Abstract
Spontaneous tumor lysis syndrome (STLS), in the absence of prior chemo or radiation therapy, is rare with solid tumors. Here, we present a case of STLS secondary to a small-cell neuroendocrine tumor of unknown origin in a 66-year-old female patient who presented with abdominal discomfort. Computed tomography (CT) abdomen showed a large tumor mass with peritoneal metastasis, and she developed renal failure from STLS, resulting in the need for hemodialysis. Due to the progressive deterioration and the comorbidities, she opted for comfort care. Timely recognition and intervention of STLS is critical. Further studies evaluating STLS in solid tumor patients are recommended.
1. Introduction
Tumor lysis syndrome (TLS) is a serious and life-threatening condition in cancer patients, caused by massive lysis of tumor cells with subsequent release of intracellular contents into the systemic circulation. It causes metabolic derangements including hyperuricemia, hyperkalemia, hyperphosphatemia, and secondary hypocalcemia, which in turn, can lead to acute renal failure, cardiac arrhythmias, seizures, and sudden death. Early diagnosis and appropriate intervention are essential to prevent grave consequences of this condition. TLS occurs most often in hematologic malignancies with high proliferation rate such as aggressive non-Hodgkin lymphomas, acute lymphoblastic leukemia (ALL), and Burkitt's leukemia/lymphoma, especially after cytotoxic chemotherapy [1–3]. It may also be seen in other hematologic malignancies such as chronic lymphocytic leukemia (CLL) [4] and less commonly in solid malignancies with high turnover and large tumor burden. Spontaneous tumor lysis syndrome (SLTS) can occur without any preceding therapeutic interventions, which has been reported in hematologic and solid tumors [5]. To our knowledge, there are reported cases of STLS in small-cell carcinoma, but all were small-cell lung cancers [6–10]. Here, we would like to report the first case of STLS in small cell neuroendocrine carcinoma of unknown origin.
2. Case Presentation
A 66-year-old African-American female with no significant past medical history presented to the emergency department (ED) with a four-week history of worsening abdominal discomfort. It was associated with the feeling of an urge to defecate without having an actual bowel movement, decreased appetite, and about six-pound weight loss over two months. She had not seen a physician for over ten years and never had a screening mammography, colonoscopy, or Pap smear. She reported chronic nonsteroidal anti-inflammatory agent (NSAID) use. Physical exam revealed stable vitals, abdominal distension, diffuse abdominal hardening, and positive shifting dullness.
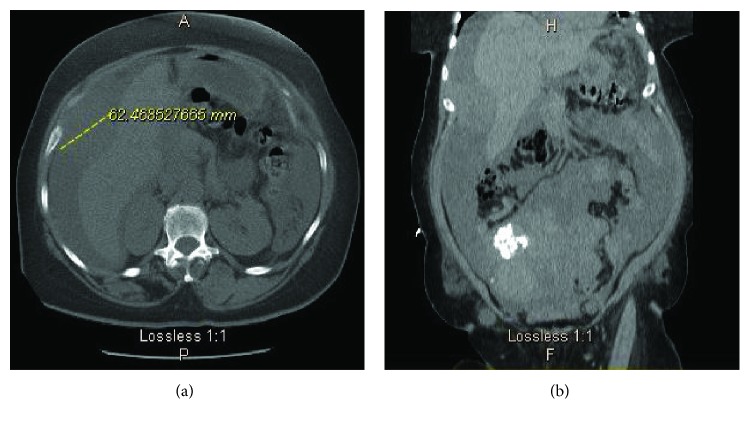
The initial labs were significant for normocytic anemia with hemoglobin (Hb) of 9.6, normal white blood cells, and creatinine of 2.57 mg/dL. The electrolytes such as potassium, phosphorous, and calcium were normal. As there was no baseline creatinine available, it was unclear if the patient has chronic kidney disease (CKD) or acute kidney injury (AKI) or AKI on CKD. Fractional Sodium Excretion (FeNa) was less than 1 indicative of prerenal cause of acute renal failure. Urine eosinophil was negative which ruled out interstitial nephritis. A computed tomogram (CT) scan of the abdomen and pelvis without contrast (Figures 1(a) and 1(b)) showed a 6.3 cm hyper dense focus adjacent to the right lobe of the liver, the large ascites, and the findings suggestive of peritoneal carcinomatosis along with diffuse body wall mass, large ascites, and grossly enlarged uterus containing multiple large partially calcified fibroids. CT chest without contrast showed a moderate left-sided pleural effusion as well as mediastinal lymph nodes measuring less than 1 cm in diameter.
