Abstract
The blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB) separate the brain and cerebrospinal fluid (CSF) from the systemic circulation and represent a barrier to the uptake of both endogenous compounds and xenobiotics into the brain. For compounds whose passive diffusion is limited due to their ionization or hydrophilicity, membrane transporters can facilitate their uptake across the BBB or BCSFB. Members of the solute carrier (SLC) and ATP-binding case (ABC) families are present on these barriers. Differences exist in the localization and expression of transport proteins between the BBB and BCSFB, resulting in functional differences in transport properties. This review focuses on the expression, membrane localization, and different isoforms present at each barrier. Diseases that affect the central nervous system including brain tumors, HIV, Alzheimer’s disease, Parkinson’s disease, and stroke affect the integrity and expression of transporters at the BBB and BCSFB and will be briefly reviewed.
Keywords: blood-brain barrier, blood-cerebral spinal fluid barrier, efflux transporters, uptake transporters
INTRODUCTION
The blood-brain barrier (BBB) and the blood-cerebrospinal fluid barrier (BCSFB) separate the brain and cerebrospinal fluid (CSF) from the systemic circulation. The BBB consists of endothelial cells that form tight junctions, which hinders the penetration of large or hydrophilic molecules, including toxins and nutrients, into the central nervous system (CNS). These endothelial cells express multiple substrate-specific transport systems, which are essential for the control of nutrient transport from the blood to the brain, as well as for transport of metabolic waste products from the brain’s interstitial fluid (ISF) into the blood (1). The BCSFB is composed of cuboidal epithelial cells known as choroid plexus epithelial cells, whose primary function is to secrete CSF. The transporters present at this barrier are involved in cell and CSF homeostasis. They are essential for the movement of nutrients into the CSF and secretion of waste products out of the CSF (2). Differences exist in the molecular composition and morphology between these barriers which result in functional differences (3).
Transport across membranes can be classified as passive diffusion, paracellular diffusion, facilitated transport, transcytosis, or active transport. The tight junctions in the BBB prevent paracellular diffusion (4). Small, lipophilic molecules and drugs, with molecular weights less than 400 Da and containing less than eight hydrogen bonds, can cross the BBB via diffusion. Molecules and drugs that do not meet these criteria can only cross the BBB via transporter-mediated processes (1). The BCSFB is less restrictive compared to the BBB, but the presence of transporters is essential in order to maintain CSF homeostasis. Transport at both membranes can be classified as uptake or efflux. Efflux transporters drive substrates out of the cell while the uptake transporters facilitate the movement of substrates into the cell. Typically, uptake of molecules is carried out by members of the solute carrier (SLC) family, while the efflux occurs against a concentration gradient by members of the ATP-binding cassette (ABC) family (5). However, some SLC transporters function as bidirectional or efflux transporters.
The SLC superfamily consists of 52 families and 395 individual transporter genes (5). Their classification into these families requires an amino acid homology of at least 25% between family members (6) and includes facilitated transporters, ion-coupled transporters, and exchangers (3). The facilitated transporters use the electrochemical potential difference in the transport of substrates, while ion-coupled transporters use a sodium or proton gradient in order to transport substrates against a concentration gradient. SLC transporters can function as bidirectional or unidirectional transporters (7). These transporters are present ubiquitously throughout the body in the BBB, choroid plexus, liver, intestine, kidney, blood-testis barrier, and placenta (6). The families that have been identified in the BBB and BCSFB are SLCO, SLC7A, SLC16A, SLC22A, SLC28A, and SLC29A (5). The SLC superfamily transports organic cations and anions, monocarboxylates, peptides, drugs and drug conjugates, steroids, and many other substrates (6,7).
The ABC family consists of 48 members that are classified into seven different families (A through G) depending their amino acid sequence and phylogeny (4). These transporters are classified as active transporters since they require an energy source, ATP, in order to transport substrates across a membrane. In some tissues, except for liver, intestine, and kidney, they are usually located on blood-facing membranes and are responsible for efflux of toxic waste or drugs (7). Members of this family that have been identified and studied in the BBB and BCSFB include ABCB1, ABCG2, and members of the ABCC subfamily.
This review focuses on transporter protein expression and localization at the BBB and BCSFB, since the main functions of these barriers are to restrict the free diffusion of molecules between the CNS and the blood and facilitate the transport of nutrients, ions, and metabolic waste products (3). There are differences in transporter expression between the BBB and BCSFB with respect to the transporter families and isoforms that are expressed, as well as their membrane localization.
BLOOD-BRAIN BARRIER
The BBB is comprised of a monolayer of non-fenestrated brain microvessel endothelial cells surrounded by pericytes, astrocytes, and neurons (8). The brain microvessel endothelial cells are joined by tight junctions that form a continuous impermeable barrier that limits paracellular flux. The BBB also has a high transendothelial electrical resistance (TEER) of 1500–2000 Ωcm2 which limits the flow of water and solutes (8). A number of in vitro BBB models have been developed to evaluate the brain penetration of drug candidates. Human BBB cell models include cryopreserved brain endothelial cells (HBMECs) and immortalized cell lines hCMEC/D3 (9) and BB19 (10). Cryopreserved brain microvascular endothelial cells (BMECs) from rats and mice are also commercially available. RBE4 cells are a rat immortalized brain microvascular endothelial cell model utilized to evaluate brain drug penetration (11). In addition, conditionally immortalized brain endothelial cell lines have been developed for rats and mice (12).
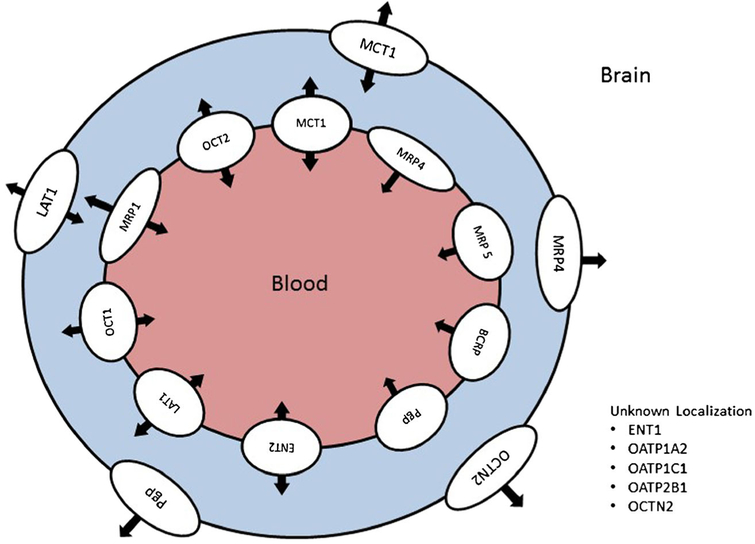
A wide range of SLC and ABC transporters are expressed in brain microvessel endothelial cells to mediate the flux of endogenous and exogenous substances across the BBB. Figure 1 illustrates human transporter expression and localization at the BBB. Species differences in transporter protein expression (quantitative and isoform differences), localization, and functional activity will be discussed in the following sections and are summarized in Table I.
Fig. 1.
Expression and localization of human drug transporters in brain capillary endothelial cells that form the blood-brain barrier
Table I.
