Abstract
Background:
Fine particulate matter (PM2.5) exposure is associated with increased morbidity and mortality, particularly for cardiovascular disease. The association between long-term exposure to PM2.5 and measures of lipoprotein subfractions remains unclear. Therefore, we examined associations between long-term PM2.5 exposure and traditional and novel lipoprotein measures in a cardiac catheterization cohort in North Carolina.
Methods:
This cross-sectional study included 6587 patients who had visited Duke University for a cardiac catheterization between 2001 to 2010 and resided in North Carolina. We used estimates of daily PM2.5 concentrations on a 1km-grid based on satellite measurements. PM2.5 predictions were matched to the address of each patient and averaged for the year prior to catheterization date. Serum lipids included HDL, LDL, and triglyceride-rich particle, and apolipoprotein B concentrations (HDL-P, LDL-P, TRL-P, and apoB, respectively). Linear and quantile regression models were used to estimate change in lipoprotein levels with each μg/m3 increase in annual average PM2.5. Models were adjusted for age, sex, race/ethnicity, history of smoking, area-level education, urban/rural status, body mass index, and diabetes.
Results:
For a 1-μg/m3 increment in PM2.5 exposure, we observed increases in total and small LDL-P, LDL-C, TRL-P, apoB, total cholesterol, and triglycerides. The percent change from the mean outcome level was 2.00% (95% CI: 1.38%, 2.64%) for total LDL-P and 2.25% (95% CI: 1.43%, 3.06%) for small LDL-P.
Conclusion:
Among this sample of cardiac catheterization patients residing in North Carolina, long-term PM2.5 exposure was associated with increases in several lipoprotein concentrations. This abstract does not necessarily reflect U.S. EPA policy.
Keywords: fine particulate matter, lipids, cardiovascular disease, air pollution
1. Introduction
Long-term air pollution exposure is associated with both cardiovascular disease (CVD) morbidity and mortality. The majority of evidence exists for associations between ambient fine particulate matter ≤2.5 μg/m3 (PM2.5) and CVD, particularly among susceptible subgroups such as older adults (Brook et al. 2010; Brook et al. 2017). In fact, about 72% of all PM-related deaths are due to ischemic heart disease and strokes (WHO 2014). Epidemiological studies have found associations between long-term PM2.5 exposure and clinical measures of atherosclerosis such as coronary artery calcification and carotid intima-media thickness (Kaufman et al. 2016; Kunzli et al. 2005). In addition to increasing systemic inflammation and oxidative stress, PM2.5 oxidizes blood lipids, which can potentially lead to progression of atherosclerosis (Araujo 2010). Assessing associations between PM2.5 and atherosclerosis risk markers may provide insight into the pathophysiological pathways by which PM causes adverse cardiovascular outcomes.
Low density lipoprotein cholesterol (LDL-C) is a well-established mediator of CVD pathogenesis and progression. LDL-C lowering therapies are major targets of CVD risk reduction strategies. However, LDL-C measures the cholesterol content of LDL particles rather than the total number of particles. Studies have suggested that LDL particles (LDL-P) concentrations—particularly small LDL-P subfractions— may be better markers of CVD risk than LDL-C per se, possibly because of the greater likelihood of smaller particles to enter the arterial wall and cause damage (Blake et al. 2002; Ip et al. 2009; Lamarche et al. 1997; Zaid et al. 2016). A few previous studies have assessed associations between PM2.5 and standard lipid measures, including LDL-C (Chuang et al. 2011; Jiang et al. 2016; Yitshak Sade et al. 2016); but very little is known about the association of PM2.5 with lipoprotein particle size and concentration.
We previously reported associations between measures of fine particulate matter and cardiovascular disease (McGuinn et al. 2017). The association between long-term air pollution and measures of lipoprotein concentrations, however, remains poorly understood. Therefore, the goal of the current study was to assess associations between annual average PM2.5 levels and circulating lipoprotein concentrations in a cohort of cardiac catheterization patients. We assessed associations with total number of LDL particle concentrations, as well as mean particle sizes (small, medium, and large) of lipoproteins, reflecting lipid content. Further, we compared associations among PM2.5 and LDL-P measures with those of standard LDL-C. We additionally assessed associations among PM2.5 and characteristics (concentration and size) of HDL (HDL-P), triglyceride-rich particle concentrations (TRL-P), and apolipoprotein B (apoB), as well as with standard lipid measures (total cholesterol and triglycerides).
2. Methods
2.1. Study Population
We used data from a cohort of patients undergoing a cardiac catheterization between 2001–2010 at Duke University Hospital in North Carolina (CATHGEN, CATHeterization GENetics Study) (Kraus et al. 2015). Participants in the current study underwent a cardiac catheterization in order to diagnose and treat coronary artery disease. The CATHGEN study collected blood samples from individuals presenting to the catheterization laboratory for concern of coronary artery disease and other cardiovascular conditions. Additional patient information came from medical records and a questionnaire administered upon enrollment in the study. Extent of coronary artery disease was assessed using the Coronary Artery Disease index. Individuals were defined as having significant coronary artery disease (CAD index >23) if they had > 75% luminal stenosis in one epicardial coronary artery (Bart et al. 1997). The CATHGEN biorepository is monitored and approved by the Duke University Institutional Review Board. Written informed consent was received from participants prior to inclusion in the study.
Complete details of the CATHGEN study population have been described previously (Kraus et al. 2015). There were 9334 total participants in the CATHGEN study population. Of those, 7116 resided in North Carolina and had geocode information available. We further excluded individuals with missing lipoprotein outcome data (n=497) and covariate data (n=32). This resulted in a final sample of 6587 participants for the current study.
