Abstract
The four-helical bundle soluble N-ethylmaleimide-sensitive fusion protein (NSF) attachment protein receptor (SNARE) complex that mediates intracellular membrane fusion events contains a highly conserved ionic layer at the center of an otherwise hydrophobic core. This layer has an undetermined function; it consists of glutamine (Q) residues in syntaxin and the two synaptosomal-associated protein of 25 kDa (SNAP-25) family helices, and an arginine (R) in vesicle-associated membrane protein (a 3Q:1R ratio). Here, we show that the ionic-layer glutamine of syntaxin is required for efficient α-SNAP and NSF-mediated dissociation of the complex. When this residue is mutated, the SNARE complex still binds to α-SNAP and NSF and is released through ATP hydrolysis by NSF, but the complex no longer dissociates into SNARE monomers. Thus, one function of the ionic layer—in particular, the glutamine of syntaxin—is to couple ATP hydrolysis by NSF to the dissociation of the fusion complex. We propose that α-SNAP and NSF drive conformational changes at the ionic layer through specific interactions with the syntaxin glutamine, resulting in the dissociation of the SNARE complex.
Intracellular membrane fusion events are mediated by proteins of the soluble N-ethylmaleimide sensitive fusion protein (NSF) attachment protein receptor (SNARE) family. Synaptic vesicle fusion requires the formation of trans-SNARE complexes between the v- or R-SNARE vesicle-associated membrane protein (VAMP) on the vesicle and the t- or Q-SNAREs syntaxin/synaptosomal-associated protein of 25 kDa (SNAP-25) on the target membrane (1). The cis-SNARE complex that forms after membrane merger (with all SNAREs now in the same membrane) acts as a receptor for α-soluble NSF attachment protein (α-SNAP), which recruits the ATPase NSF to form a 20S complex (2–4). ATP hydrolysis by NSF then dissociates the cis-SNARE complex, freeing the SNAREs for recycling and future rounds of fusion (5).
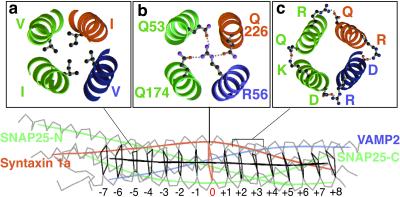
The SNARE “core complex” consists of four entwined αhelices, two from SNAP-25 and one each from syntaxin and VAMP (see Fig. 1), in which the “a” and “d” positions of the SNARE heptad repeats (Fig. 1a) form a 15-layered hydrophobic core in the center (ref. 6; Fig. 1). Interestingly, however, the central (or zero) layer is ionic rather than hydrophobic in nature (Fig. 1b). The syntaxin family invariably contains a glutamine (Q) at this position, and the SNAP-25 and VAMP families almost always harbor a glutamine and an arginine (R) residue respectively (7).
Figure 1.
Structure of the neuronal SNARE complex. Structure of the four-helical bundle of the cis-SNARE (postfusion) core complex (6), with the syntaxin 1a coil in red, VAMP2 in blue, and the two SNAP-25 coils in green. The central black polygons denote the 15 hydrophobic layers of the complex, with the central red ionic layer referred to as the zero layer. (a) A cross section of a typical (the −5) hydrophobic layer, with ball and stick structures representing the indicated amino acids. (b) The ionic layer with its three glutamines, Q226 from syntaxin, Q53 from SNAP-25 N-terminal coil, and Q174 from SNAP-25 C-terminal coil, and the arginine R56 of VAMP. The dotted lines indicate the three hydrogen bonds formed between these residues in the wild-type complex. (c) Examples of electrostatic surface interactions (dotted lines) between the SNARE coils.
Because this ionic layer is the most conserved in each of the Q- and R-SNARE families, it was predicted that the 3Q:1R ratio of SNAREs (2 SNAP-25 + 1 syntaxin:1 VAMP) within the SNARE complex must be important for SNARE-complex formation or function (7, 8). One proposed role is the proper alignment of the SNARE coils to form a correctly registered bundle, but mutation of the central glutamines in the C- and N-terminal coils of SNAP-25 does not drastically impair their ability to restore exocytosis in botulinum neurotoxin E-treated cells (botulinum neurotoxin E cleaves SNAP-25 C terminus; refs. 9 and 10). These data argue against a role in complex formation or alignment and could be consistent with the alternative hypothesis that the ionic layer facilitates the subsequent dissociation of the SNARE complex by α-SNAP and NSF (6), but so far, there has been no evidence in support of this hypothesis. Here, we provide such evidence.
Methods
Recombinant Proteins and SNARE-Complex Formation.
Recombinant SNARE proteins, rat n-sec1 and glutathione S-transferase (GST)-α-SNAP were expressed and purified with the cleavable GST-system, as described (10). Bovine his6-α-SNAP, bovine his6-NSF and his6-botulinum neurotoxin E were purified by nickel chromatography. Ionic-layer mutants were constructed by using QuickChange (Stratagene) site-directed mutagenesis PCR and verified by sequencing; the slightly slowed syntaxin Q226R mutant protein migration on gels is not caused by an altered stop-codon site. SNARE complexes were formed by mixing roughly equimolar concentrations of Syntaxin 1a full length (minus transmembrane domain) or H3 domain, SNAP-25 C- and N-terminal coils, and VAMP2 SNARE domain overnight at 4°C (unless otherwise indicated; ref. 11). CD analysis of FPLC gel-filtration purified SNAP25-N/SNAP25-C/VAMP2/Syntaxin 1a H3 complexes was performed as described (9).
NSF Dissociation of SNARE Complexes.