Expression and Localization of Drug Transporters (Human and Other Species) at the Blood-Brain Barrier
| Transporter | Localization | Expression | Activity | |
|---|---|---|---|---|
| Uptake transporters | LAT1 | L, A | Brain capillaries (h, r, m) | Characterized in rat brain capillary cellsand is situ perfusion |
| LAT2 | mRNA-h, r, m | None or minimal | ||
| MCT1 | L, A (only for rats) | Brain capillaries (h, r, m, mo, ma) and CMEC/D3 | Characterized in RBE4 cells, in situ perfusion and in vivo brain microdialysis in rats | |
| MCT8 | Brain capillaries (h, mo) | |||
| Cnt1 | Rodent brain | |||
| Cnt2 | Rodent brain | Rat brain endothelial cells | ||
| ENT1 | L (only for rats) | Brain capillaries (h, r, m) | ||
| ENT2 | Brain capillaries (h, r, m) | |||
| OAT3 | mRNA-hCMEC/D3 and BB19 | |||
| Oat2 | r | |||
| Oat3 | L, A | r, m | Rat brain | |
| OAT4 | mRNA-BB19 | |||
| OATP1A2 | brain capillaries and microvessels (h) | |||
| OATP1C1 | Brain capillaries (h) | |||
| OATP2B1 | Brain capillaries (h) | |||
| Oatp1c1 | L, A | Brain capillaries (m, r) | ||
| Oatp2b1 | Brain capillaries (m, r) | |||
| Oatp1a4 | L, A | Characterized | ||
| OCT1 | L (r, h) | Human and rat brain endothelial cells | ||
| OCT2 | L (r, h) | Human and rat brain endothelial cells | ||
| OCT3 | Protein-h | |||
| mRNA-m | ||||
| Efflux transporters | Pgp | L (h, r, m), A (h) | Brain capillaries (h, r, m) | Immortalized brain capillary cell lines, hCMEC/D3 and RBE4 |
| BCRP | L (h, m, r) | Brain capillaries, hCMEC/D3, BB19 | ||
| MRP1 | L, A (r) | Protein-hCMEC/D3 | ||
| mRNA-h, BB19 | ||||
| MRP2 | L (m, r) | mRNA-h, BB19 | Mouse | |
| MRP3 | mRNA-h | |||
| MRP4 | L, A | Protein-brain capillaries (h, m, r, mo, ma), hCEMC/D3, BB19 mRNA-m | Brain capillary endothelial cells | |
| MRP5 | L | mRNA-h, BB19 | ||
| MRP6 | mRNA-h | |||
| OCTN2 | mRNA-h, m, r | Human, rat, and mice primary brain capillary endothelial cell |
L luminal side, A abluminal side, h human, r rats, m mice, mo monkey, ma marmoset
Uptake Transporters
l-Alpha Amino Acid Transporters
l-alpha amino acid transporters (LATs) are SLC transporters belonging to families 7 and 43 with four members identified: LAT1 (SLC7A5), LAT2 (SLC7A8), LAT3 (SLC43A1), and LAT4 (SLC43A2). 4F2hc cell-surface antigen heavy chain (SLC3A2) interacts with LAT1 and LAT2 to form system L (13). LATs transport neutral amino acids, including phenylalanine, leucine, tryptophan, and tyrosine, with LAT1 transporting large amino acids and LAT2 transporting large and small amino acids (13). LAT1 is primarily expressed in the brain, placenta, and tumor tissue, while LAT2 is primarily expressed in the kidney, intestine, and colon (13).
LAT1 is expressed on the luminal and abluminal surfaces of the BBB and functions as an antiporter (13,14). Expression of LAT1 has been quantified in brain capillaries from humans (15,16), rats (17), and mice (18), but was below the limit of quantification in adult cynomolgus monkeys and human hCMEC/D3 cells (9,19). LAT1 expression was five to ten times higher in mice and rats, as compared to humans (3.00 versus 0.43–0.8 fmol/μg protein) (17,18,20) suggesting that species differences exist in brain penetration of LAT1 substrates. LAT2 mRNA is expressed at the BBB in humans, rats, and mice at a much lower level than LAT1 (21–23). Functional studies at the BBB suggest that LAT2 activity is minimal or absent (24). LAT1 is involved in the BBB transport of a number of drugs that have CNS activity including gabapentin, pregabalin, L-DOPA, and methyldopa (13,25). Functional activity of LAT1 has been characterized in isolated rat brain capillary cells and via in situ perfusion (24,26)and in isolated bovine brain capillaries (14) utilizing amino acid and drug substrates.
Monocarboxylate Transporters
Monocarboxylate transporters are SLC transporters belonging to families 5 and 16. Fourteen members of the proton-dependent family (SLC16; MCTs) and two members of the sodium-dependent family (SLC5A8, SLC5A12; SMCTs) have been identified. MCTs demonstrate a wide tissue distribution with MCT1 having ubiquitous expression (27). SMCTs demonstrate a more restricted distribution with expression primarily observed in the intestine, colon, and kidney (28). Of the 14 MCTs, eight have been functionally characterized, but only four of these have been demonstrated to function as proton-dependent transporters of monocarboxylates: MCT1 (SLC16A1), MCT2 (SLC16A7), MCT3 (SLC16A8), and MCT4 (SLC16A3). These MCTs cotransport monocarboxylates, including lactate, pyruvate, and ketone bodies, along with a proton with a stoichiometric ratio of 1:1 (29). MCT8 (SLC16A2) functions as a thyroid hormone transporter and MCT10 (SLC16A10, TAT1) transports aromatic amines (27).
Only two MCT isoforms are expressed in the brain capillaries that comprise the BBB, namely MCT1 and MCT8. MCT1 expression in brain capillaries has been quantified in humans, monkeys, marmosets, rats, and mice with species differences observed in expression levels. Rats (12.6 fmol/μg protein) and mice (23.7 fmol/μg protein) had substantially higher MCT1 expression as compared to humans (1.46 and 2.27 fmol/μg protein) (15,17,18,20). Similar MCT1 expression levels were observed in brain capillaries isolated from humans, marmosets (3.04 fmol/μg protein), and cynomolgus monkeys (1.15 fmol/μg protein) (15,17,19). Expression was also quantified in hCMEC/D3 cells (1.87 fmol/μg protein) (9) and was comparable to expression in freshly isolated human brain capillaries. Age-dependent expression of MCT1 was observed in monkeys with neonates having 3-fold higher expression than adult monkeys (19). A similar age-dependent trend was observed in rats with 17-day-old rats having 25 times higher Mct1 expression at the BBB when compared to adult rats (30). Mct1 is localized to the luminal and abluminal membranes of the BBB in rats (31,32) with no localization information available in other species. MCT8 was quantified in brain capillaries isolated from adult monkeys (1.48 fmol/μg protein) and humans (1.31 fmol/μg protein) (19,20), but was not determined in brain capillaries from other species that have been studied using quantitative proteomics. Functional activity of MCT1 has been characterized at the BBB for the drug of abuse γ-hydroxybutyrate utilizing rat RBE4 cells, in situ perfusion, and in vivo brain microdialysis in rats (33–35).
Nucleoside Transporters
Nucleoside transporters can be classified into two families: concentrative nucleoside transporters (CNTs) and equilibrative nucleoside transporters (ENTs) (36). CNTs belong to SLC family 28A, with three isoforms (CNT1, CNT2 and CNT3) identified in humans. All isoforms actively transport nucleosides against a concentration gradient and function as Na+-coupled cotransporters, but differ in their affinity for purine and pyrimidine nucleosides. CNT1 (SLC28A1) and CNT2 (SLC28A2) preferentially transport pyrimidine and purine nucleosides, respectively, while CNT3 (SLC28A3) transports both purines and pyrimidines. ENT and CNT drug substrates and their transport characteristics have been reviewed elsewhere (36).
CNTs are primarily expressed in epithelial cells primarily in the kidney and liver (36), with Cnt1 and Cnt2 mRNA expression demonstrated in the rodent brain (37). Cnt2 can be detected in rodent brain capillaries and activity has been demonstrated in cultured rat brain endothelial cells (38). However, CNT1-3 expression was below the limit of quantification in proteomic analysis of human, rat, and marmoset brain microvessels (15,17). Additionally, CNT protein expression was not quantifiable in hCMEC/D3 cells (9), suggesting that these transporters may not be involved in brain penetration of nucleosides in humans.
ENTs are SLC transporters belonging to family 29A with four isoforms identified in humans: ENT1 (SLC29A1), ENT2 (SLC29A2), ENT3 (SLC29A3), and ENT4 (SLC29A4). ENT1 and ENT2 function as facilitated carriers and mediate the uptake and efflux of nucleosides depending on the concentration gradient across the membrane (36). ENT1, ENT2, and ENT3 transport both purines and pyrimidines with broad and overlapping substrate specificities. In addition, ENT2 and ENT3 can transport nucleobases. All ENTs demonstrate a wide tissue distribution with expression observed in the liver, kidney, intestine, colon, placenta, brain, and other tissues (36,39).
Within the brain, ENT1 and ENT2 are expressed in brain capillaries of rodents and humans. ENT2 is localized to the luminal membrane of brain capillaries in rats (38), while the localization of ENT1 has not been determined. Expression of ENT1 has been quantified in brain microvessels isolated from humans, monkeys, and mice (15,19,20). Humans and monkeys had similar expression of ENT1 with approximately 0.5–0.6 fmol/μg protein (15,19), while mice demonstrated 2-fold greater expression (0.99 fmol/μg protein) using the same analytical approach (15). A separate study demonstrated higher ENT1 expression in humans (0.86 fmol/μg protein) (20) suggesting there may be substantial interindividual variability in human expression levels. Interestingly, hCMEC/D3 cells demonstrated substantially higher expression of ENT1 (5.94 fmol/μg protein), 11.7-fold higher than observed in human brain capillaries (9).
