Abstract
Background of the Article:
Hepatocellular carcinoma (HCC) is one of the most common human malignancies worldwide. Radiofrequency ablation (RFA) is considered curative option in selected patients; efficacy is severely limited by lesion size and lesions bordering a large vessel. On the other hand, transarterial chemoembolization (TACE) is not limited by lesion size and arterial occlusion of the tumor feeding vessels leads to increase the volume of the ablative zone. Combination treatments using both intraarterial liver-directed therapy and percutaneous ablation seek to overcome the disadvantages of the individual treatments alone, theoretically improving response to therapy and survival.
Material and Methods:
This is a single-center retrospectively study in which patients who received TACE plus RFA for HCC were evaluated for technical success, local tumor progression rates, distant intra and extrahepatic recurrences and survival.
Results:
The study included 22 patients, 21 patients had a solitary HCC of size 3–7 cm and one patient had three target lesions. Technical success achieved after first session of combination treatment was 100% (24/24). At 1 and 3 months follow-up 100% patients (24 target lesions) had complete response and at 6 months; 21 (87.5%) had complete response, one (4.2%) had local tumor progression and two patients (8.3%) developed progressive disease. No major difference in complication was noted. The event-free survival as shown by Kaplan–Meier graph analysis at 6 and 12 months were 90.7% and 66.4% with mean time to event-free survival was 11.1 months.
Conclusion:
The combined use of TACE and RFA is a safe and effective option in the treatment of patients with single large or multinodular HCC when surgical resection is not feasible and this approach provides better results than RFA or TACE alone.
Keywords: Hepatocellular carcinoma, radiofrequency ablation, TACE (Trans-arterial chemoembolization), TACE plus RFA
Introduction
Hepatocellular carcinoma (HCC) is one of the most common human malignancies worldwide and has an estimated diagnosis of 750,000 new cases every year. The average survival of these patients remains less than a year even after significant advancement in the management and hence the prognosis of HCC patients remains discouraging.[1]
According to the Barcelona Clinic for Liver Cancer (BCLC) staging system, surgical approaches, including surgical liver resection and liver transplantation, as well as image-guided tumor ablation, such as radiofrequency ablation (RFA), are regarded as potentially curative treatments for HCC with early stage tumor.[2] The survival rates for patients who achieved a complete response by RFA are comparable to that of patients treated by hepatic resection.[3]
Transarterial chemoembolization (TACE) is the standard of palliative care for the intermediate stages according to BCLC staging and treatment.[2] TACE is characterized in most instances by an unsatisfactory long-term outcome due to the inability to achieve complete tumor necrosis and repeated TACE is often required to completely eradicate the residual tumors. Efficiency of TACE is limited and the rate of tumor recurrence or relapse after initial remission or stable disease is very high.[4]
Ablations are considered curative options in selected patients; efficacy is severely limited by lesion size and lesions adjoining a large vessel. On the other hand, TACE is not limited by lesion size and arterial occlusion of the tumor feeding vessels leads to increase the volume of the ablative zone. In RFA alone, high rate of local recurrence may be due to residual cancer cells or microscopic satellite tumor nodules, so TACE can eradicate this peripheral viable tissue and micrometastasis. The combination of TACE and RFA has been proved to be comparable with surgical resection in overall survival prolongation for patients within the Milan criteria and also led to good results for patients with HCC exceeding the Milan criteria in many cancer centers.[5]
Combination treatments using both intraarterial liver-directed therapy and percutaneous ablation seek to overcome the disadvantages of the individual treatments alone, theoretically improving response to therapy and survival.
Materials and Methods
Patients and groups
This is a single-center retrospective study carried out at a tertiary care teaching hospital after institutional review board and ethical committee approval was obtained for all patients who underwent combination therapy for HCC. A total of 22 patients (24 target lesions) who underwent combination therapy for HCC were included in the study.
The diagnosis of HCC was made according to the American Association for the Study of Liver Disease guideline based on imaging [triplephase computed tomography (CT) or dynamic magnetic resonance imaging (MRI)] or histopathologic criteria by fine-needle aspiration (FNA)/biopsy in patients with elevated alphafetoprotein (AFP) but having atypical radiological features. Baseline blood tests like serum AFP, aspartate aminotransferase (AST), alanine aminotransferase (ALT), serum bilirubin, platelet count, INR, serum albumin, hemoglobin, TLC were done before the procedure.