Figure 1.
CT abdomen and pelvis without contrast showing a mass attached to the right lobe of the liver and peritoneal ascites. (a) Axial section. (b) Coronal section.
She underwent paracentesis and biopsy of the mass adjacent to the right liver lobe. The peritoneal fluid analysis showed red cell count (RBC) of 673,333, absolute neutrophil count of less than 250/mm3, unsuggestive of spontaneous bacterial peritonitis. Serum ascites albumin gradient (SAAG) was less than 1.1, indicating that the fluid was an exudate.
During the hospitalization, her clinical condition started deteriorating with dropping Hb. She was transfused with two units of PRBC to keep Hb above 7 g/dL. Esophageogastroduodenoscopy (EGD) showed chronic active gastritis with intestinal metaplasia and positive H. pylori but no carcinoma. She did not receive any chemotherapy or radiation therapy.
Her kidney function also continued to worsen with poor urine output (less than 200 cc per day). On day 6 of hospitalization, creatinine rose to 5.22 mg/dL. She developed metabolic acidosis with bicarbonate of 18, while the other labs showing uric acid of 14.1 mg/dL, phosphorous of 6.2, calcium of 8.7, potassium of 5, and lactate dehydrogenase (LDH) level of 1449 IU/L (above six times the upper limit of normal). The electrocardiogram showed normal sinus rhythm. She met both laboratory and clinical tumor lysis syndrome criteria with worsening creatinine and elevated uric acid and phosphorous levels.
She was given one dose of rasburicase 3 mg intravenously (IV) and started on IV hydration and renal dose-adjusted oral allopurinol. Due to her poor response to the above measures, she was started on hemodialysis after which uric acid and phosphorous levels trended down. Lactate dehydrogenase (LDH) remained persistently elevated. The progressive creatinine, uric acid, electrolyte, and LDH values were shown in Table 1.
Table 1.
Creatinine, uric acid, electrolyte, and LDH values of the patient at different time point.
| Creatinine (mg/dL) | Uric acid (mg/dL) | Potassium (mEq/L) | Phosphorous (mg/dL) | Calcium (mg/dL) | Bicarbonate (mmol/L) | LDH (IU/L) | |
|---|---|---|---|---|---|---|---|
| Day of admission | 2.57 | N/A | 3.7 | 5 | 9.0 | 25 | N/A |
| Day 1 of hospitalization | 2.83 | N/A | 3.5 | 5.3 | 8.3 | 23 | N/A |
| Day 6 (before rasburicase & allopurinol) | 5.22 | 14.2 | 4.3 | 7.3 | 8.4 | 18 | N/A |
| Day 7 (after rasburicase & allopurinol) | 5.3 | 10.7 | 5.0 | 6.2 | 8.0 | 19 | 1449 |
| After hemodialysis | 2.79 | 2.8 | 4.2 | 3 | 8.6 | 27 | 1590 |
N/A: not available.
The peritoneal fluid cytology showed atypical cells with hyperchromatic nuclei in a bloody background. The pathology of the tumor mass biopsy revealed neuroendocrine tumor, consistent with small-cell carcinoma. The hematoxylin and eosin (H&E) stain and immunohistochemical (IHC) stain patterns did not point to a specific primary site (IHC showed strongly positive synaptophysin and CD56, negative cytokeratin AE1/AE3, CD 45, chromogranin, TTF1, calcitonin, and S100, with no diastase resistant material on PAS with diastase). Figure 2 shows H&E staining, and Figure 3 shows synaptophysin staining of tumor tissue. FoundationOne study identified genomic PIK3CA amplification showing potential clinical benefit if treated with everolimus and temsirolimus. Owing to the progressive clinical deterioration and multiple embolic strokes which were diagnosed later, the patient was deemed a poor candidate for chemotherapy and she opted for comfort care. She was made hospice and passed away after.