Organic Anion Transporters
Organic anion transporters (OATs) are SLC transporters belonging to family 22 with 11 members (OAT1 through 6 and URAT1) currently identified (40,41). The OAT family has broad substrate specificity for endogenous and exogenous compounds that bear a negative charge at physiological pH. Human OAT1 to OAT4 (rodent Oat1 to Oat3) are primarily expressed in the liver, kidney, and brain, with OAT1 and OAT4 also expressed in the placenta and OAT3 expressed in skeletal muscle (40,41). Rodents express Oat5, Oat6, Oat8 (rats only), and Oat9 (mice only) that are not observed in humans and have a limited tissue distribution in rodents (primarily liver and kidney) (41).
There are distinct differences in OAT expression at the BBB. In humans, cynomologus monkeys and marmosets, OAT1 was not detected and OAT3 expression was below the limit of quantification via proteomic analysis of brain microvessel endothelial cells (15,17,19). Additionally, OAT3 expression was below the limit of quantification in hCMEC/D3 cells (9), but mRNA expression was observed in hCMEC/D3 and BB19 cells (10,20). In contrast, protein expression of Oat2 and Oat3 has been reported in rats, with Oat3 localized to the luminal and abluminal membranes (17,42). Functional activity of Oat3 was demonstrated in rat brain utilizing indoxyl sulfate transport which was inhibited by benzylpenicillin and para-aminohippurate (43), and abluminal function was shown using homovanillic acid transport (42). Oat3 expression has been demonstrated in mouse brain capillaries through proteomic analysis (18). Functional activity of Oat3 has been reported at the murine BBB through the evaluation of the brain permeation of the active form of oseltamivir (44). The authors hypothesized that Oat3 is expressed on both the luminal and abluminal membranes, which was consistent with the observed functionality (44). OAT4 mRNA has been detected in BB19 cells (10), but protein expression and localization data are unavailable.
Organic Anion Transporting Polypeptides
Organic anion transporting polypeptides (OATPs) are classified as SLCO transporters (formerly SLC21A) with 11 isoforms identified in humans and 15 rat and 14 mouse isoforms (45–47). OATPs are uptake transporters that are ATP and sodium independent and transport a diverse range of endogenous and exogenous compounds including bile salts, thyroid hormones, and polypeptides (48). OATP2A1, OATP3A1, and OATP4A1 have ubiquitous tissue distribution, while OATP1A2 (brain, kidney, liver), OATP1B1 (liver, placenta, ciliary bodies), and OATP1C1 (brain, testis) having more restricted distributions (48). OATP1B1 and OATP1B3 are only expressed in the liver (48). There are substantial species differences in OATP expression both with respect to isoforms and tissue distribution which have been discussed in detail elsewhere (48). A recent review by Ronaldson and Davis (45) details OATP/Oatp substrates and functional activity in the CNS. The present review will focus on OATP/Oatp species differences at the BBB and BCSFB.
A number of OATP isoforms are expressed at the BBB, and there are distinct species differences in BBB expression (8). In humans, OATP1A2 (SLCO1A2), OATP1C1 (SLCO1C1), and OATP2B1 are expressed in brain capillaries (16,49–51). Immuno-fluorescence staining confirmed the presence of OATP1A2 in brain microvessels and capillaries but not in astrocytes or neurons (49). Quantitative proteomic analysis was unable to quantify OATP expression in brain capillaries or hCMEC/D3 cells with all isoforms under the limit of detection (9,15); however, pathological stressors in the patients may have altered the BBB expression of OATP isoforms (45). Further functional studies are required to elucidate the contribution of OATP isoforms to substrate flux at the BBB.
Oatp1c1 and Oatp2b1 are also expressed in brain capillary endothelial cells of mice and rats (32,50,52) and are the only commonly expressed isoforms between humans and rodents. Rats express three Oatp isoforms at the BBB; Oatp1a4, Oatp1c1, and Oatp2b1. Oatp1a4 and Oatp1c1 are localized to the luminal and abluminal surfaces of the BBB (50,53), while Oatp2b1 expression is restricted to the abluminal surface (32). Oatp1a4, an ortholog of human OATP1A2, is functionally active at the BBB and mediates the brain uptake of DPDPE ([d-Pen2,5]enkephalin), pravastatin, pitavastatin, and rosuvastatin (45). A larger compliment of Oatp isoforms is observed at the mouse BBB with mRNA expression of Oatp1a1, Oatp1a4, Oatp1a5, Oatp1a6, Oatp1c1, and Oatp2b1 (52,54), with protein expression observed for Oatp1a4, Oatp1a5, and Oatp1c1 (50,54,55). Oatp expression was quantified in mouse brain capillaries with Oatp2b1 (2.11 fmol/μg protein) and Oatp1c1 (2.41 fmol/μg protein) observed (18). Compared to human brain capillaries, the amount of protein in mouse brain capillaries is at least 10-fold greater for Oatp2b1 and Oatp1c1 (15), suggesting that species differences may be observed for substrates of these transporters.
Organic Cation Transporters
Organic cation transporters (OCT) are SLC transporters belonging to family 22 with three members currently identified in humans: OCT1 (SLC22A1), OCT2 (SLC22A2), and OCT3 (SLC22A3). There are three corresponding orthologs in rats and mice, Oct1, Oct2, and Oct3. OCT1 and OCT2 have limited tissue distribution with OCT1 expressed in the liver and brain, while OCT2 is expressed in the kidney and brain (40,56). A wider tissue distribution is observed for OCT3 with expression observed in the liver, kidney, heart, lung, brain, muscle, and placenta (40). OCT1, OCT2, and OCT3 are involved in the sodium-independent electrogenic transport of organic cations and weak bases, which is bidirectional depending on the electrochemical gradient of the substrate (40).
Similar brain patterns of OCT expression are observed in humans, mice, and rats. OCT1/Oct1 and OCT2/Oct2 are localized to the luminal surface of brain capillary endothelial cells, based on confocal microscopy (56), with functional activity observed in cultured rat brain endothelial cells (56). In isolated brain capillaries from humans and rats, siRNA knockdown of OCT1/Oct1 and OCT2/Oct2 resulted in decreased uptake of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine) by 90% when both transporters were silenced (56). However, proteomic analyses in humans, monkeys, marmosets, and rats were not able to quantify OCT1 and OCT2 expression as they were below the limit of quantification (15,17,19). Additionally, OCT1/Oct1 and OCT2/Oct2 protein expression was below the limit of quantification in hCMEC/D3 cells (9). Protein expression of OCT3 has been reported in humans (16) and Oct3 mRNA expression has been observed in mice (57), but protein expression was too low to quantify utilizing LC/MS/MS approaches in all species evaluated (15,17–19).
Efflux Transporters
P-Glycoprotein
P-glycoprotein (Pgp) is an ABC transporter encoded by the MDR1 gene (ABCB1). In rats and mice, Pgp is encoded by three genes, mdr1a (Abcb1a), mdr1b (Abcb1b), and mdr2. Pgp is involved in the cellular efflux of a diverse range of compounds against a concentration gradient including antiretrovirals, anticancer agents, analgesics, anti-histamines, and antibiotics (8). Pgp is expressed in epithelial cells in the intestine, liver, lung, pancreas, kidney, and placenta (58) and may contribute to the multidrug resistance observed in tumor cells (59).