2.2. Lipoprotein Measures
Fasting plasma samples were collected at the time of the catheterization visit. Lipoprotein particle concentrations and sizes were measured by nuclear magnetic resonance (NMR) spectroscopy at LabCorp using the LP4 NMR LipoProfile (Jeyarajah et al. 2006). Particles were grouped into different sizes based on the amplitude of their lipid methyl group signals (Jeyarajah et al. 2006). Table 1 shows a description of each of the included particle concentration and subclass measures. LDL subclasses include: large (21.5 – 23 nm), medium (20.5 – 21.4 nm), and small LDL-P (19 – 20.4 nm). HDL subclasses include: large (10.3 – 12 nm), medium (8.7 – 9.5 nm), and small HDL-P (7.4 – 7.8 nm). TRL-P subclasses include: large (50–89 nm), medium (37–49 nm), and small TRL-P (30–36 nm). Total LDL-P, HDL-P, and TRL-P are calculated as the sum of the particle concentrations of the LDL, HDL, and TRL-P subclasses. Mean LDL, HDL, and TRL sizes were calculated as mass-weighted averages (Jeyarajah et al. 2006). Concentrations below the limit of detection were assigned the lowest detectable value for that particular measure.
Table 1.
Description of included lipoprotein measures
| Parameter | Description | Diameter Range (nm) |
|---|---|---|
| LDL Particle (LDL-P) Concentrations (nmol/L) | ||
| Total LDL-P | Total number of LDL particle concentrations. Sum of LDL-P subclass measures | 19–23 |
| Large LDL-P | Large LDL particle concentrations | 21.5–23 |
| Medium LDL-P | Medium LDL particle concentrations | 20.5–21.4 |
| Small LDL-P | Small LDL particle concentrations | 19–20.4 |
| HDL Particle (HDL-P) Concentrations (μmol/L) | ||
| Total HDL-P | Total number of HDL particle concentrations. Sum of HDL-P subclass measures | 7.5–13 |
| Large HDL-P | Large HDL particle concentrations | 10.3–12.0 |
| Medium HDL-P | Medium HDL particle concentrations | 8.7–9.5 |
| Small HDL-P | Small HDL particle concentrations | 7.4–7.8 |
| TG-rich Lipoprotein Particle (TRL-P) Concentrations (nmol/L) | ||
| Total TRL-P | Total number of TRL-P particle concentrations. Sum of TRL-P subclass measures | 24–240 |
| Large TRLP | Large TRL-P particle concentrations | 50–89 |
| Medium TRLP | Medium TRL-P particle concentrations | 37–49 |
| Small TRLP | Small TRL-P particle concentrations | 30–36 |
| Mean Particle Sizes (nm) | ||
| TRL size (nm) | Mass weighted mean TRL particle sizes | 30–100 |
| LDL size (nm) | Mass weighted mean LDL particle sizes | 19–22.5 |
| HDL size (nm) | Mass weighted mean HDL particle sizes | 7.4–13 |
| NMR-derived Lipid and Apolipoprotein Concentrations (mg/dL) | ||
| LDL-C | NMR derived low-density lipoprotein cholesterol | |
| HDL-C | NMR derived high-density lipoprotein cholesterol | |
| Total Triglycerides | NMR derived total triglycerides | |
| Total Cholesterol | NMR derived total cholesterol | |
| ApoB | Apolipoprotein B | |
HDL indicates high-density lipoprotein; LDL, low-density lipoprotein; NMR, nuclear magnetic resonance; TG, triglyceride.
Standard cholesterol and apolipoprotein measures were not available for the current analyses. Therefore, we used NMR-derived measures of LDL-C, HDL-C, total triglycerides, total cholesterol, and apoB. NMR-derived cholesterol measures were highly correlated with standard cholesterol measures in a separate reference population (Matyus et al. 2014) (r=0.95; information from the testing laboratory).
2.3. Exposure Assessment
We used daily PM2.5 concentrations estimated at a 1×1km spatial grid resolution for North Carolina from 2001–2010 (Di et al. 2016). The previously developed hybrid prediction model incorporated simulation outputs from a chemical transport model, satellite aerosol optical depth data, meteorological variables, and land-use terms. This prediction model additionally used a neural network to calibrate all the predictors to monitored PM2.5. Finally, the model was trained and validated with ten-fold cross-validation (R2 of 0.84).
Patient addresses on the date of their catheterization were matched to the nearest 1km grid centroid. Daily PM2.5 predictions were averaged for the year prior to each patient’s study visit date (McGuinn et al. 2017). We refer to this measure as the annual average PM2.5 value throughout the text.
2.4. Covariates
Data on covariates were obtained from the intake questionnaire and medical records. The final adjustment set identified from the directed acyclic graph included: age (continuous), sex, race/ethnicity (non-Hispanic white, African American, other), current/former smoker (yes/no), body mass index (continuous), history of diabetes, area-level education (high/low), and urban/rural status. Percentage of individuals in the block group without a high school education was obtained from the 2000 U.S. Census and used to define area-level education (U.S. Census Bureau 2000). Data on urban/rural status were obtained from the 2000 U.S. Census at the census tract level (Rural Health Research Center 2009).
2.5. Statistical Analyses
We assessed correlations between the lipoprotein outcome measures using Pearson correlation coefficients. Total and small LDL-P measures have been most consistently associated with CVD risk in recent studies (Mora et al. 2007; Zaid et al. 2016). Therefore, the main analyses focused on LDL-P particle concentrations and sizes (total, small, medium, and large), as well as standard LDL cholesterol (LDL-C). Secondary analyses included associations between PM2.5 and HDL-P and TRL-P total particle concentrations and sizes, as well as standard cholesterol measures including HDL-C, total cholesterol, total triglycerides, and apoB.
