Abstract
Cardiovascular disease remains the leading cause of morbidity and mortality for both women and men. Emerging evidence supports that ischemic heart disease (IHD) may manifest differently in women and men, in ways ranging from the clinical presentation, diagnosis, and management of disease to the basic biology and biomechanics of cardiomyocyte function and the coronary circulation. Women consistently present with a higher burden of symptoms and comorbidities as compared with men and experience worse outcomes. These data have proved perplexing given the decreased likelihood of women to demonstrate obstructive coronary artery disease (CAD) on coronary angiography. Reported sex differences have long been influenced by the practice of defining heart disease primarily as obstructive CAD, but obstructive plaque is now recognized as neither necessary nor sufficient to explain symptoms of IHD, and it is no longer adequate to tailor diagnostic and treatment strategies only to this subset of patients. To date, women remain underrepresented in guideline-changing heart disease research and trials, creating important limitations in the evidence base for cardiovascular medicine. Smaller epicardial coronary arteries in women as compared to men, coupled with differences in shear stress and inflammatory mediators over the life span, may modify the development of CAD in susceptible patients into a diffuse pattern with more contribution from coronary vasomotor dysfunction than focal obstruction. Newer studies corroborate that symptomatic women are more likely than men to present with nonobstructive CAD and coronary microvascular dysfunction. When present, these processes increase cardiovascular risk in both women and men but may constitute an especially malignant phenotype in a subset of severely affected women, with implications for the management of not only CAD but also heart failure with preserved ejection fraction. This represents a state-of-the-art review of sex differences in the coronary system, with an eye toward how diverse pathophysiological processes may contribute to IHD phenotypes prevalent in women and men. Beyond providing women and men with equitable optimal care according to current paradigms, understanding the pathophysiology of IHD beyond a conventional focus on obstructive CAD is needed to address what is likely a combination of biological as well as environmental determinants of their prognosis.
Keywords: Atherosclerosis, Cardiovascular disease, Coronary flow reserve, Coronary microvascular dysfunction, Heart failure with preserved ejection fraction, Ischemic heart disease, Nonobstructive coronary artery disease
Graphical Abstract
Artwork by Piet Michiels, Leuven, Belgium
Introduction: Sex Differences in Ischemic Heart Disease
Over the last century, cardiovascular disease (CVD) has accounted for more deaths than any other major cause of death in the United States [1] and is now the leading cause of mortality among both women and men worldwide [1–4], resulting in nearly 18 million deaths in 2015. Deaths from CVD primarily involve ischemic heart disease (IHD), such as myocardial infarction (MI) and heart failure, and also include those associated with stroke and peripheral arterial disease. Focusing within the cardiac system, approximately 630,000 Americans die from heart disease each year, representing 1 in every 4 deaths, and the numbers are similar for women and men (Fig. 1) [4]. Yet awareness of heart disease risk for women has substantially lagged that for men [5]. Heart disease—historically synonymous with coronary artery disease (CAD)—has been traditionally defined anatomically as obstructive atherosclerosis involving the epicardial coronary arteries. There is now greater understanding that IHD occurs in the presence of an inadequate blood supply to the myocardium, which may or may not result from obstructive atherosclerotic narrowing in the epicardial coronary arteries [6, 7].
Fig. 1.
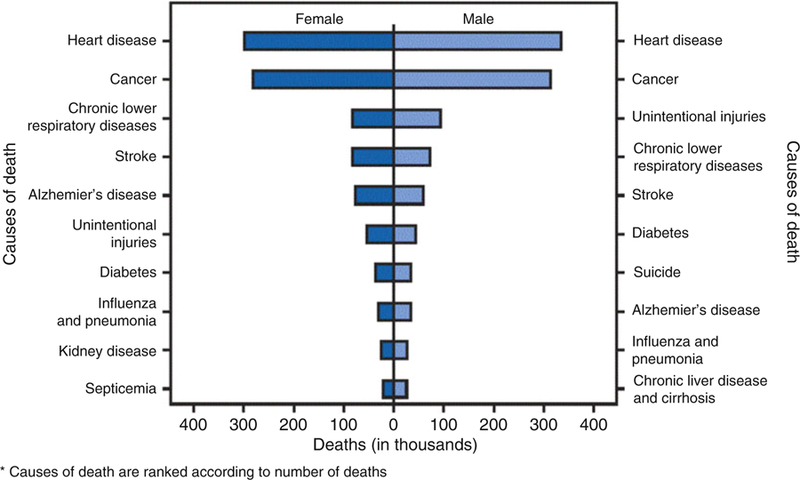
Number of deaths from ten leading causes, by sex (United States 2015). (Source: National Vital Statistics System, Centers for Disease Control and Prevention, 2015 [4])
Indeed, as demonstrated by recent national [1, 4] and global [2, 3] statistics, IHD poses a major threat to both women and men across their life spans. Emerging evidence supports that IHD may manifest differently in women and men, in ways ranging from the clinical presentation, diagnosis, and management of disease to the basic biology and biomechanics of cardiomyocyte function and the coronary circulation. This finding has led to calls to expand conventional tools developed more than a half-century ago for the diagnosis and management of (primarily obstructive) CAD to address the full spectrum of IHD impacting women as well as men. The following represents a state-of-the-art review of sex differences in the coronary system, with an eye toward how diverse pathophysiological factors and processes may contribute to IHD phenotypes prevalent in women and men.
Sex Differences in the Epidemiology of IHD: Reframing the “Gender Gap”
Over the last three decades, case fatality rates for heart disease in the United States have been similar or higher for women as compared to men (Fig. 2) [1]. Although this finding partly reflects that women outnumber men in older populations at greatest risk for IHD, women often present with a higher burden of comorbidities and experience worse IHD outcomes as compared to men. While trends in the United States suggest dramatic declines in cardiac deaths for both women and men over the last two decades, this decrease has not been uniform for all individuals, especially young women [8]. At the same time, important sex differences in the rates of IHD diagnosis, utilization of care, response to therapy, and clinical outcomes have been described [9–12]. Compared with men, women have a higher prevalence of persistent angina, nonobstructive CAD, coronary microvascular dysfunction (CMD), spontaneous coronary artery dissection, stress-induced cardiomyopathy, and heart failure with preserved ejection fraction (HFpEF) [13–21]. IHD risk factors including diabetes mellitus [22] and atrial fibrillation [23] are associated with higher rates of vascular complications in women versus men. Women presenting with acute coronary syndromes experience higher mortality as compared with men [24–27] and are referred for cardiac transplantation at later stages of heart failure [28]. There is also underutilization in women of cardiac devices [29], including implantable cardiac defibrillators [30] and cardiac resynchronization therapy (CRT) [31], despite subgroup analyses of randomized controlled trial data showing that female sex is associated with improved responsiveness to CRT [32].
Fig. 2.
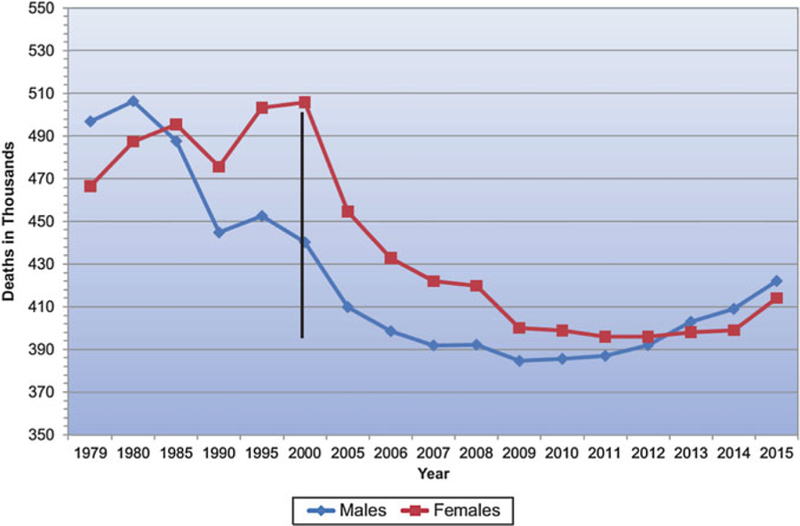
Cardiovascular disease mortality trends for males and females (United States 1979–2015). (Source: National Center for Health Statistics and National Heart, Lung, and Blood Institute [1])
Thus, the previous assumption that heart disease in women is the same as that in men, only occurring about a decade later, represents an oversimplification and underscores the importance of sex-specific research in cardiovascular care [33]. There is urgent need for randomized and comparative trial data in female as well as male patients [34]. To date, women have been underrepresented in guideline-changing CVD research and trials (Fig. 3), often comprising less than a third of enrolled patients [35]. This phenomenon has obscured important sex-specific differences in the pathobiology of disease, which are now becoming more apparent. Central among these has been the recognition that a variety of disorders, not simply obstructive CAD, may result in ischemic symptoms and worse cardiac outcomes in patients, especially women [6, 10, 36]. As such, the heart disease “gender gap” likely reflects not only inconsistencies in awareness and application of guideline-directed management of CAD in women but also fundamental limitations in how heart disease is defined and managed in the population. Beyond providing women and men with equitable optimal cardiovascular care according to current paradigms, understanding the underlying biology of IHD beyond a conventional focus on obstructive CAD is needed to address what is likely a combination of biological as well as environmental determinants of their prognosis.
Fig. 3.