Expression of MDR1/mdr1a mRNA has been demonstrated in human and rodent brain capillaries (60). Pgp expression is localized to the luminal and abluminal surfaces of brain capillaries in humans (61–64) and to the luminal surface in rats and mice (65,66). Species differences were observed in MDR1/mdr1a expression in brain capillaries with the highest expression observed for mdr1a in rats (19.1 fmol/μg protein) and mice (14.1 fmol/μg protein) (17,18). Similar MDR1 expression levels were observed in humans, cynomolgus monkeys, and marmosets (3.9–6.5 fmol/μg protein) (15,17,19,20). Age-dependent protein expression of Pgp was seen in monkeys, with adults having 0.68-fold lower expression than child monkeys (16 months of age) in isolated brain endothelial cells (19). In contrast, Pgp protein expression increases from birth to adulthood at the mouse BBB (67). Decreases in Pgp expression or inhibition of Pgp function at the BBB lead to increases in brain drug concentrations as demonstrated through the use of mdr1a/1b knockout mice and selective Pgp inhibitors (8,68). mRNA and protein expression of MDR1 were observed in hCMEC/D3 and BB19 cells (9,10), with hCMEC/D3 cells having lower MDR1 expression (3.87 fmol/μg protein) than quantified in human brain capillaries. The functional expression of Pgp has also been characterized in the human and rat immortalized brain capillary cell lines, hCMEC/D3and RBE4, utilizing a diverse range of compounds (69–72).
Breast Cancer Resistance Protein
Breast cancer resistance protein (BCRP; ABCG2) is an ABC transporter, with significant overlap in substrate specificity with Pgp, involved in the cellular efflux of sulfoconjugated organic anions and hydrophobic and amphiphilic drugs (8). However, BCRP differs from other ABC transporters in that it is a “half-transporter” with six transmembrane domains and a single nucleotide binding domain (4). BCRP expression has been demonstrated in the placenta, liver, intestine, colon, kidney, heart, and brain (73,74).
BCRP was first identified at the BBB by Galla and colleagues (75) and is localized to the luminal membrane in humans, mice, and rats (76–78). Species differences in expression of BCRP/Bcrp in brain capillaries have been demonstrated through quantitative proteomic analysis. Marmosets and monkeys had the highest BRCP expression (14–16 fmol/μg protein) (17,19), at least double the expression that was observed in humans (6.15 and 8 fmol/μg protein) (15,20). Further reduced expression of Bcrp was observed in rats and mice (4–5 fmol/μg protein) (17,18), suggesting that brain penetration of BCRP substrates would be higher in rodents and lower in monkeys and marmosets. Age-dependent BCRP protein expression was observed in cynomolgus monkeys with adults having approximately 3-fold higher expression than neonates in brain capillaries (19). In mdr1a knockout mice, Bcrp mRNA expression is increased in brain capillaries, suggesting that it compensates for the loss of Pgp (79). BCRP expression is retained in the human BBB cell culture models, hCMEC/D3 and BB19 (9,10); however, quantitative analysis of hCMEC/D3 cells showed substantially reduced expression, 2.18 versus 8 fmol/μg protein in human brain capillaries (9,15). Additionally, studies in human brain endothelial cells and RBE4 cells demonstrated a lack of functional activity utilizing mitoxantrone as a BCRP/Bcrp substrate in studies in combination with specific BCRP/Bcrp inhibitors (80).
Multidrug Resistance-Associated Proteins
Multidrug resistance-associated proteins (MRPs) are ABC transporters that are members of the ABCC family which contains 12 members: nine members are identified as MRPs (MRP1- MRP9), the cystic fibrosis transmembrane conductance regulator (CFTR), and two sulfonylurea receptors (SUR1 and SUR2) (81). MRPs are responsible for the cellular efflux of a broad range of endogenous and exogenous compounds including antiretrovirals, chemotherapeutic agents, antibiotics, and toxins (8). Differing expression patterns are observed for MRP1 through MRP6 with one or more isoforms expressed in the brain, liver, kidney, intestine, colon, heart, lung, pancreas, and placenta (81,82).
Brain capillary mRNA expression of MRPs has been demonstrated for MRP1–6 (ABCC1 to ABCC6) in humans (20,60), with MRP1, MRP4, and MRP5 localized to the luminal surface, and MRP4 to the abluminal surface, determined with immunohistochemistry (83). Protein expression has only been quantified for MRP4 in humans, rodents, marmosets, and monkey isolated brain capillaries (15,17–20), with distinct species differences observed in expression levels. MRP4 expression was similar in marmosets, monkeys, and humans (0.2–0.3 fmol/μg protein) (15,17,19,20); however, expression was approximately 10-fold higher in rats (1.59 fmol/μg protein) (17) and mice (1.60 fmol/μg protein) (18). In contrast, brain capillary expression of the remaining MRP isoforms was below the LC/MS/MS limits of quantification for all species evaluated. MRP4 protein expression was also confirmed in hCMEC/D3 cells and BB19 cells, with MRP4 expression consistent with isolated human brain capillaries in hCMEC/D3 cells (0.31 fmol/μg protein) (9). MRP1 protein expression was quantified in hCMEC/D3 cells (1.65 fmol/μg protein) (9), as well as MRP2, MRP3, and MRP5 mRNA expression (84,85), which were not quantifiable in isolated human brain capillaries. BB19 cells demonstrated mRNA expression of MRP1, MRP2, and MRP5, with only very low MRP1 and MRP2 staining observed with immunochemistry (10). In rats and mice, Mrp1 is localized on the abluminal surface differing from the luminal localization observed in humans (32,66,83). Mrp2 protein expression was demonstrated in isolated rodent brain capillaries localized to the luminal surface (86,87) indicating that species differences may be observed in brain penetration of Mrp2 substrates. Functional activity of Mrp2 was demonstrated at the mouse BBB through increased brain penetration of phenytoin in Mrp2 null mice (88). Additionally, Mrp4 null mice had increased brain penetration of adefovir suggesting Mrp4 is functionally active in brain capillary endothelial cells (89).
Novel Organic Cation Transporters
Novel organic cation transporters (OCTN) are SLC transporters belonging to family 22 with two isoforms identified in humans (OCTN1 and OCTN2) and three identified in rodents (Octn1–Octn3). OCTN1 functions as an electroneutral proton gradient-dependent antiporter that mediates substrate efflux across membranes, while OCTN2 functions as a sodium-dependent carnitine cotransporter, but can also mediate sodium-independent transport of organic cations (40). Both OCTN1 (SLC22A4) and OCTN2 (SLC22A5) have wide tissue distributions with expression observed in the liver, kidney, intestine, colon, spleen, heart, and lung (90). In the brain, mRNA expression of OCTN2 has been observed in human brain capillaries and cultured brain microvessels (20,91). Mouse and rat brain capillaries have demonstrated mRNA expression of Octn2 (57,91). Protein expression has not been observed for OCTN2 in humans, cynomolgus monkeys, marmosets, rats, or mice (15,17–19). Functional activity of OCTN2/Octn2 has been observed in primary brain capillary endothelial cell cultures from humans, rats, and mice utilizing carnitine (91). In vivo Octn2 activity was observed in rats via in situ brain perfusion of carnitine and acetyl-carnitine (91).
BLOOD-CSF BARRIER
The BCSFB is formed by the choroid plexus, which surrounds fenestrated capillaries. The choroid plexus contains modified cuboidal epithelium connected by tight junctions near the apical surface with dense microvilli on the apical membrane (3,5). The barrier is located in the lateral, third and fourth brain ventricles (5). In contrast to the BBB, the BCSFB is a leakier epithelium as evidenced by lower TEER values and leakage of plasma proteins (92). A number of choroid plexus epithelial cell culture models have been developed. Primary choroid plexus epithelial cells have been cultured from humans, rats, and pigs (93–95), as well as rat (ECPC-4; Z310) and pig (CPEC) immortalized cell lines (96–98).
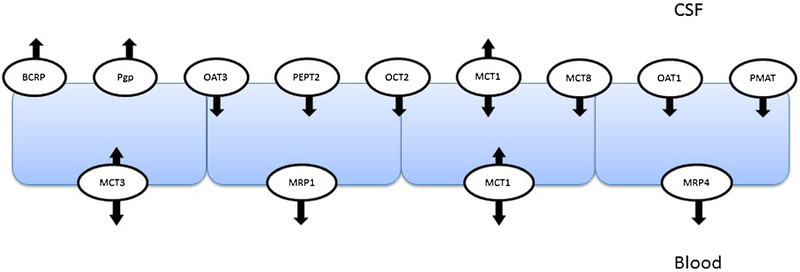
The choroid plexus has a distinct complement of SLC and ABC transporters demonstrating polarized expression involved in the uptake and efflux of drug substrates (99). Figure 2 illustrates human transporter expression and localization data available in the literature. There is limited data on SLC and ABC transporter protein expression and functional activity at the human BCSFB. This review will focus on available human data and species differences with animal models. Available data for mRNA and protein expression and species differences is summarized in Table II.
Fig. 2.