SNARE complex, α-SNAP, and NSF concentrations were estimated by SDS/PAGE, with BSA as a standard. α-SNAP was prebound to the SNARE complexes for 30 min on ice in 20 mM Hepes, pH 7.2/150 mM NaCl/1 mM DTT/0.5% Triton X-100 and either 2 mM MgCl2 or 2 mM EDTA, as indicated. NSF then was added so the final molar ratio was 1 SNARE complex:4.5 α-SNAP:1.2 NSF in the above buffer plus 2 mM ATP and 30 nM his6-botulinum neurotoxin E light chain (final concentrations); the samples were incubated at 37°C for 30 min before the addition of SDS-sample buffer for 30 min at 37°C and immediate loading onto SDS/10–15% PAGE gels without boiling. The gels then were Coomassie blue stained.
Bead-Binding Assays.
GST-syntaxin full-length wild type, Q226R, Q226A, and GST alone were purified on glutathione-agarose beads. In Fig. 4a, ≈0.8 μM (gel estimate) GST proteins were incubated with 0.3, 0.8 or 2 μM soluble n-sec1 in 50 μl buffer A (20 mM Hepes, pH 7.2/150 mM KCl/0.05% Tween-20/0.1% gelatin) for 2 h at 4°C and then were washed twice in 200 μl of buffer A alone and twice in buffer A containing 5% (vol/vol) glycerol instead of gelatin. In Fig. 5a, 1.5 μM GST-α-SNAP was preincubated with ≈1.5 μM partially purified SNARE complex and 30 μl of glutathione-agarose beads for 30 min on ice. Then, 3.15 μM NSF (preadsorbed on GST beads to reduce nonspecific binding) was added in a total of 100 μl of buffer B [25 mM Hepes, pH 7.4/150 mM KCl/0.05% Tween-20/5 mM β-mercaptoethanol/10% (vol/vol) glycerol/2 mM ATP and either 2 mM MgCl2 or 2 mM EDTA] and 30 nM botulinum neurotoxin E for 45 min on a rotator at room temperature. This treatment was followed by 4 × 750 μl washes in buffer B with 300 mM KCl. The unboiled supernatants and boiled, washed beads were analyzed by SDS/15% PAGE and Coomassie blue staining. Controls for nonspecific binding of NSF included replacing GST-α-SNAP with GST alone and omitting the SNARE complex. To ensure that MgATP alone was not responsible for detaching the complex from GST-α-SNAP, NSF was omitted from one pair of reactions (not shown).
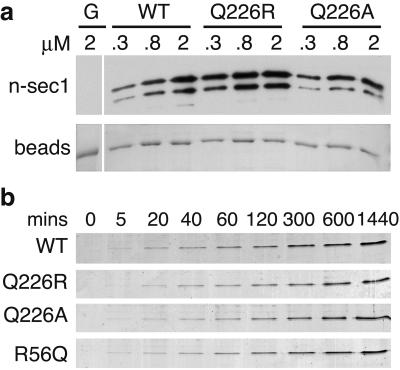
Figure 4.
The NSF dissociation defects of syntaxin Q226 mutants are not due to altered reassembly kinetics. (a) Syntaxin Q226R and Q226A are able to assume a closed conformation capable of binding n-sec1. The indicated amounts (in μM) of soluble n-sec1 were incubated with ≈0.8 μM GST-syntaxin-WT, -Q226R, or -Q226A beads, or GST alone (G) as a control for nonspecific binding. After removal of unbound n-sec1 by washing, the bound n-sec1 was detected by Western blotting (Upper). The lower band is a degradation product of n-sec1. (Lower) Ponceau S staining of the GST (G) or GST-syntaxins beads, demonstrating equal loading. (b) Time course of complex formation. Equimolar amounts (10 μM) of SNAP25-C, SNAP25-N, VAMP-WT, or R56Q, and either syntaxin-WT, -Q226R, or -Q226A were incubated on ice to form SNARE complexes for the indicated time points (in min). SDS-sample buffer then was added to prevent further SNARE-complex formation; the samples were kept at 18°C until all time points were collected before SDS/PAGE analysis (only the SNARE-complex bands are shown). For the zero time point, the sample buffer was added before the fourth SNARE coil. Syntaxin Q226R and Q226A do not assemble into complexes faster than wild-type syntaxin or VAMP R56Q.
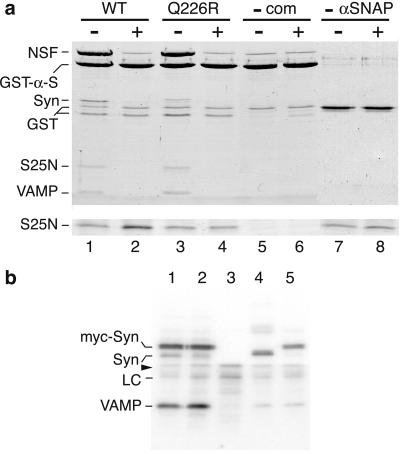
Figure 5.