Figure 2.
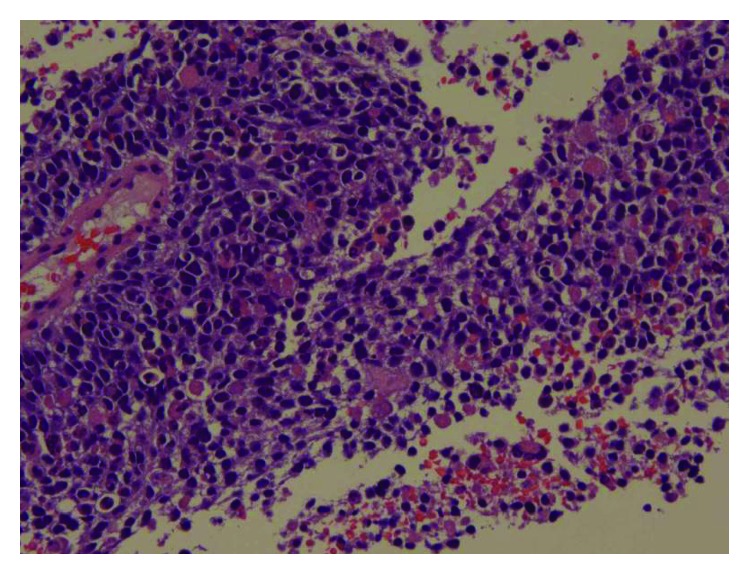
Pathology. High Power. H&E stain showing sheets of tumor cells with hyperchromatic nuclei and scant cytoplasm.
Figure 3.
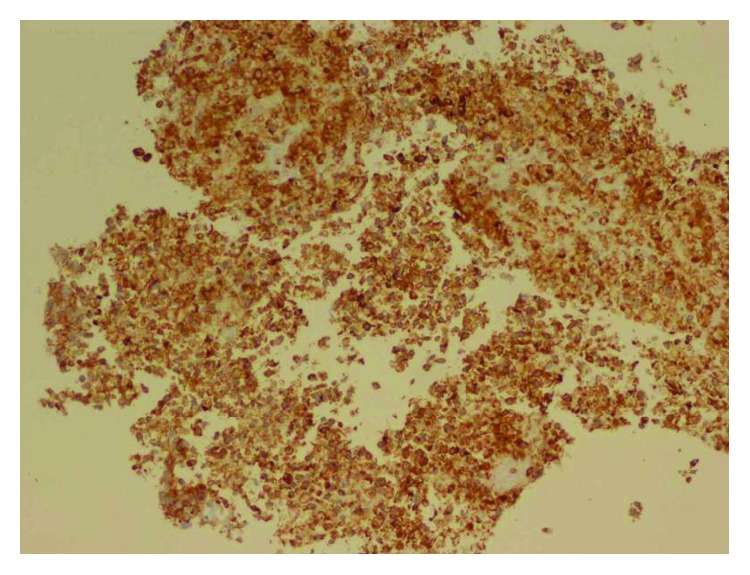
IHC (synaptophysin) stain of the tumor tissue showing positive synaptophysin confirming the tumor was neuroendocrine.
3. Discussion
Tumor lysis syndrome (TLS) is an oncologic emergency seen in about 5-20% of the malignancies [11]. It is commonly triggered after chemotherapy. Spontaneous tumor lysis syndrome (STLS) occurs without prior intervention such as chemotherapy or radiation therapy and constitutes about 15% of TLS [12, 13]. Risk factors for the development of TLS and STLS include tumor factors (extensive tumor burden or disseminated disease, rapid proliferation rate, high sensitivity to radiation or chemotherapy, and obstruction of the urinary tract by tumor compression), dehydration, infection, underlying impaired renal function, elevated uric acid level, elevated LDH level, and prior exposure to the nephrotoxic agents [14]. Our patient had multiple risk factors including large tumor mass (>10 cm), chronic NSAID use, elevated creatinine, uric acid, and LDH levels.