Associations between PM2.5 and the included lipoprotein measures were first examined using linear regression modeling. Next, because not everyone is equally responsive to PM2.5, we investigated whether PM2.5 exposure influences lipoprotein levels differently for individuals with low lipoprotein concentrations compared to those with high concentrations. Specifically, we assessed whether annual average PM2.5 was associated with percentiles of the outcome distribution in 20% increments (10th, 30th, 50th, 70th, and 90th) using quantile regression (Bind et al. 2016). For measures with >10% of concentration below the limit of detection we used three (medium LDL-P and large TRL-P) and four (medium TRL-P) percentile levels. Finally, we assessed if sex (male vs female) and race (white vs. non-white) modified associations among PM2.5 and total LDL-P concentrations by including interaction terms between these variables and PM2.5. For all analyses, results are presented as the percentage change of the mean outcome variable and 95% confidence intervals per 1-ug/m3 increase in the annual average PM2.5 level.
3. Results
3.1. Study Population
Demographic and clinical characteristics of the study population are presented in Table 2. About half of the included participants had clinically significant coronary artery disease. The majority of included CATHGEN participants were male, overweight/obese, and non-Hispanic White. About half of the study population (48%) had a history of smoking, and 29% had a history of diabetes. Urban/rural status was roughly evenly distributed, with 42% of participants residing in urban areas. The average annual average PM2.5 level for the study population was 12.5 μg/m3 (SD: 1.2), with a range of 6.9 to 17.4 μg/m3. Those included in the current analyses were slightly more likely to be non-white and female compared to those who were excluded (Supplemental Table 1).
Table 2.
Characteristics of the CATHGEN study population
| Characteristic | Total (n=6587) |
|---|---|
| Annual average PM2.5 | 12.5 ± 1.2 |
| Age at time of enrollment | 60.8 ± 12.1 |
| Body mass index (kg/m2) | 30.1 ± 7.3 |
| Coronary Artery Diseasea | |
| Yes | 2950 (50) |
| No | 2976 (50) |
| Sex | |
| Male | 3,991 (61) |
| Female | 2,596 (39) |
| Raceb | |
| Non-Hispanic white | 4,853 (74) |
| African American | 1,340 (20) |
| Other | 394 (6) |
| History of smoking | |
| Yes | 3,152 (48) |
| No | 3,435 (52) |
| History of diabetes | |
| Yes | 1,886 (29) |
| No | 4,701 (71) |
| Area level educationc | |
| Low | 2,693 (41) |
| High | 3,894 (59) |
| Urban/rural statusd | |
| Urban | 2,770 (42) |
| Rural | 3,817 (58) |
Data are reported as n (%) or mean ± SD. LDL-P, low-density lipoprotein particle concentration.
Binary measure of coronary artery disease (>23 CAD index).
Other race/ethnicity includes Native American, Hispanic, Asian, and unknown.
Low educational attainment includes those who live in block groups where ≥25% of males and females have less than a high school education.
Urban status was defined as living in a metropolitan urban core census tract.
3.2. Distribution of Lipoprotein Concentrations
Table 3 summarizes the distribution of the lipoprotein concentrations for the CATHGEN cohort. Mean lipoprotein concentrations are additionally shown for a reference population of 698 healthy adult men (n=284) and women (n=414) between the ages of 18 and 84, who were free of disease and not taking any lipid-altering drugs (Matyus et al. 2014). Lipoprotein concentrations differed between the two groups for all of the included measures, except for total LDL-P. CATHGEN participants had on average greater concentrations of small LDL-P and lower concentrations of medium and large LDL-P than the reference population. CATHGEN participants additionally had lower concentrations of total HDL-P than the reference population. The LDL particle size distribution in the CATHGEN cohort ranged from 19–23 nm, with an average size of 20.4 nm.
Table 3.
Descriptive statistics of the included lipoprotein measures
| Lipoprotein Concentration | CATHGEN | Healthy reference populationa | |||
|---|---|---|---|---|---|
| Mean ± SD | Range (5th-95th percentile) |
Mean ± SD | Range (5th-95th percentile) |
||
| LDL Particle (LDL-P) (nmol/L) |
|||||
| Total LDL-P | 1452 ± 445 | 836 – 2277 | 1454 ± 393 | 891 – 2150 | |
| Large LDL-P | 165 ± 159 | 0.07 – 464 | 309 ± 223 | 17 – 748 | |
| Medium LDL-P | 132 ± 209 | 0.02 – 554 | 676 ± 405 | 0 – 1377 | |
| Small LDL-P | 1156 ± 449 | 510 – 1973 | 469 ± 431 | 13 – 1318 | |
| HDL Particle (HDL-P) (μmol/L) |
|||||
| Total HDL-P | 18.4 ± 3.9 | 12.3 – 24.9 | 24.0 ± 3.0 | 19.2 – 29.3 | |
| Large HDL -P | 1.6 ± 1.3 | 0.4 – 4.3 | 2.5 ± 1.9 | 0.2 – 6.3 | |
| Medium HDL -P | 4.3 ± 2.6 | 1.0 – 9.2 | 7.7 ± 2.7 | 3.7 – 12.6 | |
| Small HDL -P | 12.4 ± 4.0 | 5.7 – 18.8 | 13.8 ± 3.4 | 8.1 – 19.6 | |
| TG-rich Lipoprotein Particle (TRL-P) (nmol/L) |
|||||
| Total TRL-P | 133 ± 73.8 | 30.0 – 268 | 125 ± 61.6 | 42 – 239 | |
| Large TRL-P | 2.7 ± 5.1 | 0.0 – 13.0 | 2.9 ± 6.5 | 0.0 – 12.8 | |
| Medium TRL-P | 19.4 ± 20.6 | 0.0 – 59.7 | 17.9 ± 16.2 | 0.3 – 48.4 | |
| Small TRL-P | 38.6 ± 32.5 | 0.07 – 98.8 | 56.5 ± 37.5 | 7.3 – 124 | |
| NMR-derived Lipid and Apolipoprotein (mg/dL) |
|||||
| LDL-C | 97.8 ± 31.6 | 54.1 – 155 | 111± 30.7 | 63 – 163 | |
| HDL-C | 44.3 ± 11.8 | 29.0 – 66.2 | 61.1 ± 14.4 | 41 – 88 | |
| Total triglycerides | 106 ± 83.6 | 30.2 – 249 | 119 ± 89.8 | 53 – 127 | |
| Total cholesterol | 166 ± 39.7 | 110 – 237 | 194 ± 36.5 | 116 – 209 | |
| Apolipoprotein B | 81.0 ± 24.6 | 47.2 – 126 | 87.1 ± 23.6 | 53 – 127 | |
All p-values for t-tests comparing the means between the two populations were <0.05, except for total LDL-P. HDL, high-density lipoprotein; LDL, low-density lipoprotein; NMR, nuclear magnetic resonance.