Inclusion criteria were solitary HCC of 3–7 cm or up to 3 HCC each <5 cm in size; no hepatic vascular invasion/thrombosis; no extrahepatic metastases; lesion should be visible on ultrasound (USG) or CT with a safe RFA percutaneous access; eastern cooperative oncology group performance status (ECOG PS) < 2; exclusion criteria were lesion <3 cm, contraindications to TACE/RFA, poor performance status, refractory ascites or hepatic encephalopathy, portal venous thrombosis or extra hepatic disease, previous TACE or RFA; recent esophageal/gastric variceal bleeding.
TACE procedure
Superselective conventional TACE was performed through femoral arterial access. Celiac and mesenteric arteriograms were obtained to assess arterial vascularization of the liver through the guiding catheter thereafter superselective cannulation of the segmental artery supplying the tumor was done using microcatheter [Figure 1]. Chemotherapeutic agents (mixture of 50 mg epirubicin, and 10 ml of lipiodol) were infused into the feeding arterial branches of the tumor followed by gel foam embolization to attain stasis in the feeding artery. After completion of TACE procedure RFA was done without removing the femoral arterial sheath. The sheath was kept for an easy access in case of any bleed after RFA.
Figure 1 (A-I).
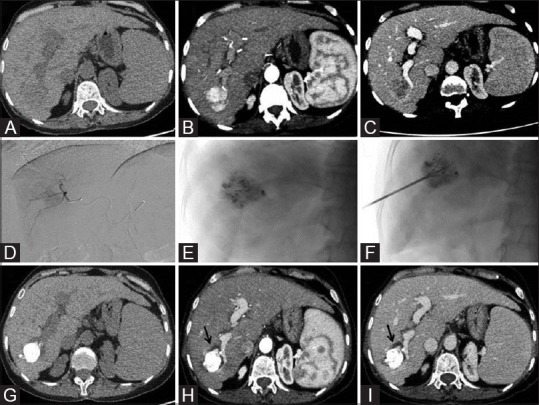
Axial triple phase CT scan [unenhanced (fig A), arterial (fig B) and delayed (fig C)] images showing a hypo dense (fig A) solitary arterial enhancing mass in segment VI of liver (fig B) which shows washout in venous phase (fig C) consistent with HCC. DSA image of super selective run show tumoral blush (fig D) and Post TACE lipiodol deposition in mass (Fig E). Under ultrasound and fluoroscopic guidance RFA multitinned electrode placed with in the mass (fig F). Post TACE plus RFA follow up triple phase CT scan images 3 month [unenhanced (fig G), arterial (fig H) and delayed (fig I)] shows complete lipiodol coverage and no any enhancement with perilesional hypodense nonenhancing ablation zone around treated mass (black arrows) most appreciable on venous phase suggestive of complete response
RFA procedure
RFA was done in same sitting immediately following TACE. All RFA procedures were performed percutaneously using USG or CT guidance with multitined expandable RFA electrode (RITA starburst XL electrode, angioDynamics) and the RF generator (RITA 1500X RF generator, AngioDynamics, Manchester, Georgia). During USG-guided RFA, position of needle was further confirmed under fluroscopy to look for position of tines within the lesion covered by lipiodol [Figure 1]. Lipiodol staining also helped in better detection of tumor on USG. Once the needle was positioned in the lesion, the target temperature of 105°C (we use the automatic temperature control mode) and the power at 150 W was set and the ablation was started. Time of ablation was set according to the size of the tumor (6 min for 2 cm, 7 min for 3 cm and 8 min for 4 cm tumor respectively); for ablating 5–6 cm tumor 4–7 cm internally cooled electrode was used with a pump for continuous saline infusion along the tines. Microbubble cloud produced while doing the RFA was monitored on USG to check the ablation zone, and the same was used as surrogate maker for complete ablation. At the end of the cool-down mode the temperature above 60°C was considered a good indication of complete tumor ablation. The needle track was also ablated while withdrawing the needle from the lesion. Postprocedure USG was done to detect any perihepatic fluid. If there was no evidence of post RFA bleed, arterial sheath was removed. Patient was kept under observation for 4 hours.
