Abstract
Objective:
To determine whether proton magnetic resonance spectroscopy (1H-MRS) can detect neurochemical changes in amyotrophic lateral sclerosis (ALS) associated with heterogeneous functional decline.
Methods:
Nineteen participants with early-stage ALS and 18 age- and sex-ratio-matched controls underwent ultra-high field 1H-MRS scans of the upper limb motor cortex and pons, ALS Functional Rating Scale-Revised (ALSFRS-R total, upper limb, bulbar) and upper motor neuron burden assessments in a longitudinal observational study design with follow-up assessments at 6 and 12 months. Slopes of neurochemical levels over time were compared between patient subgroups classified by rate of upper limb or bulbar functional decline. 1H-MRS and clinical ratings at baseline were assessed for ability to predict study withdrawal due to disease progression.
Results:
Motor cortex total N-acetylaspartate to myo-inositol ratio (tNAA/mIns) significantly declined in patients who worsened in upper limb function over the follow-up period (N=9, p=0.002). Pons glutamate+glutamine (Glx) significantly increased in patients who worsened in bulbar function (N=6, p<0.0001). Neurochemical levels did not change in patients with stable function (N=5–6) or in healthy controls (N=14–16) over time. Motor cortex tNAA/mIns and ALSFRS-R at baseline were significantly lower in patients who withdrew from follow-up due to disease progression (N=6) compared to patients who completed the 12-month scan (N=10) (p<0.001 for tNAA/mIns, p<0.01 for ALSFRS-R), with a substantially larger overlap in ALSFRS-R between groups.
Conclusion:
Neurochemical changes in motor areas of the brain are associated with functional decline in corresponding body regions. 1H-MRS was a better predictor of study withdrawal due to ALS progression than ALSFRS-R.
Keywords: amyotrophic lateral sclerosis, motor cortex, proton MRS, longitudinal, 7 tesla
INTRODUCTION
Functional decline in amyotrophic lateral sclerosis (ALS) is driven by progressive upper and lower motor neuron (UMN and LMN) degeneration. Regional variability in motor neuron degeneration contributes to heterogeneous functional decline. However, the integrity of motor neurons in the brain is not directly assessed by clinical and electrophysiological methods. Brain imaging techniques may provide sensitive and objective measures of progressive motor neuron dysfunction[1].
Proton magnetic resonance spectroscopy (1H-MRS) detects abnormal brain neurochemistry in ALS[2–6]. Reproducible findings include altered levels of motor cortex N-acetylaspartate (NAA; neuronal marker)[2, 4] and myo-inositol (mIns; putative glial marker)[4, 5] — in particular, a lower total NAA to mIns ratio (tNAA/mIns) in patients than in controls[3, 5] — as well as elevated levels of brainstem Glx (glutamate+glutamine)[4, 7].
In cross-sectional studies, tNAA/mIns and Glx levels correlate with disease severity measures, suggesting that they change longitudinally with ALS progression[4–7]. However, longitudinal studies in ALS are few and have demonstrated conflicting results regarding changes in 1H-MRS measures over time[8–12].
In this study, people with early-stage ALS and matched healthy controls were evaluated across 12 months at approximately 6-month intervals. We measured motor cortex tNAA/mIns and pons Glx levels using an optimized, ultra-high field 1H-MRS technique shown to have high test-retest reproducibility[13]. Cross-sectional findings in this cohort were reported previously[5]. Here we examined longitudinal trends and the prognostic value of 1H-MRS measures alongside scores of functional impairment and UMN burden. To identify the neurochemical changes associated with regional loss of function, we stratified patients according to their rates of upper limb and bulbar functional decline.
METHODS
Study Participants and Design
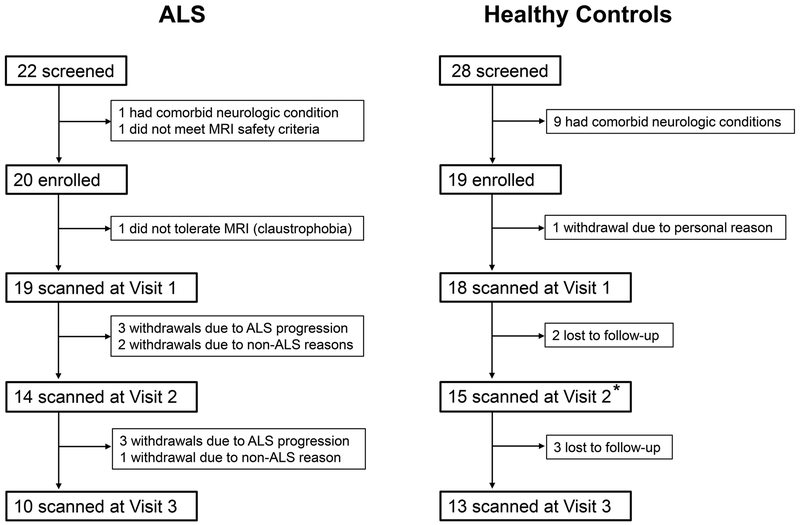
Patients who met revised El Escorial Criteria[14] for clinically possible, probable, or definite ALS were recruited from the ALS Association Certified Treatment Centers of Excellence at the University of Minnesota and Hennepin County Medical Center. Healthy control volunteers were recruited from the general public and selected to match the patient group on mean age and sex ratio. Twenty-two patients and 28 controls were screened using the following exclusion criteria: (1) presence of neurologic illnesses other than ALS, (2) inability to tolerate MRI scanning, and (3) failure to meet MRI safety requirements. After screening, 20 patients and 19 controls were enrolled after giving written informed consent (figure 1).
Figure 1.
Flow diagram of study participation. Participant withdrawals were recorded as being either due to ALS progression or due to reasons unrelated to ALS. Lost to follow-up refers to participants who discontinued follow-up but did not communicate any reason for withdrawal.
*One control participant missed Visit 2 but returned for Visit 3.