Comparison reference population includes healthy adult men (n=284) and women (n=414) between the ages of 18 and 84 (Matyus et al. 2014)
Supplemental Table 2 shows the mean LDL levels by CATHGEN participant characteristics. Several of the included characteristics differed for all three LDL measures; these include, age, sex, body mass index, area-level education, and urban/rural status. Individuals with significant coronary artery disease (CAD) had on average greater concentrations of small LDL-P; however, total LDL-P and LDL-C concentrations did not differ by CAD status.
3.3. Correlation Among Lipoprotein Concentrations
Supplemental Table 3 presents the Pearson correlation coefficients for the particle concentrations and sizes and NMR-derived lipids. There were strong correlations between total LDL particle concentrations with NMR-derived LDL-C (0.96) and total cholesterol (0.89). There were additionally strong correlations between total LDL-P and small LDL-P (0.80). NMR derived HDL-C was strongly correlated with both total (0.74) and large HDL-P (0.76), while total HDL-P was moderately correlated with small HDL-P (0.71). There were additionally positive correlations between NMR-derived total triglycerides and all of the TRL-P measures (0.74–0.84), except small TRL-P.
3.4. LDL-Related Associations with PM2.5
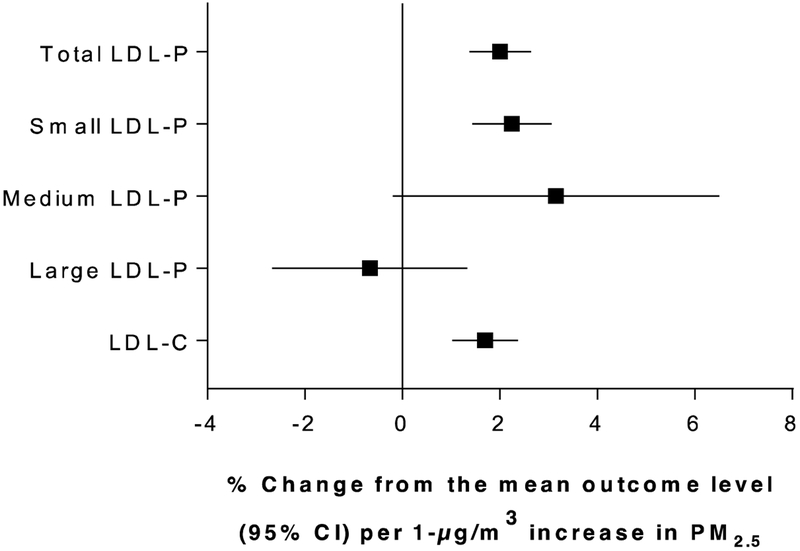
Figure 1 shows the adjusted associations among PM2.5 and measures of LDL particle concentrations and sizes, and NMR-derived LDL-C. The results are reported as the percentage change from the mean outcome level per 1-μg/m3 increase in annual average PM2.5. As seen in this figure, long-term PM2.5 exposure was most strongly associated with greater total and small LDL particle concentrations. We observed associations with medium LDL-P, however results were imprecise and the 95% confidence interval included the null. Finally, we additionally observed increases in NMR-derived LDL-C levels with each 1-μg/m3 increase in annual average PM2.5. The results for LDL-C were similar in effect size to the total and small LDL-P results.
Figure 1.
Associations between 1-μg/m3 increases in annual average PM2.5 and measures of total particle concentrations and sizes of LDL-P and NMR-derived LDL-C. Results are adjusted for race/ethnicity, history of smoking, sex, age, area level education, urban/rural status, body mass index, and diabetes.
We conducted stratified analyses in order to assess if the association between PM2.5 and total LDL-P differed by sex and race. In stratified models, we observed stronger associations between PM2.5 and LDL-P levels for women (% change for women: 2.53%, 95% CI 1.55%, 3.50%) compared to men (% change for men: 1.67%, 95% CI 0.84%, 2.51%), however the interaction was not significant. We additionally observed slight differences for whites vs. non-whites in our study population (% change for whites: 2.26%, 95% CI 1.55%, 2.97%; non-whites: 1.34%, 95% CI −0.12%, 2.79%), although this interaction was also not significant.
