Abstract
Background:
Pesticide applicators are at risk of developing neurological symptoms and neurobehavioral deficits. This risk may increase if the applicator chews stimulant plants like khat.
Objective:
To examine the sociodemographic and exposure determinants of neurological symptoms presentation, neurobehavioral performance, and cholinesterase activity among pesticide applicators in a vector control unit, Saudi Arabia.
Methods:
In a cross-sectional study, 30 pesticide applicators and 32 non-applicators from a vector control unit in Jazan region, Saudi Arabia, were studied. The study participants completed an exposure and medical questionnaire, and a neurobehavioral test battery. Their blood samples were also tested for the measurement of butyryl cholinesterase (BChE).
Results:
The mean blood BChE level was no significantly different between the applicators and non-applicators. Working in pesticide application and chewing khat were significant predictors of the neurological symptoms presentation and neurobehavioral deficits among the study participants. Each factor was associated with about 40% of the symptoms included in the questionnaire. Exposure to pyrethroids was significantly associated with a decrement in symbol digit test latency, tapping (TAP) non-preferred hand, and TAP alternating hands measures, representing the executive and motor speed/coordination functions. Khat chewing was associated with TAP preferred and non-preferred hands and serial digit learning measures, representing the memory and motor speed/coordination functions.
Conclusion:
It seems that being exposed to pyrethroids and chewing khat are associated with neurological and neurobehavioral drawbacks among pesticide applicators.
Keywords: Neurological manifestations, Catha, Pyrethrins, Insect Vectors, Saudi Arabia
TAKE-HOME MESSAGE
Exposure to pesticides and khat chewing affect the neurological health of pesticide applicators in vector control units.
Longitudinal follow up and biological assay of pesticide and khat biomarkers are required to characterize exposure and estimating dose-response relationship between the exposure and effect.
Designing an interventional study to reduce pesticide exposure and khat chewing is warranted.
Introduction
Vector control units, under the management of the preventive section of the Ministry of Health, are bodies responsible for implementing malaria control plans in Saudi Arabia. Various types of pesticides have been used to control the transmitting mosquitoes (primarily Anopheles sergentii and A. arabiensis) over the years. For indoor residual spraying (IRS), type II pyrethroids, eg, lambda-cyhalothrin and deltamethrin, are the most commonly used pesticides.1 Larvicides, particularly diflubenzuron, methoprene, and pyriproxyfen, are applied to control vector breeding sites.2 Pyrethroids, mainly type I pyrethroids, eg, bifenthrin and bioallethrin, are used for space spraying; so does cyphenothrin, a type II pyrethroids.3
Workers exposed to pyrethroids are subjected to various types of health effects. Cutaneous paresthesia is the commonest health symptom due to topical exposure to synthetic pyrethroids.4 The severity of paresthesia ranges from a mild itch to a stinging sensation in the exposed area with progression to numbness. The relationship between dermal exposure to pyrethroid compounds and development of paresthesia is not consistent across studies, especially when applying multiple pyrethroid compounds.5,6 Other neurological symptoms reported among workers exposed to pyrethroids include dizziness,7,8 eye and nose irritation, arthralgia, blurred vision, headache, skin irritation,5,8,9 and fatigue10. Spraymen exposed to deltamethrin also experience muscle tremor or feel numbness localized to the area of skin contamination.11 In addition, flight attendants exposed to permethrin report having hyperactivity, anxiety, or irritability.9 Workers exposed to higher levels of pyrethroids experience additional clinical manifestations, such as listlessness, muscular fasciculation, and mild disturbance of consciousness, all of which are indicative of moderate acute pyrethroid poisoning.12,13
There is a rarity of reports addressing the neurobehavioral performance of those working with pyrethroids. A Canadian study reported a positive association between the pyrethroid metabolite, 3-(2,2-dichlorovinyl)-2,2-dimethylcyclopropane carboxylic acid (cis-DCCA), and increased total difficulties score as reported by parents on the Strengths and Difficulties Questionnaire.14 Horton and colleagues found that piperonyl butoxide—a synergist that potentiates the insecticidal action of pyrethroids collected during the 3rd trimester of pregnancy—was associated with a significantly lower Bayley Mental Development Index scores at age 3. However, these scores were not significantly associated with the specific pyrethroid metabolites: cis- or trans-permethrin level measured in maternal and cord plasma.15 In contrary to the few studies conducted on humans, numerous animal studies have been carried out reporting a wide range of neurobehavioral defects.16-22 The most prominent defect is altered motor activity, which is more potent with cyano-pyrethroids than with non-cyano compounds.23 Impaired coordination after exposure to deltamethrin in rats16 and mice,17 and after exposure to permethrin and cypermethrin in male and female Long-Evans rats were also reported.18 Other reported impairments include neuromuscular weakness, tremors, learning and memory problems, and disturbed somatosensory response.18-22
Khat chewing is a common habit in the southwestern Saudi Arabia. In a recent study, it was reported that the prevalence of chewing khat is 33.2% among Jazan population.24 Inhabitants in this area chew khat for its pleasant amphetamine-like effects caused by its cathinone.25 It is reported that khat chewing is associated with neurological symptoms, eg, impaired concentration, fine tremors, headache, and impaired motor coordination,26 as well as deficits in the neurobehavioral performance, eg, inhibitory control, impairments in working memory and cognitive flexibility.27-29 Studies conducted by the authors investigating the neurological symptoms and neuropsychological performance among Jazan population showed that learning, motor speed/coordination, and set-shifting/response inhibition functions are the neurobehavioral function mostly affected in khat chewers, that more than half of the khat chewers showed psychological dependence, and that neurological symptoms were more prevalent among khat-dependent chewers.30,31
Pesticide applicators working in vector control units in southwestern Saudi Arabia are exposed to pyrethroids; they may also chew khat. To the best of our knowledge, there are no studies that have investigated neurological health effects among pesticide applicators working in vector control units in Saudi Arabia. We hypothesized that both application of pesticide and chewing khat would put the workers at a higher risk of developing neurological symptoms and neurobehavioral deficits. This study was conducted to test this hypothesis.
