Abstract
The sesquiterpene–coumarin ether samarcandone provided a suitable framework to replace the apocarotenoid A–C ring system of strigol (1), replicating, after linking to a butenolide moiety, the activity of the natural phytohormone on Nrf2 and also showing potent NF-kB inhibitory activity, overall modulating two critical pathways of inflammation and cancer.
Keywords: Strigolactones, terpenes, Nrf2, NF-κB
Small-molecule endogenous hormones modulate basic plant functions like growth, differentiation, and reproduction as well as their response to abiotic- and biotic stress. Ethylene was the first member of this structurally heterogeneous class to be identified, and the inventory has then significantly expanded to include as major members indoleacetic and abscisic acids, brassinosteroids, cytokinins, gibberellins, jasmonoids, salicylic acid, and strigolactones (SLs). In addition to their role in plant physiology, certain plant hormones can also bind mammalian targets, or even be produced by mammalian cells, as exemplified by abscisic acid.1 Furthermore, plant hormones can also serve as a scaffold for drug discovery, as shown by aspirin and, more recently, by the cyclin-dependent kinase inhibitors olomoucine and roscovitine, whose structure was inspired by cytokinins, a class of N6-substituted adenine derivatives.2 Despite these interesting clues, the potential of plant hormones to serve a lead structure for drug discovery has not yet been systematically evaluated. This gap has provided a rationale to explore the potential of strigolactones (SLs), the most recent addition to the plant hormone inventory, to modulate mammalian targets of medical relevance.
Strigolactones (SLs) are C15 apocarotenoid dilactones involved in shoot- and root architecture and in plant responses to environmental stress. Strigol (1), the first member of this family, was isolated in 1966, and the inventory of strigolactonoids now includes more than 15 additional analogues. SLs are characterized by a tricyclic scaffold linked to a butenolide D ring by an enolic oxymethine. There is convincing evidence that the reactive CD ring system is responsible for the plant hormone activity of SLs, which is mediated by covalent binding to a reactive cysteine residue in their macromolecular targets.3 Synthetic and natural SLs have been extensively investigated as germination stimulants of parasitic weed seeds as well as biopesticides for crop protection,4 but only limited knowledge exist on their involvement in animal cell function and their potential cross-kingdom activity, despite promising results in the realm of anticancer drug discovery.5 Within the possible mammalian targets of SLs, the transcription factor Nrf2 (nuclear factor (erythroid-derived 2)-like 2) seemed of particular relevance because of its sensitivity to nucleophilic trapping and its role in the regulation of many cytoprotective enzymes involved in the adaptive oxidative stress response.6 Nrf2 is the target of dimethyl fumarate, a compound used in the management of multiple sclerosis,7 and of bardoxolone methyl, currently under phase III clinical study for the management of pulmonary hypertension.8
In the event, strigolactone (1) turned out to
be a
potent activator of the Nrf2 pathway (EC50 = 1.2 ±
0.9 μM), providing a rationale for investigating the structure–activity
relationship of this chemotype. Strigol and SLs in general have a
very limited availability, and we therefore attempted to identify
a surrogate of the apocarotenoid A–C ring system of the natural
hormone within more easily available isoprenoids. To this purpose,
analogues where ring D is implanted in various isoprenoids scaffolds
were designed. Two series of analogues were prepared, differing for
the way ring D and the isoprenoid core are linked (oxymethine- or
oxygen tether), and all compounds were then comparatively evaluated
with strigol (1) for their capacity to modulate the activity
of Nrf2. All compounds were also investigated for their capacity to
inhibit NF-κB, another transcription factor sensitive to electrophilic
modulation. Although strigol was inactive in this assay, dual modulators
of Nrf2 and Nf-κB hold great pharmacological potential,7,8 and we hope to discover compounds with this bioactivity profile.
Compounds with an oxymethine tethered were built by Claisen formylation of an isoprenoid ketone and then coupling with the bromofuranone 2 (Table 1). The reaction was stereoselective regarding the configuration of the oxymethine linker, with the predictable exclusive formation of the E-isomer, evident from the downfield shift of the oxymethine (δ ca. 7.40), diagnostic of a syn-relationship with the carbonyl. Thus, the nucleophilic displacement reaction occurred with formal inversion of configuration of the enol double bond, which was in the intramolecularly hydrogen-bonded Z-configuration in the starting enol (compounds B, Table 1). In all cases, an almost equimolecular mixture of isomers at the furanone C-5 carbon was, however, obtained. The diastereomeric mixture was difficult to separate, and all compounds were assayed as such.
Table 1. Synthesis of Homoterpeno-strigoids (A) and Terpenostrigoids (B).