3.5. HDL-P, TRL-P, and NMR-derived Lipids Associations
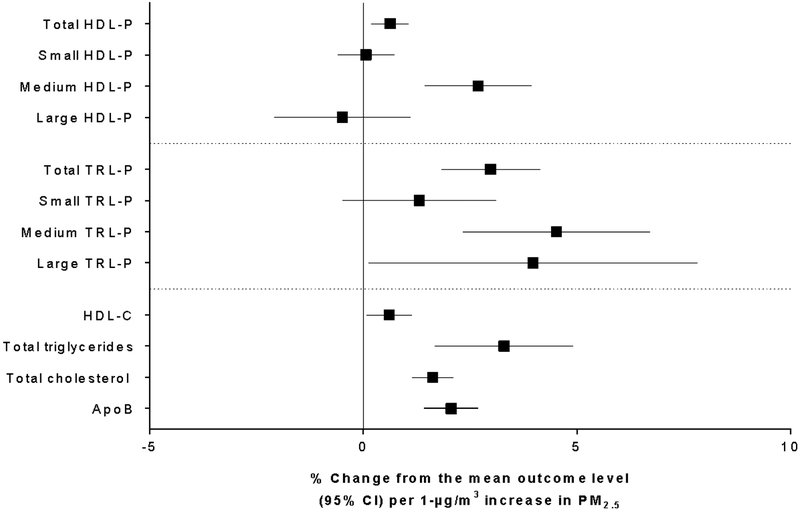
Figure 2 shows the adjusted associations among PM2.5 and HDL-P and TRL-P total particle concentrations and sizes, as well as NMR-derived HDL-C, total triglycerides, total cholesterol, and apoB. There were consistent elevated associations among long-term PM2.5 and triglyceride-rich particle concentrations and sizes, particularly for total and medium TRL-P. These associations were similar to those for NMR-derived total triglyceride. There were less consistent associations between PM2.5 and the HDL-P measures. However, there were more significant associations for total and medium HDL-P, as well as HDL-C. Finally, we observed greater total cholesterol and apoB concentrations associated with each 1-μg/m3 increase in annual average PM2.5.
Figure 2.
Associations between 1-μg/m3 increases in annual average PM2.5 and measures of HDL-P, TRL-P, and NMR-derived HDL-C, total triglycerides, total cholesterol, and ApoB. Results are adjusted for race/ethnicity, history of smoking, sex, age, area level education, urban/rural status, body mass index, and diabetes.
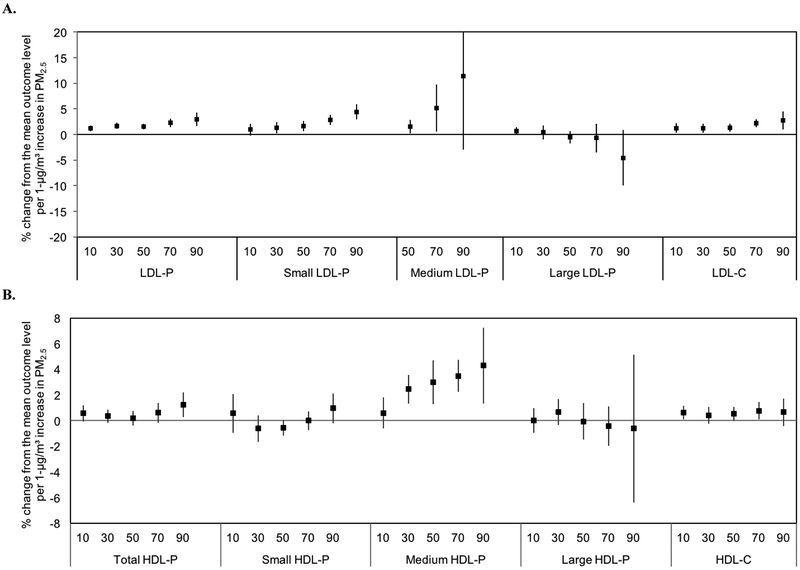
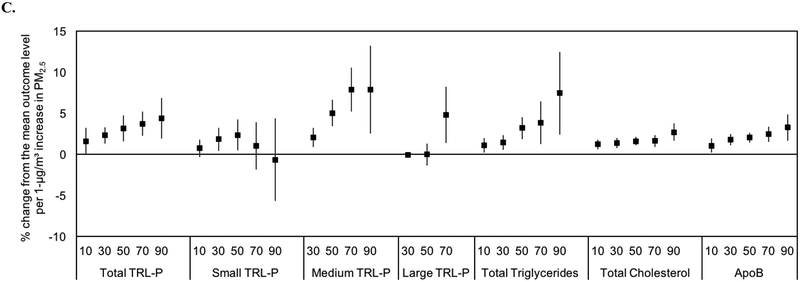
3.6. Quantile Regression Analyses
We additionally investigated whether PM2.5 exposure influences lipoprotein levels differently for individuals with low concentrations compared to those with greater lipoprotein concentrations. Figure 3 (A–C) shows the associations between PM2.5 by six quantiles of the lipoprotein outcome distribution (with breaks at the 10th, 30th, 50th, 70th, and 90th percentiles). The associations were heterogeneous across quantiles. There was an increasing trend for total, small, and medium LDL-P, and LDL-C (Figure 3A). The percentage change from the mean outcome level per 1-μg/m3 increase in PM2.5 ranged from 1.22% (10th percentile) to 2.94% (90th percentile) for total LDL-P and 0.94% (10th percentile) to 4.41% (90th percentile) for small LDL-P.
Figure 3.
Associations between 1-μg/m3 increases in annual average PM2.5 and quantiles of the distribution of lipoprotein measures. A, total particle concentrations and sizes of LDL-P and NMR-derived LDL-C. B, total particle concentrations and sizes of HDL-P and NMR-derived HDL- C. C, total particle concentrations and sizes of TRL-P and NMR-derived triglycerides, total cholesterol, and ApoB. Results are adjusted for race/ethnicity, history of smoking, sex, age, area level education, urban/rural status, body mass index, and diabetes.