Material and Methods
Participants
This is a cross-sectional study of neurological symptoms, neurobehavioral performance, and cholinesterase activity among pesticide applicators working in vector control units in Jazan region, Saudi Arabia. There are 10 units in Jazan region responsible for controlling, detecting and treatment of patients with malaria. A unit was chosen at random to conduct the study, which was carried on in between March and August 2015 at Horoub vector control unit in Jazan region. The unit has about 60 pesticide applicators and 70 administrative employees. All applicators were invited to participant in the study as the “exposed group;” the administrative staff were invited as “non-exposed” group. All applicators in the vector control unit were male. Inclusion criteria included working as a pesticide applicator or an administrative job in Horoub vector control center, aged between 18 and 60 years, and having a minimum education of reading and writing. Participants with a history of neurological disease were excluded from the study. After applying the inclusion and exclusion criteria, the final study sample included 30 applicators and 32 non-applicators. The main reason for not participating was lacking the ability to read and write. There were no significant differences between the study participants and others who did not participate with regard to the sociodemographic characteristics, age, smoking habit, khat chewing, and employment history. This sample size was enough to detect a difference with an effect size of 0.74 between pesticide applicators and non-applicators reported earlier in a similar community.32
Pesticide Application
Vector control activities in Saudi Arabia are performed through an integrated management program that covers areas where malaria is prevalent, particularly the southwestern part of the country that includes Jazan and Asir regions. These activities are undertaken by the vector control units under the supervision of the Ministry of Health and include combatting the anopheles mosquitoes, primarily A. sergentii and A. arabiensis, responsible for transmission of malaria, surveying people to diagnose and treat patients with malaria, and performing health education and health awareness programs. There are three main ways to control mosquitoes responsible for transmission of the malaria parasite: (A) using larvicides through spraying the breeding foci of mosquitoes to control larvae by insect growth regulators, predominantly diflubenzuron, methoprene, and pyriproxyfen.2 This method is widely used all over the Kingdom, except mosquito-free areas. (B) using indoor residual spraying (IRS) with insecticides that have a residual effect. This method is used in areas with a high density of malaria mosquitoes. Deltamethrin or lambda-cyhalothrin were used and protected 83% of the 3.15 million people at risk between 1999 and 2011.1 (C) space spraying with pyrethroids, eg, bifenthrin, bioallethrin, and cyphenothrin, is employed in areas with a high prevalence of malaria (ultralow-volume and thermal fogging).33
Pesticide applicators in the vector control units work interchangeably among these various types of pesticide application and are not assigned to work in a specific type of application. It is also obvious that the two types of pyrethroids have been used—type I includes bifenthrin and bioallethrin, and type II includes deltamethrin, lambda-cyhalothrin, and cyphenothrin.
Procedures
Each participant was interviewed and asked to complete a questionnaire about medical and work history. The questionnaire included detailed questions regarding occupational exposure, eg, type of pesticide application, the applied pesticides, the task they performed during pesticide application, years of working in pesticide application, and days worked a week and hours per day. The participants were screened for a set of neurological symptoms to identify the presence of autonomic, motor, cognitive, behavioral, and other general symptoms. The symptoms' questionnaire was developed from the Q16 questionnaire,34 which was previously applied to the same community and detected neurological defects among khat chewers and non-chewers in Jazan region.31 The classification and categorization of the symptoms have been described previously in a study that employed this screening procedure to Egyptian adolescent pesticide applicators.35
The neurobehavioral performance of the study group was evaluated by a computerized neurobehavioral test battery—The Behavioral Assessment and Research System (BARS).36 Participants were tested for memory, attention/short memory, executive, motor speed/coordination, and information processing speed functions. The assessed functions, known to be affected by exposure to pesticides, were employed to detect the neurobehavioral deficits among pesticide applicators.30,37,38 The test battery included match-to-sample (MTS) and serial digit learning (SDL) tests to evaluate memory function; digit span forward (DSF) and digit span backward (DSB) tests to examine attention/short-term memory function; symbol digit (SDT) latency test to assess the executive function; tapping (TAP) preferred and TAP non-preferred and TAP alternative tests to evaluate motor speed/coordination function; and the simple reaction test (SRT) latency to measure information processing function. These tests have been described in details elsewhere.39 BARS system has simple step-by-step instructions that suit lower levels of education so that participants can listen to and understand them easily. Each test has a training part before starting the actual test.36
Five mL of venous blood were drawn from each participant to measure the butyryl cholinesterase levels (BChE). The analysis was performed at the laboratories of the Faculty of Medicine, Jazan University. Cholinesterase hydrolyzes butyryl thiocholine to give thiocholine and butyrate. The reaction between thiocholine and dithiobis-nitrobenzoate (DTNB) produces 2-nitro-mercaptobenzoate, a yellow chemical complex the concentration of which can be measured at a wavelength of 405 nm by photometry.40,41
Ethics
The study was approved by the IRB at the Faculty of Medicine, Jazan University on February 2015. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Each participant signed a consent form after learning about the objective of the study and the procedures involved. Testing and data collection were performed in the clinic of Horoub vector control unit.