Post treatment assessment and followup
LFT, CBC, INR, and serum LDH(lactate dehydogenage) were done at day 1 after the procedure in all patients. Investigations were repeated as per the requirement and hospital course of the patients. Treatment response was evaluated at 1 month, 3 months and 6 months using the modified response evaluation criteria in solid tumors (mRECIST). At least 6 months follow-up was available for all patients and 11 of 22 patients had >1 year follow-up. During the follow-up period, laboratory tests, including serum AFP, albumin, bilirubin, AST, ALT, INR, hemogram, and a triple phase CT/MRI were performed.
Outcome measure
The technical success (complete ablation) after first session; local tumor progression (LTP) rates; distant intra and extrahepatic recurrences were recorded. The technical success was defined as complete replacement of viable tumor tissue by non-enhancing necrotic (ablated) tissue at 1 month follow-up imaging [Figure 1]. The LTP was diagnosed when a follow-up imaging demonstrated interval development/growth of the tumor along the margin of the ablation zone. The distant intra and extrahepatic recurrence was defined as a new lesion with similar characteristics, having no contact with the ablated tumor in the liver or extrahepatic in location. The event-free survival referred to the time period between procedure and appearance of an event.
Statistical analysis
The local therapeutic efficacy in terms of technique effectiveness, complication, and LTP was assessed on a tumor basis. Mann–Whitney test, repeated measure analysis followed by post hoc comparison by Bonferroni method or Friedman test as appropriate were used for analysis. The event-free survival was calculated by using the Kaplan–Meier method. All statistical analyses were performed using a statistical software program (SPSS 20; SPSS, IMB Corp. Limited, Armonk). A P value of ≤ 0.05 was considered to indicate a significant difference.
Observations and Results
Characteristics of patients [Table 1]
Table 1.
Baseline characteristic of patients (n=22)
| Variable | Baseline (Mean±SD) |
|---|---|
| Age (years) | 61.27±7.2 |
| Sex | |
| M | 21 (95.5%) |
| F | 1 (4.5%) |
| Etiology of cirrhosis | |
| HCV | (27.3%) |
| HBV | 4 (18.2%) |
| Ethanol | 7 (31.8%) |
| NASH | 5 (22.7%) |
| Child class | |
| A | 12 (54.5%) |
| B | 10 (45.5%) |
| Segments involved by HCC | 1 (4.2%)/1 (4.2%)/1 (4.2%)/6 (25%)/5 |
| 1/3/4/5/6/7/8 | (20.8%)/2 (8.3%)/8 (33.3%) |
| Tumor size | |
| 3-5 cm | 20 (83.3%) |
| 5-7 cm | 4 (16.7%) |
| Maximum tumor diameter (cm) | 4.1±0.91 |
| Child score | 7±1.27 |
| MELD score | 14.55±4.44 |
| AFP (ng/ml) | 58.68±86.13 |
| Serum Bilirubin (mg/dL) | 1.14±0.51 |
| SGOT (IU/L) | 62.82±51.20 |
| SGPT (IU/L) | 44.59±46.39 |
| Serum Albumin (g/dL) | 3.5±0.38 |
| INR | 1.18±0.11 |
| Platelets | 126.09±86.13 |
The study included 22 patients (95% males), 21 patients had a solitary HCC of size 3–7 cm, and one patient had three target lesions. Most common etiology of chronic liver disease was ethanol intake (31.8%) followed by hepatitis C virus (HCV) infection (27.3%), nonalcoholic steatohepatitis (NASH) (22.7%) and hepatitis B virus (HBV) infection (18.2%). Mean Child Pugh score and MELD score were 7 ± 1.27 and 14.45 ± 4.44 respectively. Four lesions (16.7%) had diameter range of 5–7 cm and rest 20 lesions in range of 3-5 cm with mean tumor diameter being 4.1 ± 0.91 cm. Lesions with size less than 3 cm were excluded from this study. Segment VIII (33.3%) was most common location with 45.83% lesions being close to vessel (abutting a branch of hepatic artery/portal vein/hepatic vein). Mean serum AFP level was 58.68 ± 86.13 and mean serum bilirubin was 1.14 ± 0.51 mg/dL.
Technical success
At 1 month follow-up all target lesions showed complete response suggestive of 100% technical success.
Treatment response and follow-up [Table 2]
Table 2.