Participants underwent MRI and clinical assessments at baseline (Visit 1) and returned for repeat assessments after approximately 6 and 12 months (Visits 2 and 3). Sample size was estimated from a preliminary analysis of 5 patients who completed baseline and 6-month follow-up exams and showed decline in motor cortex tNAA/mIns levels (SD of the difference between baseline and 6 months = 0.2). Twenty patients scanned longitudinally gave 85% power to detect a motor cortex tNAA/mIns decline of 0.13 using paired t-test (α-level = 0.05).
Clinical Assessments
At Visit 1, each participant underwent a neurologic examination by a neuromuscular neurologist. A UMN burden score for each upper limb was generated using a modification of a rating system described previously[15]. The following signs were tallied for a score out of 5: pathologically brisk reflexes (biceps, triceps, and finger flexors), Hoffman’s sign, and upper limb spasticity.
Functional impairment was measured at all 3 visits using the ALS Functional Rating Scale-Revised (ALSFRS-R) and its region-specific subscales[16]. The total ALSFRS-R score was used as a measure of global disability. The bulbar subscore equaled the sum of ALSFRS-R questions 1 to 3, and the upper limb subscore equaled the sum of questions 4 to 6. Disease duration was calculated as the interval from the date of symptom onset to the date of the MRI exam in months. Progression rate was estimated with the following formula: (48 - ALSFRS-R total)/disease duration. Clinical staging was abstracted from the ALSFRS-R using an algorithm developed by King’s College London (Stage 1 mild ‒ Stage 4 advanced)[17]. Cognitive and behavioral status was assessed at all visits using the Edinburgh Cognitive and Behavioral ALS Screen (ECAS)[18]. Scores on the total ECAS and its ALS-specific component were recorded. Current riluzole use was documented to evaluate its potential effects on longitudinal neurochemical trends. All clinical assessments were performed within one week of the MRI exam.
1H-MRS Protocol
All 1H-MRS data were acquired as described in our previous report on Visit 1 results[5]. Briefly, studies were performed on a 7 tesla (T) whole body Siemens MAGNETOM scanner using a 16-channel head transceiver array coil with B1+ shimming[19]. T1-weighted MPRAGE images were acquired for 1H-MRS voxel placement. Voxels were manually placed in the upper limb region of the primary motor cortex (22 × 22 × 22 mm3) and the pons (16 × 16 × 16 mm3). In patients, the motor cortex voxel was positioned in the hemisphere contralateral to the more clinically affected upper limb. For patients who did not have upper limb involvement at the time of Visit 1, the hemisphere was selected contralateral to the side of the body with greater overall disease involvement. In controls, hemispheres were chosen such that the ratio of left- to right-sided scans was comparable in both cohorts. Metabolite spectra were acquired from each voxel using semi-LASER[20] (repetition time TR = 5 s, echo time TE = 26 ms, 64 averages). Unsuppressed water reference spectra were collected for metabolite quantification. Total scan duration was approximately one hour, with the pons acquisition conducted during the second half of the session.
Spectral post-processing[21] and quantification (LCModel version 6.3–0G)[22] were performed as described previously[5] using automated methods to eliminate observer bias. Signal-to-noise ratio (SNR) was measured on summed metabolite spectra by dividing the height of the NAA peak at 2.01 ppm by the root mean square of the noise measured from −4 to −2 ppm. Linewidth was measured as the full-width at half-maximum of the water reference signal. To minimize bias during quality assessment, spectra with SNR < 25 were excluded from quantification analysis. The LCModel basis set contained model spectra for macromolecules and 19 metabolites: alanine, aspartate, ascorbate, glycerophosphocholine, phosphocholine, creatine, phosphocreatine, γ-aminobutyric acid, glucose, glutamine (Gln), glutamate (Glu), glutathione, mIns, lactate, NAA, N-acetylaspartylglutamate (NAAG), phosphoethanolamine, scyllo-inositol, and taurine. Metabolite concentrations in tissue were determined by water scaling and adjusted for cerebrospinal fluid (CSF) within the voxel[13]. In the motor cortex, we analyzed the total NAA (tNAA: NAA+NAAG) to mIns ratio, a potential marker and predictor of progressive neurodegeneration[3]. This measure correlated significantly with ALSFRS-R[5] and UMN burden[6] in earlier cross-sectional studies, including our published analysis of Visit 1 data. In the pons, we analyzed Glx (Glu+Gln), which also correlated with disease severity measures in cross-sectional studies[4, 7].
Measurement reliability was assessed using Cramér-Rao Lower Bounds (CRLB) estimates from LCModel. Only those metabolites quantified with mean CRLB ≤ 20% were examined longitudinally. In the pons, individual concentrations for Gln and Glu were not reported since Gln did not pass the CRLB criterion[5]. Additionally, correlated metabolites (r < −0.5) were analyzed only as sums (e.g., tCho [phosphocholine+glycerophosphocholine], tCr [creatine+phosphocreatine], and tNAA).
Statistical Analysis
All analyses were performed using R version 3.4.2. Comparisons of binary data (sex, hemisphere scanned, and riluzole use) were performed using Fisher’s exact tests. Other measures (e.g., age, clinical scores, and spectral quality parameters) were compared between groups at each visit using Student’s t-tests. Using all participants who completed 2 or 3 visits, linear mixed-effects models estimated time trends (linear slopes) in motor cortex tNAA/mIns, pons Glx, and clinical measures in whole cohorts and in patient subgroups. Age and sex were not included as covariates due to their similarity by design between cohorts. Mean slope estimates in patients were tested against no change over time and against mean slope estimates in controls. Patients were classified into those with stable function and those with declining function (defined as a negative slope in ALSFRS-R subscore over the observation period). To examine the effect of riluzole on neurochemical trends, patients were also divided into riluzole users and non-users.
Motor cortex tNAA/mIns and pons Glx levels at Visit 1 were evaluated for their ability to predict study withdrawal due to ALS progression. Student’s t-tests were used to compare these levels between patients who withdrew due to disease progression and patients who completed the study. Withdrawals due to non-ALS reasons were excluded.