Statistical Analysis
SPSS® for Windows® ver 22.0 (SPSS Inc, Chicago, IL, USA) was used for data analysis. Normally distributed continuous variables, eg, age, years of education, and days and hours of pesticide application, were presented as means and SD. Student's t test was used to examine the difference between means of two normally distributed groups. Non-normally distributed continuous variables, eg, BChE and number of symptoms, were presented as median and interquartile range (IQR). The difference between two non-normally distributed variables was assessed by Mann-Whitney U test. Categorical variables, eg, smoking, khat chewing, and the studied symptoms, were described as number and percentage. c2 test was used to examine the differences in distribution of categorical variables.
Symptoms were recorded as experiencing the symptoms “never,” “once,” or “more than once.” As the majority of participants reported the symptoms as “never” or “more than once,” and also for the purpose of the logistic regression analysis, the responses were re-categorized into two categories: “more than once” and “infrequent;” infrequent category included “never” and “once.” A logistic regression model was employed to control for the confounders that might have a role in symptom presentation. In addition to job status, khat chewing and years of education were included in the model when they had a univariate significant relationship with the neurological symptoms. A multiple linear regression analysis was also used to estimate the differences in average performance between applicators and non-applicators, taking into account the effect of khat chewing, age, and education on the neurobehavioral performance. Holm's correction of p values was used to adjust for multiple comparisons. As with logistic regression, the models for each neurobehavioral measure included in addition to job status, variables with a significant relationship in univariate analysis. For one variable, tapping non-preferred hand, the interaction term of job status and khat chewing was also included in the model.
Result
Demographic Characteristics of Study Participants
The mean age of applicators was 33.3 (SD 8.5) years, non-significantly (p=0.7) different from that in non-applicators (33.9, SD 8.4 years). Non-applicators were educated for a mean of 11.1 (SD 3.5) years, significantly (p=0.002) higher than that for applicators (8.5, SD 2.7 years). Applicators had a mean experience of pesticide applications of 8.1 (SD 3.6) years. They worked for a mean of 4.9 (SD 0.7) days a weeks, 5.9 (SD 1.5) hours a day. The frequency of smoking was significantly (p=0.008) higher in non-applicators compared to applicators. The distributions of marriage and khat chewing were not significantly different between the studied groups (Table 1).
| Table 1: Frequency of some demographic variables in the studied groups | ||||
| Variable | Non-applicators n (%) (n=32) | Applicators n (%) (n=30) | p value | |
| Marital status | Married | 25 (86) | 30 (88) | 0.8 |
| Single | 4 (14) | 4 (12) | ||
| Smoking | No | 16 (55) | 29 (85) | 0.008 |
| Yes | 13 (45) | 5 (15) | ||
| Khat chewing | No | 14 (48) | 21 (66) | 0.2 |
| Yes | 15 (52) | 11 (34) | ||
Butyryl Cholinesterase (BChE) Levels
There was no significant (p=0.7) difference between the blood BChE level between applicators and non-applicators (Fig 1).
Figure 1.
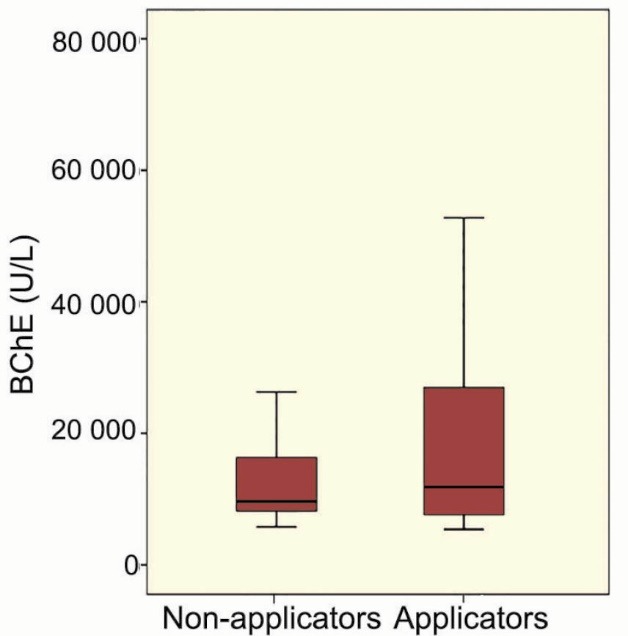
Butyryl cholinesterase levels of the study groups (applicators and non-applicators)
Neurological Symptoms
Logistic regression analysis results for each neurological symptom including primarily the job status (applicator vs non-applicator), khat chewing (chewer vs non-chewer), and years of education, are presented in Table 2. The frequency of each neurological symptom and the impact of job and khat chewing statuses on the development of the symptom are also presented in Table 2. Out of the 24 symptoms studied, applicators reported higher frequencies in 20 symptoms than non-applicators; the difference was significant for 10 symptoms (ORs ranging from 4.1 to 61.1). Khat chewing was significantly associated with nine symptoms (ORs ranging from 1.4 to 92.0) (Table 2). Years of education was a significant predictor for only one symptom, difficulty seeing at night (OR 1.5, 95% CI 1.1 to 2.0).