Expression and localization of human drug transporters in choroid plexus epithelial cells that form the blood-CSF barrier
Table II.
Expression and Localization of Drug Transporters (Human and Other Species) at the Blood-CSF Barrier
| Transporter | Localization | Expression | Activity | |
|---|---|---|---|---|
| Uptake transporters | LAT2 | Protein-r, m | ||
| mRNA-r | ||||
| MCT1 | A (r), B (r) | |||
| MCT3 | B | Protein-r | ||
| MCT8 | A (h, r, m) | Characterized | ||
| Cnt2 | A (r) | Protein-r | ||
| mRNA-r | ||||
| CNT3 | mRNA-h, r | |||
| ENT1 | B (r) | Protein-h, r | ||
| mRNA-h, r | ||||
| ENT2 | A (r), B (r) | Protein-r | Active in choroid plexus segments | |
| mRNA-h, r | ||||
| ENT3 | mRNA-h | |||
| OAT1 | A (h, r) | mRNA-m | ||
| OAT3 | A (h, r) | mRNA-m | Characterized in rats and mice | |
| Oat2 | mRNA-r, m | |||
| Oat4 | mRNA-r | |||
| OATP1C1 | A (h), B (h) | |||
| OATP3A1 | Protein-h | |||
| OATP3A4 | B (h) | |||
| Oatp1a4 | B (r) | Protein-m | ||
| mRNA-r, m | ||||
| Oatp1a1 | A (r) | |||
| Oatp1a3 | A (r) | |||
| Oatp1a5 | A (r, m) | Protein-m | Characterized in rats | |
| mRNA-r, m | ||||
| Oatp1a6 | mRNA-m | |||
| Oatp2a1 | A (r) | |||
| Oatp2b1 | A (r) | |||
| Oatp1c1 | A (r, m), B (r, m) | Protein-m | ||
| mRNA-m | ||||
| Oct2 | A (r) | Protein-r | Characterized in rat | |
| mRNA-r | ||||
| Oct3 | Protein-r | |||
| mRNA-1 | ||||
| PMAT | A (h, m) | Protein-h, m | Characterized in mice | |
| mRNA-h, m | ||||
| MATE1 | Protein-h | |||
| Pept1 | mRNA-r | |||
| Pept2 | A (r, m) | mRNA-r | Characterized in mice | |
| Efflux transporters | Pgp | A (h) | Protein-h, r, m | Studied in CPEC cells |
| mRNA-h, r, m | ||||
| mdr1a | mRNA-m | |||
| mdr1b | ||||
| BCRP | A (h, m) | h, m, r | Confirmed in rat choroid plexus Z310 cells | |
| MRP1 | B (h, m, r, p) | Protein-h, m | In vivo mice and CPEC cells | |
| mRNA-h, r, m | ||||
| MRP2 | mRNA-h | |||
| MRP3 | mRNA-h | |||
| MRP4 | B (h, m, r) | Protein-h, m | ||
| mRNA-h, r, m | ||||
| MRP5 | B (h) | mRNA-h | ||
| MRP6 | mRNA-h, r |
B basolateral side, A abluminal side, h human, r rats, m mice, p porcine
Uptake Transporters
l-Alpha Amino Acid Transporters
Species differences exist in LAT/Lat expression in the choroid plexus with Lat2 mRNA expression observed in rats and mice. Lat2 mRNA expression was observed in the choroid plexus via in situ hybridization; however, protein expression was not evaluated (100). Expression of Lat2 was quantified in rat choroid plexus (1.61 fmol/μg protein), while human LAT2 expression was under the limit of quantification (101). The quantification limit for LAT2 was 2.08 fmol/μg protein (101), so human expression may be similar to rats, but comparison is hindered by the higher limit of quantification. Functional activity of LAT2/Lat2 in choroid plexus epithelial cells has not been evaluated.
Monocarboxylate Transporters (MCTs)
In humans, MCT1 is expressed to a similar extent in choroid plexus epithelial cells (3.47 fmol/μg protein) and brain capillaries (2.27 fmol/μg protein) (15,101). Mct1 (Slc16a1) protein expression has been demonstrated in rat choroid plexus epithelial cells with low expression observed on the apical and basolateral membranes (30). Roberts et al. also observed faint and diffuse Mct1 staining in rat choroid plexus through in vivo biotinylation; however, Mct1 did not appear to be localized to the plasma membrane (32). MCT3 (SLC16A8), which has the most restricted expression pattern of all MCT isoforms, has been detected in the choroid plexus and is localized to the basolateral membrane (102). Mct3 expression was quantifiable in the rat choroid plexus (2.75 fmol/μg protein); however, expression was below the limit of quantification in human samples (101). In contrast to the observed expression patterns at the BBB, MCT8 (SLC16A2) has much greater expression in choroid plexus epithelial cells in humans, rats, and mice and is localized to the apical membrane (50,103–105). During the rat fetal period, Mct8 is highest in the choroid plexus likely due to its role in thyroid hormone transport (106). In isolated human choroid plexus, MCT8 was expressed to a lower extent than MCT1 with an expression level of 1.65 fmol/μg protein (101). Functional activity has not been demonstrated for MCT1 in choroid plexus epithelia cells. Loss of MCT8 (Allan-Herndon-Dudley syndrome) leads to reduced CSF concentrations of free T4 demonstrating functional activity of MCT8 at the BCSFB (107).
Nucleoside Transporters
In the choroid plexus epithelium, mRNA and protein expression of CNTs and ENTs has been observed in humans and rodents; however, there are species differences in the isoforms that are expressed. In humans, CNT3 and ENT1, ENT2, and ENT3 mRNA are expressed at the choroid plexus with ENT3 having the highest transcript expression (108). Functional studies suggest that ENT2 is active in choroid plexus epithelial segments, with no activity of ENT1 (108). Contradicting the functional studies, proteomic analysis of ENT expression in the human choroid plexus was able to quantify ENT1 expression (2.49 fmol/μg protein), but ENT2 expression was below the limit of quantitation (LQ = 1.49 fmol/μg protein) (101). In the same quantitative proteomic analysis, all CNT isoforms were below their limits of quantification. ENT3 is located intracellularly on lysosomal membranes and therefore would not contribute to drug transport across the choroid plexus (8). In rats, mRNA expression of Ent1, Ent2, Cnt2, and Cnt3 is observed in choroid plexus epithelia cells (38,109,110), with protein expression observed for Ent1, Ent2, and Cnt2 (38). Ent1 is localized to the basolateral (blood-facing) membrane, while Ent2 was demonstrated to be localized to both the apical and basolateral membranes in choroid plexus epithelia cells (38). In contrast, Cnt2 is localized to the apical membrane (38).
Organic Anion Transporters (OATs)
The expression of multiple OATs has been demonstrated at the BCSFB in humans, rats, and mice (5). In humans, OAT1 and OAT3 are localized to the cytoplasmic membrane and cytosol of choroid plexus epithelial cells based on immunohistochemical staining (111). Localization of Oat1 and Oat3 to the apical membrane had been observed in the rat choroid plexus (112,113). Quantitative analysis demonstrated species differences in OAT3/Oat3 protein expression with rats having 2-fold higher expression than humans, with neither rats nor humans having quantifiable protein expression of OAT1/Oat1 (101,112). Functional expression of Oat3 was demonstrated in rats utilizing transport of p-aminohippurate and benzylpenicillin and known Oat3 inhibitors (112). In addition to Oat1/Oat3, mRNA expression of Oat2 and Oat4 has been seen in the rat choroid plexus (114,115); however, protein expression was not quantifiable by proteomic analysis (101), and membrane localization remains unknown. mRNA expression of Oat1, Oat2, and Oat3 in the choroid plexus has been observed in mice (113,114), with Oat3 knockout mice demonstrating reduced transport of Oat3 substrates confirming its functional expression at this barrier (114). In addition, functional expression of Oat1 was retained in Oat3 knockout mice, and functional expression of Oat3 was retained in Oat1 knockout mice supporting the overlap in substrate specificities between these isoforms (114).