Tumor response (No of target lesions-24)
| Response | 6 months (n=24) | 1 year (n=13) | ||
|---|---|---|---|---|
| Number | Percentage | Number | Percentage | |
| Complete Response | 21 | 87.5 | 11 | 84.6 |
| Local tumor progression | 1 | 4.2 | 1 | 7.6 |
| Distant intrahepatic new lesion | 2 | 8.3 | 1 | 7.6 |
At 1 and 3 months follow-up 100% patients (24 target lesions) had complete response. At 6 months, 21 (87.5%) had complete response, while 1 (4.2%) had LTP. The patient with LTP had a large lesion of tumor diameter of 5.6 cm at the presentation. Two patients (8.3%) developed progressive disease; one of these patients had multifocal HCC at initial presentation. These patients underwent RFA for new lesions and had complete response at 1 month follow-up. Eleven patients with 13 target lesions had 1 year follow-up; 11 (84.6%) had complete response and one patient (7.6%) had LTP having maximum tumor diameter of 5.1 cm at presentation. One patient had progressive disease in the form of distant intrahepatic new lesion.
The event-free survival as shown by Kaplan–Meier graph analysis at 6 months and 12 months were 90.7% and 66.4% with mean time to event-free survival being 11.1 months [Figure 2].
Figure 2.
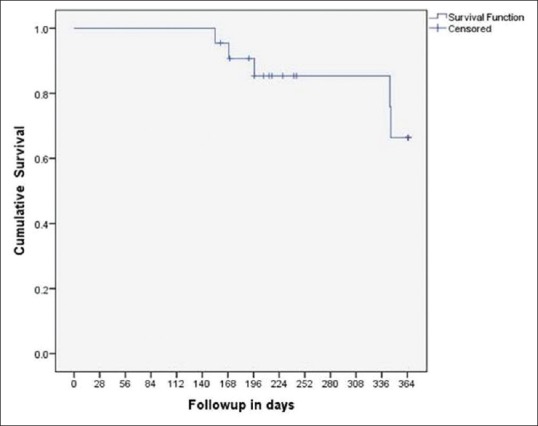
Kaplan-Meier graph shows event free survival
In patients with complete response mean AFP level showed reduction over time [Table 3]. In patients with LTP mean AFP level showed reduction up to 3 months follow-up and increase thereafter. In patients with progressive disease mean AFP level was higher at baseline and during follow-up, as compared to patients with complete response. This indicates that patients with higher AFP level had aggressive HCC with more chances of disease progression over time [Figure 3].
Table 3.
AFP levels
| Time | Patients with complete response (n=17) (mean±SD) | Patients with progressive disease (n=5) (mean±SD) |
|---|---|---|
| Pre procedure | 50.41±86.41 | 110.69±121.25 |
| 1 month | 15.41±20.92 | 51.78±64.0 |
| 3 months | 8.83±9.13 | 38.41±41.80 |
| 6 months | 8.04±8.63 | 40.30±43.14 |
| 1 year | 5.58±3.94 | 42.13±50.79 |
Figure 3.

Correlation of AFP and Response rate with time show that in patients with complete response mean AFP level showed reduction over time, however in patients with progressive disease mean AFP level showed reduction up to 3 months follow up and thereafter increases
Of 12 patients having Child A status, 1 (8.3%) patient developed progressive disease, while 4 patients with Child B status developed progressive disease. This is attributable to poor status of liver disease in Child B patients [Table 4]. No statistically significant differences were seen in term of baseline INR, Platelet count, serum bilirubin, serum albumin, AST, and ALT in patients with complete response and progressive disease.
Table 4.
Frequency of events according to CTP class
| CTP class | Complete response | Progressive disease | Total |
|---|---|---|---|
| A | 11 | 1 | 12 |
| B | 6 | 4 | 10 |
| Total | 17 | 5 | 22 |
Complications
No major complication except post embolization syndrome was noted in 50% patients. They were managed symptomatically by antipyretics and antiemetics. Persistent pain at RFA local site was noted in 31.8%, which was managed by application of Fentanyl transdermal patch. One patient developed mild perihepatic hematoma after RFA. CT angiography was done for identifying the source of bleed in this patient but did not reveal any active bleed and the patient was managed conservatively with resolution of hematoma on follow-up. One patient required repeat hospitalization after 10 days. He had worsening jaundice and abdominal distension. On ultrasound, he had mild ascites. All patients had raised serum LDH level after the procedure and the mean LDH value on post-procedure day 1 was 916.59 ± 351.93.