| Table 2: Frequency of neurological symptoms in studied participants stratified by job status (applicators vs nonapplicators). Using logistic regression analysis, independent effects of job status as well as khat chewing on developing the symptoms are also presented. | ||||
| n (%) | OR (95% CI) | |||
| Neurologic symptoms | Non-applicators (n=29) | Applicators (n=32) | Job status | Khat chewing |
| Dizziness | 1 (3) | 13 (41) | 61.1 (5.1 to 743.2) | 16.7 (5.1 to 743.2) |
| Feeling anxious | 7 (24) | 16 (50) | 8.3 (1.6 to 43.1) | 16.6 (3.2 to 86.1) |
| Nausea and vomiting | 2 (7) | 6 (19) | 3.1 (0.6 to 16.9) | — |
| Feeling tired | 6 (21) | 15 (47) | 5.5 (1.4 to 21.0) | 5.0 (1.3 to 18.7) |
| Excessive sweating | 4 (14) | 11 (34) | 4.1 (1.0 to 16.6) | — |
| Difficulty seeing at night | 3 (10) | 10 (31) | 13.0 (1.8 to 92.4) | — |
| Being absent-minded | 6 (21) | 8 (20) | 2.1 (0.5 to 8.0) | 6 (1.5 to 23.8) |
| Headache | 8 (28) | 23 (72) | 14.7 (3.0 to 72.7) | 8.5 (1.7 to 42.4) |
| Loss of appetite | 5 (17) | 11 (34) | 20.0 (2.1 to 195.4) | 92.0 (7.9 to 1068.3) |
| Fast heart rate | 3 (10) | 2 (6) | 0.6 (0.1 to 3.7) | — |
| Difficulty with balance | 1 (3) | 4 (13) | 4.0 (0.4 to 38.1) | — |
| Blurred vision | 6 (21) | 8 (25) | 1.3 (0.4 to 4.3) | — |
| Difficulty in concentration | 2 (7) | 8 (25) | 12.8 (1.5 to 108.3) | 1.4 (1.0 to 2.0) |
| Numbness | 3 (10) | 14 (44) | 20.2 (3.1 to 131.8) | — |
| Momentary loss of consciousness | 5 (17) | 8 (25) | 2.6 (0.6 to 10.3) | — |
| Feeling irritable | 5 (17) | 8 (25) | 1.6 (0.5 to 5.6) | 5.3 (1.3 to 21.7) |
| Shaking or trembling of hands | 1 (3) | 8 (25) | 23.2 (2.2 to 239.8) | 14.4 (2.2 to 95.7) |
| Difficulty in falling asleep | 6 (21) | 4 (13) | 0.5 (0.1 to 2.1) | — |
| Weakness in arms and legs | 2 (7) | 5 (16) | 5.4 (0.9 to 33.3) | — |
| Change in smell and taste | 3 (10) | 5 (16) | 1.6 (0.3 to 7.4) | — |
| Feeling depressed | 5 (17) | 5 (16) | 0.9 (0.2 to 3.5) | — |
| Involuntary movements | 2 (7) | 3 (9) | 1.4 (0.2 to 9.0) | — |
| Excessive salivation | 1 (3) | 2 (6) | 2.5 (0.2 to 31.5) | — |
| Tinnitus | 3 (10) | 6 (19) | 2.0 (0.5 to 8.9) | — |
The applicators reported a significantly (p=0.02) higher median (5.0, IQR 9.8) number of symptoms than non-applicators (2, IQR 4.8).
Neurobehavioral Performance of the Studied Groups
Age, years of education, khat chewing, years of working in pesticide application, BChE level, smoking, and the job status (applicator or non-applicator) were considered in the regression models that predict the neurobehavioral performance of the study population. The performance of applicators vs non-applicators is presented in Table 3 by reporting the adjusted means of both groups as obtained from the regression model. Other factors were included in the model if they had a significant association with the neurobehavioral measure in univariate analysis.
| Table 3: Neurobehavioral performance of applicators and non-applicators, showing also the effect of khat chewing, years of education, and age (only included in the regression model if they are significantly associated with the neurobehavioral measure in univariate analysis) | |||||
|
Neurobehavioral outcomes |
Mean (SD) | Regression coefficient (95% CI) | |||
| Non-applicators (n=28) | Applicators (n=30) | Job status | Khat chewing | ||
| Memory | MTS score | 10.6 (3.2) | 10.1 (4.9) | -0.6 (-2.4 to 1.2) | — |
| SDL score | 18.4 (4.2) | 17.8 (4.9) | -0.5 (-2.3 to 1.2) | -4.2 (-6.0 to -2.5) | |
| Attention/shortterm memory | DSF | 5.4 (1.6) | 5.4 (2.2) | -0.03 (-0.8 to 0.8) | -0.7 (-1.5 to 0.05) |
| DSB | 4.4 (1.1) | 4.4 (1.6) | -0.05 (-0.6 to 0.5) | -0.5 (-1.1 to 0.02) | |
| Executive function | SDT latency*† | 4135.1 (2913.5) | 4736.6 (1602.6) | 601.5 (14.7 to 1188.3) | — |
| Motor speed/coordination | TAP preferred | 97.2 (2.1) | 97.8 (2.2) | 0.08 (-0.8 to 0.9) | -1.6 (-2.4 to -0.7) |
| TAP non-preferred‡ | 94.5 (3.2) | 92.2 (3.3) | -3.6 (-5.2 to -2.0) | -3.6 (-5.3 to -2.0) | |
| TAP alternative | 53.5 (4.2) | 51.2 (6.0) | -2.3 (-4.6 to -0.1) | — | |
| Information processing speed SRT latency*§ | 273.9 (488.4) | 309.3 (220.2) | 35.4 (-45.2 to 116.0) | — | |
|
*These are latency measures, which means higher values are worse †Years of education is included in the regression model (b=-92.0, 95% CI ‑184.0 to 0.003) ‡The interaction term of group×khat chewing is also included (b= 3.0, 95% CI 0.5 to 5.4) §Age is included in the regression model (b=5.8, 95% CI 0.7 to 11.0) | |||||
Applicators performed worse than non-applicators on six neurobehavioral measures. This lower performance was only significant in three measures—SDT latency, TAP non-preferred hand, and TAP alternating hands. Applicators performed significantly lower than non-applicators in only one neurobehavioral measure, Tapping non-preferred hand, after Holm's correction being applied (p=0.009).