Organic Anion Transporting Polypeptides (OATPs)
Species differences exist in the OATP/Oatp isoforms that are expressed at the BCSFB, with rodents having a broader range of Oatps expressed. In humans, three OATP isoforms have been observed in choroid plexus epithelial cells, OATP1C1, OATP3A1, and OATP3A4 (5,101). OATP1C1 is localized to the apical and basolateral membranes while OATP3A4 is localized to the basolateral membrane (50,116). Rats have the largest compliment of Oatp isoforms identified at BCSFB with protein expression of nine isoforms observed in choroid plexus epithelial cells (5). Oatp1a4 is localized to the basolateral membrane and Oatp1a1, Oatp1a3, Oatp1a5, Oatp2a1, and Oatp2b1 are localized to the apical membrane of rat choroid plexus epithelial cells (32,53,117–119). In contrast to other rat Oatp isoforms in choroid plexus epithelial cells, Oatp1c1 is expressed at both the apical and basolateral membranes (50). Studies suggest that Oatp1a5 is the most abundant on rat choroid plexus with its’ mRNA 500-fold higher than in the liver, kidney, and ileum (110). Quantitative differences exist between humans and rats for OATP/Oatp expression. Oatp1c1 is much greater in rats (6.66 fmol/μg protein) with human OATP1C1 expression in choroid plexus epithelia below the limit of quantitation (LQ = 0.156 fmol/μg protein) (101). In contrast, OATP3A1 protein expression was quantified in human choroid plexus (0.641 fmol/μg protein), while expression was not quantifiable in rats (101). Additionally, all other OATP/Oatp isoforms were below protein quantitation limits in both humans and rats (101). Mice express four Oatp isoforms in choroid plexus epithelial cells, Oatp1a4, Oatp1a5, Oatp1a6 (mRNA only), and Oatp1c1 (5). Consistent with isoform localization in rats, Oatp1a5 is expressed at the apical membrane and Oatp1c1 is localized to both the apical and basolateral membranes (50,54). Functional activity of Oatp1a5 at the BCSFB was confirmed in conditionally immortalized rat choroid plexus epithelial cells via estrone-3-sulfate transport and known Oatp1a5 inhibitors (119).
Organic Cation Transporters
Transport of organic cations at the BCSFB demonstrates species differences in protein expression and the primary transporter involved. Expression and functional activity of OCTs is not observed at the human BCSFB; however, Oct2 and Oct3 mRNA expression has been demonstrated at the rat choroid plexus (120). Oct2 expression is localized to the apical membrane with functional activity confirmed through in vitro choline transport in intact choroid plexus (120). In contrast, organic cation transport in the human choroid plexus is mediated by the plasma membrane monoamine oxidase transporter (PMAT; SLC29A4) and multidrug and toxic compound extrusion protein-1 (MATE1; SLC47A1) (121). MATE1 expression was quantified in human choroid plexus (8.61 fmol/μg protein) with expression under the limit of quantitation in rats (101). Localization of MATE1 in the choroid plexus has not been evaluated. mRNA and protein expression of PMAT has been demonstrated in the human and mouse choroid plexus with expression localized to the apical membrane (121). Protein expression of PMAT was quantified in human choroid plexus (0.288 fmol/μg protein), with expression below the limit of quantitation in rats (101). Functional activity was confirmed in ex vivo uptake studies evaluating MPP+, 5-HT, and dopamine transport in isolated choroid plexus tissues from normal and Pmat knockout mice (121).
Peptide Transporters
Peptide transporters are SLC transporters belonging to family 15 with four isoforms identified in humans: PEPT1 (SLC15A1), PEPT2 (SLC15A2), PHT1 (SLC15A4), and PHT2 (SLC15A3). These transporters function as proton-coupled oligopeptide symporters with the uptake of small peptides coupled to the efflux of a proton (122). The various isoforms have broad and overlapping substrate specificity and transport di- and tri-peptides and peptidomimetic drug substrates (8). PEPT1 and PEPT2 are expressed in the intestine, kidney, liver, and brain, with expression in choroid plexus epithelia cells (123). PHT1 is expressed in the brain, intestine, retina, and placenta, while PHT2 is expressed in the lung, spleen, and thymus (123).
Pept1 and Pept2 mRNA expression has been identified in the rat choroid plexus epithelium (110,124) with Pept2 having the highest transcript expression (110). Rat Pept2 mRNA expression in the choroid plexus was greater than observed in the liver, kidney, and ileum (110). Protein expression of Pept2 is localized to the apical membrane of the choroid plexus epithelial cells in rats and mice (124–126). In rats, Pept2 expression in the choroid plexus was 3.06 fmol/μg protein, while PEPT2 was below the limit of quantification in humans (101), suggesting that there are species differences in the functionality of PEPT2/Pept2 at the BCSFB. Functional activity of Pept2 at the BCSFB was confirmed in Pept2 knockout mice who demonstrated altered uptake of cefadroxil from the CSF into choroid plexus epithelial cells (127).
Efflux Transporters
P-Glycoprotein
Pgp mRNA and protein are expressed at the BCSFB in humans, rats, and mice, with expression of mdr1a and mdr1b in rats and mdr1a in mice (5). Pgp (MDR1) is localized to the apical side of the choroid plexus in humans (128). MDR1/mdr1a protein expression in choroid plexus epithelial cells is substantially lower than expression in brain capillaries in both humans and rats. Rat mdr1a expression in the choroid plexus is 0.320 fmol/μg protein, which is approximately 15-fold lower than expression levels in brain capillaries (19.1 fmol/μg protein) (17,101). In vivo biotinylation studies were not able to detect rat Pgp in choroid plexus epithelial cells (32). Pascale et al. (129) demonstrated a significant age-dependent increase in Pgp mRNA and protein expression from 3 to 36 months in the choroid plexus epithelium of male rats. The difference between BBB and BCSFB protein expression is less in humans with around 3-fold lower expression in choroid plexus epithelial cells (15,101). Mdr1a expression in the mouse choroid plexus epithelia has been observed at the mRNA level, but there is a lack of protein expression and localization data (130). In CPEC cells, minimal Pgp expression was observed on the apical membrane with the majority of protein expression observed in sub-apical compartments (97). Functional activity of Pgp was evaluated in CPEC cells utilizing rhodamine 123 and verapamil with no directional transport observed suggesting that Pgp is not active in these isolated choroid plexus cells (97).
Breast Cancer Resistance Protein
BCRP/Bcrp protein expression is observed in the choroid plexus of humans, mice, and rats (5,101). Quantitative analysis indicated that humans have 2-fold higher expression of BCRP in choroid plexus epithelial cells when compared to rats (0.706 versus 0.333 fmol/μg protein) (101). These expression levels are significantly lower than those observed at the BBB, and the interspecies differences are also much less. BCRP/Bcrp is localized to the apical membrane of the choroid plexus in humans and mice (75,78). Functional activity of Bcrp was confirmed in the rat choroid plexus utilizing Z310 cells and sulfasalazine transport with Ko143, a Bcrp specific inhibitor, altering sulfasalazine flux (131).
Multidrug Resistance Protein
At the human BCSFB, mRNA expression for MRP1 through MRP6 has been observed in epithelial cells; however, protein expression has only been observed for MRP1, MRP4, and MRP5 (128,132,133). Both MRP4 and MRP5 protein expression has been localized to the basolateral membrane in choroid plexus epithelial cells (32). In rats a similar mRNA expression pattern is seen (110), with protein expression for observed Mrp1, Mrp4, and Mrp6 (32,134). Expression of Mrps at the mouse choroid plexus is limited to Mrp1 and Mrp4 with both mRNA and protein expression observed (133,135). MRP1/Mrp1 and MRP4/Mrp4 have been quantified in choroid plexus epithelial cells from rats and humans with higher protein expression observed in rats for both transporters (4-fold higher and 2-fold higher, respectively) (101). MRP1 protein expression is higher in the BCSFB, as compared to the BBB where expression was below quantification limits. MRP4 expression is also higher at the BCSFB with approximately 2–3-fold higher protein expression in choroid plexus epithelial cells (15,101). In contrast, Mrp4 protein expression in rat choroid plexus epithelial cells and brain capillaries was similar (17,101). Localization of MRP1/Mrp1 and MRP4/Mrp4 is consistent between species with localization on the basolateral membrane (32,133,136,137). Mrp1 mRNA and protein expression was observed in freshly culture porcine choroid plexus and CPEC cells with localization on the basolateral membrane (97). Functionally activity of Mrp1 was established in CPEC cells utilizing fluorescein-methotrexate (97) and in vivo in mice lacking Mrp1 with the increased CSF accumulation of the Mrp1 substrate etoposide (135).