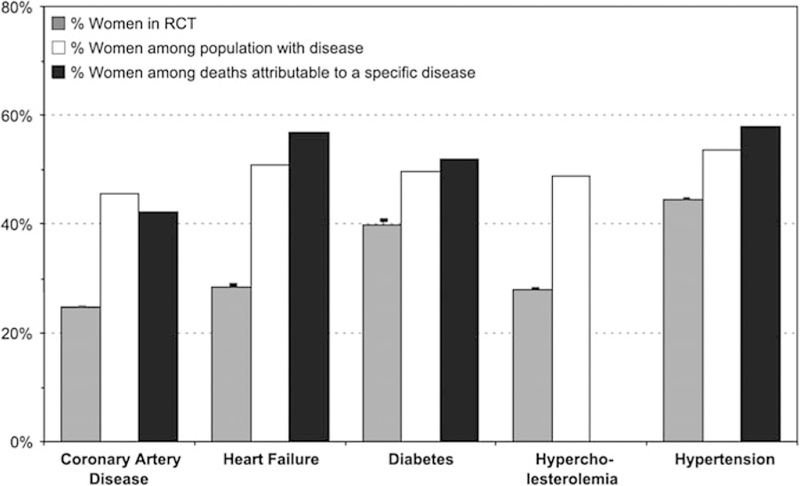
Percentage of women enrolled in randomized clinical trials (RCTs) of cardiovascular disease, compared with relevant disease and death statistics. (Reproduced with permission [35])
Sex Differences in Risk Factors of IHD
Traditional IHD disease risk factors include older age, smoking, hypertension, hyperlipidemia, obesity, insulin resistance, and a family history of premature atherosclerosis. These factors play important roles in the development of IHD in both women and men, although the prevalence of certain risk factors differ between the sexes, and some are stronger predictors of IHD in women. The overall incidence of IHD in women lags that in men by about a decade, suggesting loss of a cardioprotective effect in postmenopausal women [37] that remains incompletely understood. Differences in the pattern of smoking between women and men have decreased over time [1], and smoking appears to confer a 25% increased adjusted relative risk of major adverse cardiovascular events (MACE) in women versus men, as reported in a meta-analysis of 86 prospective trials encompassing 3.9 million participants [38].
Systolic blood pressure rises steeply in older women, and mild-moderate hypertension has been associated with more cardiovascular complications in women than men (risk-factor-adjusted hazard ratio of 2.5 versus 1.6 in women and men, respectively) [39]. Nonetheless, no apparent sex difference was observed in the relationship of systolic blood pressure and MACE in a contemporary meta-analysis of 124 cohort studies of 1.2 million individuals, 44% of whom were women [40]. Although female lipid profiles worsen with menopause, elevated total cholesterol seems to confer a clinically similar (albeit statistically lower) risk of MACE in women as in men, according to a recent meta-analysis of 97 cohort studies including over 1 million individuals [41]. Despite a higher prevalence of obesity in nonwhite women as compared to nonwhite men [1], no significant sex differences have been observed between increasing body mass index (BMI) and adjusted risk of MACE [42].
In contrast, although the prevalence of diabetes mellitus is now similar in women and men, robust evidence exists for a greater excess risk of IHD among diabetic women than men. The relative risk for fatal IHD associated with diabetes was 40–50% higher in women than men, as reported in meta-analyses of up to 64 prospective cohort studies of nearly 860,000 diabetic patients [22, 43]. Diabetes appears to nullify any cardioprotective effects associated with younger age in women. The transition from normoglycemia to overt diabetes in women appears to accompany a greater decline in health than in men, such that women who develop diabetes experience a heavier burden of risk factors, including a higher BMI, than diabetic men.
In summary, while the effects of hypertension, hyperlipidemia, and obesity appear mostly similar between the sexes, prolonged smoking and diabetes seem significantly more hazardous for women than men. The mechanisms underlying these observed sex differences in risk factor effects are not well understood. Besides menopause, additional female-specific or female-predominant risk factors for IHD include: (1) pregnancy-related complications [44], such as gestational diabetes, pregnancy-induced hypertension, and preeclampsia; (2) emotional stress [45], such as that linked to stress cardiomyopathy [46]; and (3) autoimmune disorders characterized by chronic inflammation, such as systemic lupus erythematosus and rheumatoid arthritis [47], which underscore the pathological role of inflammation in atherosclerosis [48, 49].
Sex Differences in Clinical Presentation, Diagnosis and Management of IHD
Early reported sex differences [50] in the clinical presentation, diagnosis and management of IHD have been influenced by the long-standing practice of defining heart disease as obstructive CAD and tailoring diagnostic and treatment strategies to this subset of patients. We now recognize that obstructive CAD, as defined anatomically on coronary angiography, is neither necessary nor sufficient to explain symptoms of IHD, which commonly include angina and dyspnea in both women and men [51, 52]. Indeed, women present more frequently than men with symptoms of angina [14] but are less likely to manifest anatomic obstructive CAD. In a contemporary cohort of 11,223 symptomatic patients (42% women) referred for non-urgent coronary angiography, one-third of men, but two-thirds of women, had no obstructive CAD (Fig. 4), and these patients still experienced elevated risk of MACE (Fig. 5) [15]. Among patients with stable angina who are found to have obstructive CAD, sex differences also exist in the extent and severity of disease, with women less likely than men to have obstructive multivessel disease [36,53]. Consistent sex differences in angiographic findings have been demonstrated not only in stable IHD but also in patients presenting with acute coronary syndromes, including unstable angina and MI [5456]. In autopsy evaluations of patients who died of IHD, women also demonstrated less extensive and less obstructive CAD than men, despite pathologic evidence of MI [57], with more evidence of plaque erosion than plaque rupture [58]. In addition, in patients with obstructive CAD, women are less likely than men to demonstrate coronary collateralization [59]. Despite consistently documented lower angiographic disease burden and more often preserved left ventricular (LV) function as compared with men, women with IHD have similar adverse outcomes [1, 60].
Fig. 4.
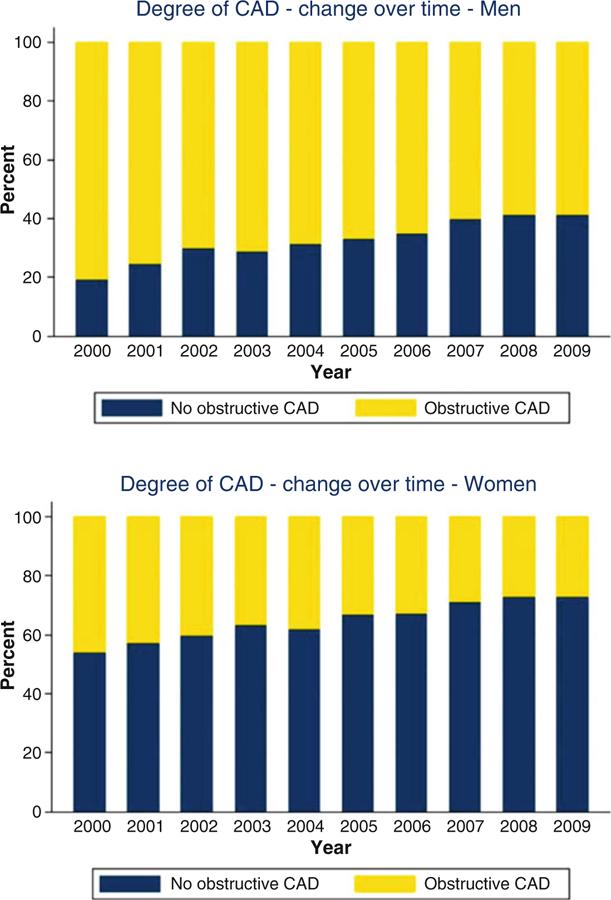
Degree of coronary artery disease (CAD) by sex and year of invasive coronary angiography examination. (Reproduced with permission [15])
Fig. 5.
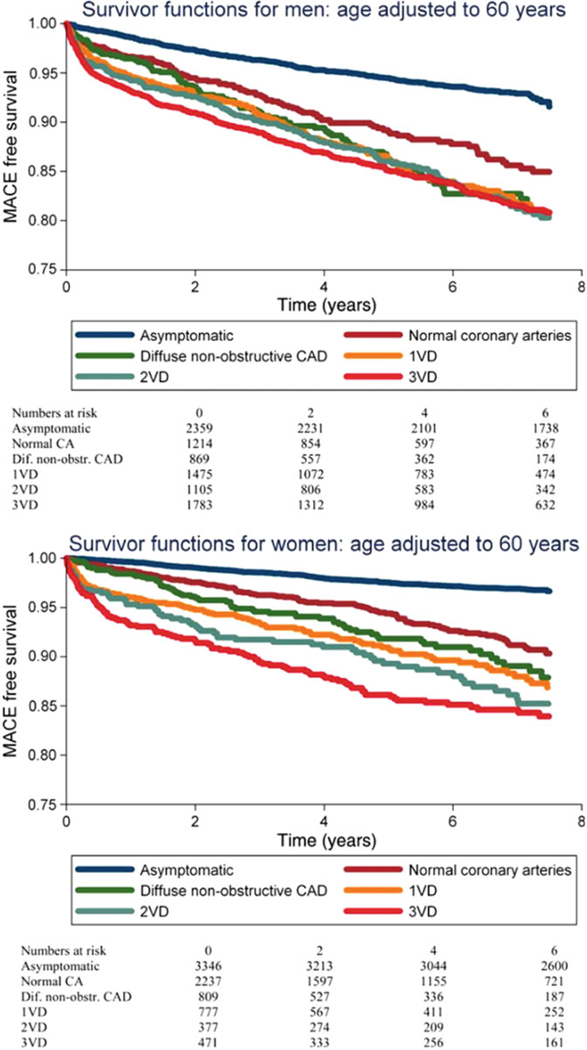
Major adverse cardiovascular event (MACE)-free survival by sex and vessel disease (VD) involvement on invasive coronary angiography. (Reproduced with permission [15])
Due to a greater symptom burden and rate of functional disability in women, coupled with a lower prevalence of obstructive CAD by coronary angiography, the evaluation of IHD in women as compared with men can present unique challenges to clinicians. The accuracy of standard noninvasive diagnostic testing for ischemia, such as stress testing with exercise electrocardiography, echocardiography, or nuclear myocardial perfusion imaging (MPI), can vary significantly when evaluated against a gold standard of finding anatomic obstructive CAD. This has been particularly relevant for women, whose symptoms are less likely to be explained by findings on coronary angiography and whose abnormal stress tests in the absence of obstructive CAD are more likely to be interpreted as “false positives” [61, 62]. Yet, women with angina and confirmed ischemia have increased mortality from IHD [63]. As discussed later on, the recent application of modern clinical diagnostic tools, such as cardiac computed tomography angiography (CCTA) and positron emission tomography (PET), is changing the paradigm of how disease is diagnosed [64, 65], broadening definitions of CAD and ischemia, respectively, to better reflect pathological phenotypes more prevalent in certain patients, especially women.