Khat chewing was another factor with a significant impact on neurobehavioral performance of the studied groups; khat chewers recorded significantly lower performance than non-chewers in three neurobehavioral measures—TAP preferred and non-preferred hands, and SDL score. These three measures were still significant after adjusting the p values using Holm's correction. Age was positively associated with SRT latency, while the relationship between years of education and SDT latency was borderline (p=0.05) (Table 3).
Discussion
Our study included about half of the pesticide applicators (exposed group) and administrators not working in pesticide application (control group) of a vector control unit. The applicators performed three types of application activities—larviciding, space spraying, and indoor residual spraying. Throughout these activities, they were exposed primarily to a mixture of type I (bifenthrin and bioallethrin) and type II (lambda-cyhalothrin, deltamethrin, cyphenothrin, and cypermethrin) pyrethroids. They were also exposed to the growth regulators—diflubenzuron, methoprene, and pyriproxyfen. Although blood BChE levels were not significantly different between the two groups, the applicators had a higher prevalence of many neurological symptoms and performed worse on neurobehavioral tests in comparison to non-applicators.
More than half of the non-applicators and one-third of applicators were khat chewers. Khat chewing was another major predictor of neurological symptoms and neurobehavioral performance in the studied groups. The neurological symptoms and neurobehavioral deficits associated with khat chewing were similar to those associated with pesticide application.
Neurological Symptoms
Results showed that pesticide application and khat chewing had a significant association with developing neurological symptoms. Pesticide application had a significant relationship with 10 neurological performance while khat chewing significantly related to nine symptoms. Pesticide application was significantly associated with symptoms that represent various neurological domains: behavioral (feeling anxious), autonomic (excessive sweating, loss of appetite), cognitive (difficulty in concentration), sensory (numbness, difficulty seeing at night), motor (shaking or trembling of hands), and general symptoms (dizziness, headache, feeling tired). The ORs of these symptoms ranged from 4.1 to 61.1 (Table 2). These results agreed with previous studies reporting paresthesia and skin irritation among workers exposed to pyrethroids, especially cyano-pyrethroids.4-6,9,42 Other neurological symptoms were also reported in previous studies. Those included dizziness,7 headache,5,9 fatigue,10 muscle tremors,11 hyperactivity, anxiety, irritability, and tingling in hands, feet, etc.9
Khat chewing is a common in Saudi Arabia, especially in southern regions where one-third of the population chew khat.24 This was reflected in our sample—about one-third of applicators and more than half of non-applicators were khat chewers. People chew khat due to its psychostimulant effects induced by the cathinone found in the plant.25 We found that khat chewing was significantly associated with symptoms representing the following neurological domains: behavioral (feeling anxious, feeling irritable), autonomic (loss of appetite), cognitive (difficulty in concentration, being absentminded), motor (shaking or trembling of hands), and general symptoms (dizziness, headache, feeling tired). The ORs of these symptoms ranged from 1.4 to 92.0 (Table 2). These results were in accordance with our report that examined the neurological symptoms among khat chewers in the same community and found that these symptoms are more frequent among dependent rather than non-dependent chewers.31 Other studies reported anxiety, irritability, hyperactivity, restlessness, insomnia,43 impaired concentration, headache, migraine, mydriasis, impaired motor coordination, and fine tremors,26 among khat chewers.
Neurobehavioral Performance
Few studies have examined the neurobehavioral performance of workers exposed only to pyrethroid pesticides.14,44 On the other hand, there are many studies examining these effects among workers exposed to a mixture of pesticides, including pyrethroids.30,3,2,37,38,45,46 In the current study, pesticide applicators were exposed to a mixture of pyrethroid compounds for many years. Therefore, our study sample was a unique group studied for the neurobehavioral performance after long-term exposure to pyrethroids. They were also examined for another factor that might influence the workers' neurobehavioral performance, khat chewing. Pesticide applicators performed significantly worse than non-applicators in two neurobehavioral functions—executive function (SDT latency measure) and motor speed/coordination function (tapping preferred and non-preferred hands). These functions are among those that are mostly affected when workers are exposed to a group of pesticides including pyrethroids.37,38 This also confirmed the animal studies that found the most prominent neurobehavioral deficit after exposure to pyrethroids is altered motor activity. The deficit happens more often with exposure to cyano-pyrethroids compared to non-cyano-pyrethroids.23
Khat chewing was another major factor that had a significant impact on neurobehavioral outcomes; it was significantly associated with SDL score, a measure of the memory function, and tapping preferred and non-preferred hand, measures of the motor speed/coordination function. This was in accordance with the results of neurobehavioral performance of a group of khat chewers in the Jazan region, where khat chewing was significantly associated with measures representing learning and motor speed/coordination functions.39 These deficits were also reported among khat chewers in other populations.25,28,47
Mechanism of Neurotoxicity
There was no significant difference in the mean blood BChE levels between the applicators and non-applicators. This was in agreement with many studies showing that exposure to pyrethroids is not associated with inhibition of cholinesterase activity, especially with chronic exposure.48-50 This indicated that pyrethroids might not similar to organophosphorus pesticides in terms of its mechanism of inhibiting BChE to produce the neurological health effects. Instead, other mechanisms were suggested including prolonged opening of voltage-sensitive sodium channels, reported as the main mechanism of these neurological impairments.51 Animal studies provide more mechanisms including oxidative stress and inhibition of oxidative biomarkers, eg, superoxide dismutase, catalase, glutathione peroxidase, glutathione reductase, and glutathione-S-transferase.52 Recent reports connect the neurotoxicity of pyrethroids to the dopaminergic and serotonergic systems.53,54 The latter mechanism might also be the mechanism of khat-induced neurotoxicity.55,56
In spite of the strengths of our study in terms of having this unique homogenous group with a long history of exposure to pyrethroids, and characterizing neurological symptoms and neurobehavioral performance using a well-established questionnaire and computerized test battery with the advantage of the step-by-step instructions, the study was cross-sectional. This study design is not appropriate to examine the temporal relationship between exposure and neurological outcomes. There is a need to evaluate the biological metabolites of the used pyrethroids to study the effect of different levels of exposure to pyrethroids on the neurological performance. A prospective longitudinal design is required to examine this temporal relationship between exposure and effect. Measuring the pyrethroid metabolites to study the dose-response relationship between exposure and effect is also warranted. This would precisely define the exposure load among those pesticide applicators, and accurately determine the neurological impact of both exposure to pyrethroids and khat chewing.