Linear mixed models were also used to examine longitudinal change in the absolute concentrations of metabolites quantified with mean CRLB ≤ 20%. This exploratory analysis did not include Type 1 error corrections for multiple testing.
RESULTS
Cohort Characteristics
The demographics and clinical features of participants at each visit are displayed in table 1. Participant exclusions and withdrawals are shown in figure 1.
Table 1.
Cohort Characteristics: Demographics, Clinical Features, and Spectral Quality*
| Visit 1 | Visit 2 | Visit 3 | ||||
|---|---|---|---|---|---|---|
| ALS | Controls | ALS | Controls | ALS | Controls | |
| Sample size n | 19 | 18 | 14 | 15 | 10 | 13 |
| Time of exam after Visit 1, months | ‒ | ‒ | 6.6 ± 1.1 | 6.3 ± 0.8 | 12.8 ± 1.4 | 12.3 ± 1.0 |
| Sex ratio, male:female | 10:9 | 10:8 | 8:6 | 7:8 | 6:4 | 6:7 |
| Age, years | 57 ± 9 (31–74) |
57 ± 9 (30–69) |
58 ± 10 (32–71) |
59 ± 7 (48–69) |
58 ± 11 (32–70) |
58 ± 10 (31–69) |
| Brain hemisphere scanned, left:right | 8:11 | 8:10 | 5:9 | 7:8 | 3:7 | 6:7 |
| Riluzole use, yes:no | 8:11 | ‒ | 7:7 | ‒ | 4:6 | ‒ |
| Disease duration, months | 40.3 ± 43.1 (3.5–148.1) |
‒ | 47.1 ± 49.4 (9.9–154.2) |
‒ | 43.2 ± 41.8 (15.9–156.5) |
‒ |
| Progression rate, points per month | 0.37 ± 0.32 (0.03–1.43) |
‒ | 0.32 ± 0.20 (0.03–0.65) |
‒ | 0.41 ± 0.27 (0.03–1.01) |
‒ |
| King’s Disease Stage (no. of patients) | Stage 1 (6) Stage 2 (6) Stage 3 (6) Stage 4 (1) |
‒ | Stage 1 (4) Stage 2 (6) Stage 3 (2) Stage 4 (2) |
‒ | Stage 1 (1) Stage 2 (2) Stage 3 (2) Stage 4 (5) |
‒ |
|
ALSFRS-R Total Score (0 most severe ‒ 48 normal) |
39.8 ± 5.6 (26–45) |
‒ | 39.7 ± 4.1 (33–47) |
‒ | 36.4 ± 5.9 (26–45) |
‒ |
|
ALSFRS-R Upper Limb Subscore
(0 most severe ‒ 12 normal) |
8.7 ± 3.2 (0–12) |
‒ | 9.3 ± 1.6 (7–12) |
‒ | 8.4 ± 1.8 (5–12) |
‒ |
|
ALSFRS-R Bulbar Subscore
(0 most severe ‒ 12 normal) |
10.3 ± 1.9 (6–12) |
‒ | 9.7 ± 2.8 (4–12) |
‒ | 8.8 ± 3.8 (0–12) |
‒ |
|
Contralateral Upper Limb UMN Score** (0 normal ‒ 5 most severe) |
1.9 ± 1.7 (0–5) |
‒ | ‒ | ‒ | 1.9 ± 2.0 (0–5) |
‒ |
|
ECAS Total Score (0 most severe ‒ 136 normal) |
115.3 ± 6.5 (99–127) |
119.3 ± 8.8 (103–129) |
116.7 ± 8.2 (106–135) |
123.0 ± 7.1 (107–132) |
117.5 ± 6.6 (106–129) |
122.4 ± 7.2 (108–134) |
|
ECAS ALS-Specific Subscore (0 most severe ‒ 100 normal) |
86.3 ± 4.9 (74–95) |
‒ | 87.2 ± 6.3 (77–100) |
‒ | 86.6 ± 5.1 (80–97) |
‒ |
| Motor Cortex Spectra SNR | 205 ± 37 | 201 ± 34 | 215 ± 48 | 198 ± 45 | 208 ± 26 | 202 ± 44 |
| Motor Cortex Spectra Linewidth, Hz | 11 ± 2 | 11 ± 2 | 10 ± 2 | 11 ± 2 | 10 ± 2 | 11 ± 2 |
| Pons Spectra SNR | 45 ± 10 | 53 ± 17 | 51 ± 10 | 56 ± 16 | 52 ± 10 | 62 ± 12 |
| Pons Spectra Linewidth, Hz | 16 ± 2 | 15 ± 2 | 14 ± 2 | 15 ± 2 | 15 ± 2 | 15 ± 2 |
Data are given as counts or mean ± SD and range. Comparisons were made between patients and controls using Fisher’s exact tests (sex ratio and brain hemisphere scanned) and unpaired, two-tailed Student’s t-tests. Total ECAS scores trended lower in patients compared with controls at each visit. All other parameters were not different between groups.
Abbreviations: ALSFRS-R = ALS Functional Rating Scale-Revised; UMN = upper motor neuron; ECAS = Edinburgh Cognitive and Behavioral ALS Screen.
Pons spectra were acquired from fewer participants (see Results: 1H-MRS Data Completeness and Quality).
Neuromuscular examinations were conducted at Visits 1 and 3. Contralateral Upper Limb UMN burden was assessed in all patients at Visit 1 and in 7 patients at Visit 3.
Patients and controls at Visit 1 were well-matched on age-range, sex ratio, and hemisphere scanned. Matching on age-range and sex ratio was retained across Visits 2 and 3. At Visit 1, most patients (12 out of 19, 63%) were in King’s disease stages 1 or 2. This proportion was similar at Visit 2 (10/14 = 72%) and lower at Visit 3 (3/10 = 30%). Mean ALSFRS-R scores also indicated that the patient cohort was mildly disabled at Visits 1 and 2 and more severely disabled at Visit 3. UMN burden scores were determined for the upper limb contralateral to the scanned hemisphere. Mean scores indicated mild UMN dysfunction in 19 patients at Visit 1 and in 7 patients who returned at Visit 3. Total ECAS scores were lower in patients compared to controls at all visits, but the differences did not reach statistical significance.