Management strategies for IHD, with the goal of ameliorating symptoms and improving survival, have also largely been the same for women and men while focused predominantly on obstructive CAD. As a function of this, symptomatic women are less likely than men to be candidates for invasive approaches, such as percutaneous coronary intervention (PCI), which targets flow-limiting obstructive coronary artery stenoses, and particularly coronary artery bypass grafting (CABG), which is most often reserved for multivessel obstructive disease. These individuals may present with severe ischemia or acute MI prior to being diagnosed with nonobstructive coronary arteries (phenomena recently described as INOCA [66] and MINOCA [67, 68], respectively), comprising an even higher-risk subset of patients. Furthermore, women undergoing PCI or standard treatment for acute MI demonstrate increased risk of bleeding and vascular complications as compared to men [69, 70]. This finding suggests that the beneficial impact of invasive interventions, particularly in stable IHD, may be lower in women if not tailored to key biological differences involving not only their burden of obstructive atherosclerosis, but also the size of their vessels, effective circulating volumes, and metabolism of drugs.
Separate from differences in biology, there have been multiple reports of sex differences in the use of evidence-based interventions, including contemporary guideline-directed medical therapies (GDMT) [10]. In a 2002 survey of 3779 patients (42% female) with stable angina, women were less likely than men to receive optimal secondary prevention with antiplatelet and lipid-lowering therapies, even after angiographic documentation of disease [53]. At 1-year follow-up, women with confirmed CAD were less likely than men to report complete resolution of angina and more than twice as likely to suffer death or nonfatal MI, even after multivariable adjustment for baseline risk factors including age, the presence of diabetes, abnormal LV function and severity of CAD, or interim revascularization. In 49,358 older patients hospitalized for CAD from 2003 to 2009, women (47%) were less likely than men to receive optimal GDMT at discharge and more likely to have higher mortality if they received suboptimal care [71]. Of note, authors found that the sex disparity in mortality disappeared with optimal care, and up to 69% of the excess mortality observed in older women could potentially be reduced by providing equitable optimal care, including timely initiation of aspirin, lipid-lowering medications and smoking cessation counseling. This finding is important because sex differences in response to medical therapies have been described, for example, greater coronary atheroma regression in response to high-intensity statins [72, 73] and greater benefit in cardiovascular outcomes with lesser degrees of LDL reduction [74] in women compared with men. These data underscore that women (and men) may especially benefit from a tailored approach that (1) achieves not only equitable standards of quality care as currently defined, (2) but also addresses fundamental differences in their biological and pathophysiologic manifestations of IHD.
Sex Differences in Coronary Anatomy and Function
Sex Differences in Coronary Artery Size and Blood Flow
The coronary arterial system represents a continuous network of functionally distinct vessel segments of decreasing size (Fig. 6) [75, 76]. The proximal large epicardial coronary arteries (400 µm to 2–5 mm in diameter) give way to small prearterioles (100–400 µm) and smaller intramural arterioles (<100 µm), which interface directly with the coronary capillary bed (<10 µm). The epicardial arteries have a primary capacitance function and exhibit minimal resistance to coronary flow under normal conditions, with their diameter primarily regulated by shear stress. In contrast, the prearterioles and arterioles make up most of the resistance circuit of the heart. Specifically, the prearterioles serve a critical role in the autoregulation of coronary blood flow via changes in vascular tone in response to local shear stress and luminal pressure changes, and the arterioles are instrumental in the matching of myocardial oxygen demand with blood supply via changes in perfusion of the low-resistance coronary capillary bed in response to local tissue metabolism [77, 78].
Fig. 6.
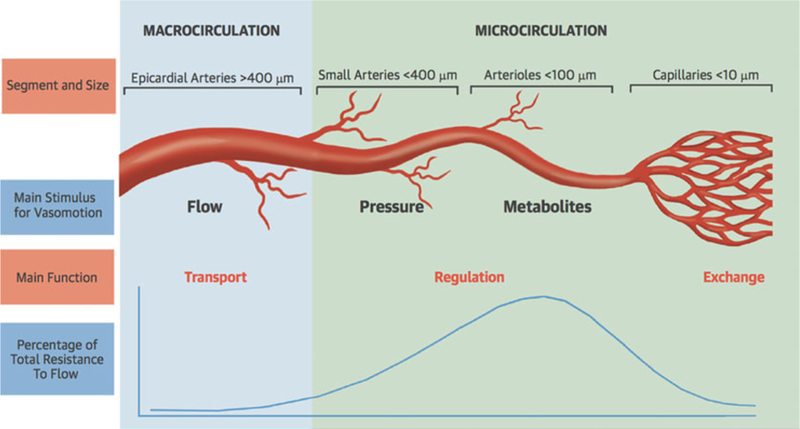
Schematic of the anatomy and function of the coronary circulation. (Reproduced with permission [76])
Women have smaller epicardial coronary arteries than men, even after accounting for body habitus and LV mass [79–81]. In 710 patients (46% female) being evaluated for suspected CAD with CCTA and found to have limited coronary artery calcium scores (CAC, <100), women demonstrated significantly smaller diameters of all major epicardial coronary arteries, and these differences persisted after multivariable adjustment for age, BMI, body surface area, and LV mass [80]. Myocardial perfusion, or coronary blood flow, is typically also higher in women than in men at both rest and under hyperemic conditions [16, 82, 83]. In 1218 patients (67% female) being evaluated for suspected CAD with stress testing using PET and found to have no evidence of myocardial perfusion defects, women demonstrated higher coronary blood flows at both rest and peak stress when averaged over the entire left ventricle [16], resulting in a similar global coronary flow reserve (CFR) when compared to men.
With changes in blood flow, coronary arteries demonstrate an intrinsic tendency to maintain a given level of shear stress by endothelial-dependent dilatation [84]. Endothelial shear stress is dynamic and can change in response to plaque formation and vascular remodeling [85]. Low endothelial shear stress has been implicated as a catalyst for focal lipid accumulation, inflammation, oxidative stress, matrix breakdown, and pathologic expansive remodeling with associated plaque instability [86, 87]. Gould and others [88] have hypothesized that higher coronary blood flow in women, coupled with their smaller coronary arteries, may result in clinically significant higher endothelial shear stress conditions, which may contribute to sex differences in susceptibility to coronary atherosclerosis. This phenomenon may be especially relevant earlier in the life cycle prior to the withdrawal of estrogens, which may interact with pressure-and shear stress-dependent mechanisms of arteriolar vasomotor function to impact upon release of endothelial mediators including nitric oxide, prostaglandins, and endothelium-derived hyperpolarizing factor [89]. The burden of coronary atherosclerosis is indeed lower in women than in men, particularly at younger ages. The distribution of CAC stratified by age in 9341 asymptomatic study participants (40% female, median age 54 ± 10 years) is illustrated in Fig. 7, with women demonstrating a slower rate of rise in CAC over the life span [90]. A similar pattern has been shown in asymptomatic cohorts from the Framingham Heart Study [91] and the Multi-Ethnic Study of Atherosclerosis [92].
Fig. 7.
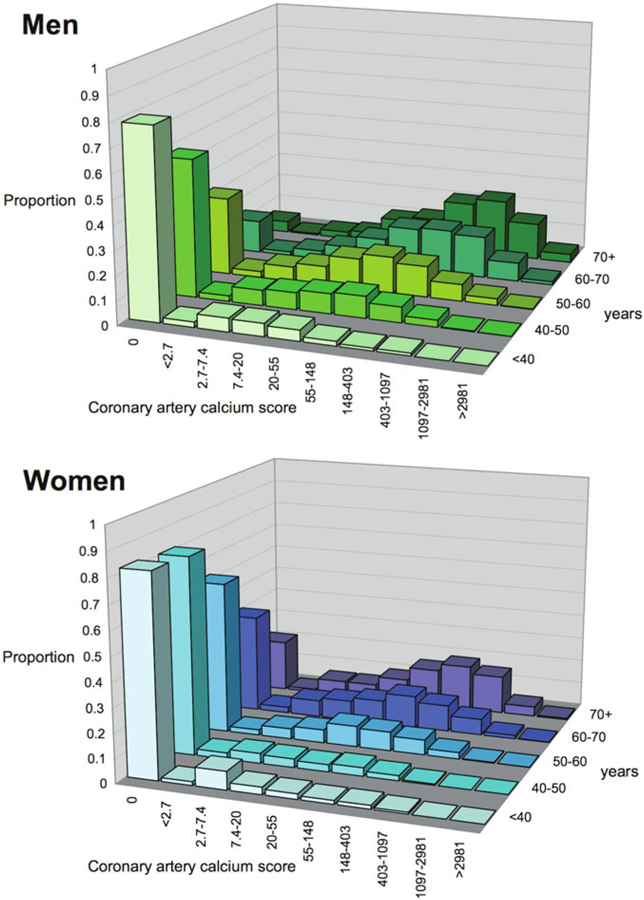
Distribution of coronary artery calcium scores (logarithmic scale) by sex and age in asymptomatic individuals. (Reproduced with permission [90])
Differences in shear stress and their associated effects on mechanoreceptor-induced intracellular cascades may affect not only the susceptibility to, but also the anatomical pattern of CAD in women and men. While the mitigating effects of high shear stress on atheroma progression may not entirely prevent the development of CAD in susceptible patients, they may modify it into a diffuse pattern of disease with decreased contributions from focal obstruction. Such an effect would be consistent with previously described clinical and pathological observations of sex differences in the presentation of IHD.
Nonobstructive Coronary Atherosclerosis and Coronary Microvascular Dysfunction
Growing data [15, 17, 36, 53–60, 93–99] support that the pathophysiology of IHD in women may vary as compared to that in men. Although women frequently have less anatomical obstructive CAD, they do not necessarily experience fewer IHD events. A major contributor to this apparent paradox may be vascular dysfunction in the form of abnormal coronary reactivity, which often coexists with diffuse, nonobstructive atherosclerosis and endothelial dysfunction [17, 36, 100]. Invasive coronary angiography using visual assessments of epicardial coronary luminal patency with X-ray and contrast dye remains a cornerstone of modern cardiovascular care for the diagnosis of obstructive CAD. However, this technique has limited ability to identify diffuse atherosclerosis and small-vessel dysfunction, which may contribute to MACE including acute coronary syndromes, heart failure and death from plaque erosion, impaired vaso-reactivity, and CMD with resultant myocardial ischemia [54, 56–58]. The addition of invasive fractional flow reserve, an assessment of the pressure drop across a focal epicardial stenosis, to coronary angiography has proven beneficial to identify lesion-specific ischemia and guide revascularization, [101] but may miss the integrated contribution of diffuse atherosclerosis and small-vessel disease to myocardial ischemia [102, 103]. It is now well recognized that a normal coronary angiogram is not synonymous with a normal coronary circulation. Testing for IHD, especially in women, is currently moving beyond testing for the presence or absence of obstructive epicardial CAD, which represents only one of several possible contributors to myocardial ischemia.