Ionic-layer mutants form complexes with α-SNAP and NSF but are not dissociated upon ATP hydrolysis. (a) NSF binds to syntaxin Q226R-containing complexes via α-SNAP. Partially purified syntaxin (1.5 μM) Q226R complex (lanes 3 and 4) or wild-type complex (lanes 1, 2, 7, and 8) was incubated with 1.5 μM GST-α-SNAP and glutathione beads with ATP for 30 min on ice, followed by the addition of 3.15 μM NSF for 45 min at room temperature in EDTA (−) or MgCl2 (+) buffer. Half the supernatant was analyzed by SDS/PAGE without boiling (Lower, showing the SNAP25-N band only), whereas the beads were washed and boiled before loading (Upper). As specificity controls, the SNARE complex (lanes 5 and 6) and α-SNAP (lanes 7 and 8; GST alone was used instead) were omitted. The lower GST band is a cleaved contaminant from the purification of the SNAREs and α-SNAP. (b) Syntaxin Q226R is present in SNARE complexes to a greater extent than wild-type syntaxin in vivo. PC12 cells were transfected for 64 h with untagged VAMP alone (lanes 3 and 4) or with either myc-tagged syntaxin-wild type (lane 1) or -Q226R (lane 2). Total postnuclear membranes were extracted with Triton X-100 and immunoprecipitated with an anti-myc antibody (lanes 1–3, 5) or an anti-syntaxin antibody (lane 4) and protein A Sepharose. The washed immunoprecipitates were Western blotted with anti-syntaxin (above the arrowhead) and anti-VAMP (below the arrowhead) antibodies and quantified by phosphorimaging after [125I]secondary antibody labeling. A total of 1.43 times (± 0.16 SE; n = 3; P < 0.05) more VAMP coimmunoprecipitated with syntaxin Q226R (lane 2) than wild type (lane 1). Lane 5, lysates from cells separately transfected with VAMP and myc-syntaxin wild type were mixed before membrane extraction to show that no SNARE complexes formed during the immunoprecipitation procedure. Syn, syntaxin; LC, antibody light chain. The gel shown is representative of three independent experiments.
Immunoprecipitation of SNARE Complexes in Transfected PC12 Cells.
Two confluent 15-cm dishes of PC12 cells were electroporated (230V, 960 μF) with 35 μg of VAMP DNA and/or with 40 μg myc-Syntaxin wild type or Q226R in pcDNA3 and replated in two 15-cm dishes for 64 h along with one coverslip [for immunofluorescence to check transfection efficiency (≈20%) and distribution of the syntaxin and VAMP proteins (which were normal)]. Cells were then homogenized 25× in 2 ml HB buffer (50 mM Tris⋅Cl, pH 7.4/150 mM NaCl/10 mM sucrose/1 mM EDTA/1 mM EGTA/1 mM DTT/1 mM PMSF/2 μg/ml leupeptin/4 μg/ml aprotinin/0.7 μg/ml pepstatin A) in a Teflon-glass homogenizer and spun at 1,000 × g for 15 min to produce a postnuclear supernatant. The supernatant then was centrifuged at 100,000 × g for 1 h, and the resultant membrane pellet was solubilized in 0.5 ml of IP buffer (HB + 1% Triton X-100) for 2 h at 4°C. After pelleting the insoluble material (30 min at 100,000 × g), 2 μg of anti-myc antibody 9E10 or anti-syntaxin HPC1 was added for 1 h followed by 30 μl of 50% (vol/vol) protein-A Sepharose and 4 μg of goat anti-mouse Fc region linker antibody for 2 h at 4°C. The supernatants were methanol-precipitated and resuspended in sample buffer to verify equal SNARE expression levels (not shown), while the beads were washed 4× in IP buffer and 2× in HB buffer lacking sucrose, DTT, and protease inhibitors. The bound proteins were boiled and analyzed by Western blotting, detected with 125I-labeled secondary antibodies, and quantitated by phosphorimaging (IQMAC V1.2).
Results
Syntaxin Q226 Is Required for SNARE-Complex Dissociation by NSF.
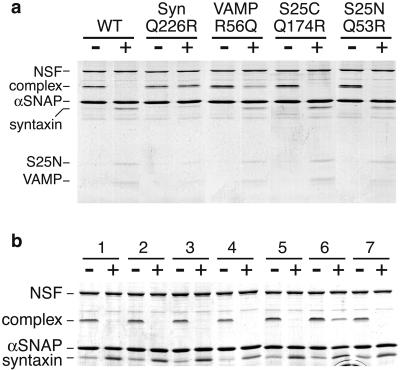
We tested the ability of mutated ionic-layer complexes of neuronal SNAREs [syntaxin 1a Q226, SNAP25-N Q53, SNAP25-C Q174, and VAMP2 R56 (Fig. 1b), all mutated to the opposite R or Q residue or to A] to undergo dissociation by NSF and α-SNAP in the presence of MgATP. To avoid complications caused by SNARE-complex reassembly, we used full-length syntaxin, because its N-terminal domain interacts with the core (H3) domain, significantly slowing SNARE-complex formation (12–14). Furthermore, as SDS-resistant SNARE complexes cannot form when any coil is cleaved by neurotoxins (15), we also included botulinum neurotoxin E to cleave the dissociated (but not complexed) SNAP-25 C-terminal helix (15). Under conditions where the wild-type complex was readily dissociated, any complex containing a syntaxin Q226R or Q226A coil could not be fully dissociated (Fig. 2a and Table 1), even if the 3Q:1R ratio was maintained by also swapping VAMP's R56 to Q (complex #6, Table 1). VAMP2 R56Q and R56A-containing complexes also were not completely (>95%) dissociated within 30 min (Fig. 2a and Table 1). Mutation of the glutamines of either SNAP-25 helix had no effect on NSF dissociation in our assay.
Figure 2.