TLS occurs after the lysis of tumor cells with massive release of the intracellular contents such as nucleic acids, phosphorous, and potassium into the blood. The released nucleic acids are catabolized into xanthine and hypoxanthine, then into uric acid by the enzyme xanthine oxidase. Uric acid causes renal endothelial cell dysfunction and inflammation secondary to the inflammatory cytokine release and local ischemia secondary to vascular smooth muscle vasoconstriction. Elevated uric acid also precipitates in the renal tubules, resulting in acute uric acid nephropathy and acute kidney injury [3, 15–17]. Rapid potassium release into the circulation, together with inadequate potassium excretion from impaired renal function, can give rise to severe hyperkalemia predisposing to muscle cramps as well as cardiac arrhythmias and cardiac arrest [4]. Malignant cells have phosphate concentrations up to four times as high as the normal cells. Hyperphosphatemia increases the calcium-phosphate product (serum calcium concentration multiplied by serum phosphate concentration) that results in calcium phosphate deposition in the renal tubules, further worsening the acute kidney injury. Utilization of calcium in the above process causes hypocalcemia which can in turn lead to tetany, seizures, psychiatric disturbances, and cardiac arrhythmias [4, 18–20].
In STLS, it is postulated that the rapid tumor growth and burden which outgrows the blood supply gives rise to the necrosis of the tumor cells and the release of the intracellular contents [21]. As the rapidly growing tumor cells can reutilize the released phosphorous (unlike the tumor cells after chemotherapy), hyperphosphatemia is less common in STLS [22–24].
Cairo-Bishop Criterion is commonly used for the diagnosis of TLS. Laboratory TLS was defined by the presence of at least two of the abnormal laboratory values in Table 2 which is present three days before or seven days after the initiation of chemotherapy. Clinical TLS was defined by the presence of laboratory TLS plus any of the following: creatinine >1.5 times the upper limit of normal (ULN), cardiac arrhythmias, seizures, or death. Table 3 [21] demonstrates the grading of the clinical TLS. Our patient met both laboratory and clinical criteria for TLS with elevated phosphorous, uric acid, and serum creatinine levels.
Table 2.
Cairo-Bishop definition of laboratory tumor lysis syndrome.
| Variable | Value |
|---|---|
| Uric acid | ≥8 mg/dL or 25% increase from baseline |
| Phosphorous | ≥4.6 mg/dL or 25% increase from baseline |
| Potassium | ≥6 mEq/L or 25% increase from baseline |
| Calcium | ≤7 mg/dL or 25% decrease from baseline |
Table 3.
Cairo-Bishop clinical tumor lysis syndrome grading.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
|---|---|---|---|---|---|---|
| Creatinine | None | >1.5 times ULN | 1.5-3 times ULN | >3-6 times ULN | >6 times ULN | Death |
| Cardiac arrhythmias | None | No intervention needed | Nonurgent medical intervention indicated | Symptomatic and incompletely controlled medially or with device (e.g., defibrillator) | Life threatening (e.g., arrhythmia with hypotension, heart failure) | Death |
| Seizures | None | None | One generalized seizure, seizures controlled by anticonvulsants, or infrequent focal motor seizures not interfering with ADL | Seizures in which consciousness is altered; poorly controlled seizure disorder, with breakthrough generalized seizures despite medical intervention | Seizure which is prolonged, repetitive or difficult to control | Death |
Prevention is the mainstay in the management of TLS. Patients at risk for the development of TLS should have close clinical and laboratory monitoring with the avoidance of nonsteroidal anti-inflammatory agents (NSAIDs), iodinated contrast materials, and other nephrotoxic agents. Intravenous hydration with the use of hypouricemic agents (allopurinol or Rasburicase) should be instituted in high-risk patients [25, 26].
