinal Chemistry Research. 2010;19(8):948–961. doi: 10.1007/s00044-009-9241-5. [DOI] [Google Scholar]
- 77.Shabrova E. V., Tarnopolsky O., Singh A. P., Plutzky J., Vorsa N., Quadro L. Insights into the molecular mechanisms of the anti-atherogenic actions of flavonoids in normal and obese mice. PLoS ONE. 2011;6(10) doi: 10.1371/journal.pone.0024634.e24634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Mahomoodally M. F., Subratty A. H., Gurib-Fakim A., Choudhary M. I., Nahar Khan S. Traditional medicinal herbs and food plants have the potential to inhibit key carbohydrate hydrolyzing enzymes in vitro and reduce postprandial blood glucose peaks in vivo. The Scientific World Journal. 2012;2012:9. doi: 10.1100/2012/285284.285284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohamed E. A. H., Siddiqui M. J. A., Ang L. F., et al. Potent α-glucosidase and α-amylase inhibitory activities of standardized 50% ethanolic extracts and sinensetin from Orthosiphon stamineus Benth as anti-diabetic mechanism. BMC Complementary and Alternative Medicine. 2012;12(1):176. doi: 10.1186/1472-6882-12-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.He Q., Lv Y., Yao K. Effects of tea polyphenols on the activities of α-amylase, pepsin, trypsin and lipase. Food Chemistry. 2006;101(3):1178–1182. doi: 10.1016/j.foodchem.2006.03.020. [DOI] [Google Scholar]
- 81.Usman L. A., Zubair M. F., Adebayo S. A., Oladosu I. A., Muhammad N. O., Akolade J. Chemical composition of leaf and fruit essential oils of Hoslundia opposite Vahl grown in Nigeria. American-Eurasian Journal Agricultural and Environmental Science. 2010;8(1):40–43. [Google Scholar]
- 82.Akolade J. O., Usman L. A., Okereke O. E., Muhammad N. O. Antidiabetic potentials of essential oil extracted from the leaves of hoslundia opposita vahl. Journal of Medicinal Food. 2014;17(10):1122–1128. doi: 10.1089/jmf.2013.0118. [DOI] [PubMed] [Google Scholar]
- 83.Ahmed D., Kumar V., Sharma M., Verma A. Target guided isolation, in-vitro antidiabetic, antioxidant activity and molecular docking studies of some flavonoids from Albizzia Lebbeck Benth. bark. BMC Complementary and Alternative Medicine. 2014;14(1):155. doi: 10.1186/1472-6882-14-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are included within the article.