1H-MRS Data Completeness and Quality
Motor cortex metabolite spectra were acquired at each visit, and all datasets subsequently passed quality criteria. In contrast, fewer pons datasets were obtained. Thirteen pons acquisitions were incomplete due to participant discomfort during the latter half of the exam. Seven pons datasets were excluded due to SNR < 25. In total, usable pons spectra were acquired from 13 patients and 15 controls at Visit 1, 9 patients and 13 controls at Visit 2, and 9 patients and 10 controls at Visit 3.
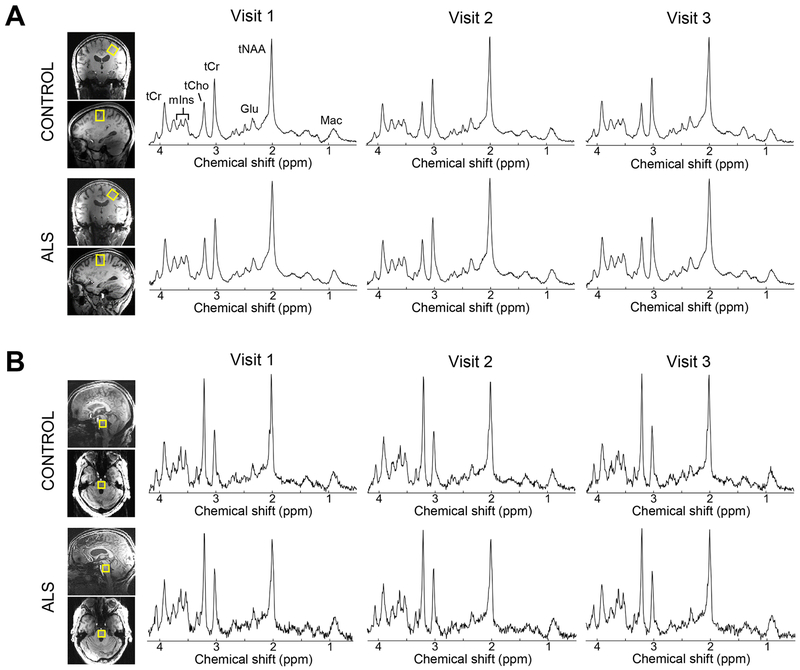
Motor cortex and pons metabolite spectra that satisfied quality criteria had high SNR that was reproducible across visits (table 1; figure 2). The mean coefficient of variation in SNR across visits was 9.0% for motor cortex spectra and 12.4% for pons spectra. Motor cortex spectra had significantly higher SNR and narrower linewidths than pons spectra (both p < 0.0001). Patient and control spectra did not differ in SNR or linewidth.
Figure 2.
Proton spectra obtained from the motor cortex and pons of a patient and healthy control. Upper limb motor cortex spectra (A) and pons spectra (B) had high SNR that was reproducible across visits (semi-LASER: TR/TE = 5000/26 ms, 64 averages). Spectral SNR was not different between patients and controls using unpaired Student’s t-test. Spectra are weighted with a 5-Hz Gaussian function for display purposes. Abbreviations: Glu, glutamate; Mac; macromolecules; mIns, myo-inositol; tCho, phosphocholine+glycerophosphocholine; tCr, creatine+phosphocreatine; tNAA, N-acetylaspartate+N-acetylaspartylglutamate.
For metabolites that satisfied mean CRLB ≤ 20%, motor cortex tNAA was measured with the lowest mean CRLB, and motor cortex Glc+Tau was measured with the highest (supplementary figure). CRLB for individual measurements ranged from 1% to 27% across the metabolites.
Analyses of Longitudinal Changes
In patients, ALSFRS-R total scores declined over the follow-up period at an average rate of −0.46 points per month (p < 0.0001; table 2). ALSFRS-R upper limb and bulbar subscores also declined (p < 0.0001 and p = 0.04, respectively). Cognitive-behavioral and UMN burden scores for the contralateral upper limb did not change.
Table 2.
Linear Trends in Clinical and Spectroscopy Measures*
| ALS | Controls | |||||
|---|---|---|---|---|---|---|
| Clinical Measures** | Mean change per month | SE | p-value | Mean change per month | SE | p-value |
| ALSFRS-R Total | −0.46 | 0.07 | < 0.0001 | ‒ | ‒ | ‒ |
| ALSFRS-R Upper Limb | −0.13 | 0.03 | < 0.0001 | ‒ | ‒ | ‒ |
| ALSFRS-R Bulbar | −0.13 | 0.06 | 0.04 | ‒ | ‒ | ‒ |
| Contralateral Upper Limb UMN | −0.07 | 0.04 | 0.16 | ‒ | ‒ | ‒ |
| ECAS ALS-Specific | 0.08 | 0.11 | 0.48 | ‒ | ‒ | ‒ |
| 1H-MRS Measures | Mean change per year | SE | p-value | Mean change per year | SE | p-value |
| Motor cortex tNAA/mIns | −0.126 | 0.049 | 0.01 | −0.030 | 0.047 | 0.53 |
| Pons Glx, μmol/g | 0.570 | 0.178 | < 0.01 | 0.414 | 0.308 | 0.19 |
P-values are from tests against null change over time.
Abbreviations: SE = standard error; ALSFRS-R = ALS Functional Rating Scale-Revised; UMN = upper motor neuron burden; ECAS = Edinburgh Cognitive and Behavioral ALS Screen.
Slope analyses were performed on longitudinal data from participants who completed two or three visits. The sample size was 14 patients and 16 controls for all analyses, except for the following: ALSFRS-R bulbar and pons Glx analyses (12 patients, 14 controls) and contralateral upper limb UMN burden analysis (7 patients).
Please refer to Table 1 for scale ranges.