Once TLS develops, the goal of therapy is to normalize the serum values of the released intracellular solutes and to prevent or lessen the acute kidney injury. Patients should be started on aggressive intravenous hydration to improve renal blood flow and to avoid the deposition of urate and calcium phosphate crystals. Laboratory values including electrolytes, uric acid, and creatinine should be monitored every 4 to 6 hours. Hyperkalemia can be treated with oral potassium-sparing agents (e.g., sodium polystyrene), temporary measures such as glucose plus insulin or beta-agonists, and calcium gluconate to prevent cardiac arrhythmias if there are electrocardiogram abnormalities [27]. Hyperphosphatemia may be managed with the restriction of oral phosphorous intake and noncalcium phosphate binders. For hypocalcemia, calcium supplementation should be avoided unless there are severe symptoms of hypocalcemia such as tetany or cardiac dysrhythmias as added calcium can worsen the calcium phosphate deposition in the renal tubules [28]. Urate-lowering agents such as allopurinol or rasburicase should be given for hyperuricemia. Allopurinol prevents uric acid formation by inhibiting xanthine oxidase enzyme, but there is a risk of the accumulation of xanthine and hypoxanthine with resultant xanthine crystal deposition in the renal accumulations. Rasburicase, instead, converts the already formed uric acid into a more soluble allantoin and can lower uric acid level more effectively and more quickly. So, rasburicase is often recommended when there is evidence of renal failure or persistently elevated uric acid level. Since it has been introduced, rasburicase has effectively lowered the requirement for hemodialysis in patients at high risk for TLS. However, rasburicase can cause hemolysis in patients with glucose 6-phosphate deficiency and is contraindicated in those patients [23, 25, 26, 29]. Urinary alkalinization is not currently recommended [30].
Hemodialysis or hemofiltration is indicated if the patient develops severe oliguria or anuria, intractable fluid overload, persistent hyperkalemia, hyperphosphatemia-induced hypokalemia, or there is failure to response to the medical therapy [30–32]. In our patient, one dose of rasburicase was given, followed by allopurinol, but she had to be started on hemodialysis as she was persistently oliguric and there was little improvement in the laboratory parameters with the medical therapy.
The development of TLS is associated with delay in further chemotherapy, increased morbidity and mortality, increased length of hospital stay, and increased healthcare costs. About 15% of TLS can be fatal [3, 33, 34]. Although rare, STLS in solid tumors seems to be associated with high mortality. In a literature review of the case reports of STLS in solid tumors, 17 out of 26 cases resulted in mortality [10]. The exact incidence and mortality of STLS in solid tumors is uncertain, owing to the lack of larger studies.
4. Conclusion
Spontaneous tumor lysis syndrome (STLS) is a rare form of TLS which occurs in the absence of any prior interventions such as chemo or radiation therapy. Although rarer in solid tumors, when it occurs, STLS can lead to serious clinical consequences including death. Awareness of the clinical and laboratory risk factors, along with the close monitoring in patients at risk, is necessary for early intervention and prevention of unwanted complications. Further studies evaluating STLS in solid tumors are also warranted.
Acknowledgments
Special thanks to Dr. Kenneth Wind for providing the pictures of the histological slides.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
References
- 1.Hande K. R., Garrow G. C. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. The American Journal of Medicine. 1993;94(2):133–139. doi: 10.1016/0002-9343(93)90174-N. [DOI] [PubMed] [Google Scholar]
- 2.Wossmann W., Schrappe M., Meyer U., Zimmermann M., Reiter A. Incidence of tumor lysis syndrome in children with advanced stage Burkitt’s lymphoma/leukemia before and after introduction of prophylactic use of urate oxidase. Annals of Hematology. 2003;82(3):160–165. doi: 10.1007/s00277-003-0608-2. [DOI] [PubMed] [Google Scholar]