The relationships for HDL were less consistent. However, there was an increasing trend for medium HDL-P (Figure 3B). Finally, there was an increasing trend across quantiles for several of the TRL-P measures and NMR-derived total triglycerides (Figure 3C). The percentage change from the mean outcome level per 1-μg/m3 increase in PM2.5 ranged from 1.58% (10th percentile) to 4.36% (90th percentile) for total TRL-P and 1.09% (10th percentile) to 7.42% (90th percentile) for total triglycerides.
4. Discussion
Among this sample of cardiac catheterization patients residing in North Carolina and undergoing coronary angiography, long-term ambient air PM2.5 exposure was associated with increases in several standard and novel blood lipoprotein concentrations. The use of NMR technology is an emerging way to characterize lipoprotein particle concentrations. While the clinical advantages of these new measures when compared with standard cholesterol measures remain debated (Allaire et al. 2017; Steffen et al. 2015), NMR-based measures of blood lipoprotein particle size and concentration are predictive of future cardiovascular events in a cardiac catheterization cohort (May et al. 2016), and offer a novel and more refined approach to measuring the relationship between long-term PM2.5 exposure and changes in blood lipoprotein composition. We found associations between long-term PM2.5 and total and small LDL-P, and NMR-derived LDL-C. Additionally, our findings showed elevated associations between PM2.5 and measures of TRL-P and NMR-derived total cholesterol and triglycerides.
To the best of our knowledge, this is the first study to examine the impact of long-term PM2.5 exposure on LDL particle concentrations and sizes. Our findings showed significant associations for total and small LDL-P concentrations. We additionally observed associations with medium LDL-P. However, the results were imprecise and 41% of the medium LDL-P measures were below the LOD, thus these specific findings should be interpreted with caution. Our findings between PM2.5 and elevated total and small LDL-P concentrations were similar for NMR-derived LDL-C, which is expected given the strong correlations between total LDL-P and NMR-derived LDL-C in our study population. Other studies have found strong correlations between total LDL-P and LDL-C using standard methods, although correlations were not as strong as those observed in our study between LDL-P and NMR-derived LDL-C (Matyus et al. 2014). The positive correlations between total LDL-P and small LDL-P is additionally expected given that small LDL-P makes up the majority of total LDL-P concentrations in this study population. This positive correlation between total and small LDL-P has additionally been observed in previous studies (Zaid et al. 2016).
A few previous studies have assessed associations between long-term air pollution exposure and LDL using standard LDL cholesterol measures. Studies in China (Jiang et al. 2016), Taiwan (Chuang et al. 2011), California (Chen et al. 2016), and Israel (Yitshak Sade et al. 2016) have found consistent associations between intermediate and long-term air pollution exposure and elevated LDL-C. Findings from these studies showed inconsistent effects for short-term exposures. Using particle concentrations to assess the association between air pollution and LDL levels may be more clinically meaningful given that total particle number is thought to potentially be a better predictor of cardiovascular disease than standard cholesterol measures alone, particularly for individuals with discordant LDL-P/LDL-C levels (Otvos et al. 2011; Zaid et al. 2016). Further, previous studies have specifically found smaller dense LDL particles to be strongly associated with coronary artery disease (Blake et al. 2002; Kuller et al. 2002; Lamarche et al. 1997), possibly because smaller dense particles can more easily move between the endothelial cells and are more easily oxidized, thus increasing susceptibility to atherosclerosis. Therefore, these particle concentration measures may be a better predictor of potential adverse outcomes associated with PM2.5 exposure than traditional measures of blood cholesterol and may also help in understanding the underlying mechanisms between PM2.5 and cardiovascular disease.
We additionally found associations between long-term PM2.5 exposure and increases in triglyceride-rich particle concentrations and sizes, as well as with NMR-derived total triglycerides and cholesterol. This is consistent with a few previous studies that found associations between long-term air pollution exposure and increased levels of standard measures of total triglycerides and cholesterol (Shanley et al. 2016; Sorensen et al. 2015). Elevated triglyceride levels are independently associated with increased risk of cardiovascular events (Rosenson et al. 2014). Moreover, recent genetic studies suggest that triglycerides may also have a causal relationship with cardiovascular disease (Holmes et al. 2015; Triglyceride Coronary Disease Genetics et al. 2010). Thus, in addition to being associated with a more atherogenic LDL particle profile, long-term PM2.5 exposure may shift the triglyceride-rich particle profile to potentially atherogenic subclasses.
Long-term PM2.5 exposure was also associated with increased apolipoprotein B concentrations. Although LDL accounts for approximately 90% of apoB particles, apoB is also found on other atherogenic lipoproteins such as very low-density lipoproteins and intermediate density lipoproteins and, as such, apoB concentrations predict cardiovascular risk better than LDL-C alone (Sniderman et al. 2011).
We observed inconsistent associations with HDL particle concentrations and sizes. Our results showed increased levels of total and medium HDL-P and HDL-C with each 1-μg/m3 increase in annual average PM2.5, and null associations with small and large HDL-P concentrations. Bell et al. (2017) assessed associations between short, intermediate, and long-term air pollutant exposure and measures of HDL-C and HDL-P. Findings from their study showed inverse associations between intermediate and long-term air pollution and HDL-C and HDL-P. Mean total HDL-P and HDL-C levels in their study population were 34.0 μmol/L and 51.0 mg/dL, respectively, which are on average higher than the observed levels in our study population.
The Clean Air Act mandates that air quality standards be set to protect susceptible populations (EPA 2007). Several previous studies have shown that not everyone is equally responsive to air pollution (Sacks et al. 2011). Thus, it is important for epidemiological studies to assess the health effects of air pollution exposure in potentially sensitive populations. Therefore, we investigated whether everyone in the CATHGEN population was equally responsive to PM2.5 using quantile regression. The advantage of using quantile regression is that we were able to use the entire distribution of the lipoprotein concentrations, in order to identify participants who were most and least responsive. Our results showed an increasing trend for several of the LDL, TRL-P and total triglyceride measures, with the greatest percent change in the highest percentiles of these outcome measures. This is consistent with a recent study that found an increasing trend between black carbon and PM2.5 with standard measures of LDL and triglycerides using quantile regression (Bind et al. 2016).