In conclusion, exposure to pyrethroids and khat chewing had a neurological and neurobehavioral health impact on pesticide applicators working in a vector control unit, Jazan, Saudi Arabia. Each factor was significantly associated with about 40% of the symptoms included in the questionnaire. Exposure to pyrethroids was also significantly associated with a decrement in executive and motor speed/coordination functions, while khat chewing was associated with memory and motor speed/coordination functions.
Acknowledgments
Authors thank the Ministry of Health administration for great collaboration to accomplish this work, especially the officials of Horoub vector control unit, Mr. Mohamed Elbakkary, Dr. Ali Elhazmy, and Mr. Yehia Elgheithy. We also thank the pesticide applicators and employees participated in the study. The authors also thank the Scientific Research Deanship, Jazan University in Saudi Arabia for the financial support of this grant number 1434-M5-12.
Conflicts of Interest
None declared.
Financial Support
This study was funded by the scientific research deanship, Jazan University, grant # 1434-M5-12.
Cite this article as: Ismail AA, Almalki M, Agag A, et al. Pesticide application and khat chewing as predictors of the neurological health outcomes among pesticide applicators in a vector control unit, Saudi Arabia. Int J Occup Environ Med 2018;9:32-44. doi: 10.15171/ijoem.2018.1160
References
- 1. WHO. World Malaria Report: 2011: World Health Organization, 2011. Available from www.who.int/malaria/world_malaria_report_2011/en/ (Accessed August 27, 2017).
- 2.Coleman M, Al-Zahrani MH, Coleman M. et al. A country on the verge of malaria elimination--the Kingdom of Saudi Arabia. PLoS One. 2014;9:e105980. doi: 10.1371/journal.pone.0105980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Sarar AS, Al-Shahrani D, Hussein HI. et al. Evaluation of Cold and Thermal Fogging Spraying Methods for Mosquito Control. Neotrop Entomol. 2014;43:85–9. doi: 10.1007/s13744-013-0180-y. [DOI] [PubMed] [Google Scholar]
- 4.Tucker SB, Flannigan SA. Cutaneous effects from occupational exposure to fenvalerate. Arch Toxicol. 1983;54:195–202. doi: 10.1007/BF01239203. [DOI] [PubMed] [Google Scholar]
- 5.Kolmodin-Hedman B, Akerblom M, Flato S, Alex G. Symptoms in forestry workers handling conifer plants treated with permethrin. Bull Environ Contam Toxicol. 1995;55:487–93. doi: 10.1007/BF00196026. [DOI] [PubMed] [Google Scholar]
- 6.Flannigan SA, Tucker SB, Key MM. et al. Synthetic pyrethroid insecticides: a dermatological evaluation. Br J Ind Med. 1985;42:363–72. doi: 10.1136/oem.42.6.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flannigan SA, Tucker SB. Variation in cutaneous perfusion due to synthetic pyrethroid exposure. Br J Ind Med. 1985;42:773–6. doi: 10.1136/oem.42.11.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walters JK, Boswell LE, Green MK. et al. Pyrethrin and pyrethroid illnesses in the Pacific northwest: a five-year review. Public Health Rep. 2009;124:149–59. doi: 10.1177/003335490912400118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sutton PM, Vergara X, Beckman J. et al. Pesticide illness among flight attendants due to aircraft disinsection. Am J Ind Med. 2007;50:345–56. doi: 10.1002/ajim.20452. [DOI] [PubMed] [Google Scholar]
- 10.Wieseler B, Kuhn K, Leng G, Idel H. Effects of pyrethroid insecticides on pest control operators. Bull Environ Contam Toxicol. 1998;60:837–44. doi: 10.1007/s001289900703. [DOI] [PubMed] [Google Scholar]
- 11.Wang SJ, Zheng QL, Yu L. et al. Health survey among farmers exposed to deltamethrin in the cotton fields. Ecotoxicol Environ Saf. 1988;15:1–6. doi: 10.1016/0147-6513(88)90037-1. [DOI] [PubMed] [Google Scholar]
- 12.Chen SY, Zhang ZW, He FS. et al. An epidemiological study on occupational acute pyrethroid poisoning in cotton farmers. Br J Ind Med. 1991;48:77–81. doi: 10.1136/oem.48.2.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He F, Sun J, Han K. et al. Effects of pyrethroid insecticides on subjects engaged in packaging pyrethroids. Br J Ind Med. 1988;45:548–51. doi: 10.1136/oem.45.8.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oulhote Y, Bouchard MF. Urinary metabolites of organophosphate and pyrethroid pesticides and behavioral problems in Canadian children. Environ Health Perspect. 2013;121:1378–84. doi: 10.1289/ehp.1306667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horton MK, Rundle A, Camann DE. et al. Impact of prenatal exposure to piperonyl butoxide and permethrin on 36-month neurodevelopment. Pediatrics. 2011;127:e699–706. doi: 10.1542/peds.2010-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Manna S, Bhattacharyya D, Mandal TK, Dey S. Neuropharmacological effects of deltamethrin in rats. J Vet Sci. 2006;7:133–6. doi: 10.4142/jvs.2006.7.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pham HC, Navarro-Delmasure C, Pham HC. et al. Toxicological studies of deltamethrin. Int J Tissue React. 1984;6:127–33. [PubMed] [Google Scholar]