Over the last decade, the clinical integration of advanced diagnostic imaging tools is helping to redefine IHD and highlight the importance of nonobstructive CAD and CMD. Noninvasive approaches using CCTA and PET, for example, have provided very sensitive assessments for the evaluation of anatomic atherosclerotic plaque and functional ischemia, respectively. Regarding atherosclerotic plaque, recent observational studies have revealed stepwise incremental risk for future adverse events in patients along a continuum of both severity (i.e., mild, moderate, or severe stenosis) and extent (i.e., number of involved segments or vessels) of CAD [15, 104–106]. Accelerated by the growth of noninvasive CCTA, which both increases test sensitivity for the diagnosis of CAD and enables characterization of plaque morphology, mounting evidence now supports that (1) the presence of any atherosclerotic plaque, obstructive or not, portends increased risk of events and (2) the higher the overall plaque burden that is present, the higher the risk. From the international multicenter CONFIRM registry, in which 23,854 consecutive patients without known CAD (33% asymptomatic) underwent CCTA between 2005 and 2009, per-patient nonobstructive and obstructive CAD conferred increased risk of mortality in a stepwise manner as compared with absence of CAD, which was associated with very low risk [106]. In a subsequent sex-specific subgroup analysis of patients with no or nonobstructive CAD (n = 18,158), nonobstructive CAD was associated with a modest adjusted risk of MACE (composite of death and nonfatal MI) that was similarly increased in both women and men [107]. Further, in a smaller subset of patients with longer follow-up (n = 5632 followed for 5 years, 30% with nonobstructive CAD), there was no interaction of sex on the association between per-vessel extent of obstructive CAD and MACE, and authors concluded that exploratory analyses of atherosclerotic burden did not identify sex-specific patterns predictive of MACE [108]. Thus, women and men with comparable risk and extent of CAD had comparable prognosis. Nonetheless, prevalent patterns of disease do differ between women and men, with more symptomatic women than men manifesting nonobstructive rather than obstructive CAD [15, 60], with important implications for diagnosis and management.
Neither conventional angiography nor CCTA can detect CMD, which is defined not anatomically, but functionally as a reduced CFR in the absence of flow-limiting CAD. Global CFR, calculated as the ratio of hyperemic to rest absolute myocardial blood flow averaged over the left ventricle, is an integrated marker of coronary vasomotor dysfunction that measures the hemodynamic effects of focal, diffuse, and small-vessel CAD on myocardial tissue perfusion [82, 109] and has emerged as an important prognostic imaging marker of cardiovascular risk. Observational data have consistently shown that CFR measurements using PET MPI distinguish patients at low or high risk for MACE, including cardiac death [110–113], beyond comprehensive clinical assessment, LVEF, myocardial perfusion defects, low-level troponin elevation [96], or plaque severity on invasive coronary angiography [114]. In parallel to findings with atherosclerotic plaque, evidence now supports that (1) the existence of impaired CFR, whether in the presence or absence of obstructive CAD (e.g., CMD), portends increased risk of cardiovascular events and (2) the more severely impaired the overall global CFR, the higher the risk. Whereas a CFR >2 effectively excluded high-risk angiographic CAD and was associated with low rates of annualized cardiac death [115], event rates for patients with CFR <2 increased exponentially as CFR decreased [7, 112, 116]. Because CFR is a measure of not only the effects of epicardial CAD but also of diffuse atherosclerosis and CMD on myocardial tissue perfusion, worse prognosis in patients with CFR <2 may be related to coronary vasomotor dysfunction arising from a mix of pathophysiologic CAD phenotypes.
These findings highlight the morbidity associated with diffuse atherosclerosis and CMD. Similar to CTA findings for nonobstructive CAD, cardiac PET MPI studies support that CMD is common in symptomatic patients, affecting approximately 50% of patients with normal MPI and LVEF referred for testing [16]. Furthermore when present, CMD was associated with MACE independently of sex (Fig. 8) [16]. That is to say, both women and men with CMD (CFR <2 in the presence of normal cardiac perfusion) experienced worse outcomes, although this phenotype was twice as prevalent in women as in men. This result was consistent even in patients with a CAC score of 0 [16], and global CFR, but not CAC, provided significant incremental risk stratification over clinical risk score for prediction of MACE [117]. Thus, symptomatic patients who do not demonstrate regional ischemia associated with flow-limiting CAD may have diffuse atherosclerosis and CMD for which a more sensitive, quantitative assessment of ischemia may better diagnose abnormalities and identify novel targets for systemic therapies. Although not a uniquely female disorder, this pattern of abnormalities may be more prognostically useful in women because they often occur absent obstructive CAD, and may be especially relevant in those with diabetes and/or metabolic syndrome, MINOCA, INOCA, and HFpEF.
Fig. 8.
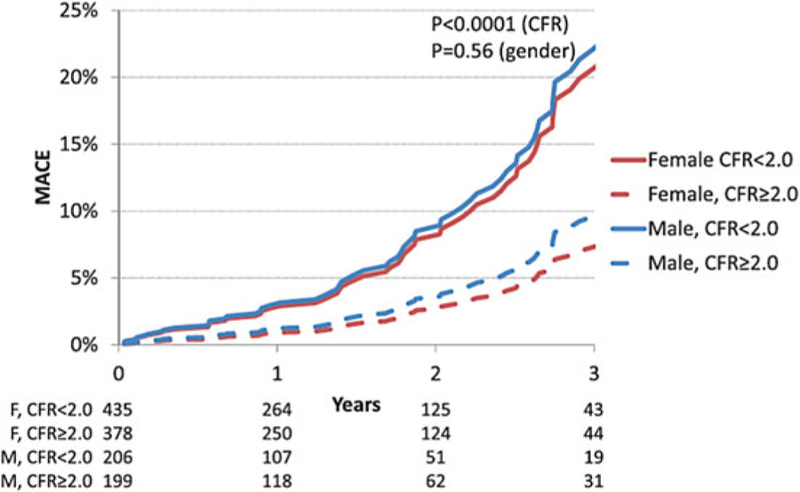
Adjusted cumulative rate of major adverse cardiac events (MACE) by sex and coronary flow reserve (CFR) in patients with normal myocardial perfusion. (Reproduced with permission [16])
There is also emerging evidence that women with very low CFR may be at an especially elevated risk of cardiovascular events. In symptomatic patients referred for invasive coronary angiography after PET MPI, women had a lower pretest probability of CAD and a lower burden of obstructive CAD relative to men, but were not protected from MACE (Fig. 9a, b) [36], consistent with previously described epidemiologic trends. Whereas outcomes for men were more closely associated with presence or absence of severely obstructive CAD (Fig. 9c, d), in women, only those with impaired CFR demonstrated a significantly increased adjusted risk of MACE (Fig. 9e, f). The excess cardiovascular risk in women relative to men referred for coronary angiography was independently associated with and mediated by impaired CFR, not obstructive CAD (p for interaction = 0.04, Fig. 10a). Whereas most men with severely impaired CFR were found to have >1 vessel CAD on coronary angiography, most women with similarly impaired CFR demonstrated <1 vessel CAD (Fig. 10b). In adjusted analysis, approximately 40% of this observed differential effect of sex on outcomes was mediated by CFR [36].
Fig. 9.
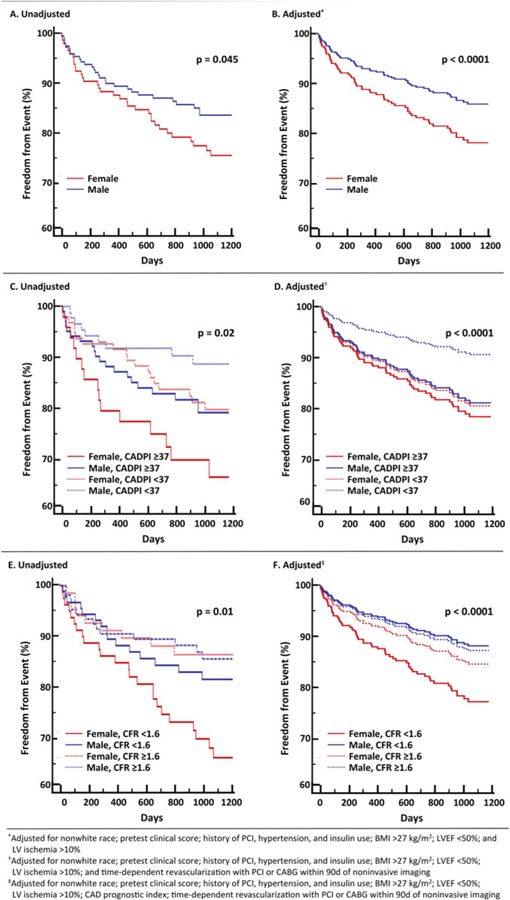
Freedom from major adverse cardiovascular events (MACE) according to sex (a and b), sex and angiographic disease (c and d), or sex and coronary flow reserve (e and f) in patients referred for myocardial perfusion imaging and invasive coronary angiography. (Reproduced with permission [36])
Fig. 10.