Mutation of the ionic layer of syntaxin and VAMP, but not SNAP-25, affects complex dissociation by NSF. (a) ≈2 μM purified SNARE complexes (containing full-length syntaxin) with one coil mutated by swapping glutamine for arginine or vice-versa, as indicated, were incubated for 30 min on ice with 4.5-fold molar excess of α-SNAP, followed by the addition of 1.2 molar ratios of NSF in the presence (+) or absence (−) of MgATP for 30 min at 37°C before SDS/PAGE analysis without boiling (see Methods). The complex containing syntaxin Q226R was not dissociated and the VAMP R56Q-containing complex was only partially dissociated, whereas the SNAP25-C (S25C) Q174R and SNAP25-N (S25N) Q53R complexes were dissociated as efficiently as wild-type complexes (WT). (b) The NSF dissociation defect is specific for the ionic layer of syntaxin. ≈2 μM SNARE complexes were dissociated in vitro as in a, but they contained full-length syntaxin mutated in different layers (16): 1, wild-type; 2, R198A/I202A (−7 layer); 3, L205A/I209A (−6/−5 layers); 4, L212A/F216A (−4/−3 layers); 5, I230A/I233A (+1/+2 layers); 6, V223A/Q226A (−1/0 layers); 7, A240V/V244A (+4/+5 layers).
Table 1.
Summary of SNARE mutants tested for NSF-mediated dissociation
| Complex | VAMP (R56) | SYNTAXIN (Q226) | SNAP25-N (Q53) | SNAP25-C* (Q174) | Q:R ratio | NSF† DIS. | Tm‡ (CD,SDS) |
|---|---|---|---|---|---|---|---|
| Swaps | |||||||
| 1 | V2 | S1 | S25N | S25C | 3:1 | + | 94, 78 |
| 2 | R56Q | S1 | S25N | S25C | 4:0 | +/− | 88, 69 |
| 3 | V2 | Q226R | S25N | S25C | 2:2 | − | 70, 66§ |
| 4 | V2 | S1 | Q53R | S25C | 2:2 | + | 97, 82 |
| 5 | V2 | S1 | S25N | Q174R | 2:2 | + | 93, 72 |
| 6 | R56Q | Q226R | S25N | S25C | 3:1 | − | 91 |
| 7 | R56Q | S1 | S25N | Q174R | 3:1 | +/− | 90 |
| 8 | R56Q | S1 | Q53R | S25C | 3:1 | +/− | 84 |
| 9 | V2 | Q226R | S25N | Q174R | 1:3 | − | |
| 10 | V2 | Q226R | Q53R | S25C | 1:3 | − | |
| 11 | V2 | S1 | Q53R | Q174R | 1:3 | + | 91, 74 |
| 12 | R56Q | Q226R | S25N | Q174R | 2:2 | − | |
| 13 | R56Q | Q226R | Q53R | S25C | 2:2 | − | |
| 14 | R56Q | S1 | Q53R | Q174R | 2:2 | +/− | |
| 15 | V2 | Q226R | Q53R | Q174R | 0:4 | − | |
| 16 | R56Q | Q226R | Q53R | Q174R | 1:3 | − | 89 |
| 17 | V2 | Q226E | S25N | S25C | 2:1 | + | |
| 18 | V2 | Q226N | S25N | S25C | 2:1 | + | |
| Nonpolar | |||||||
| 19 | R56A | S1 | S25N | S25C | 3:0 | +/− | nd, 66 |
| 20 | V2 | Q226A | S25N | S25C | 2:1 | − | 60, 76§ |
| 21 | V2 | S1 | Q53A | S25C | 3:0 | + | 94, 70 |
| 22 | V2 | S1 | S25N | Q174A | 3:0 | + | nd, 70 |
| 23 | V2 | S1 | Q53A | Q174A | 1:1 | + | |
| 24 | R56A | S1 | S25N | Q174A | 2:0 | +/− | |
| 25 | R56A | S1 | Q53A | Q174A | 1:0 | +/− | |
| 26 | R56A | Q226A | Q53A | Q174A | 0:0 | − | |
| 27 | R56L | S1 | S25N | S25C | 3:0 | +/− | |
| 28 | V2 | Q226I | S25N | S25C | 2:1 | +/− | |
| 29 | V2 | S1 | Q53I | S25C | 2:1 | + | |
| 30 | V2 | S1 | S25N | Q174I | 2:1 | + | 90, 64 |
| 31 | R56L | Q226I | Q53I | Q174I | 0:0 | − | |
| Syntaxins | |||||||
| 32 | V2 | R198A/I202A | S25N | S25C | 3:1 | + | |
| 33 | V2 | L205A/I209A | S25N | S25C | 3:1 | + | |
| 34 | V2 | L212A/F216A | S25N | S25C | 3:1 | + | |
| 35 | V2 | I230A/I233A | S25N | S25C | 3:1 | + | |
| 36 | V2 | V223A/Q226A | S25N | S25C | 2:1 | +/− | |
| 37 | V2 | A240V/V244A | S25N | S25C | 3:1 | + | |
| 38 | V2 | S3 | S25N | S25C | 3:1 | + | |
| 39 | V2 | S4 | S25N | S25C | 3:1 | + | 86 |
| 40 | V2 | S4 Q234R | S25N | S25C | 2:2 | − | |
| 41 | V2 | S7 | S25N | S25C | 3:1 | + | |
| 42 | V2 | S13 | S25N | S25C | 3:1 | + | 74 |
| S25Ns | |||||||
| 43 | V2 | S1 | V36A/S39A | S25C | 3:1 | + | 97, 75 |
| 44 | V2 | S1 | L50A | S25C | 3:1 | + | 87, 65 |
| 45 | V2 | S1 | L57A/I60A | S25C | 3:1 | + | 79 |
| 46 | V2 | S1 | M64A/I67A | S25C | 3:1 | + | 84 |
| S25Cs | |||||||
| 47 | V2 | S1 | S25N | E143A/E145A | 3:1 | + | nd, 78 |