- 18.McDaniel KL, Moser VC. Utility of a neurobehavioral screening battery for differentiating the effects of two pyrethroids, permethrin and cypermethrin. Neurotoxicol Teratol. 1993;15:71–83. doi: 10.1016/0892-0362(93)90065-v. [DOI] [PubMed] [Google Scholar]
- 19.Nishimura M, Obana N, Yagasaki O, Yanagiya I. Involvement of adrenergic and serotonergic nervous mechanisms in allethrin-induced tremors in mice. J Toxicol Sci. 1984;9:131–42. doi: 10.2131/jts.9.131. [DOI] [PubMed] [Google Scholar]
- 20. EMEA. α-Cypermethrin: European Agency for the Evaluation of Medicinal Products, 2003. Available from www.ema.europa.eu/docs/en_GB/document_library/Work_programme/2010/01/WC500046819.pdf (Accessed August 27, 2017).
- 21.Cagen SZ, Malley LA, Parker CM. et al. Pyrethroid-mediated skin sensory stimulation characterized by a new behavioral paradigm. Toxicol Appl Pharmacol. 1984;76:270–9. doi: 10.1016/0041-008x(84)90008-5. [DOI] [PubMed] [Google Scholar]
- 22.Hossain MM, Richardson JR. Mechanism of pyrethroid pesticide-induced apoptosis: role of calpain and ER stress pathways. Toxicol Sci. 2011;122:512–25. doi: 10.1093/toxsci/kfr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolansky MJ, Harrill JA. Neurobehavioral toxicology of pyrethroid insecticides in adult animals: a critical review. Neurotoxicol Teratol. 2008;30:55–78. doi: 10.1016/j.ntt.2007.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Mahfouz MS, Rahim BE, Solan YM. et al. Khat Chewing Habits in the Population of the Jazan Region, Saudi Arabia: Prevalence and Associated Factors. PLoS One. 2015;10:e0134545. doi: 10.1371/journal.pone.0134545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffman R, Al'Absi M. Khat use and neurobehavioral functions: suggestions for future studies. J Ethnopharmacol. 2010;132:554–63. doi: 10.1016/j.jep.2010.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Motarreb A, Baker K, Broadley KJ. Khat: pharmacological and medical aspects and its social use in Yemen. Phytother Res. 2002;16:403–13. doi: 10.1002/ptr.1106. [DOI] [PubMed] [Google Scholar]
- 27.Colzato LS, Ruiz MJ, van den Wildenberg WP. et al. Long-term effects of chronic khat use: impaired inhibitory control. Front Psychol. 2010;1:219. doi: 10.3389/fpsyg.2010.00219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colzato LS, Ruiz MJ, van den Wildenberg WP, Hommel B. Khat use is associated with impaired working memory and cognitive flexibility. PLoS One. 2011;6:e20602. doi: 10.1371/journal.pone.0020602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colzato LS, Ruiz MJ, van den Wildenberg WP, Hommel B. Khat use is associated with increased response conflict in humans. Hum Psychopharmacol. 2012;27:315–21. doi: 10.1002/hup.2229. [DOI] [PubMed] [Google Scholar]
- 30.Ismail AA, Bodner TE, Rohlman DS. Neurobehavioral performance among agricultural workers and pesticide applicators: a meta-analytic study. Occup Environ Med. 2012;69:457–64. doi: 10.1136/oemed-2011-100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Setouhy M, Alsanosy RM, Alsharqi A, Ismail AA. Khat Dependency and Psychophysical Symptoms among Chewers in Jazan Region, Kingdom of Saudi Arabia. Biomed Res Int. 2016;2016:2642506. doi: 10.1155/2016/2642506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abdel Rasoul GM, Abou Salem ME, Mechael AA. et al. Effects of occupational pesticide exposure on children applying pesticides. Neurotoxicology. 2008;29:833–8. doi: 10.1016/j.neuro.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 33.Sebai ZA. Malaria in Saudi Arabia. Trop Doct. 1988;18:183–8. doi: 10.1177/004947558801800415. [DOI] [PubMed] [Google Scholar]
- 34.Lundberg I, Hogberg M, Michelsen H. et al. Evaluation of the Q16 questionnaire on neurotoxic symptoms and a review of its use. Occup Environ Med. 1997;54:343–50. doi: 10.1136/oem.54.5.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan K, Ismail AA, Abdel Rasoul G. et al. Longitudinal assessment of chlorpyrifos exposure and self-reported neurological symptoms in adolescent pesticide applicators. BMJ Open. 2014;4:e004177. doi: 10.1136/bmjopen-2013-004177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rohlman DS, Gimenes LS, Eckerman DA. et al. Development of the Behavioral Assessment and Research System (BARS) to detect and characterize neurotoxicity in humans. Neurotoxicology. 2003;24:523–31. doi: 10.1016/s0161-813x(03)00023-8. [DOI] [PubMed] [Google Scholar]