CHANGES IN EXPRESSION/FUNCTION OF BBB AND BCSFB TRANSPORTERS WITH DISEASE STATES
Several diseases affect the delivery of drugs to the brain by altered expression of drug transporters on the BBB and BCSFB, including HIV, Alzheimer’s disease, Parkinson’s disease, and stroke (please refer to other articles in this Theme Issue on CNS Barriers in Health and Disease). Research has primarily focused on efflux transporters, which can prevent pharmaceutical agents from reaching sufficient brain concentrations to elicit the desired effect.
Available treatments for brain tumors are surgery followed by chemotherapy and/or radiotherapy; however, even with this treatment regimen the survival rate is low due to the presence of a multidrug resistance phenotype (MDR) characterized by overexpression of efflux transporters. Most chemotherapy agents are substrates of ABC transporters, which contribute to the MDR phenotype due to increased drug efflux in cancer cells (8,138). On et al. (139) demonstrated that after 12 days of brain tumor implantation in Balb/c mice, the BBB integrity is compromised increasing the permeability of small molecules. This was confirmed by MRI analysis of Gad-DTPA contrast agent brain uptake, which was significantly enhanced 12 days post-implantation (139). However, Pgp expression in brain capillary endothelial cells was not different in brain regions containing tumor as compared to tumor free brain regions (139).
HIV is a retrovirus that has the ability to compromise the immune system by infecting immune cells, like monocytes, CD4+T lymphocytes, dendritic cells, and macrophages and leads to acquired immunodeficiency syndrome (AIDS) in humans. It infects the CNS right after transmission, causing neuropathological complications (140). As the infection progress, it results in HIV-endocephalitis (HIVE) which is characterized by multinucleated giant cells, elevated infiltration of monocytes/macrophages into the brain, myelin pallor, reactive astrocytosis, and microglial nodules (8,140). HIV infection, especially HIVE, results in increased inflammatory and neurotoxic cytokines, including interferon, tumor necrosis factor alpha and interleukin-1β; this chronic inflammation can lead to alterations in brain capillary endothelial cells and BBB integrity thereby increasing the leakiness of the BBB (8,140). The presence of pro-inflammatory cytokines results in decreased Pgp expression in astrocytes, while glial expression is increased (8). In primary mouse brain capillary endothelial cells, Pgp and MRP1 mRNA and protein expression were increased in the presence of HIV Tat protein, with no changes observed for MRP2–MRP7 (141,142). Protease inhibitors utilized in HIV therapy have also been shown to induce Pgp protein expression at the BBB (69). Increased protein expression of MRP1 and Pgp at the BBB further limits the brain penetration of antiretrovirals and may contribute to the variability in response to HIV therapies (140). BCSFB integrity, analyzed by the CSF albumin/serum albumin quotient (QAlb = AlbCSF/Albserum), was reduced in HIV patients but not in uninfected controls; however, there is a lack of information on transporter expression in choroid plexus epithelial cells in HIV infection (143).
Alzheimers’s disease (AD) is a neurodegenerative disorder that causes decreased mental and memory processing (8). Accumulation of amyloid-β (Aβ) plaques and neurofibrillary tangles are characteristic neurological changes observed in AD. Transport of Aβ from the brain to blood is mediated by Pgp (8). Decreased Pgp expression has been associated with accumulation of Aβ and age progression, confirming the role of this transporter at the BBB (8,144,145). Immunohistochemical analyses of Pgp and Aβ expression in human brain samples indicated that they have an inverse relationship (146).
Parkinson’s disease (PD) is the second most common neurodegenerative disease and is characterized by progressive loss of dopamine neurons in the substania nigra leading to motor impairment, muscle rigidity, and tremor (8,147). PD etiology is related to environmental or genetic factors, with disease progression related to a decrease in the ability to detoxify neurotoxins via Pgp. Genetic polymorphisms in the MDR1 gene have been associated with PD. Bartels et al. (148) studied the uptake of verapamil, a Pgp substrate, and reported that the functional activity of Pgp changes with disease progression likely due to increased Pgp expression. A decrease in Pgp expression was observed with PD progression, with an initial increase in Pgp during early disease stages when compared with healthy individuals (148). Decreases in MDR1 mRNA expression in the brains of patients with Parkinson disease compared to healthy controls have also been observed (8,147).
Studies have demonstrated a time-dependent increase in Pgp expression at the BBB after induced ischemia in rats (149). Deprivation from oxygen and glucose when the blood flow is interrupted during ischemic stroke causes irreversible damage to the ischemic core and surrounding tissue (8). Functional activity of Pgp has been studied using Rh123 and nimodipine, with decreased concentrations observed after 4 h and elevated ratios observed after 6 h after the ischemic event, demonstrating that even with an increase in protein expression, the elevated Pgp functional activity was not enough to prevent agents from penetrating through the disrupted BBB (149). Ennis et al. (150) demonstrated in three different ischemia models that the BCSFB is disrupted earlier as compared to the BBB. Ischemia disrupts the BCSFB enhancing the movement of compounds from the blood to areas close to the ventricular system (150,151).
SUMMARY
The BBB is comprised of a monolayer of non-fenestrated brain microvessel endothelial cells surrounded by pericytes, astrocytes, and neurons and joined by tight junctions that form a continuous impermeable barrier that limits paracellular flux. A wide range of SLC and ABC transporters are expressed at the BBB to mediate the flux of endogenous and exogenous substances across the BBB. The BCSFB, on the other hand, is formed from the choroid plexus and contains modified cuboidal epithelium connected by tight junctions. In contrast with the BBB, the BCSFB represents a leakier barrier and is characterized with a distinct complement of SLC and ABC transporters. In vitro and in vivo models of the BBB and BCSFB have been developed in order to study the expression, membrane localization, and functionality of transporters. Human models of the BBB include isolated brain microvessel endothelial cells and immortalized cell lines hCMEC/D3 and BB19. For other species, including mouse and rat, there are commercially available cryopreserved brain microvascular endothelium cells, immortalized brain microvascular endothelial cells (RBE4), and immortalized brain endothelial cell lines. For BCSFB, primary choroid plexus epithelial cells have been cultured from humans, rats, and pigs, as well as rat (ECPC-4; Z310) and pig (CPEC) immortalized cell lines. This review highlights the reported expression, localization, and species differences in the SLC and ABC transporters at the BBB and BCSFB. Neurological diseases including brain tumors, HIV, Alzheimer’s disease, Parkinson’s disease, and stroke affect the integrity of the BBB and BCSFB and the expression of transporters at these CNS barriers.
ACKNOWLEDGEMENTS
MEM received funding support from the National Institutes of Health National Institute on Drug Abuse [Grant R01DA023223]. VRC was supported in part by a Research Supplement to promote Diversity in Health-Related Research from NIDA. MAF received funding support from the University of the Pacific.