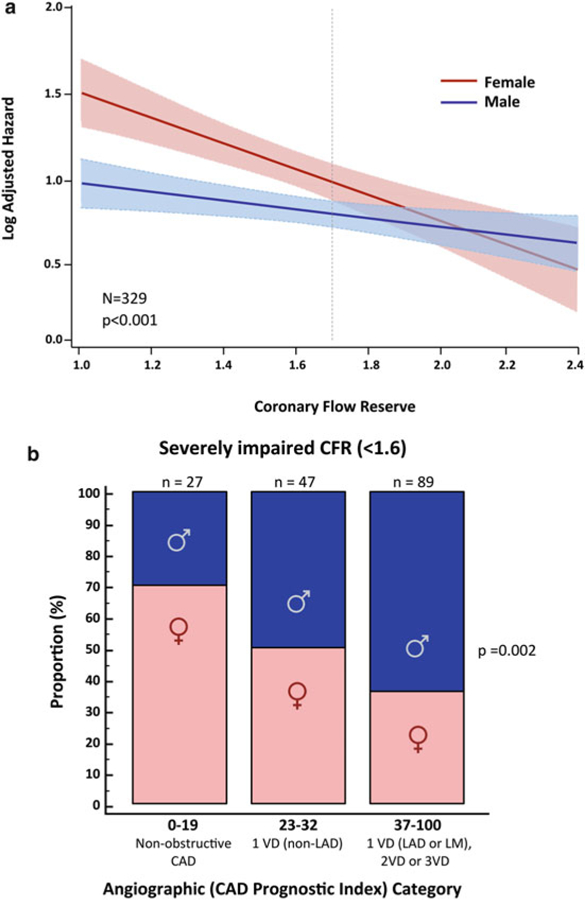
Log adjusted hazard for major adverse cardiovascular events (MACE) by sex and coronary flow reserve (CFR), (a) Patients with severely impaired CFR (<1.6) by angiographic disease and sex categories, (b) CAD indicates coronary artery disease, VD vessel disease, LAD left anterior descending artery, LM left main artery. (Reproduced with permission [36])
A very low CFR may represent a crucial link to understanding the hidden biological risk of IHD among women. Previous data [114] support that revascularization, especially by CABG, in certain individuals with severely impaired CFR may be beneficial. That sex differences on outcomes of cardiovascular events are amplified in those with severely impaired CFR further suggests that certain patients (i.e., with very low CFR and less obstructive CAD, a phenotype more prevalent in women and less amenable to focal revascularization) may be at especially high risk (Fig. 11) [36]. Instead of being interpreted as demonstrating a “false positive” (or in some cases negative) traditional ischemic evaluation, patients with impaired CFR and less obstructive CAD may be at significantly increased CVD risk despite having access to revascularization, a tool fundamentally targeted to the management of obstructive CAD. Thus, while providing optimal, equitable guideline-directed care remains a critical goal for managing women with IHD, doing so according to current paradigms may be insufficient to address what is likely a combination of biological as well as environmental determinants of their prognosis. Additional research is needed to determine precisely what is optimal care for this subset of vulnerable patients with a predominance of women.
Fig. 11.
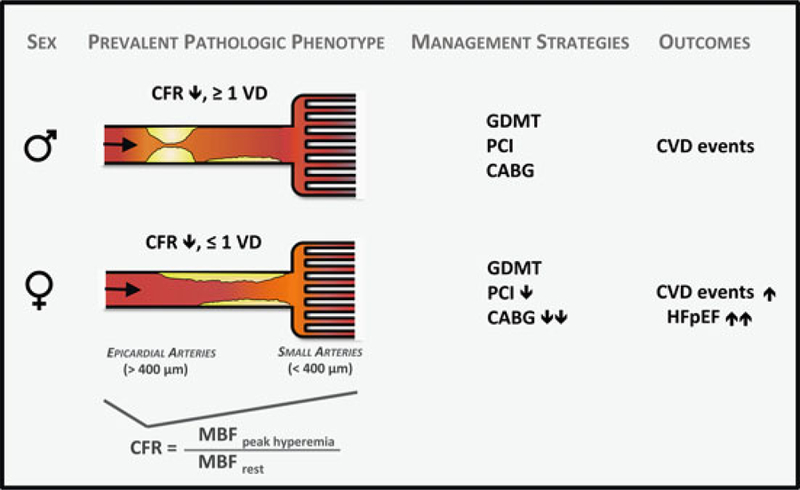
Conceptual model of prevalent pathological phenotypes in women and men with ischemic heart disease and possible impact on cardiovascular management strategies and outcomes. CABG indicates coronary artery bypass surgery, CFR coronary flow reserve, CVD cardiovascular disease, GDMT guideline-directed medical therapy, MBF myocardial blood flow, PCI percutaneous coronary intervention, and VD vessel disease. (Reproduced with permission [36])
Remaining Knowledge Gaps and Future Directions
We have come to recognize that for a majority of patients seeking an explanation for their angina in the cardiac catheterization laboratory, no significantly obstructing lesion are found [118], and a substantial proportion of these patients are women. Nonetheless, these patients may still have significant atherosclerosis and ischemia, and their prognosis is not necessarily benign. Women as compared to men commonly present with higher rates of baseline comorbidities, including not only older age but also hypertension, diabetes mellitus, obesity, chronic renal failure, peripheral vascular disease, HFpEF, and inflammatory diseases such as rheumatoid arthritis [10, 25–27, 54–56]. Many of these disease processes are associated with diffuse atherosclerosis and microvascular ischemia, and parallel themes are emerging between nonobstructive CAD and CMD. In particular, two key findings regarding these entities in symptomatic patients have emerged: (1) they are common in both women and men but more prevalent in women, and (2) they are associated with similarly increased risk of IHD events in both women and men, except for in patients with severely impaired CFR, where women demonstrate even greater risk [36].
This latter subgroup is particularly interesting and represents a clinically unmet need that is ripe for future investigation. Specifically, in cases where impaired CFR stems not from obstructive CAD (with no opportunity for revascularization to mitigate CVD risk), a novel therapeutic strategy to systemically target IHD may be warranted. These cases of severe CMD, which often coexist with nonobstructive CAD, may provide a clue as to a common mechanism underlying IHD risk in both women and men. Such a mechanism may involve inflammation [119], endothelial dysfunction [120], and increased cardiomyocyte oxygen demand with ensuing microvascular ischemia, myocardial injury [96], and impaired cardiac mechanics [97, 121]. Thus, clearer understanding of the relationship between coronary vasomotor dysfunction and CAD comorbid conditions, including insulin resistance and heart failure, may guide development of novel systemic therapies to harness the benefit of more “complete revascularization” [114, 122–125] in a manner not defined by anatomy alone. As such, new imaging tools may represent important biomarkers not only for prospective studies evaluating the role of ischemia and revascularization, but also of novel anti-inflammatory [126, 127], lipid-lowering [128], glucose-lowering [129], and neurohormonal-modulating [130] agents on cardiovascular outcomes.
Conclusion
While prominent sex differences are apparent in certain anatomical measures of CAD, IHD represents a continuum of disease in women and men, with both anatomical and functional manifestations. The integration of functional and anatomical parameters associated with CAD (i.e., quantification of coronary flow reserve with visualization of anatomic atherosclerotic plaque) may enhance our understanding of sex differences in cardiac risk and lead to improved algorithms for diagnosing and treating women and men with IHD. Many of the hypothesis-generating studies reviewed here illustrate the importance of performing sex-specific analyses to advance beyond current limitations in cardiovascular care. Insights like these may inform future trials and, possibly, the implementation of sex-specific thresholds within clinical guidelines for more optimal patient management. Doing so may lead us to a more complete understanding of the pathobiology of IHD and its manifestations in large subsets of patients, reframing sex-specific medicine as a form of precision medicine with the goal of improving outcomes for all.
Acknowledgments
Funding This work was supported by NIH K23HL135438 to Dr. Taqueti.
Footnotes
Disclosures None.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, de Ferranti SD, Ferguson JF, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Lutsey PL, Matchar DB, Matsushita K, Mussolino ME, Nasir K, O’Flaherty M, Palaniappan LP, Pandey DK, Reeves MJ, Ritchey MD, Rodriguez CJ, Roth GA, Rosamond WD, Sampson UKA, Satou GM, Shah SH, Spartano NL, Tirschwell DL, Tsao CW, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation 2018;137:e67–492. [DOI] [PubMed] [Google Scholar]
- 2.Atlas Writing Group, Timmis A, Townsend N, Gale C, Grobbee R, Maniadakis N, Flather M, Wilkins E, Wright L, Vos R, Bax J, Blum M, Pinto F, Vardas P. European society of cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508–79. [DOI] [PubMed] [Google Scholar]
- 3.The top 10 causes of death - World Health Organization, 2015. Fact sheet, updated Jan 2017. http://www.who.int/mediacentre/factsheets/fs310/en/
- 4.Number of deaths from 10 leading causes, by sex — National Vital Statistics System, United States, 2015. Morb Mortal Wkly Rep 2017;66:413 10.15585/mmwr.mm6615a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosca L, Hammond G, Mochari-Greenberger H, Towfighi A, Albert MA, American Heart Association Cardiovascular D, Stroke in W, Special Populations Committee of the Council on Clinical Cardiology CoE, Prevention CoCNCoHB. Fifteen-year trends in awareness of heart disease in women: results of a 2012 American heart association national survey. Circulation 2013;127:1254–63. e1251–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marzilli M, Merz CN, Boden WE, Bonow RO, Capozza PG, Chilian WM, DeMaria AN, Guarini G, Huqi A, Morrone D, Patel MR, Weintraub WS. Obstructive coronary atherosclerosis and ischemic heart disease: an elusive link! J Am Coll Cardiol 2012;60:951–6. [DOI] [PubMed] [Google Scholar]
- 7.Taqueti VR, Di Carli MF. Clinical significance of noninvasive coronary flow reserve assessment in patients with ischemic heart disease. Curr Opin Cardiol 2016;31:662–9. [DOI] [PubMed] [Google Scholar]
- 8.Wilmot KA, O’Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation 2015;132:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wenger NK. Clinical characteristics of coronary heart disease in women: emphasis on gender differences. Cardiovasc Res 2002;53:558–67. [DOI] [PubMed] [Google Scholar]
- 10.Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol 2009;54:1561–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bairey Merz CN. Sex, death, and the diagnosis gap. Circulation 2014;130:740–2. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs AK. Coronary intervention in 2009: are women no different than men? Circ Cardiovasc Interv 2009;2:69–78. [DOI] [PubMed] [Google Scholar]
- 13.Gulati M, Cooper-DeHoff RM, McClure C, Johnson BD, Shaw LJ, Handberg EM, Zineh I, Kelsey SF, Arnsdorf MF, Black HR, Pepine CJ, Merz CN. Adverse cardiovascular outcomes in women with nonobstructive coronary artery disease: a report from the women’s ischemia syndrome evaluation study and the St James women take heart project. Arch Intern Med 2009;169:843–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hemingway H, Langenberg C, Damant J, Frost C, Pyorala K, Barrett-Connor E. Prevalence of angina in women versus men: a systematic review and meta-analysis of international variations across 31 countries. Circulation 2008;117:1526–36. [DOI] [PubMed] [Google Scholar]
- 15.Jespersen L, Hvelplund A, Abildstrom SZ, Pedersen F, Galatius S, Madsen JK, Jorgensen E, Kelbaek H, Prescott E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur Heart J 2012;33:734–44. [DOI] [PubMed] [Google Scholar]