| 48 | V2 | S1 | S25N | D147A/E148A | 3:1 | + | nd, 79 |
| 49 | V2 | S1 | S25N | L150A/L153A | 3:1 | + | 91, 76 |
| 50 | V2 | S1 | S25N | I157A/L160A | 3:1 | + | 88, 73 |
| 51 | V2 | S1 | S25N | R161A | 3:1 | + | 94, 76 |
| 52 | V2 | S1 | S25N | M167A/I171A | 3:1 | + | 88, 65 |
| 53 | V2 | S1 | S25N | N169A | 3:1 | + | |
| 54 | V2 | S1 | S25N | E170A | 3:1 | + | |
| 55 | V2 | S1 | S25N | D172A | 3:1 | + | |
| 56 | V2 | S1 | S25N | E170A/D172A | 3:1 | + | nd, 74 |
| 57 | V2 | S1 | S25N | R176A | 3:1 | + | |
| 58 | V2 | S1 | S25N | I178A/I181A | 3:1 | + | 85, 62 |
| 59 | V2 | S1 | S25N | D179A | 3:1 | + | |
| 60 | V2 | S1 | S25N | R180A | 3:1 | + | 91 |
| 61 | V2 | S1 | S25N | E183A | 3:1 | + | 95, 77 |
| 62 | V2 | S1 | S25N | D186A | 3:1 | + | 95, 75 |
| 63 | V2 | S1 | S25N | N188A | 3:1 | + | 97 |
| 64 | V2 | S1 | S25N | N188A/I192A | 3:1 | + | 91, 69 |
| 65 | V2 | S1 | S25N | D193A | 3:1 | + | nd, 75 |
| 66 | V2 | S1 | S25N | R198A | 3:1 | + | nd, 78 |
| 67 | V2 | S1 | S25N | D186A/D193A | 3:1 | + | 93 |
| 68 | V2 | S1 | S25N | D186A/D193A/D179A | 3:1 | + | 90 |
The indicated SNARE complexes were formed in vitro, with any mutated SNARE coils shown in bold type, and subjected to dissociation by α-SNAP and NSF for 30 min at 37°C. Where indicated, complexes were also analyzed for thermostability (Tm).
Underlined S25C mutations were not previously published in Chen et al., 1999 (9); see instead Chen et al., 2001 (33).
NSF dissociation: +, ≥95%; −, ≤ 5%; +/−, 6–94% dissociated.
CD and SDS/PAGE analysis of complex thermostabilities was as in Chen et al., 1999 (9), using the H3 domain of syntaxin. Where only one figure is shown, it represents the CD data, unless preceded by nd (not determined).
These syntaxin H3 complexes were not SDS-resistant, so full-length syntaxin was employed for the SDS analysis instead, as this made no difference to the Tm of the wild-type syntaxin.
To examine the effect of mutated syntaxin- and VAMP-containing complexes in more detail, we measured a time course of dissociation (Fig. 3a). The VAMP mutations had only a minor effect on the dissociation rate, increasing the half-time from 3 min (wild-type) to 7 (for R56A) or 8 (for R56Q) min, with 1–3 h required for full dissociation (Fig. 3b). The syntaxin Q226A mutation had a stronger effect, increasing the half-time to 20 min and requiring >3 h for full dissociation, whereas the Q226R mutation did not permit more than 36% (±9% SD) dissociation even after 6–12 h (Fig. 3b and data not shown). Control experiments showed that NSF remained fully active for more than 6 h at 37°C (data not shown).
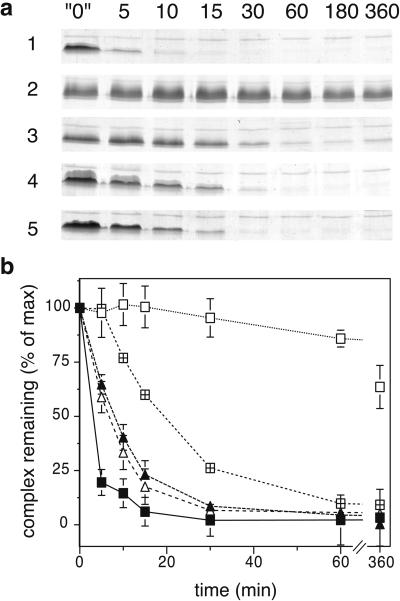
Figure 3.
Time course dissociation of ionic-layer mutant complexes. (a) Time course of NSF-mediated dissociation. Recombinant SNARE complexes (≈6 μM) bearing the indicated mutations in the ionic layer of one of the coils were subject to in vitro dissociation as in Fig. 2a, except that the 37°C incubation with NSF and α-SNAP was carried out for different times, as shown (in min). The “0” min time point represents 360 min at 37°C in the presence of NSF with EDTA-ATP instead of Mg-ATP, showing that the complex did not spontaneously dissociate. The gels, showing only the SNARE-complex bands, are representative of at least five independent experiments. 1, WT; 2, Syn Q226R; 3, Syn Q226A; 4, VAMP R56Q; 5, VAMP R56A. (b) SNARE-complex bands at each time point from three experiments such as that shown in a were quantitated by densitometry and the results were plotted as a percentage of the amount of complex at the 0 min time point. Error bars represent the standard deviation of the mean. The half time of dissociation of each mutant complex is in parentheses (in min): ■, WT (3 min); □, Syn Q226R (>720 min); ⊞, Syn Q226A (20 min); ▴, VAMP R56Q (8 min); ▵, VAMP R56A (7 min).