- 3.Annemans L., Moeremans K., Lamotte M., et al. Incidence, medical resource utilisation and costs of hyperuricemia and tumour lysis syndrome in patients with acute leukaemia and non-Hodgkin’s lymphoma in four European countries. Leukemia & Lymphoma. 2003;44(1):77–83. doi: 10.1080/1042819021000054661. [DOI] [PubMed] [Google Scholar]
- 4.Cheson B. D., Frame J. N., Vena D., Quashu N., Sorensen J. M. Tumor lysis syndrome: an uncommon complication of fludarabine therapy of chronic lymphocytic leukemia. Journal of Clinical Oncology. 1998;16(7):2313–2320. doi: 10.1200/JCO.1998.16.7.2313. [DOI] [PubMed] [Google Scholar]
- 5.Hsu H. H., Chan Y. L., Huang C. C. Acute spontaneous tumor lysis presenting with hyperuricemic acute renal failure: clinical features and therapeutic approach. Journal of Nephrology. 2004;17(1):50–56. [PubMed] [Google Scholar]
- 6.Boonpheng B., Murtaza G., Ginn D. Spontaneous tumor lysis syndrome in a patient with metastatic small cell lung cancer: a case report. Case Reports in Oncology. 2017;10(1):392–395. doi: 10.1159/000474937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dean R. K., Subedi R., Lee M. Spontaneous tumor lysis syndrome in small cell lung cancer. Baylor University Medical Center Proceedings. 2018;31(1):79–80. doi: 10.1080/08998280.2017.1391042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dhakal P., Rai M. P., Thrasher M., Sharma M. Spontaneous tumour lysis syndrome in small cell lung cancer: a rare phenomenon. BMJ Case Reports. 2018;2018 doi: 10.1136/bcr-2018-224512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanchustambham V., Saladi S., Patolia S., Stoeckel D. Spontaneous tumor lysis syndrome in small cell lung cancer. Cureus. 2017;9(2, article e1017) doi: 10.7759/cureus.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weerasinghe C., Zaarour M., Aranout S., Garcia G., Dhar M. Spontaneous tumor lysis syndrome in small-cell lung cancer: a rare complication. World Journal of Oncology. 2015;6(5):464–471. doi: 10.14740/wjon946w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tosi P., Barosi G., Lazzaro C., et al. Consensus conference on the management of tumor lysis syndrome. Haematologica. 2008;93(12):1877–1885. doi: 10.3324/haematol.13290. [DOI] [PubMed] [Google Scholar]
- 12.Vodopivec D. M., Rubio J. E., Fornoni A., Lenz O. An unusual presentation of tumor lysis syndrome in a patient with advanced gastric adenocarcinoma: case report and literature review. Case Reports in Medicine. 2012;2012:12. doi: 10.1155/2012/468452.468452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kekre N., Djordjevic B., Touchie C. Spontaneous tumour lysis syndrome. Canadian Medical Association Journal. 2012;184(8):913–916. doi: 10.1503/cmaj.111251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weeks A. C., Kimple M. E. Spontaneous tumor lysis syndrome: a case report and critical evaluation of current diagnostic criteria and optimal treatment regimens. Journal of Investigative Medicine High Impact Case Reports. 2015;3(3) doi: 10.1177/2324709615603199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seegmiller J. E., Laster L., Howell R. R. Biochemistry of uric acid and its relation to gout. The New England journal of medicine. 1963;268(13):712–716. doi: 10.1056/NEJM196303282681306. [DOI] [PubMed] [Google Scholar]
- 16.Kang D. H., Park S. K., Lee I. K., Johnson R. J. Uric acid–induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. Journal of the American Society of Nephrology. 2005;16(12):3553–3562. doi: 10.1681/ASN.2005050572. [DOI] [PubMed] [Google Scholar]
- 17.Graves E., Culligan D. Tumour lysis syndrome: new territory for a familiar foe? British Journal of Haematology. 2015;169(5):609–610. doi: 10.1111/bjh.13402. [DOI] [PubMed] [Google Scholar]
- 18.Gomez G. A., Han T. Acute tumor lysis syndrome in prolymphocytic leukemia. Archives of Internal Medicine. 1987;147(2):375–376. doi: 10.1001/archinte.1987.00370020193063. [DOI] [PubMed] [Google Scholar]