Discussion
Combination treatment theoretically improve the response to therapy by increasing ablation zone and eradicating the micro metastases around the tumor and indirectly improve the survival. Performing TACE before RFA shows lipiodol deposition within the tumor and helps in precise placement of RFA needle within the tumor.
Technical success was achieved in all patients (100%) in this study. At 1 and 3 months follow-up complete response was achieved in all (100%) target lesions. None of the patient had LTP or distant intra/extrahepatic recurrence at 1 and 3 months follow-up. This result shows immense benefit of combination therapy as compared to TACE or RFA alone. As per available literature, its results are better than TACE alone, RFA alone or surgical resection at short term follow-up. Out of all target lesions, 45.83% lesions were close to vessel (abutting a branch of hepatic artery/portal vein/hepatic vein). Achievement of complete tumor ablation in these patients proves the benefit of preventing heat sink effect by combination therapy. We found 87.5% complete response in the target lesions at 6 months follow-up, 4.2% target lesions developed LTP while 8.3% developed progressive disease in the form of new lesion in distant intrahepatic site. At 1 year follow-up (13 target lesions), 84.6% had complete response, 7.6% had LTP, 7.6% had progressive disease in the form of distant intrahepatic new lesion. LTP in our study can be attributed to large tumor size (both patients had tumor size >5 cm), lesions close to vessels, elongated shape, incomplete tumor capsule and irregular margins. Previous studies have reported LTP rate after RFA in early stage HCC as high as 15.2–41.0% over median follow-up periods ranging from 16.0 to 38.0 months. In comparison, the LTP rates after TACE-RFA have generally been lower than those of RFA alone, with previously published rates of 2.9%–14.5% over median follow-up periods of 37.0–50.0 months.[6,7,8,9,10] Kim et al.[7] observed LTP in 40% of treated lesions in the combined treatment group and 70% in the RFA-alone group. The 2-year local progression-free survival rate after RFA has been reported to be 74.1% for small (<3 cm) HCC, but only 38.3% for medium (3–5 cm) and large (5–7 cm) HCC.[11] Strong evidence was shown by Carmi et al., in favor of combination treatment for 3 to 5 cm lesions. They showed that response rates, local tumor recurrence rates, and patient survival were much better with combination therapy.[12] LTP is related to locoregional treatment efficacy. Development of distant intra- or extrahepatic new lesion is attributable to underlying liver disease and tumor biology as well. It is not affected by locoregional treatments.
Event-free survival at 6 and 12 months was 90.7% and 66.4% and mean time to event-free survival was 11.1 months. According to Kagawa et al., RFA combined with TACE is an efficient and safe treatment that provides overall survival rates similar to those achieved with surgical resection. In their study, the probabilities of overall survival at 1, 3, and 5 years were similar between the TACE plus RFA and resection groups (TACE plus RFA, 100%, 94.8%, and 64.6%; resection, 92.5%, 82.7%, and 76.9%, respectively).[10] According to Pan et al., treatment by TACE-RFA conferred an overall survival rate comparable with that of surgical resection in patients within the up-to-seven criteria.[13] Liu et al. performed meta-analysis of seven randomized-controlled trials. They showed that RFA plus TACE significantly improved the survival rates of patients with HCC at 1 and 3 years compared with RFA alone.[9] Yang et al. in their meta-analysis concluded that use of TACE plus RFA for intermediate stage hepatocellular carcinoma can attain higher tumor response rates and improve survival rates than TACE alone.[8] According to Xie et al., the 1-, 2-, 3-, 4- and 5-year overall survival rates after RFA and TACE treatment were 97.5%, 89.4%, 84.2%, 80.4% and 78.7%, respectively.[14]
In patients with progressive disease mean AFP level was higher at baseline and during follow-up, as compared to patient with complete response. Child B patients showed more chances of disease progression as compared to Child A (40% vs 8.3%). This indicates that patients with higher AFP level and higher child score have advanced liver disease and aggressive tumor behavior leading to higher chances of disease progression over time.