Motor cortex tNAA/mIns levels significantly declined in patients over the follow-up period (p = 0.01) and did not in controls (p = 0.53). Additionally, pons Glx levels significantly increased in patients (p = 0.003) and did not in controls (p = 0.19). The difference between patients and controls in their rates of change in these measures did not reach statistical significance (tNAA/mIns: p = 0.16; Glx: p = 0.66). Rates of change were also not significantly different between riluzole users and non-users (half of patients were riluzole users in each longitudinal analysis). An exploratory analysis of absolute concentrations for other neurochemicals revealed no significant changes over time in patients or controls for either region (supplementary figure).
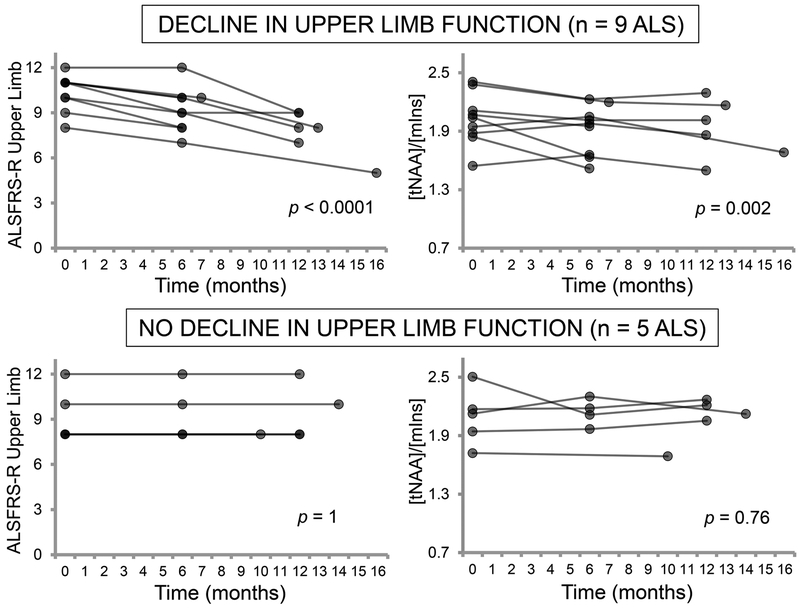
To determine whether clinical subgroups drove the longitudinal changes in neurochemical levels, we stratified patients by progression of regional functional impairment. Of the 14 patients with usable longitudinal motor cortex 1H-MRS data, 9 experienced decline in ALSFRS-R upper limb subscores during the follow-up period (p < 0.0001; average rate = −0.21 points/month), while 5 remained stable (p = 1; average rate = 0 points/month). Motor cortex tNAA/mIns levels dropped significantly over time in the patients with declining upper limb function (p = 0.002) but did not change in those who were stable (p = 0.76; figure 3). The functionally declining patients showed a significantly greater rate of decline in tNAA/mIns compared to controls (p = 0.04), while functionally stable patients did not (p = 0.94). The proportion of riluzole users was not significantly different between the functionally declining and functionally stable subgroups (5 out of 9 patients versus 2 out of 5 patients; Fisher’s test).
Figure 3.
Longitudinal changes in motor cortex tNAA/mIns are related to progression of upper limb function. Patients who had declining ALSFRS-R upper limb scores over the follow-up period experienced decreasing tNAA/mIns over the same time window (both p < 0.01). In contrast, patients who had stable ALSFRS-R upper limb scores showed no change in tNAA/mIns over time. Analyses were performed on data from participants who completed two or three visits. P-values are from a test of the mean slope against no change over time.
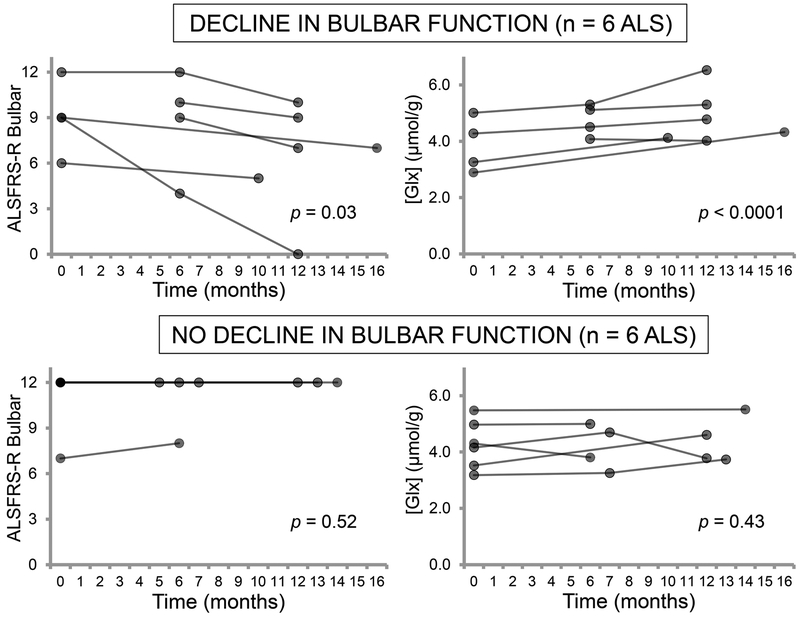
Of the 12 patients with usable longitudinal pons 1H-MRS data, 6 experienced decline in ALSFRS-R bulbar subscores during the follow-up period (p = 0.03; average rate = −0.26 points/month), while 6 remained stable (p = 0.52; average rate = 0.01 points/month). Pons Glx levels increased significantly over time in the patients with declining bulbar function (p < 0.0001) and did not change in those who were stable (p = 0.43; figure 4). Both the functionally declining and functionally stable patients did not differ significantly from controls in their rate of change in Glx (p = 0.15 and 0.61, respectively). The proportion of riluzole users was equal in the functionally declining and functionally stable subgroups (3 out of 6 patients in each).
Figure 4.
Longitudinal changes in pons Glx levels are related to progression of bulbar function. Patients who had declining ALSFRS-R bulbar scores over the follow-up period experienced increasing Glx levels in the pons over the same time window (p < 0.0001). In contrast, patients who had stable ALSFRS-R bulbar scores showed no change in pons Glx levels over time. Analyses were performed on data from participants who completed two or three visits. P-values are from a test of the mean slope against no change over time.