- 16.Murthy VL, Naya M, Taqueti VR, Foster CR, Gaber M, Hainer J, Dorbala S, Blankstein R, Rimoldi O, Camici PG, Di Carli MF. Effects of sex on coronary microvascular dysfunction and cardiac outcomes. Circulation 2014;129:2518–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pepine CJ, Anderson RD, Sharaf BL, Reis SE, Smith KM, Handberg EM, Johnson BD, Sopko G, Bairey Merz CN. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the national heart, lung and blood institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol 2010;55:2825–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis SE, Holubkov R, Conrad Smith AJ, Kelsey SF, Sharaf BL, Reichek N, Rogers WJ, Merz CN, Sopko G, Pepine CJ, Investigators W. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am Heart J 2001;141:735–41. [DOI] [PubMed] [Google Scholar]
- 19.Scantlebury DC, Borlaug BA. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr Opin Cardiol 2011;26:562–8. [DOI] [PubMed] [Google Scholar]
- 20.Templin C, Ghadri JR, Diekmann J, Napp LC, Bataiosu DR, Jaguszewski M, Cammann VL, Sarcon A, Geyer V, Neumann CA, Seifert B, Hellermann J, Schwyzer M, Eisenhardt K, Jenewein J, Franke J, Katus HA, Burgdorf C, Schunkert H, Moeller C, Thiele H, Bauersachs J, Tschope C, Schultheiss HP, Laney CA, Rajan L, Michels G, Pfister R, Ukena C, Bohm M, Erbel R, Cuneo A, Kuck KH, Jacobshagen C, Hasenfuss G, Karakas M, Koenig W, Rottbauer W, Said SM, Braun-Dullaeus RC, Cuculi F, Banning A, Fischer TA, Vasankari T, Airaksinen KE, Fijalkowski M, Rynkiewicz A, Pawlak M, Opolski G, Dworakowski R, MacCarthy P, Kaiser C, Osswald S, Galiuto L, Crea F, Dichtl W, Franz WM, Empen K, Felix SB, Delmas C, Lairez O, Erne P, Bax JJ, Ford I, Ruschitzka F, Prasad A, Luscher TF. Clinical features and outcomes of takotsubo (stress) cardiomyopathy. N Engl J Med 2015;373:929–38. [DOI] [PubMed] [Google Scholar]
- 21.Tweet MS, Hayes SN, Pitta SR, Simari RD, Lerman A, Lennon RJ, Gersh BJ, Khambatta S, Best PJ, Rihal CS, Gulati R. Clinical features, management, and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–88. [DOI] [PubMed] [Google Scholar]
- 22.Huxley R, Barzi F, Woodward M. Excess risk of fatal coronary heart disease associated with diabetes in men and women: meta-analysis of 37 prospective cohort studies. BMJ 2006;332:73–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies. BMJ 2016;532:h7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Champney KP, Frederick PD, Bueno H, Parashar S, Foody J, Merz CN, Canto JG, Lichtman JH, Vaccarino V, Investigators N. The joint contribution of sex, age and type of myocardial infarction on hospital mortality following acute myocardial infarction. Heart 2009;95:895–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bucholz EM, Butala NM, Rathore SS, Dreyer RP, Lansky AJ, Krumholz HM. Sex differences in long-term mortality after myocardial infarction: a systematic review. Circulation 2014;130:757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gupta T, Kolte D, Khera S, Agarwal N, Villablanca PA, Goel K, Patel K, Aronow WS, Wiley J, Bortnick AE, Aronow HD, Abbott JD, Pyo RT, Panza JA, Menegus MA, Rihal CS, Fonarow GC, Garcia MJ, Bhatt DL. Contemporary sex-based differences by age in presenting characteristics, use of an early invasive strategy, and inhospital mortality in patients with non-ST-segment-elevation myocardial infarction in the United States. Circ Cardiovasc Interv 2018;11: e005735. [DOI] [PubMed] [Google Scholar]
- 27.Khera S, Kolte D, Gupta T, Subramanian KS, Khanna N, Aronow WS, Ahn C, Timmermans RJ, Cooper HA, Fonarow GC, Frishman WH, Panza JA, Bhatt DL. Temporal trends and sex differences in revascularization and outcomes of ST-segment elevation myocardial infarction in younger adults in the United States. J Am Coll Cardiol 2015;66:1961–72. [DOI] [PubMed] [Google Scholar]
- 28.Regitz-Zagrosek V, Petrov G, Lehmkuhl E, Smits JM, Babitsch B, Brunhuber C, Jurmann B, Stein J, Schubert C, Merz NB, Lehmkuhl HB, Hetzer R. Heart transplantation in women with dilated cardiomyopathy. Transplantation 2010;89:236–44. [DOI] [PubMed] [Google Scholar]
- 29.Taqueti VR, Bairey Merz CN. Sex-specific precision medicine: targeting CRT-D and other cardiovascular interventions to those most likely to benefit. Eur Heart J 2017;38:1495–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007;298:1525–32. [DOI] [PubMed] [Google Scholar]
- 31.Zusterzeel R, Selzman KA, Sanders WE, Canos DA, O’Callaghan KM, Carpenter JL, Pina IL, Strauss DG. Cardiac resynchronization therapy in women: US food and drug administration meta-analysis of patient-level data. JAMA Intern Med 2014;174:1340–8. [DOI] [PubMed] [Google Scholar]
- 32.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W, M-CTI. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med 2009;361:1329–38. [DOI] [PubMed] [Google Scholar]
- 33.Wenger NK, Ouyang P, Miller VM, Bairey Merz CN. Strategies and methods for clinical scientists to study sex-specific cardiovascular health and disease in women. J Am Coll Cardiol 2016;67:2186–8. [DOI] [PubMed] [Google Scholar]
- 34.Maas AH, van der Schouw YT, Regitz-Zagrosek V, Swahn E, Appelman YE, Pasterkamp G, Ten Cate H, Nilsson PM, Huisman MV, Stam HC, Eizema K, Stramba-Badiale M. Red alert for women’s heart: the urgent need for more research and knowledge on cardiovascular disease in women: proceedings of the workshop held in Brussels on gender differences in cardiovascular disease, 29 September 2010. Eur Heart J 2011;32:1362–8. [DOI] [PubMed] [Google Scholar]
- 35.Melloni C, Berger JS, Wang TY, Gunes F, Stebbins A, Pieper KS, Dolor RJ, Douglas PS, Mark DB, Newby LK. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes 2010;3:135–42. [DOI] [PubMed] [Google Scholar]
- 36.Taqueti VR, Shaw LJ, Cook NR, Murthy VL, Shah NR, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation 2017;135:566–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med 1999;340:1801–11. [DOI] [PubMed] [Google Scholar]
- 38.Huxley RR, Woodward M. Full hazards of smoking and benefits of stopping for women. Lancet 2013;381:96–8. [DOI] [PubMed] [Google Scholar]
- 39.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D. Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 2001;345:1291–7. [DOI] [PubMed] [Google Scholar]
- 40.Peters SA, Huxley RR, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke 2013;44:2394–401. [DOI] [PubMed] [Google Scholar]
- 41.Peters SA, Singhateh Y, Mackay D, Huxley RR, Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: a systematic review and meta-analysis. Atherosclerosis 2016;248:123–31. [DOI] [PubMed] [Google Scholar]
- 42.Emerging Risk Factors C, Wormser D, Kaptoge S, Di Angelantonio E, Wood AM, Pennells L, Thompson A, Sarwar N, Kizer JR, Lawlor DA, Nordestgaard BG, Ridker P, Salomaa V, Stevens J, Woodward M, Sattar N, Collins R, Thompson SG, Whitlock G, Danesh J. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet 2011;377:1085–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peters SA, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014;57:1542–51. [DOI] [PubMed] [Google Scholar]
- 44.Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA. Cardiovascular health after maternal placental syndromes (champs): population-based retrospective cohort study. Lancet 2005;366:1797–803. [DOI] [PubMed] [Google Scholar]
- 45.Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O’Connor CM, Velazquez EJ, Jiang W, Investigators R. Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the remit study. J Am Coll Cardiol 2014;64:1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation 2008;118:397–409. [DOI] [PubMed] [Google Scholar]
- 47.Symmons DP, Gabriel SE. Epidemiology of CVD in rheumatic disease, with a focus on RA and SLE. Nat Rev Rheumatol 2011;7:399–408. [DOI] [PubMed] [Google Scholar]
- 48.Taqueti VR, Ridker PM. Inflammation, coronary flow reserve, and microvascular dysfunction: moving beyond cardiac syndrome X. JACC Cardiovasc Imaging 2013;6:668–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taqueti VR, Ridker PM. Lipid-lowering and anti-inflammatory benefits of statin therapy: more than meets the plaque. Circ Cardiovasc Imaging 2017;10:e006676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med 1979;300:1350–8. [DOI] [PubMed] [Google Scholar]
- 51.Kreatsoulas C, Shannon HS, Giacomini M, Velianou JL, Anand SS. Reconstructing angina: cardiac symptoms are the same in women and men. JAMA Intern Med 2013;173:829–31. [DOI] [PubMed] [Google Scholar]
- 52.Canto JG, Goldberg RJ, Hand MM, Bonow RO, Sopko G, Pepine CJ, Long T. Symptom presentation of women with acute coronary syndromes: myth vs reality. Arch Intern Med 2007;167:2405–13. [DOI] [PubMed] [Google Scholar]
- 53.Daly C, Clemens F, Lopez Sendon JL, Tavazzi L, Boersma E, Danchin N, Delahaye F, Gitt A, Julian D, Mulcahy D, Ruzyllo W, Thygesen K, Verheugt F, Fox KM, Euro Heart Survey I. Gender differences in the management and clinical outcome of stable angina. Circulation 2006;113:490–8. [DOI] [PubMed] [Google Scholar]
- 54.Hochman JS, Tamis JE, Thompson TD, Weaver WD, White HD, Van de Werf F, Aylward P, Topol EJ, Califf RM. Sex, clinical presentation, and outcome in patients with acute coronary syndromes. Global use of strategies to open occluded coronary arteries in acute coronary syndromes IIB investigators. N Engl J Med 1999;341:226–32. [DOI] [PubMed] [Google Scholar]
- 55.Hochman JS, McCabe CH, Stone PH, Becker RC, Cannon CP, DeFeo-Fraulini T, Thompson B, Steingart R, Knatterud G, Braunwald E. Outcome and profile of women and men presenting with acute coronary syndromes: a report from TIMI IIIB. TIMI investigators. Thrombolysis in myocardial infarction. J Am Coll Cardiol 1997;30:141–8. [DOI] [PubMed] [Google Scholar]