To verify whether the defect in NSF dissociation was specific to the ionic layer of syntaxin, we examined complexes containing syntaxin mutated at other (hydrophobic) heptad repeat positions (16). With the exception of the −1/0 layer mutant that includes the ionic layer (V223A/Q226A), all of these syntaxin mutants were fully dissociated by NSF (Fig. 2b) as were complexes substituted with syntaxins 3, 4, 7, and 13 (Table 1, complex #38, #39, #41, #42). Furthermore, a whole panel of mutations at hydrophobic or surface positions along the SNAP-25 C- and N-terminal helices (9, 10) had no effect on complex dissociation (Table 1, #43–#68). Thus, whereas 42 combinations of mutations throughout three helices of the SNARE complex are tolerated, it seems that specifically the ionic-layer glutamine of syntaxin (and to a much lesser extent VAMP) is important for NSF-mediated dissociation.
To understand further the requirement for glutamine at the ionic position of syntaxin, we substituted it with the structurally similar glutamate and asparagine residues and found that these did permit complex dissociation within 30 min (Table 1, #17 and #18). These two residues, like glutamine, contain an electrophilic carbonyl group, which may be important for the dissociation mechanism. Syntaxin 4, complexed with SNAP-25 and VAMP2, also did not dissociate when substituted at its ionic layer (Q234R). (The cognate syntaxin 4 complex (where SNAP-23 replaces SNAP-25; ref. 17), could not be examined because it is not SDS-resistant (11)). Thus the requirement for glutamine at the ionic layer of syntaxin is likely to be general rather than unique to syntaxin 1, as suggested by the conservation of this residue.
We examined the possibility that the defect in syntaxin Q226R complex dissociation was caused by faster complex reassembly than the wild type. Mammalian n-sec1 binds the closed conformation of syntaxin (18–20) in which the N terminus binds its own H3 domain and hinders SNARE-complex assembly (12, 20). The syntaxin Q226R mutant bound n-sec1 as well as, if not better than, wild-type syntaxin, suggesting that syntaxin is not locked in an open conformation that could account for faster complex reassembly (Fig. 4a). Furthermore, the time course of complex formation in vitro revealed that the complexes containing syntaxin Q226R, Q226A, or VAMP R56Q did not form faster than the wild-type complex (Fig. 4b). Thus, the dissociation defects of these mutants are not a secondary effect of accelerated complex reassociation.
Syntaxin Q226R Forms the 20S Complex but Is Not Dissociated by NSF.
Syntaxin mutated to alanine at both Q226 and V223 (at the 0 and −1 layers of the SNARE complex, respectively) has been shown to be defective in α-SNAP binding (16), which might account for the lack of complex dissociation seen here, because NSF requires α-SNAP to bind to SNARE complexes (4). However, free syntaxin Q226R and Q226A retained the ability to bind to α-SNAP as well as did wild-type syntaxin in GST pull-down assays (data not shown), suggesting that the previously shown defect (16) was caused by the −1 layer V223A mutation. More importantly, the syntaxin Q226R mutant SNARE complex was able to form a 20S (i.e., SNARE complex:α-SNAP:NSF) complex as efficiently as wild type in vitro, as assayed by SNARE complex-dependent NSF binding to α-SNAP on glutathione beads in the presence of ATP/EDTA (compare lanes 1 and 3 in Fig. 5a). NSF binding was specific because there was only minimal binding to GST-α-SNAP in the absence of SNARE complex (lane 5) and no binding to GST alone (lane 7). Furthermore, in the presence of MgATP, both wild-type and Q226R SNARE complexes were removed from the GST-α-SNAP beads, and NSF binding was reduced to background levels (compare lanes 2 and 4 with 6). However, analysis of the unbound fraction indicated that unlike the wild-type complex, the Q226R complex was not dissociated into monomers. This result is shown in Fig. 5a (Lower) by the increase in intensity of the SNAP25-N band from lanes 1 to 2 compared with the lack of increase between lanes 3 and 4. Thus, the Q226R dissociation defect is not due to the inability to bind α-SNAP and NSF, nor to the ATP-dependent release of the SNARE complex from α-SNAP by NSF, but rather is due to the subsequent disassembly process of the SNARE complex itself.
To investigate whether the in vitro syntaxin Q226R dissociation defect was also manifest in vivo, we expressed myc-tagged syntaxin wild type and Q226R in PC12 cells and quantified the amount of VAMP that was coimmunoprecipitated from a membrane extract by using an anti-myc tag antibody (Fig. 5b). The ratio of VAMP to syntaxin was significantly greater (1.43 times ± 0.16 SE; n = 3; P < 0.05 by Student's t test) with the Q226R mutant syntaxin than with wild type (compare lanes 1 and 2 in Fig. 5b) under conditions where the total VAMP levels were equal (data not shown). This result is consistent with a dissociation defect in cells resulting in greater steady-state SNARE-complex levels. The immunoprecipitation was specific, because no VAMP immunoprecipitated with the anti-myc antibody in the absence of myc-syntaxins (lane 3). Furthermore, the SNARE complexes formed in the cells rather than in the immunoprecipitation buffers, because separately transfected VAMP and myc-syntaxin wild-type (lane 5) or Q226R (data not shown) cells only resulted in endogenous levels of VAMP coprecipitating with myc-syntaxin when mixed in the lysis buffer before the membrane extraction step (compare with the endogenous syntaxin immunoprecipitate in lane 4). Thus, the data are consistent with a syntaxin Q226R dissociation defect in vivo.