Our study is not without limitations. First, standard cholesterol measures were not available for the current analyses. We relied on NMR-derived LDL-C, HDL-C, total triglyceride, and total cholesterol measures, which were highly correlated with standard cholesterol measures in a separate reference population. Next, we made use of an area level PM2.5 measure, thus resulting in some amount of exposure misclassification. However, the use of modeled air pollution data at a fine spatial resolution should help to reduce this error. Further, spatial clustering is of concern in air pollution analyses such as these. In our assessment of the data we did not observe any significant clustering. Additionally, we may have inadequately controlled for all potential confounders as we did not have information on medication use, including lipid-lowering drugs, or measures of individual-level SES.
Catheterization patients represent a selective population; thus, results may not be generalizable to the general population. For instance, in comparing levels of lipoprotein concentrations from CATHGEN with those from a healthy reference population we found significant differences between the two groups for all measures, except total LDL-P (Supplementary Table 1). Whether similar changes in lipoprotein particle profiles with long-term PM2.5 exposure occur in healthier individuals remains to be explored. An additional concern is the potential for selection bias. It is theoretically possible that air pollution exposure could impact selection into the study if people who reside in areas of higher air pollution are more likely to have a heart condition, and thus more likely to need an intervention such as catheterization. Factors, such as race/ethnicity and SES, could additionally impact selection into the study and are associated with air pollution exposure. We adjusted for race/ethnicity and an area-level SES measure in our analyses.
To our knowledge, this is the first study to link long-term PM2.5 exposure with measures of LDL particle concentration and size. A few previous studies have assessed associations with standard cholesterol measures; however, it has recently been suggested that novel measures of particle concentrations may be more clinically relevant to assigning risk of atherosclerosis. Further, we used an improved exposure assessment method that helped to minimize measurement error by combining GEOS-Chem predictions with AOD data, monitored values of PM2.5, meteorological values, and land use terms at a fine spatial resolution. Finally, CATHGEN is an at-risk population with cardiovascular disease, a group known to be especially susceptible to PM2.5.
In conclusion, we found associations between long-term PM2.5 exposure and several of the included lipoprotein measures, including total and small LDL-P and apoB. The results of our study may help in understanding the underlying mechanisms between PM2.5 exposure and cardiovascular disease.
Supplementary Material
Acknowledgements
We would like to acknowledge and thank all CATHGEN staff and participants for making this work possible.
Sources of Funding
This research was supported by intramural research funding by the US EPA; Health Effects Institute (Research Agreement #4946- RFPA10–3/14–7); National Institute of Environmental Health Sciences (award number T32ES007018); and an appointment to the Internship/Research Participation Program at Office of Research and Development (National Health and Environmental Effects Research Laboratory), U.S. Environmental Protection Agency, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and EPA.
Footnotes
Disclosures
The authors declare no conflict of interest.
Disclaimer
The research described in this article has been reviewed by the Environmental Protection Agency and approved for publication. The contents of this article do not necessarily represent Agency policy nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Allaire J, Vors C, Couture P, Lamarche B. 2017. LDL particle number and size and cardiovascular risk: Anything new under the sun? Curr Opin Lipidol 28:261–266. [DOI] [PubMed] [Google Scholar]
- Araujo JA. 2010. Particulate air pollution, systemic oxidative stress, inflammation, and atherosclerosis. Air Qual Atmos Health 4:79–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bart BA, Shaw LK, McCants CB Jr., Fortin DF, Lee KL, Califf RM, et al. 1997. Clinical determinants of mortality in patients with angiographically diagnosed ischemic or nonischemic cardiomyopathy. J Am Coll Cardiol 30:1002–1008. [DOI] [PubMed] [Google Scholar]
- Bell G, Mora S, Greenland P, Tsai M, Gill E, Kaufman JD. 2017. Association of air pollution exposures with high-density lipoprotein cholesterol and particle number: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 37:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bind MA, Peters A, Koutrakis P, Coull B, Vokonas P, Schwartz J. 2016. Quantile regression analysis of the distributional effects of air pollution on blood pressure, heart rate variability, blood lipids, and biomarkers of inflammation in elderly American men: The Normative Aging Study. Environ Health Perspect 124:1189–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake GJ, Otvos JD, Rifai N, Ridker PM. 2002. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation 106:1930–1937. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. 2010. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation 121:2331–2378. [DOI] [PubMed] [Google Scholar]
- Brook RD, Newby DE, Rajagopalan S. 2017. Air pollution and cardiometabolic disease: An update and call for clinical trials. Am J Hypertens. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, et al. 2016. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care 39:547–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang KJ, Yan YH, Chiu SY, Cheng TJ. 2011. Long-term air pollution exposure and risk factors for cardiovascular diseases among the elderly in Taiwan. Occup Environ Med 68:64–68. [DOI] [PubMed] [Google Scholar]