- 56.Berger JS, Elliott L, Gallup D, Roe M, Granger CB, Armstrong PW, Simes RJ, White HD, Van de Werf F, Topol EJ, Hochman JS, Newby LK, Harrington RA, Califf RM, Becker RC, Douglas PS. Sex differences in mortality following acute coronary syndromes. JAMA 2009;302:874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smilowitz NR, Sampson BA, Abrecht CR, Siegfried JS, Hochman JS, Reynolds HR. Women have less severe and extensive coronary atherosclerosis in fatal cases of ischemic heart disease: an autopsy study. Am Heart J 2011;161:681–8. [DOI] [PubMed] [Google Scholar]
- 58.Arbustini E, Dal Bello B, Morbini P, Burke AP, Bocciarelli M, Specchia G, Virmani R. Plaque erosion is a major substrate for coronary thrombosis in acute myocardial infarction. Heart 1999;82:269–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Z, Pericak-Vance MA, Goldschmidt-Clermont P,Seo D, Wang L, Rundek T, Beecham GW. Coronary collateralization shows sex and racial-ethnic differences in obstructive artery disease patients. PLoS One 2017;12:e0183836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shaw LJ, Shaw RE, Merz CN, Brindis RG, Klein LW, Nallamothu B, Douglas PS, Krone RJ, McKay CR, Block PC, Hewitt K, Weintraub WS, Peterson ED, American College of Cardiology-National Cardiovascular Data Registry I. Impact of ethnicity and gender differences on angiographic coronary artery disease prevalence and in-hospital mortality in the American College of Cardiology-National Cardiovascular Data Registry. Circulation 2008;117:1787–801. [DOI] [PubMed] [Google Scholar]
- 61.Taqueti VR, Dorbala S, Wolinsky D, Abbott B, Heller GV, Bateman TM, Mieres JH, Phillips LM, Wenger NK, Shaw LJ. Myocardial perfusion imaging in women for the evaluation of stable ischemic heart disease: state-of-the-evidence and clinical recommendations. J Nucl Cardiol 2017;24:1402–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mieres JH, Gulati M, Bairey Merz N, Berman DS, Gerber TC, Hayes SN, Kramer CM, Min JK, Newby LK, Nixon JV, Srichai MB, Pellikka PA, Redberg RF, Wenger NK, Shaw LJ, American Heart Association Cardiac Imaging Committee of the Council on Clinical C, Cardiovascular I, Intervention Committee of the Council on Cardiovascular R, Intervention. Role of noninvasive testing in the clinical evaluation of women with suspected ischemic heart disease: a consensus statement from the American Heart Association. Circulation 2014;130:350–79. [DOI] [PubMed] [Google Scholar]
- 63.Hemingway H, McCallum A, Shipley M, Manderbacka K, Martikainen P, Keskimaki I. Incidence and prognostic implications of stable angina pectoris among women and men. JAMA 2006;295:1404–11. [DOI] [PubMed] [Google Scholar]
- 64.Taqueti VR, Dorbala S. The role of positron emission tomography in the evaluation of myocardial ischemia in women. J Nucl Cardiol 2016;23:1008–15. [DOI] [PubMed] [Google Scholar]
- 65.Paul TK, Sivanesan K, Schulman-Marcus J. Sex differences in nonobstructive coronary artery disease: recent insights and substantial knowledge gaps. Trends Cardiovasc Med 2017;27:173–9. [DOI] [PubMed] [Google Scholar]
- 66.Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Beltrame JF. Assessing patients with myocardial infarction and nonobstructed coronary arteries (MINOCA). J Intern Med 2013;273:182–5. [DOI] [PubMed] [Google Scholar]
- 68.Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation 2017;135:1490–3. [DOI] [PubMed] [Google Scholar]
- 69.Lansky AJ, Hochman JS, Ward PA, Mintz GS, Fabunmi R, Berger PB, New G, Grines CL, Pietras CG, Kern MJ, Ferrell M, Leon MB, Mehran R, White C, Mieres JH, Moses JW, Stone GW, Jacobs AK, American College of Cardiology F, American Heart A. Percutaneous coronary intervention and adjunctive pharmacotherapy in women: a statement for healthcare professionals from the American Heart Association. Circulation 2005;111:940–53. [DOI] [PubMed] [Google Scholar]
- 70.Mehran R, Pocock SJ, Nikolsky E, Clayton T, Dangas GD, Kirtane AJ, Parise H, Fahy M, Manoukian SV, Feit F, Ohman ME, Witzenbichler B, Guagliumi G, Lansky AJ, Stone GW. A risk score to predict bleeding in patients with acute coronary syndromes. J Am Coll Cardiol 2010;55:2556–66. [DOI] [PubMed] [Google Scholar]
- 71.Li S, Fonarow GC, Mukamal KJ, Liang L, Schulte PJ, Smith EE, DeVore A, Hernandez AF, Peterson ED, Bhatt DL. Sex and race/ethnicity-related disparities in care and outcomes after hospitalization for coronary artery disease among older adults. Circ Cardiovasc Qual Outcomes 2016;9: S36–44. [DOI] [PubMed] [Google Scholar]
- 72.Puri R, Nissen SE, Nicholls SJ. Statin-induced coronary artery disease regression rates differ in men and women. Curr Opin Lipidol 2015;26:276–81. [DOI] [PubMed] [Google Scholar]
- 73.Puri R, Nissen SE, Shao M, Ballantyne CM, Barter PJ, Chapman MJ, Erbel R, Libby P, Raichlen JS, Uno K, Kataoka Y, Nicholls SJ. Sex-related differences of coronary atherosclerosis regression following maximally intensive statin therapy: insights from saturn. JACC Cardiovasc Imaging 2014;7:1013–22. [DOI] [PubMed] [Google Scholar]
- 74.Truong QA, Murphy SA, McCabe CH, Armani A, Cannon CP, Group TS. Benefit of intensive statin therapy in women: results from prove IT-TIMI 22. Circ Cardiovasc Qual Outcomes 2011;4:328–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med 2007;356:830–40. [DOI] [PubMed] [Google Scholar]
- 76.De Bruyne B, Oldroyd KG, Pijls NHJ. Microvascular (dys) function and clinical outcome in stable coronary disease. J Am Coll Cardiol 2016;67:1170–2. [DOI] [PubMed] [Google Scholar]
- 77.Chilian WM. Coronary microcirculation in health and disease. Summary of an NHLBI workshop. Circulation 1997;95:522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Crea F, Camici PG, Bairey Merz CN. Coronary microvascular dysfunction: an update. Eur Heart J 2013. [DOI] [PMC free article] [PubMed]
- 79.Dickerson JA, Nagaraja HN, Raman SV. Gender-related differences in coronary artery dimensions: a volumetric analysis. Clin Cardiol 2010;33:E44–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hiteshi AK, Li D, Gao Y, Chen A, Flores F, Mao SS, Budoff MJ. Gender differences in coronary artery diameter are not related to body habitus or left ventricular mass. Clin Cardiol 2014;37:605–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kucher N, Lipp E, Schwerzmann M, Zimmerli M, Allemann Y, Seiler C. Gender differences in coronary artery size per 100 g of left ventricular mass in a population without cardiac disease. Swiss Med Wkly 2001;131:610–5. [DOI] [PubMed] [Google Scholar]
- 82.Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision-making. J Am Coll Cardiol 2013;62:1639–53. [DOI] [PubMed] [Google Scholar]
- 83.Johnson NP, Gould KL. Integrating noninvasive absolute flow, coronary flow reserve, and ischemic thresholds into a comprehensive map of physiological severity. JACC Cardiovasc Imaging 2012;5:430–40. [DOI] [PubMed] [Google Scholar]
- 84.Lupi A, Buffon A, Finocchiaro ML, Conti E, Maseri A, Crea F. Mechanisms of adenosine-induced epicardial coronary artery dilatation. Eur Heart J 1997;18:614–7. [DOI] [PubMed] [Google Scholar]
- 85.Koskinas KC, Feldman CL, Chatzizisis YS, Coskun AU, Jonas M, Maynard C, Baker AB, Papafaklis MI, Edelman ER, Stone PH. Natural history of experimental coronary atherosclerosis and vascular remodeling in relation to endothelial shear stress: a serial, in vivo intravascular ultrasound study. Circulation 2010;121:2092–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chatzizisis YS, Coskun AU, Jonas M, Edelman ER, Feldman CL, Stone PH. Role of endothelial shear stress in the natural history of coronary atherosclerosis and vascular remodeling: molecular, cellular, and vascular behavior. J Am Coll Cardiol 2007;49:2379–93. [DOI] [PubMed] [Google Scholar]
- 87.Koskinas KC, Sukhova GK, Baker AB, Papafaklis MI, Chatzizisis YS, Coskun AU, Quillard T, Jonas M, Maynard C, Antoniadis AP, Shi GP, Libby P, Edelman ER, Feldman CL, Stone PH. Thin-capped atheromata with reduced collagen content in pigs develop in coronary arterial regions exposed to persistently low endothelial shear stress. Arterioscler Thromb Vasc Biol 2013;33:1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patel MB, Bui LP, Kirkeeide RL, Gould KL. Imaging microvascular dysfunction and mechanisms for female-male differences in cad. JACC Cardiovasc Imaging 2016;9:465–82. [DOI] [PubMed] [Google Scholar]
- 89.Huang A, Kaley G. Gender-specific regulation of cardiovascular function: estrogen as key player. Microcirculation 2004;11:9–38. [DOI] [PubMed] [Google Scholar]
- 90.Pletcher MJ, Tice JA, Pignone M, McCulloch C, Callister TQ, Browner WS. What does my patient’s coronary artery calcium score mean? Combining information from the coronary artery calcium score with information from conventional risk factors to estimate coronary heart disease risk. BMC Med 2004;2:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffmann U, Massaro JM, Fox CS, Manders E, O’Donnell CJ. Defining normal distributions of coronary artery calcium in women and men (from the Framingham heart study). Am J Cardiol 2008;102:1136–41. 1141.e1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the multi-ethnic study ofatherosclerosis (MESA). Circulation 2006;113:30–7. [DOI] [PubMed] [Google Scholar]
- 93.Bairey Merz CN, Shaw LJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Pepine CJ, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: Part II: Gender differences in presentation, diagnosis, and outcome with regard to gender-based pathophysiology of atherosclerosis and macrovascular and microvascular coronary disease. J Am Coll Cardiol 2006;47:S21–9. [DOI] [PubMed] [Google Scholar]