Discussion
We propose that α-SNAP/NSF specifically act upon the ionic layer, in particular, the glutamine of syntaxin. SNARE-complex disassembly likely proceeds by means of at least two steps: (i) binding of α-SNAP/NSF to the SNARE complex, followed by (ii) an NSF-dependent conformational change that leads to the release of α-SNAP and NSF concomitant with dissociation of the SNARE complex. α-SNAP/NSF binding and release are not affected by the syntaxin Q226R mutation, whereas the dissociation of the SNARE complex is affected. This glutamine residue likely transmits the conformational change of NSF/α-SNAP into disruption of the SNARE complex at the ionic layer; when mutated, α-SNAP/NSF fall off without accomplishing the SNARE-complex dissociation event. The carbonyl group of glutamine is likely important in this process, perhaps in a rearrangement of the hydrogen bonds, because syntaxin Q226E and Q226N mutated complexes were able to dissociate. Because the hydrogen bonds are, for the most part, buried within the interior of the SNARE bundle, α-SNAP/NSF are likely to perturb the local environment by means of a conformational change to achieve this. Once the ionic layer is disrupted, water can enter at this position to promote further complex disassembly, as suggested (6). The R56 residue of VAMP also may facilitate the disassembly step, because slightly slower dissociation was observed when this residue was mutated.
That syntaxin is the most important feature of the SNARE complex with respect to NSF dissociation is supported by previous evidence showing that syntaxin alone and a syntaxin-SNAP25 binary complex are also substrates for α-SNAP/NSF (3, 21, 22). In addition, the yeast syntaxin Q226R equivalents (Sso1 Q224R or Sso2 Q228R) secrete and grow slowly and only at low temperatures. This finding is consistent with a possible defect in dissociation, although it was not directly examined (23, 24). NSF-mediated dissociation clearly is not related to complex thermostability, because the syntaxin Q226 mutants (#3 and #20) are less thermostable than wild type yet are more resistant to dissociation. Furthermore, restoration of the 3Q:1R ratio of the ionic layer in the syntaxin-VAMP R-Q swap (complex #6) recovers thermostability but not dissociation. This swap mutant also suggests that the ionic layer 3Q:1R ratio itself is not vital for dissociation, which is supported by the observation that several non-3Q:1R combinations did dissociate; the critical determinant is the presence of glutamine 226 on the syntaxin helix (Table 1). This finding was unexpected, because the yeast syntaxin Q-R growth defect seems to be rescued by a complementary R-Q mutation in VAMP (Snc2p R52Q; refs. 23 and 24), although the cells are more sensitive to mutations in other layers, revealing defects in the SNARE complex that are not manifest in the growth phenotype. NSF-mediated dissociation may be more efficient in vivo than in the purified in vitro system, as evidenced by the only 1.43-fold increase in SNARE-complex levels in our syntaxin-Q226R transfected cells compared with an over 200-fold decrease in our dissociation rate in vitro. This result might be due to the presence of regulatory proteins or cofactors that are absent from our system. In addition, the transmembrane domain of syntaxin (lacking in our system) has been shown to greatly enhance α-SNAP binding (21, 25), which is a limiting factor in our dissociation assay (data not shown). A recent report also proposed that the cysteine residues in the loop of SNAP25, which was absent from our in vitro complexes, enhance complex dissociation in vivo (26). Thus, it is possible that the yeast Q-R swap mutant maintains a level of SNARE-complex dissociation that is sufficient for normal secretory activity, despite our inability to detect it in our purified system.
Functional evidence in a mammalian system for the importance of the ionic layer includes that mutation Q174L in SNAP-25 C terminus reduces the sustained, but not the burst, phase of catecholamine release from adrenal chromaffin cells (8), perhaps because the sustained phase requires recycling of SNARE complexes by NSF-mediated dissociation, although a role in SNARE-complex alignment is also plausible. We previously found no effect of mutating Q174 or Q53 (the glutamine of the SNAP-25 N-terminal helix) on exocytosis (involving SNARE-complex formation and correct alignment) in PC12 cells (9, 10), and in this study, we were unable to detect any defect in dissociation when either was mutated to R, A, or I. Additionally, we found that the rate of formation of an SDS-resistant complex where the ionic layer is replaced by the +2 layer (complex #31 in Table 1) was indistinguishable from that of the wild-type complex (data not shown), and a complex with all of the ionic residues mutated to alanine (#26) also formed, suggesting that the ionic layer cannot be essential for helical bundle register. However, it is possible that subtle changes in SNARE-complex dissociation or assembly kinetics are more readily detectable on the time scale of seconds of chromaffin cell capacitance measurements. We suggest that the glutamine residues of SNAP-25 function in dissociation to provide a hydrophilic environment for syntaxin Q226 and VAMP R56 while maintaining optimal packing of the layer. Because the hydrophobic layers also have specific packing arrangements, it is likely that they also contribute to the correct alignment of the bundle, which is supported by the yeast Q-R swap mutant being less tolerant of mutations in these layers (24). Thus, whereas all of the layers likely contribute to the register of the SNARE complex, only the ionic layer is important for its dissociation.
We conclude that ATP hydrolysis by NSF is coupled to SNARE-complex dissociation through the ionic layer, specifically by means of the conserved glutamine residue in syntaxin. Although complex dissociation may not be the only function of the ionic layer, it likely accounts for the highly conserved and polar nature of these residues.
Acknowledgments
We are grateful to Dr. James Rothman for the α-SNAP and NSF constructs and the late Dr. Heiner Niemann for the botulinum neurotoxin E light chain construct. We thank Drs. Andy May, Bill Weis, and Lino Gonzalez, Jr., for helpful discussions and comments on the manuscript. S.J.S and B.Y.Y were supported by grants to R.H.S. from the Howard Hughes Medical Institute.