- Di Q, Kloog I, Koutrakis P, Lyapustin A, Wang Y, Schwartz J. 2016. Assessing PM2.5 exposures with high spatiotemporal resolution across the continental United States. Environ Sci Technol 50:4712–4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, et al. 2015. Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J 36:539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S, Lichtenstein AH, Chung M, Lau J, Balk EM. 2009. Systematic review: Association of low-density lipoprotein subfractions with cardiovascular outcomes. Ann Intern Med 150:474–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyarajah EJ, Cromwell WC, Otvos JD. 2006. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med 26:847–870. [DOI] [PubMed] [Google Scholar]
- Jiang S, Bo L, Gong C, Du X, Kan H, Xie Y, et al. 2016. Traffic-related air pollution is associated with cardio-metabolic biomarkers in general residents. Int Arch Occup Environ Health 89:911–921. [DOI] [PubMed] [Google Scholar]
- Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, et al. 2016. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi-Ethnic Study of Atherosclerosis and Air Pollution): A longitudinal cohort study. Lancet 388:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus WE, Granger CB, Sketch MH Jr., Donahue MP, Ginsburg GS, Hauser ER, et al. 2015. A guide for a cardiovascular genomics biorepository: The CATHGEN experience. J Cardiovasc Transl Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuller L, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, et al. 2002. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the cardiovascular health study. Arterioscler Thromb Vasc Biol 22:1175–1180. [DOI] [PubMed] [Google Scholar]
- Kunzli N, Jerrett M, Mack WJ, Beckerman B, LaBree L, Gilliland F, et al. 2005. Ambient air pollution and atherosclerosis in Los Angeles. Environ Health Perspect 113:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche B, Tchernof A, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, et al. 1997. Small, dense low-density lipoprotein particles as a predictor of the risk of ischemic heart disease in men. Prospective results from the Quebec Cardiovascular Study. Circulation 95:69–75. [DOI] [PubMed] [Google Scholar]
- Matyus SP, Braun PJ, Wolak-Dinsmore J, Jeyarajah EJ, Shalaurova I, Xu Y, et al. 2014. NMR measurement of LDL particle number using the Vantera Clinical Analyzer. Clin Biochem 47:203–210. [DOI] [PubMed] [Google Scholar]
- May HT, Anderson JL, Winegar DA, Rollo J, Connelly MA, Otvos JD, et al. 2016. Utility of high density lipoprotein particle concentration in predicting future major adverse cardiovascular events among patients undergoing angiography. Clin Biochem 49:1122–1126. [DOI] [PubMed] [Google Scholar]
- Mora S, Szklo M, Otvos JD, Greenland P, Psaty BM, Goff DC Jr., et al. 2007. LDL particle subclasses, LDL particle size, and carotid atherosclerosis in the multi-ethnic study of atherosclerosis (MESA). Atherosclerosis 192:211–217. [DOI] [PubMed] [Google Scholar]
- McGuinn LA, Ward-Caviness C, Neas LM, Schneider A, Di Q, Chudnovsky A, et al. 2017. Fine particulate matter and cardiovascular disease: Comparison of assessment methods for long-term exposure. Environ Res 159:16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otvos JD, Mora S, Shalaurova I, Greenland P, Mackey RH, Goff DC Jr. 2011. Clinical implications of discordance between low-density lipoprotein cholesterol and particle number. J Clin Lipidol 5:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenson RS, Davidson MH, Hirsh BJ, Kathiresan S, Gaudet D. 2014. Genetics and causality of triglyceride-rich lipoproteins in atherosclerotic cardiovascular disease. J Am Coll Cardiol 64:2525–2540. [DOI] [PubMed] [Google Scholar]
- Rural Health Research Center. 2009. Rural-Urban Commuting Area Codes (RUCAS). Available: http://depts.washington.edu/uwruca/ [accessed 16 December 2017].
- Sacks JD, Stanek LW, Luben TJ, Johns DO, Buckley BJ, Brown JS, et al. 2011. Particulate matter-induced health effects: who is susceptible? Environ Health Perspect 119:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanley RP, Hayes RB, Cromar KR, Ito K, Gordon T, Ahn J. 2016. Particulate air pollution and clinical cardiovascular disease risk factors. Epidemiology 27:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniderman AD, Williams K, Contois JH, Monroe HM, McQueen MJ, de Graaf J, et al. 2011. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes 4:337–345. [DOI] [PubMed] [Google Scholar]
- Sorensen M, Hjortebjerg D, Eriksen KT, Ketzel M, Tjonneland A, Overvad K, et al. 2015. Exposure to long-term air pollution and road traffic noise in relation to cholesterol: A cross-sectional study. Environ Int 85:238–243. [DOI] [PubMed] [Google Scholar]
- Steffen BT, Guan W, Remaley AT, Paramsothy P, Heckbert SR, McClelland RL, et al. 2015. Use of lipoprotein particle measures for assessing coronary heart disease risk post-American Heart Association/American College of Cardiology guidelines: The Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol 35:448–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triglyceride Coronary Disease Genetics C, Emerging Risk Factors C, Sarwar N, Sandhu MS, Ricketts SL, Butterworth AS, et al. 2010. Triglyceride-mediated pathways and coronary disease: collaborative analysis of 101 studies. Lancet 375:1634–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. 2000. Summary Files 1 and 3. Available: https://www.census.gov/census2000/sumfile1.html [accessed 2 October 2017].
- U.S. EPA (Environmental Protection Agency). 2007. The Plain English Guide to the Clean Air Act EPA-456/K-07–001. Available: https://www.epa.gov/sites/production/files/2015-08/documents/peg.pdf [accessed 16 October 2017].
- WHO (World Health Organization). 2016. Ambient (outdoor) Air Quality and Health. http://www.Who.Int/mediacentre/factsheets/fs313/en/ [accessed 7 December 2017].
- Yitshak Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. 2016. The association between air pollution exposure and glucose and lipids levels. J Clin Endocrinol Metab 101:2460–2467. [DOI] [PubMed] [Google Scholar]
- Zaid M, Miura K, Fujiyoshi A, Abbott RD, Hisamatsu T, Kadota A, et al. 2016. Associations of serum LDL particle concentration with carotid intima-media thickness and coronary artery calcification. J Clin Lipidol 10:1195–1202 e1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.