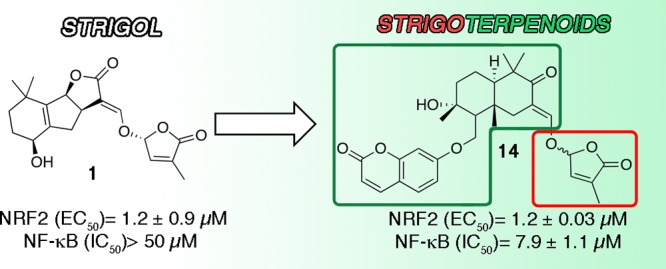
Strigol (1) EC50: NRF2 (EC50) 1.2 ± 0.9;
NF-κB (IC50) > 50.
The strigoids obtained from pentacyclic triterpene ketones (3–9) were totally devoid of activity, suggesting that the isoprenoid scaffold was too large to access the site hosting the reactive thiol group of Nrf2, and similar results were observed for the inhibition of NF-κB. However, bicyclic and monocyclic isoprenoid ketones afforded active strigoids, some of which showed potency similar to strigol, with EC50 in the one-digit micromolar range (Table 1). Within p-menthane derivatives, the cross-conjugated dienones 11 and 12, obtained from, respectively, carvone and pulegone, were significantly more potent than the enone 10, derived from menthone (EC50 = 1.6 ± 0.3 μM and 5 ± 0.2 μM vs 17.8 ± 1,7 μM, respectively), suggesting that electronic factors are important for activity. Also 13, derived from α-thujone, was significantly active (EC50 = 1.9 ± 0.6 μM), and one-digit micromolar activity was also retained in the bicyclic strigoids 14 (EC50 = 1.2 ± 0.03 μM), and 15 (EC50 = 2.5 ± 0.2 μM), derived, respectively, from the sesquiterpene coumarin ether samarcandone and the triterpenoid mirranone B.
The second series of terpenostrigoids was obtained by condensing the butenolide lactol 22 with a series of isoprenoid alcohols according to the Feringa protocol (heating at 120 °C in the absence of solvent)9 (Table 1). Also in this case, the reaction gave a mixture of diastereomeric furanones (16–21) that were assayed as such. Overall, the oxygen-tethered terpenostrigoids were one order of magnitude less potent than the oxymethine-linked homoterpeno-strigoids.
Strigol (1) could activate Nrf2, but had no effect on TNFα-induced NF-κB activation, another upstream regulatory process sensitive to thiol trapping that critically relies on the presence of cysteine as an on/off switch. This profile was replicated by all active compounds we identified with the exception of the bicyclic drimane coumarin ether 14, that could, surprisingly, also inhibit NF-κB activity at low μM concentrations (IC50 = 7.9 μM). This dual profile of activity is interesting, and was further investigated. We first clarified the Nrf2 activation mechanism, which can be electrophilic (direct thiol trapping) or oxidative (oxidation of the cysteine sulfur atom) and mediated by reactive oxygen species (ROS). To this purpose, we investigated the relationship between the induction of Nrf2 activity and the increase of cellular ROS. Figure 1A shows that, in contrast to tert-butyl hydroperoxide (TBHP), 14 was unable to affect the intracellular levels of ROS. Interestingly, pretreatment with N-acetyl cysteine (NAC) inhibited the activity of 14 on Nrf2 activation (Figure 1B). NAC is a scavenger of oxygen free radicals and a precursor of l-cysteine. Since 14 was not able to induce ROS, NAC might react with its coumarin moiety, which has Michael-acceptor properties,10 to generate an inactive adduct.
Figure 1.

Compound 14 activates Nrf2 without inducing ROS. (A) ROS production in HaCaT cells. Images were obtained after 3 h of treatment. (B) Nrf2 transcription activity was analyzed in HaCaT-ARE-Luc. Cells were treated with 14 in absence or presence of NAC (15 mM) at the doses indicated during 6 h.
Next, we investigated the effect of 14 on the canonical pathway of NF-κB activation by analyzing the steady-state levels of phosphorylated IκBα and p65 (a subunit of the more common form of NF-κB heterodimers). Both IκBα and p65 proteins are phosphorylated by the IκB kinase β (IKKβ), which is activated by TNFα through the so-called canonical pathway. The drimane strigoid 7 clearly inhibited the phosphorylation of both IκBα and p65 induced by TNFα in NIH-3T3-KBF-Luc cells. Phosphorylation of IκBα is required for its degradation, and we found that 14 could also prevent TNFα-induced IκBα degradation (Figure 2A).
Figure 2.
Effects of 14 on NF-κB activation. (A) Levels of NF-κB proteins expression and phosphorylation by immunoblot. (B) IKKβ-induced NF-κB activation is inhibited by 14.
Furthermore, 14 could also inhibit specifically the NF-κB activation induced by overexpression of IKKβ (Figure 2B). Taken together, these observations suggest that 14 could directly interact with the Cys-179 of this kinase and inhibit its activity.