Prediction of Patient Withdrawal
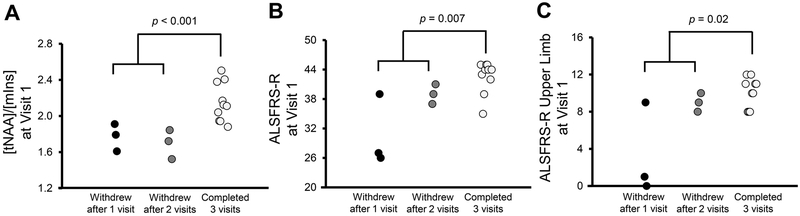
We compared motor cortex tNAA/mIns levels at Visit 1 between 6 patients who withdrew from the study due to ALS progression and 10 patients who returned for all follow-up visits. Levels of tNAA/mIns were significantly lower in people who withdrew than in those who completed all visits (p < 0.001; figure 5A).
Figure 5.
Comparison of motor cortex tNAA/mIns and ALSFRS-R at Visit 1 between study withdrawers and completers. Patient subgroups were divided according to study withdrawal and completion. All 3 measures were significantly different between patients who withdrew due to ALS progression and patients who completed all visits (p < 0.05; unpaired, two-tailed t-tests). However, the most significant difference between groups was observed in the tNAA/mIns comparison (p < 0.001).
Compared with those who completed all visits, patients who withdrew due to ALS progression also had lower ALSFRS-R total and upper limb scores at Visit 1 (p = 0.007 and 0.02, respectively). However, overlap in the clinical scores between withdrawers and completers was substantially larger than the overlap in their tNAA/mIns levels (figure 5B, 5C).
Pons Glx levels at Visit 1 did not display predictive value.
DISCUSSION
This longitudinal study revealed changes in brain neurochemical levels over one year in people with ALS. These neurochemical changes were regionally related to functional decline (i.e., upper limb motor cortex tNAA/mIns and upper limb function, pons Glx and bulbar function). Additionally, motor cortex tNAA/mIns was a significant predictor of study withdrawal due to ALS progression.
Most 1H-MRS studies in ALS to date have been cross-sectional and typically found lower tNAA and higher mIns levels in the motor cortex in patients compared to healthy controls[23]. Reduction in tNAA indicates neuronal loss and dysfunction, while elevation in mIns suggests gliosis[24]. Hence, the tNAA/mIns ratio may be a robust, pathologically relevant biomarker for ALS, especially since UMN degeneration in the disease is often accompanied by glial activation and proliferation[25]. Here we report that motor cortex tNAA/mIns decreases longitudinally in patients, which extends previous cross-sectional findings[3, 5, 6] and supports tNAA/mIns as a measure of progressive neurodegeneration in ALS.
The longitudinal 1H-MRS literature in ALS has been inconsistent. Although a few studies reported declines in motor cortex tNAA or its ratios (i.e., tNAA/tCho, tNAA/tCr)[2, 9], other studies obtained negative results[8, 10–12]. Notably, these studies did not examine whether neurochemical changes in the scanned area of motor cortex are related to functional decline in the represented body region. Investigating this question is meaningful because the heterogeneity of disease spread in ALS may be driven by differences among patients in their pattern of motor cortex degeneration. In our stratification analysis, tNAA/mIns in the upper limb motor cortex declined in patients with worsening upper limb function but did not change in patients who were functionally stable. This suggests that tNAA/mIns in the upper limb motor cortex reflects the integrity of UMNs that control upper limb function. More broadly, however, our finding also indicates that the degeneration of distinct regions of the motor cortex is variable in ALS. This may explain the mixed longitudinal results in literature, which were all obtained from analyses of small areas within the motor cortex. Furthermore, focal motor cortex neurochemical changes appear to have functional consequences according to the cortical somatotopic map. This regional relationship has also been suggested in a previous cross-sectional study, which reported that upper limb corticospinal tract tNAA/tCho correlated significantly with finger-tapping rate but not with foot-tapping or bulbar muscle syllable repeat rates[26].
In our analysis of absolute concentrations, motor cortex tNAA and mIns did not significantly change over time in patients with ALS. Therefore, the decline in tNAA/mIns is probably driven by associated disease processes that cause small but opposite changes in the two concentrations. The measurement of the tNAA/mIns ratio likely confers additional robustness in detecting neurodegenerative changes. Notably, changes in tNAA/mIns were detected while observing no changes in clinical UMN burden over time. This reinforces the understanding that clinical scores based upon reflexes and tone are an imperfect surrogate for UMN degeneration. The physiology that underlies clinical UMN signs is complex, and the presentation of these signs may also be obscured by advanced lower motor neuron signs[27].
We also observed that motor cortex tNAA/mIns may be prognostic, with the potential to identify patients who are likely to withdraw from a study due to progressive decline. This finding is especially relevant to clinical trial design, which would benefit greatly from accurate prognostic information during participant selection[28]. Relatedly, low cingulate cortex tNAA/mIns in normal individuals predicts the conversion to mild cognitive impairment and Alzheimer’s disease[29, 30]. Together these studies suggest that tNAA/mIns has prognostic value for conditions that show both neuronal degeneration and gliosis.
The sum Glx is frequently reported in 1H-MRS literature due to the difficulty in reliably separating the overlapping resonances of Glu and Gln. Alterations in Glx levels have been interpreted primarily as alterations in Glu, which accounts for approximately 80% of the sum in healthy brain regions[31]. In cross-sectional studies, authors reported elevated Glx in the brainstem in patients with ALS and suggested that this supports the role of excess Glu neurotransmission in motor neuron death[4, 7]. Our previously published analysis of Visit 1 data showed no differences in pons Glx levels between patients with relatively early-stage ALS and healthy controls[5]. However, longitudinal follow-up of these patients revealed increases in pons Glx levels over the subsequent year. These increases are likely unrelated to riluzole’s purported antiglutamatergic activity[32], since trends in Glx were not different between riluzole users and non-users. Our findings suggest that pons Glx levels are normal in early-stage ALS and rise as the disease transitions to later stages. Consistently, the mean disability scores of the cohorts that were previously reported to have elevated Glx levels[4, 7] were more severe than our cohort at Visit 1. Longitudinal increases in Glx may reflect progressive abnormalities in Glu metabolism in ALS[33] or increases in astroglial Gln[34]. It may also indirectly support Glu excitotoxicity as a pathogenic mechanism, although this interpretation is tenuous as the Glu signal in 1H-MRS principally arises from the intracellular compartment[35].