- 94.Shaw LJ, Bairey Merz CN, Pepine CJ, Reis SE, Bittner V, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Wessel TR, Arant CB, Pohost GM, Lerman A, Quyyumi AA, Sopko G, Investigators W. Insights from the NHLBI-sponsored women’s ischemia syndrome evaluation (WISE) study: Part I: Gender differences in traditional and novel risk factors, symptom evaluation, and gender-optimized diagnostic strategies. J Am Coll Cardiol 2006;47:S4–S20. [DOI] [PubMed] [Google Scholar]
- 95.von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA, National Heart L, Blood I. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the national heart, lung, and blood institute-sponsored women’ s ischemia syndrome evaluation (WISE). Circulation 2004;109:722–5. [DOI] [PubMed] [Google Scholar]
- 96.Taqueti VR, Everett BM, Murthy VL, Gaber M, Foster CR, Hainer J, Blankstein R, Dorbala S, Di Carli MF. Interaction of impaired coronary flow reserve and cardiomyocyte injury on adverse cardiovascular outcomes in patients without overt coronary artery disease. Circulation 2015;131:528–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018; 39:840–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arbustini E, Dal Bello B, Morbini P, Grasso M, Diegoli M, Fasani R, Pilotto A, Bellini O, Pellegrini C, Martinelli L, Campagna C, Gavazzi A, Specchia G, Vigano M, Roberts WC. Frequency and characteristics of coronary thrombosis in the epicardial coronary arteries after cardiac transplantation. Am J Cardiol 1996;78:795–800. [DOI] [PubMed] [Google Scholar]
- 99.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE, Investigators C. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the clinical outcomes utilizing revascularization and aggressive drug evaluation (Courage) trial nuclear substudy. Circulation 2008;117:1283–91. [DOI] [PubMed] [Google Scholar]
- 100.Naya M, Murthy VL, Blankstein R, Sitek A, Hainer J, Foster C, Gaber M, Fantony JM, Dorbala S, Di Carli MF. Quantitative relationship between the extent and morphology of coronary atherosclerotic plaque and downstream myocardial perfusion. J Am Coll Cardiol 2011;58:1807–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van’t Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF, Investigators FS. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med 2009;360:213–24. [DOI] [PubMed] [Google Scholar]
- 102.Johnson NP, Gould KL, Di Carli MF, Taqueti VR. Invasive FFR and noninvasive CFR in the evaluation of ischemia: what is the future? J Am Coll Cardiol 2016;67:2772–88. [DOI] [PubMed] [Google Scholar]
- 103.Motwani M, Motlagh M, Gupta A, Berman DS, Slomka PJ. Reasons and implications of agreements and disagreements between coronary flow reserve, fractional flow reserve, and myocardial perfusion imaging. J Nucl Cardiol 2018;25:104–19. [DOI] [PubMed] [Google Scholar]
- 104.Bittencourt MS, Hulten E, Ghoshhajra B, O’Leary D, Christman MP, Montana P, Truong QA, Steigner M, Murthy VL, Rybicki FJ, Nasir K, Gowdak LH, Hainer J, Brady TJ, Di Carli MF, Hoffmann U, Abbara S, Blankstein R. Prognostic value of nonobstructive and obstructive coronary artery disease detected by coronary computed tomography angiography to identify cardiovascular events. Circ Cardiovasc Imaging 2014;7:282–91. [DOI] [PubMed] [Google Scholar]
- 105.Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA 2014;312:1754–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS, Investigators C. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter CONFIRM (coronary CT angiography evaluation for clinical outcomes: an international multicenter registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol 2011;58:849–60. [DOI] [PubMed] [Google Scholar]
- 107.Leipsic J, Taylor CM, Gransar H, Shaw LJ, Ahmadi A, Thompson A, Humphries K, Berman DS, Hausleiter J, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chow BJ, Cury RC, Delago AJ, Dunning AL, Feuchtner GM, Hadamitzky M, Kaufmann PA, Lin FY, Chinnaiyan KM, Maffei E, Raff GL, Villines TC, Gomez MJ, Min JK. Sex-based prognostic implications of nonobstructive coronary artery disease: results from the international multicenter CONFIRM study. Radiology 2014;273:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schulman-Marcus J, o Hartaigh B, Gransar H, Lin F, Valenti V, Cho I, Berman D, Callister T, DeLago A, Hadamitzky M, Hausleiter J, Al-Mallah M, Budoff M, Kaufmann P, Achenbach S, Raff G, Chinnaiyan K, Cademartiri F, Maffei E, Villines T, Kim YJ, Leipsic J, Feuchtner G, Rubinshtein R, Pontone G, Andreini D, Marques H, Shaw L, Min JK. Sex-specific associations between coronary artery plaque extent and risk of major adverse cardiovascular events: the CONFIRM long-term registry. JACC Cardiovasc Imaging 2016;9:364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gould KL. Does coronary flow trump coronary anatomy? JACC Cardiovasc Imaging 2009;2:1009–23. [DOI] [PubMed] [Google Scholar]
- 110.Fukushima K, Javadi MS, Higuchi T, Lautamaki R, Merrill J, Nekolla SG, Bengel FM. Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med 2011;52:726–32. [DOI] [PubMed] [Google Scholar]
- 111.Herzog BA, Husmann L, Valenta I, Gaemperli O, Siegrist PT, Tay FM, Burkhard N, Wyss CA, Kaufmann PA. Longterm prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol 2009;54:150–6. [DOI] [PubMed] [Google Scholar]
- 112.Murthy VL, Naya M, Foster CR, Hainer J, Gaber M, Di Carli G, Blankstein R, Dorbala S, Sitek A, Pencina MJ, Di Carli MF. Improved cardiac risk assessment with noninvasive measures of coronary flow reserve. Circulation 2011;124:2215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ziadi MC, Dekemp RA, Williams KA, Guo A, Chow BJ, Renaud JM, Ruddy TD, Sarveswaran N, Tee RE, Beanlands RS. Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol 2011;58:740–8. [DOI] [PubMed] [Google Scholar]
- 114.Taqueti VR, Hachamovitch R, Murthy VL, Naya M, Foster CR, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Global coronary flow reserve is associated with adverse cardiovascular events independently of luminal angiographic severity and modifies the effect of early revascularization. Circulation 2015;131:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Naya M, Murthy VL, Taqueti VR, Foster CR, Klein J, Garber M, Dorbala S, Hainer J, Blankstein R, Resnic F, Di Carli MF. Preserved coronary flow reserve effectively excludes high-risk coronary artery disease on angiography. J Nucl Med 2014;55:248–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murthy VL, Lee BC, Sitek A, Naya M, Moody J, Polavarapu V, Ficaro EP, Di Carli MF. Comparison and prognostic validation of multiple methods of quantification of myocardial blood flow with 82Rb PET. J Nucl Med 2014;55:1952–8. [DOI] [PubMed] [Google Scholar]
- 117.Naya M, Murthy VL, Foster CR, Gaber M, Klein J, Hainer J, Dorbala S, Blankstein R, Di Carli MF. Prognostic interplay of coronary artery calcification and underlying vascular dysfunction in patients with suspected coronary artery disease. J Am Coll Cardiol 2013;61:2098–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med 2010;362:886–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Recio-Mayoral A, Rimoldi OE, Camici PG, Kaski JC. Inflammation and microvascular dysfunction in cardiac syndrome X patients without conventional risk factors for coronary artery disease. JACC Cardiovasc Imaging 2013;6:660–7. [DOI] [PubMed] [Google Scholar]
- 120.Di Carli MF, Bianco-Batlles D, Landa ME, Kazmers A, Groehn H, Muzik O, Grunberger G. Effects of autonomic neuropathy on coronary blood flow in patients with diabetes mellitus. Circulation 1999;100:813–9. [DOI] [PubMed] [Google Scholar]
- 121.Paulus WJ, Tschope C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol 2013;62:263–71. [DOI] [PubMed] [Google Scholar]
- 122.Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. The bypass angioplasty revascularization investigation (BARI) investigators. N Engl J Med 1996;335:217–25. [DOI] [PubMed] [Google Scholar]
- 123.Farkouh ME, Domanski M, Sleeper LA, Siami FS, Dangas G, Mack M, Yang M, Cohen DJ, Rosenberg Y, Solomon SD, Desai AS, Gersh BJ, Magnuson EA, Lansky A, Boineau R, Weinberger J, Ramanathan K, Sousa JE, Rankin J, Bhargava B, Buse J, Hueb W, Smith CR, Muratov V, Bansilal S, King S 3rd, Bertrand M, Fuster V, Investigators FT. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012;367:2375–84. [DOI] [PubMed] [Google Scholar]
- 124.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med 2009;360:2503–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW, Investigators S. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009;360:961–72. [DOI] [PubMed] [Google Scholar]
- 126.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida-Simiti-L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ, Group CT. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med 2017;377:1119–31. [DOI] [PubMed] [Google Scholar]
- 127.Everett BM, Pradhan AD, Solomon DH, Paynter N, Macfadyen J, Zaharris E, Gupta M, Clearfield M, Libby P, Hasan AA, Glynn RJ, Ridker PM. Rationale and design of the cardiovascular inflammation reduction trial: a test of the inflammatory hypothesis of atherothrombosis. Am Heart J 2013;166:199–207. e115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, Committee FS, Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med 2017;376:1713–22. [DOI] [PubMed] [Google Scholar]
- 129.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ, Broedl UC, Inzucchi SE, Investigators E-RO. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med 2015;373:2117–28. [DOI] [PubMed] [Google Scholar]
- 130.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators P-H, Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]