In conclusion, we have identified a sesquiterpene-coumarin strigoid (14) that not only replicates the activity of natural strigol (1) on the activation of Nrf2, but also targets the NF-kB pro-inflammatory pathway. Within terpeno-strigoids, the activation of Nrf2 was sensitive to the size of the isoprenoid moiety, tolerating mono- and bicyclic systems but not more complex polycyclic constructs, while the inhibition of NF-κB was specific of 14. The cross-talk between inflammation and the oxidative response plays an important role in cancer, and compounds capable to modulate both pathways are interesting leads to prevent and treat malignancies, qualifying 14 for further studies.11
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00604.
Materials and methods; original spectroscopic data for the novel compounds (PDF)
Author Contributions
‡ These authors contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
The authors declare no competing financial interest.
Supplementary Material
References
- Zocchi E.; Hontecillas R.; Leber A.; Einerhand A.; Carbo A.; Bruzzone S.; Tubau-Juni N.; Philipson N.; Zoccoli-Rodriguez V.; Sturia L.; Bassaganya-Riera J. Abscisic Acid: A Novel Nutraceutical for Glycemic Control. Front. Nutr. 2017, 4, 24. 10.3389/fnut.2017.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer L.; Raymond E. Roscovitine and Other Purines as Kinase Inhibitors. From Starfish Oocytes to Clinical Trials. Acc. Chem. Res. 2003, 36, 417–425. 10.1021/ar0201198. [DOI] [PubMed] [Google Scholar]
- de Saint Germain A.; Clavé G.; Badet-Denisot M. A.; Pillot J. P.; Cornu D.; Le Caer J. P.; Burger M.; Pelissier F.; Retailleau P.; Turnbull C.; Bonhomme S.; Chory J.; Rameau C.; Boyer F. D. An histidine covalent receptor and butenolide complex mediates strigolactone perception. Nat. Chem. Biol. 2016, 12, 787–794. 10.1038/nchembio.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Screpanti C.; Yoneyama K.; Bouwmeester H. J. Strigolactones and parasitic weed management 50 years after the discovery of the first natural strigolactone strigol: status and outlook. Pest Manage. Sci. 2016, 72, 2013–2015. 10.1002/ps.4436. [DOI] [PubMed] [Google Scholar]
- Pollock C. B.; Koltai H.; Kapulnik Y.; Prandi C.; Yarden R. I. Strigolactones: a novel class of phytohormones that inhibit the growth and survival of breast cancer cells and breast cancer stem-like enriched mammosphere cells. Breast Cancer Res. Treat. 2012, 134, 1041–55. 10.1007/s10549-012-1992-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuadrado A.; Manda G.; Hassan A.; Alcaraz M. J.; Barbas C.; Daiber A.; Ghezzi P.; León R.; López M. G.; Oliva B.; Pajares M.; Rojo A. I.; Robledinos-Antón N.; Valverde A. M.; Guney E.; Schmidt H. H. H. W. Transcription Factor NRF2 as a Therapeutic Target for Chronic Diseases: A Systems Medicine Approach. Pharmacol. Rev. 2018, 70, 348–383. 10.1124/pr.117.014753. [DOI] [PubMed] [Google Scholar]
- Montes Diaz G.; Hupperts R.; Fraussen J.; Somers V. Dimethyl fumarate treatment in multiple sclerosis: Recent advances in clinical and immunological studies. Autoimmun. Rev. 2018, 17, 1240–1250. 10.1016/j.autrev.2018.07.001. [DOI] [PubMed] [Google Scholar]
- Cheng Y.; Gong Y.; Qian S.; Mou Y.; Li H.; Chen X.; Kong H.; Xie W.; Wang H.; Zhang Y.; Huang Z. Identification of a Novel Hybridization from Isosorbide 5-Mononitrate and Bardoxolone Methyl with Dual Activities of Pulmonary Vasodilation and Vascular Remodeling Inhibition on Pulmonary Arterial Hypertension Rats. J. Med. Chem. 2018, 61, 1474–1482. 10.1021/acs.jmedchem.7b01153. [DOI] [PubMed] [Google Scholar]
- Feringa B. L.; De Lange B.; De Jong J. C. Synthesis of enantiomerically pure.gamma.-(menthyloxy)butenolides and (R)- and (S)-2-methyl-1,4- butanediol. J. Org. Chem. 1989, 54, 2471–2475. 10.1021/jo00271a050. [DOI] [Google Scholar]
- Avonto C.; Taglialatela-Scafati O.; Pollastro F.; Minassi A.; Di Marzo V.; De Petrocellis L.; Appendino G. An NMR spectroscopic method to identify and classify thiol-trapping agents: revival of Michael acceptors for drug discovery?. Angew. Chem., Int. Ed. 2011, 50, 467–471. 10.1002/anie.201005959. [DOI] [PubMed] [Google Scholar]
- Khurana N.; Sikka S. C. Targeting Crosstalk between Nrf-2, NF-κB and Androgen Receptor Signaling in Prostate Cancer. Cancers 2018, 10, 352. 10.3390/cancers10100352. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.