The stratification analysis in patients showed that elevation in pons Glx is related to decline in bulbar function. This finding was somewhat surprising, given that the ALSFRS-R bulbar subscore is a rating of functions governed largely by medullary motor neurons (i.e., speech, swallowing, and salivation)[17]. The pons voxel in this study contains cranial motor nuclei and corticospinal fibers that are less directly involved in controlling these functions. However, it also includes corticobulbar projections to the medullary motor nuclei as well as extrapyramidal tracts that greatly influence axial muscle tone[36]. Moreover, pons Glx may indirectly reflect disease burden in the medulla, since degenerative changes are likely to affect both brainstem regions simultaneously due to their anatomic proximity.
The regional association between neurochemical changes and functional decline suggests that 1H-MRS can inform us about disease spread in ALS. The pattern of disease spread is a subject of intense interest and, to date, has relied largely upon longitudinal clinical examinations and post-mortem histopathology[37, 38]. Unlike these methods, 1H-MRS may provide direct in vivo information on disease progression within and between brain regions, e.g., by tracking neurochemical changes in different areas of motor cortex that control bulbar, upper limb, or lower limb function. Specifically, 1H-MRS may be able to determine how motor areas of the brain are sequentially involved in ALS, which would help explain patterns of functional decline.
Our longitudinal cohort was small, with 10 patients undergoing scanning at all three visits. Additionally, the rate of ALSFRS-R decline in our cohort was approximately half the rate typically observed in natural history studies[39]. We detected statistically significant longitudinal changes in motor cortex tNAA/mIns and pons Glx levels despite these factors, which suggests that these measures are highly sensitive to disease progression in the brain.
A potential limitation of this study is that it was conducted at ultra-high field, which has limited availability in the clinical setting. In order to achieve high measurement precision[13], we utilized an optimized 1H−MRS technique at 7T. In our earlier work, comparisons of neurochemical levels between patients with ALS and controls yielded similar results whether 3T edited or 7T unedited 1H-MRS was performed[5]. Here we expect that unedited 1H-MRS experiments at 3T will confirm our present findings because tNAA/mIns and Glx each have comparable measurement precision at both field strengths. Specifically, the mean coefficients of variation for these measures at 3T and 7T are each under 5% as calculated from prior test-retest data from the cortex[13].
Overall, this study shows that neurochemical changes in motor areas of the brain reflect disease progression in corresponding areas of the body. Subsequent studies may confirm the relationship between neurochemical changes and functional decline for other motor cortex regions, such as the lower limb and cranial representations. Ultimately, 1H-MRS may provide outcome measures for monitoring and predicting disease progression in future therapeutic trials. In natural history studies, 1H-MRS may also be useful for mapping the course of cerebral degeneration.
Supplementary Material
ACKNOWLEDGEMENTS
The authors are grateful to all study participants for their generous contribution of time and effort. The authors also acknowledge Valerie Ferment and Susan Rolandelli, RN, for study coordination, and Pamela Droberg, NP, for her assistance with recruitment.
FUNDING
This work was supported by the Bob Allison Ataxia Research Center, the Curt O’Hagan ALS-PLS and ALS-Lou Gehrig Disease funds of the University of Minnesota Foundation, and the National Institute of Neurological Disorders and Stroke (NINDS) grant R01 NS080816. The Center for Magnetic Resonance Research is supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) grant P41 EB015894 and the Institutional Center Cores for Advanced Neuroimaging award P30 NS076408. This study was also supported by the National Center for Advancing Translational Sciences (NCATS) award UL1TR000114. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
COMPETING INTERESTS
IC, DKD, LEE, MM, GM, and GG report no disclosures. DW consults for Acceleron Pharma and receives research support from the ALS Association, Acceleron Pharma, PHARNext, and FLEX Pharma. GO received research support from Takeda Pharmaceuticals, Inc. and NeuroVia, Inc.
ETHICS APPROVAL
All study participants provided written informed consent. The study was approved by the Institutional Review Board: Human Subjects Committee of the University of Minnesota.