Conclusion
The combined use of TACE and RFA is a safe and effective option in the treatment of patients with HCC. Combined treatments may be considered an alternative treatment modality in patients with single large or multinodular HCC when surgical resection is not feasible. In particular, this approach seems to provide better results than RFA and TACE alone for the treatment of large HCC exceeding 3 cm in size, significantly improving the efficacy, quality of life and long-term survival of patients. Finally, it could also expand the indication for RFA to previously contraindicated “complex cases”, in which the application of RFA alone entails an increased risk of complications (like lesions close to vessels).
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: An update. Hepatology. 2011;53:1020–2. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.N’Kontchou G, Mahamoudi A, Aout M, Ganne-Carrie N, Grando V, Coderc E, et al. Radiofrequency ablation of hepatocellular carcinoma: Long-term results and prognostic factors in 235 Western patients with cirrhosis. Hepatology. 2009;50:1475–83. doi: 10.1002/hep.23181. [DOI] [PubMed] [Google Scholar]
- 4.Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: State of the art. World J Gastroenterol. 2016;22:1935–42. doi: 10.3748/wjg.v22.i6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takuma Y, Takabatake H, Morimoto Y, Toshikuni N, Kayahara T, Makino Y, et al. Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology. 2013;269:927–37. doi: 10.1148/radiol.13130387. [DOI] [PubMed] [Google Scholar]
- 6.Song MJ, Bae SH, Lee JS, Lee SW, Song DS, You CR, et al. Combination transarterial chemoembolization and radiofrequency ablation therapy for early hepatocellular carcinoma. Korean J Intern Med. 2016;31:242–52. doi: 10.3904/kjim.2015.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim JH, Won HJ, Shin YM, Kim SH, Yoon HK, Sung KB, et al. Medium-sized (3. 1-5.0 cm) hepatocellular carcinoma: Transarterial chemoembolization plus radiofrequency ablation versus radiofrequency ablation alone. Ann Surg Oncol. 2011;18:1624–9. doi: 10.1245/s10434-011-1673-8. [DOI] [PubMed] [Google Scholar]
- 8.Yang D-j, Luo K-l, Liu H, Cai B, Tao G-q, Su X-f, et al. Meta-analysis of transcatheter arterial chemoembolization plus radiofrequency ablation versus transcatheter arterial chemoembolization alone for hepatocellular carcinoma Oncotarget. 2017;8:2960–70. doi: 10.18632/oncotarget.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Wang ZG, Fu SY, Li AJ, Pan ZY, Zhou WP, et al. Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg. 2016;103:348–56. doi: 10.1002/bjs.10061. [DOI] [PubMed] [Google Scholar]
- 10.Kagawa T, Koizumi J, Kojima S, Nagata N, Numata M, Watanabe N, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma: Comparison with surgical resection. Cancer. 2010;116:3638–44. doi: 10.1002/cncr.25142. [DOI] [PubMed] [Google Scholar]
- 11.Shibata T, Isoda H, Hirokawa Y, Arizono S, Shimada K, Togashi K. Small hepatocellular carcinoma: Is radiofrequency ablation combined with transcatheter arterial chemoembolization more effective than radiofrequency ablation alone for treatment? Radiology. 2009;252:905–13. doi: 10.1148/radiol.2523081676. [DOI] [PubMed] [Google Scholar]
- 12.Carmi L, Georgiades C. Combination percutaneous and intraarterial therapy for the treatment of hepatocellular carcinoma: A review. Semin Intervent Radiol. 2010;27:296–301. doi: 10.1055/s-0030-1261788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pan T, Mu LW, Wu C, Wu XQ, Xie QK, Li XS, et al. Comparison of Combined Transcatheter Arterial Chemoembolization and CT-guided Radiofrequency Ablation with Surgical Resection in Patients with Hepatocellular Carcinoma within the Up-to-seven Criteria: A Multicenter Case-matched Study. J Cancer. 2017;8:3506–13. doi: 10.7150/jca.19964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H, Wang H, An W, Ma W, Qi R, Yang B, et al. The efficacy of radiofrequency ablation combined with transcatheter arterial chemoembolization for primary hepatocellular carcinoma in a cohort of 487 patients. PLoS One. 2014;9:e89081. doi: 10.1371/journal.pone.0089081. [DOI] [PMC free article] [PubMed] [Google Scholar]