- 19.Boles J.-M., Mialon P., Garre M., et al. Acute renal failure caused by extreme hyperphosphatemia after chemotherapy of an acute lymphoblastic leukemias. Cancer. 1984;53(11):2425–2429. doi: 10.1002/1097-0142(19840601)53:11<2425::AID-CNCR2820531111>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 20.Kanfer A., Richet G., Roland J., Chatelet F. Extreme hyperphosphataemia causing acute anuric nephrocalcinosis in lymphosarcoma. BMJ. 1979;1(6174):1320–1321. doi: 10.1136/bmj.1.6174.1320-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cairo M. S., Bishop M. Tumour lysis syndrome: new therapeutic strategies and classification. British Journal of Haematology. 2004;127(1):3–11. doi: 10.1111/j.1365-2141.2004.05094.x. [DOI] [PubMed] [Google Scholar]
- 22.Vaisban E., Mosenzon O., Kolin M., Horn Y., Braester A. Spontaneous tumor lysis syndrome in solid tumors: really a rare condition? The American Journal of the Medical Sciences. 2003;325(1):38–40. doi: 10.1097/00000441-200301000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Wilson F. P., Berns J. S. Tumor lysis syndrome: new challenges and recent advances. Advances in Chronic Kidney Disease. 2014;21(1):18–26. doi: 10.1053/j.ackd.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wollner A., Shalit M., Brezis M. Tumor genesis syndrome. Hypophosphatemia accompanying Burkitt’s lymphoma cell leukemia. Mineral and Electrolyte Metabolism. 1986;12(3):173–175. [PubMed] [Google Scholar]
- 25.Coiffier B., Mounier N., Bologna S., et al. Efficacy and safety of rasburicase (recombinant urate oxidase) for the prevention and treatment of hyperuricemia during induction chemotherapy of aggressive non-Hodgkin’s lymphoma: results of the GRAAL1 (Groupe d'Etude des Lymphomes de l'Adulte Trial on Rasburicase Activity in Adult Lymphoma) study. Journal of Clinical Oncology. 2003;21(23):4402–4406. doi: 10.1200/jco.2003.04.115. [DOI] [PubMed] [Google Scholar]
- 26.Cortes J., Moore J. O., Maziarz R. T., et al. Control of plasma uric acid in adults at risk for tumor Lysis syndrome: efficacy and safety of rasburicase alone and rasburicase followed by allopurinol compared with allopurinol alone—results of a multicenter phase III study. Journal of Clinical Oncology. 2010;28(27):4207–4213. doi: 10.1200/JCO.2009.26.8896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maxwell A. P., Linden K., O'Donnell S., Hamilton P. K., McVeigh G. E. Management of hyperkalaemia. The Journal of the Royal College of Physicians of Edinburgh. 2013;43(3):246–251. doi: 10.4997/JRCPE.2013.312. [DOI] [PubMed] [Google Scholar]
- 28.Mirrakhimov A. E., Voore P., Khan M., Ali A. M. Tumor lysis syndrome: a clinical review. World Journal of Critical Care Medicine. 2015;4(2):130–138. doi: 10.5492/wjccm.v4.i2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Band P. R., Silverberg D. S., Henderson J. F., et al. Xanthine nephropathy in a patient with lymphosarcoma treated with allopurinol. The New England Journal of Medicine. 1970;283(7):354–357. doi: 10.1056/NEJM197008132830708. [DOI] [PubMed] [Google Scholar]
- 30.Coiffier B., Altman A., Pui C. H., Younes A., Cairo M. S. Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence-based review. Journal of Clinical Oncology. 2008;26(16):2767–2778. doi: 10.1200/JCO.2007.15.0177. [DOI] [PubMed] [Google Scholar]
- 31.Jones G. L., Will A., Jackson G. H., Webb N. J. A., Rule S., the British Committee for Standards in Haematology Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. British Journal of Haematology. 2015;169(5):661–671. doi: 10.1111/bjh.13403. [DOI] [PubMed] [Google Scholar]
- 32.Howard S. C., Jones D. P., Pui C. H. The tumor lysis syndrome. The New England Journal of Medicine. 2011;364(19):1844–1854. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Locatelli F., Rossi F. Incidence and pathogenesis of tumor lysis syndrome. Contributions to Nephrology. 2005;147:61–68. doi: 10.1159/000082543. [DOI] [PubMed] [Google Scholar]
- 34.Candrilli S., Bell T., Irish W., Morris E., Goldman S., Cairo M. S. A comparison of inpatient length of stay and costs among patients with hematologic malignancies (excluding Hodgkin disease) associated with and without acute renal failure. Clinical Lymphoma and Myeloma. 2008;8(1):44–51. doi: 10.3816/CLM.2008.n.003. [DOI] [PubMed] [Google Scholar]