Abbreviations
- NSF
N-ethylmaleimide-sensitive fusion protein
- SNARE
soluble NSF attachment protein receptor
- VAMP
vesicle-associated membrane protein
- SNAP-25
synaptosomal-associated protein of 25 kDa
- α-SNAP
α-soluble NSF attachment protein
- GST
glutathione S-transferase
References
- 1.Lin R C, Scheller R H. Annu Rev Cell Dev Biol. 2000;16:19–49. doi: 10.1146/annurev.cellbio.16.1.19. [DOI] [PubMed] [Google Scholar]
- 2.Söllner T, Whiteheart S W, Brunner M, Erdjument-Bromage H, Geromanos S, Tempst P, Rothman J E. Nature (London) 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 3.McMahon H T, Südhof T C. J Biol Chem. 1995;270:2213–2217. doi: 10.1074/jbc.270.5.2213. [DOI] [PubMed] [Google Scholar]
- 4.Misura K M, May A P, Weis W I. Curr Opin Struct Biol. 2000;10:662–671. doi: 10.1016/s0959-440x(00)00151-2. [DOI] [PubMed] [Google Scholar]
- 5.Hanson P I, Heuser J E, Jahn R. Curr Opin Neurobiol. 1997;7:310–315. doi: 10.1016/s0959-4388(97)80057-8. [DOI] [PubMed] [Google Scholar]
- 6.Sutton R B, Fasshauer D, Jahn R, Brunger A T. Nature (London) 1998;395:347–353. doi: 10.1038/26412. [DOI] [PubMed] [Google Scholar]
- 7.Fasshauer D, Sutton R B, Brunger A T, Jahn R. Proc Natl Acad Sci USA. 1998;95:15781–15786. doi: 10.1073/pnas.95.26.15781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei S, Xu T, Ashery U, Kollewe A, Matti U, Antonin W, Rettig J, Neher E. EMBO J. 2000;19:1279–1289. doi: 10.1093/emboj/19.6.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y A, Scales S J, Patel S M, Doung Y C, Scheller R H. Cell. 1999;97:165–174. doi: 10.1016/s0092-8674(00)80727-8. [DOI] [PubMed] [Google Scholar]
- 10.Scales S J, Chen Y A, Yoo B Y, Patel S M, Doung Y C, Scheller R H. Neuron. 2000;26:457–464. doi: 10.1016/s0896-6273(00)81177-0. [DOI] [PubMed] [Google Scholar]
- 11.Yang B, Gonzalez L, Jr, Prekeris R, Steegmaier M, Advani R J, Scheller R H. J Biol Chem. 1999;274:5649–5653. doi: 10.1074/jbc.274.9.5649. [DOI] [PubMed] [Google Scholar]
- 12.Nicholson K L, Munson M, Miller R B, Filip T J, Fairman R, Hughson F M. Nat Struct Biol. 1998;5:793–802. doi: 10.1038/1834. [DOI] [PubMed] [Google Scholar]
- 13.Parlati F, Weber T, McNew J A, Westermann B, Söllner T H, Rothman J E. Proc Natl Acad Sci USA. 1999;96:12565–12570. doi: 10.1073/pnas.96.22.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Munson M, Chen X, Cocina A E, Schultz S M, Hughson F M. Nat Struct Biol. 2000;7:894–902. doi: 10.1038/79659. [DOI] [PubMed] [Google Scholar]
- 15.Hayashi T, McMahon H, Yamasaki S, Binz T, Hata Y, Südhof T C, Niemann H. EMBO J. 1994;13:5051–5061. doi: 10.1002/j.1460-2075.1994.tb06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kee Y, Lin R C, Hsu S C, Scheller R H. Neuron. 1995;14:991–998. doi: 10.1016/0896-6273(95)90337-2. [DOI] [PubMed] [Google Scholar]
- 17.Flaumenhaft R, Croce K, Chen E, Furie B, Furie B C. J Biol Chem. 1999;274:2492–2501. doi: 10.1074/jbc.274.4.2492. [DOI] [PubMed] [Google Scholar]
- 18.Misura K M, Scheller R H, Weis W I. Nature (London) 2000;404:355–362. doi: 10.1038/35006120. [DOI] [PubMed] [Google Scholar]
- 19.Yang B, Steegmaier M, Gonzalez L C, Jr, Scheller R H. J Cell Biol. 2000;148:247–252. doi: 10.1083/jcb.148.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dulubova I, Sugita S, Hill S, Hosaka M, Fernandez I, Sudhof T C, Rizo J. EMBO J. 1999;18:4372–4382. doi: 10.1093/emboj/18.16.4372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hanson P I, Otto H, Barton N, Jahn R. J Biol Chem. 1995;270:16955–16961. doi: 10.1074/jbc.270.28.16955. [DOI] [PubMed] [Google Scholar]
- 22.Matveeva E, Whiteheart S W. FEBS Lett. 1998;435:211–214. doi: 10.1016/s0014-5793(98)01071-0. [DOI] [PubMed] [Google Scholar]
- 23.Katz L, Brennwald P. Mol Biol Cell. 2000;11:3849–3858. doi: 10.1091/mbc.11.11.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ossig R, Schmitt H D, de Groot B, Riedel D, Keränen S, Ronne H, Grubmüller H, Jahn R. EMBO J. 2000;19:6000–6010. doi: 10.1093/emboj/19.22.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lewis J L, Dong M, Earles C A, Chapman E R. J Biol Chem. 2001;276:15458–15465. doi: 10.1074/jbc.M011687200. [DOI] [PubMed] [Google Scholar]
- 26.Washbourne P, Cansino V, Mathews J R, Graham M, Burgoyne R D, Wilson M C. Biochem J. 2001;357:625–634. doi: 10.1042/0264-6021:3570625. [DOI] [PMC free article] [PubMed] [Google Scholar]