- 37.Rohlman DS, Ismail AA, Abdel-Rasoul G. et al. Characterizing exposures and neurobehavioral performance in Egyptian adolescent pesticide applicators. Metab Brain Dis. 2014;29:845–55. doi: 10.1007/s11011-014-9565-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rohlman DS, Ismail AA, Rasoul GA. et al. A 10-month prospective study of organophosphorus pesticide exposure and neurobehavioral performance among adolescents in Egypt. Cortex. 2016;74:383–95. doi: 10.1016/j.cortex.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ismail AA, El Sanosy RM, Rohlman DS, El-Setouhy M. Neuropsychological functioning among chronic khat users in Jazan region, Saudi Arabia. Subst Abus. 2014;35:235–44. doi: 10.1080/08897077.2013.832469. [DOI] [PubMed] [Google Scholar]
- 40.Kendel M, Bottger R. A kinetic method for determination of the activity of pseudocholinesterase. Klin Wochenscher. 1967;45:325–7. doi: 10.1007/BF01747115. [DOI] [PubMed] [Google Scholar]
- 41.den Blaauwen DH, Poppe WA, Tritschler W. [Cholinesterase (EC 3118) with butyrylthiocholine-iodide as substrate: references depending on age and sex with special reference to hormonal effects and pregnancy] J Clin Chem Clin Biochem. 1983;21:381–6. [in German]. [PubMed] [Google Scholar]
- 42.Knox JM 2nd, Tucker SB, Flannigan SA. Paresthesia from cutaneous exposure to a synthetic pyrethroid insecticide. Arch Dermatol. 1984;120:744–6. [PubMed] [Google Scholar]
- 43.Cox G, Rampes H. Adverse effects of khat: a review. Adv Psychiatr Treat. 2003;9:456–63. [Google Scholar]
- 44.Muller-Mohnssen H, Hahn K. [A new method for early detection of neurotoxic diseases (exemplified by pyrethroid poisoning)] Gesundheitswesen. 1995;57:214–22. [in German]. [PubMed] [Google Scholar]
- 45.Farahat TM, Abdelrasoul GM, Amr MM. et al. Neurobehavioural effects among workers occupationally exposed to organophosphorous pesticides. Occup Environ Med. 2003;60:279–86. doi: 10.1136/oem.60.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cole DC, Carpio F, Julian J. et al. Neurobehavioral outcomes among farm and nonfarm rural Ecuadorians. Neurotoxicol Teratol. 1997;19:277–86. doi: 10.1016/s0892-0362(97)00019-6. [DOI] [PubMed] [Google Scholar]
- 47.Feyissa AM, Kelly JP. A review of the neuropharmacological properties of khat. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1147–66. doi: 10.1016/j.pnpbp.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 48.Zhang ZW, Sun JX, Chen SY. et al. Levels of exposure and biological monitoring of pyrethroids in spraymen. Br J Ind Med. 1991;48:82–6. doi: 10.1136/oem.48.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sudjaroen Y. Biochemical and hematological status of pesticide sprayers in Samut Songkhram, Thailand. Annals of Tropical Medicine and Public Health. 2015;8:186–90. [Google Scholar]
- 50.Aroonvilairat S, Kespichayawattana W, Sornprachum T. et al. Effect of pesticide exposure on immunological, hematological and biochemical parameters in thai orchid farmers- a cross-sectional study. Int J Environ Res Public Health. 2015;12:5846–61. doi: 10.3390/ijerph120605846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bradberry SM, Cage SA, Proudfoot AT, Vale JA. Poisoning due to pyrethroids. Toxicol Rev. 2005;24:93–106. doi: 10.2165/00139709-200524020-00003. [DOI] [PubMed] [Google Scholar]
- 52.Ali Z. Neurotoxic Effect of Lambda-Cyhalothrin, A Synthetic Pyrethroid Pesticide: Involvement of Oxidative Stress And Protective Role of Antioxidant Mixture. New York Sci J. 2012;5:93–103. [Google Scholar]
- 53.Rodriguez JL, Ares I, Castellano V. et al. Effects of exposure to pyrethroid cyfluthrin on serotonin and dopamine levels in brain regions of male rats. Environ Res. 2016;146:388–94. doi: 10.1016/j.envres.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 54.Ansari RW, Shukla RK, Yadav RS. et al. Involvement of dopaminergic and serotonergic systems in the neurobehavioral toxicity of lambda-cyhalothrin in developing rats. Toxicol Lett. 2012;211:1–9. doi: 10.1016/j.toxlet.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Wagner GC, Preston K, Ricaurte GA. et al. Neurochemical similarities between d,l-cathinone and d-amphetamine. Drug Alcohol Depend. 1982;9:279–84. doi: 10.1016/0376-8716(82)90067-9. [DOI] [PubMed] [Google Scholar]
- 56.Kalix P, Glennon RA. Further evidence for an amphetamine-like mechanism of action of the alkaloid cathinone. Biochem Pharmacol. 1986;35:3015–9. doi: 10.1016/0006-2952(86)90380-1. [DOI] [PubMed] [Google Scholar]