REFERENCES
- 1.Simon NG, Turner MR, Vucic S, et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann Neurol 2014;76:643–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pioro EP, Antel JP, Cashman NR, et al. Detection of cortical neuron loss in motor neuron disease by proton magnetic resonance spectroscopic imaging in vivo. Neurology 1994;44:1933–8. [DOI] [PubMed] [Google Scholar]
- 3.Kalra S, Hanstock CC, Martin WR, et al. Detection of cerebral degeneration in amyotrophic lateral sclerosis using high-field magnetic resonance spectroscopy. Arch Neurol 2006;63:1144–8. [DOI] [PubMed] [Google Scholar]
- 4.Foerster BR, Pomper MG, Callaghan BC, et al. An imbalance between excitatory and inhibitory neurotransmitters in amyotrophic lateral sclerosis revealed by use of 3-T proton magnetic resonance spectroscopy. JAMA Neurol 2013;70:1009–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheong I, Marjańska M, Deelchand DK, et al. Ultra-High Field Proton MR Spectroscopy in Early-Stage Amyotrophic Lateral Sclerosis. Neurochem Res 2017;42:1833–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atassi N, Xu M, Triantafyllou C, et al. Ultra high-field (7tesla) magnetic resonance spectroscopy in Amyotrophic Lateral Sclerosis. PLoS One 2017;12:e0177680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pioro EP, Majors AW, Mitsumoto H, et al. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology 1999;53:71–9. [DOI] [PubMed] [Google Scholar]
- 8.Mitsumoto H, Ulug AM, Pullman SL, et al. Quantitative objective markers for upper and lower motor neuron dysfunction in ALS. Neurology 2007;68:1402–10. [DOI] [PubMed] [Google Scholar]
- 9.Pohl C, Block W, Karitzky J, et al. Proton magnetic resonance spectroscopy of the motor cortex in 70 patients with amyotrophic lateral sclerosis. Arch Neurol 2001;58:729–35. [DOI] [PubMed] [Google Scholar]
- 10.Rule RR, Suhy J, Schuff N, et al. Reduced NAA in motor and non-motor brain regions in amyotrophic lateral sclerosis: a cross-sectional and longitudinal study. Amyotroph Lateral Scler Other Motor Neuron Disord 2004;5:141–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unrath A, Ludolph AC, Kassubek J. Brain metabolites in definite amyotrophic lateral sclerosis. A longitudinal proton magnetic resonance spectroscopy study. J Neurol 2007;254:1099–106. [DOI] [PubMed] [Google Scholar]
- 12.van der Graaff MM, Lavini C, Akkerman EM, et al. MR spectroscopy findings in early stages of motor neuron disease. AJNR Am J Neuroradiol 2010;31:1799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Terpstra M, Cheong I, Lyu T, et al. Test-retest reproducibility of neurochemical profiles with short-echo, single-voxel MR spectroscopy at 3T and 7T. Magn Reson Med 2016;76:1083–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brooks BR, Miller RG, Swash M, et al. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 2000;1:293–9. [DOI] [PubMed] [Google Scholar]
- 15.Woo JH, Wang S, Melhem ER, et al. Linear associations between clinically assessed upper motor neuron disease and diffusion tensor imaging metrics in amyotrophic lateral sclerosis. PLoS One 2014;9:e105753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 1999;169:13–21. [DOI] [PubMed] [Google Scholar]
- 17.Balendra R, Jones A, Jivraj N, et al. Estimating clinical stage of amyotrophic lateral sclerosis from the ALS Functional Rating Scale. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:279–84. [DOI] [PubMed] [Google Scholar]
- 18.Abrahams S, Newton J, Niven E, et al. Screening for cognition and behaviour changes in ALS. Amyotroph Lateral Scler Frontotemporal Degener 2014;15:9–14. [DOI] [PubMed] [Google Scholar]
- 19.Emir UE, Auerbach EJ, Van De Moortele PF, et al. Regional neurochemical profiles in the human brain measured by (1)H MRS at 7 T using local B(1) shimming. NMR Biomed 2012;25:152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Öz G, Tkáč I. Short-echo, single-shot, full-intensity proton magnetic resonance spectroscopy for neurochemical profiling at 4 T: validation in the cerebellum and brainstem. Magn Reson Med 2011;65:901–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deelchand DK, Adanyeguh IM, Emir UE, et al. Two-site reproducibility of cerebellar and brainstem neurochemical profiles with short-echo, single-voxel MRS at 3T. Magn Reson Med 2015;73:1718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 1993;30:672–9. [DOI] [PubMed] [Google Scholar]
- 23.Verstraete E, Foerster BR. Neuroimaging as a New Diagnostic Modality in Amyotrophic Lateral Sclerosis. Neurotherapeutics 2015;12:403–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Öz G, Alger JR, Barker PB, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology 2014;270:658–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Radford RA, Morsch M, Rayner SL, et al. The established and emerging roles of astrocytes and microglia in amyotrophic lateral sclerosis and frontotemporal dementia. Front Cell Neurosci 2015;9:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Govind V, Sharma KR, Maudsley AA, et al. Comprehensive evaluation of corticospinal tract metabolites in amyotrophic lateral sclerosis using whole-brain 1H MR spectroscopy. PLoS One 2012;7:e35607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swash M Why are upper motor neuron signs difficult to elicit in amyotrophic lateral sclerosis? J Neurol Neurosurg Psychiatry 2012;83:659–62. [DOI] [PubMed] [Google Scholar]
- 28.Chio A, Logroscino G, Hardiman O, et al. Prognostic factors in ALS: A critical review. Amyotroph Lateral Scler 2009;10:310–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarci K, Weigand SD, Przybelski SA, et al. MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurology 2013;81:126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Waragai M, Moriya M, Nojo T. Decreased N-Acetyl Aspartate/Myo-Inositol Ratio in the Posterior Cingulate Cortex Shown by Magnetic Resonance Spectroscopy May Be One of the Risk Markers of Preclinical Alzheimer’s Disease: A 7-Year Follow-Up Study. J Alzheimers Dis 2017;60:1411–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tkáč I, Öz G, Adriany G, et al. In vivo 1H NMR spectroscopy of the human brain at high magnetic fields: metabolite quantification at 4T vs. 7T. Magn Reson Med 2009;62:868–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doble A The pharmacology and mechanism of action of riluzole. Neurology 1996;47:S233–41. [DOI] [PubMed] [Google Scholar]
- 33.Van Den Bosch L, Van Damme P, Bogaert E, et al. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim Biophys Acta 2006;1762:1068–82. [DOI] [PubMed] [Google Scholar]
- 34.Ramadan S, Lin A, Stanwell P. Glutamate and glutamine: a review of in vivo MRS in the human brain. NMR Biomed 2013;26:1630–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Danbolt NC. Glutamate uptake. Prog Neurobiol 2001;65:1–105. [DOI] [PubMed] [Google Scholar]
- 36.Mukherjee A, Chakravarty A. Spasticity mechanisms - for the clinician. Front Neurol 2010;1:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brettschneider J, Del Tredici K, Toledo JB, et al. Stages of pTDP-43 pathology in amyotrophic lateral sclerosis. Ann Neurol 2013;74:20–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology 2009;73:805–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qureshi M, Schoenfeld DA, Paliwal Y, et al. The natural history of ALS is changing: improved survival. Amyotroph Lateral Scler 2009;10:324–31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.