REFERENCES
- 1.Zhao Z, Nelson AR, Betsholtz C, Zlokovic BV. Establishment and dysfunction of the blood-brain barrier. Cell. 2015;163(5):1064–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Keep RF, Smith DE. Choroid plexus transport: gene deletion studies. Fluids Barriers CNS. 2011;8(1):26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Redzic Z. Molecular biology of the blood-brain and the blood-cerebrospinal fluid barriers: similarities and differences. Fluids Barriers CNS. 2011;8(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chaves C, Shawahna R, Jacob A, Scherrmann JM, Decleves X. Human ABC transporters at blood-CNS interfaces as determinants of CNS drug penetration. Curr Pharm Des. 2014;20(10):1450–62. [DOI] [PubMed] [Google Scholar]
- 5.Stieger B, Gao B. Drug transporters in the central nervous system. Clin Pharmacokinet. 2015;54(3):225–42. [DOI] [PubMed] [Google Scholar]
- 6.Girardin F. Membrane transporter proteins: a challenge for CNS drug development. Dialogues Clin Neurosci. 2006;8(3):311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strazielle N, Ghersi-Egea JF. Efflux transporters in blood-brain interfaces of the developing brain. Front Neurosci. 2015;9:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashraf R, Ronaldson PT, Bendayan R. Drug transport in the brain In: You G, Morris ME, editors. Drug transporters: molecular characterization and role in drug disposition. Hoboken, NJ: John Wiley & Sons; 2014. p. 273–301. [Google Scholar]
- 9.Ohtsuki S, Ikeda C, Uchida Y, Sakamoto Y, Miller F, Glacial F, et al. Quantitative targeted absolute proteomic analysis of transporters, receptors and junction proteins for validation of human cerebral microvascular endothelial cell line hCMEC/D3 as a human blood-brain barrier model. Mol Pharm. 2013;10(1):289–96. [DOI] [PubMed] [Google Scholar]
- 10.Kusch-Poddar M, Drewe J, Fux I, Gutmann H. Evaluation of the immortalized human brain capillary endothelial cell line BB19 as a human cell culture model for the blood-brain barrier. Brain Res. 2005;1064(1–2):21–31. [DOI] [PubMed] [Google Scholar]
- 11.Roux F, Durieu-Trautmann O, Chaverot N, Claire M, Mailly P, Bourre JM, et al. Regulation of gamma-glutamyl transpeptidase and alkaline phosphatase activities in immortalized rat brain microvessel endothelial cells. J Cell Physiol. 1994;159(1):101–13. [DOI] [PubMed] [Google Scholar]
- 12.Terasaki T, Hosoya K. Conditionally immortalized cell lines as a new in vitro model for the study of barrier functions. Biol Pharm Bull. 2001;24(2):111–8. [DOI] [PubMed] [Google Scholar]
- 13.del Amo EM, Urtti A, Yliperttula M. Pharmacokinetic role of L-type amino acid transporters LAT1 and LAT2. Eur J Pharm Sci. 2008;35(3):161–74. [DOI] [PubMed] [Google Scholar]
- 14.Boado RJ, Li JY, Nagaya M, Zhang C, Pardridge WM. Selective expression of the large neutral amino acid transporter at the blood-brain barrier. Proc Natl Acad Sci U S A. 1999;96(21):12079–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchida Y, Ohtsuki S, Katsukura Y, Ikeda C, Suzuki T, Kamiie J, et al. Quantitative targeted absolute proteomics of human blood-brain barrier transporters and receptors. J Neurochem. 2011;117(2):333–45. [DOI] [PubMed] [Google Scholar]
- 16.Geier EG, Chen EC, Webb A, Papp AC, Yee SW, Sadee W, et al. Profiling solute carrier transporters in the human blood-brain barrier. Clin Pharmacol Ther. 2013;94(6):636–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoshi Y, Uchida Y, Tachikawa M, Inoue T, Ohtsuki S, Terasaki T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J Pharm Sci. 2013;102(9):3343–55. [DOI] [PubMed] [Google Scholar]
- 18.Kamiie J, Ohtsuki S, Iwase R, Ohmine K, Katsukura Y, Yanai K, et al. Quantitative atlas of membrane transporter proteins: development and application of a highly sensitive simultaneous LC/MS/MS method combined with novel in-silico peptide selection criteria. Pharm Res. 2008;25(6):1469–83. [DOI] [PubMed] [Google Scholar]
- 19.Ito K, Uchida Y, Ohtsuki S, Aizawa S, Kawakami H, Katsukura Y, et al. Quantitative membrane protein expression at the blood-brain barrier of adult and younger cynomolgus monkeys. J Pharm Sci. 2011;100(9):3939–50. [DOI] [PubMed] [Google Scholar]
- 20.Shawahna R, Uchida Y, Decleves X, Ohtsuki S, Yousif S, Dauchy S, et al. Transcriptomic and quantitative proteomic analysis of transporters and drug metabolizing enzymes in freshly isolated human brain microvessels. Mol Pharm. 2011;8(4):1332–41. [DOI] [PubMed] [Google Scholar]
- 21.Pineda M, Fernandez E, Torrents D, Estevez R, Lopez C, Camps M, et al. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. J Biol Chem. 1999;274(28):19738–44. [DOI] [PubMed] [Google Scholar]
- 22.Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, et al. LAT2, a new basolateral 4F2hc/CD98-associated amino acid transporter of kidney and intestine. J Biol Chem. 1999;274(49):34948–54. [DOI] [PubMed] [Google Scholar]
- 23.Segawa H, Fukasawa Y, Miyamoto K, Takeda E, Endou H, Kanai Y. Identification and functional characterization of a Na+-independent neutral amino acid transporter with broad substrate selectivity. J Biol Chem. 1999;274(28):19745–51. [DOI] [PubMed] [Google Scholar]
- 24.Killian DM, Chikhale PJ. Predominant functional activity of the large, neutral amino acid transporter (LAT1) isoform at the cerebrovasculature. Neurosci Lett. 2001;306(1–2):1–4. [DOI] [PubMed] [Google Scholar]
- 25.Dickens D, Webb SD, Antonyuk S, Giannoudis A, Owen A, Radisch S, et al. Transport of gabapentin by LAT1 (SLC7A5). Biochem Pharmacol. 2013;85(11):1672–83. [DOI] [PubMed] [Google Scholar]
- 26.Kido Y, Tamai I, Uchino H, Suzuki F, Sai Y, Tsuji A. Molecular and functional identification of large neutral amino acid transporters LAT1 and LAT2 and their pharmacological relevance at the blood-brain barrier. J Pharm Pharmacol. 2001;53(4):497–503. [DOI] [PubMed] [Google Scholar]
- 27.Morris ME, Felmlee MA. Overview of the proton-coupled MCT (SLC16A) family of transporters: characterization, function and role in the transport of the drug of abuse gamma-hydroxybutyric acid. AAPS J. 2008;10(2):311–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ganapathy V, Thangaraju M, Gopal E, Martin PM, Itagaki S, Miyauchi S, et al. Sodium-coupled monocarboxylate transporters in normal tissues and in cancer. AAPS J. 2008;10(1):193–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vijay N, Morris ME. Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des. 2014;20(10):1487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leino RL, Gerhart DZ, Drewes LR. Monocarboxylate transporter (MCT1) abundance in brains of suckling and adult rats: a quantitative electron microscopic immunogold study. Brain Res Dev Brain Res. 1999;113(1–2):47–54. [DOI] [PubMed] [Google Scholar]
- 31.Gerhart DZ, Enerson BE, Zhdankina OY, Leino RL, Drewes LR. Expression of monocarboxylate transporter MCT1 by brain endothelium and glia in adult and suckling rats. Am J Phys. 1997;273(1 Pt 1):E207–13. [DOI] [PubMed] [Google Scholar]
- 32.Roberts LM, Black DS, Raman C, Woodford K, Zhou M, Haggerty JE, et al. Subcellular localization of transporters along the rat blood-brain barrier and blood-cerebral-spinal fluid barrier by in vivo biotinylation. Neuroscience. 2008;155(2):423–38. [DOI] [PubMed] [Google Scholar]
- 33.Roiko SA, Vijay N, Felmlee MA, Morris ME. Brain extracellular gamma-hydroxybutyrate concentrations are decreased by L-lactate in rats: role in the treatment of overdoses. Pharm Res. 2013;30(5):1338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bhattacharya I, Boje KM. GHB (gamma-hydroxybutyrate) carrier-mediated transport across the blood-brain barrier. J Pharmacol Exp Ther. 2004;311(1):92–8. [DOI] [PubMed] [Google Scholar]
- 35.Roiko SA, Felmlee MA, Morris ME. Brain uptake of the drug of abuse gamma-hydroxybutyric acid in rats. Drug Metab Dispos. 2012;40(1):212–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kong W, Engel K, Wang J. Mammalian nucleoside transporters. Curr Drug Metab. 2004;5(1):63–84. [DOI] [PubMed] [Google Scholar]
- 37.Lu H, Chen C, Klaassen C. Tissue distribution of concentrative and equilibrative nucleoside transporters in male and female rats and mice. Drug Metab Dispos. 2004;32(12):1455–61. [DOI] [PubMed] [Google Scholar]
- 38.Redzic ZB, Biringer J, Barnes K, Baldwin SA, Al-Sarraf H, Nicola PA, et al. Polarized distribution of nucleoside transporters in rat brain endothelial and choroid plexus epithelial cells. J Neurochem. 2005;94(5):1420–6. [DOI] [PubMed] [Google Scholar]
- 39.Govindarajan R, Bakken AH, Hudkins KL, Lai Y, Casado FJ, Pastor-Anglada M, et al. In situ hybridization and immunolocalization of concentrative and equilibrative nucleoside transporters in the human intestine, liver, kidneys, and placenta. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1809–22. [DOI] [PubMed] [Google Scholar]
- 40.Koepsell H, Endou H. The SLC22 drug transporter family. Pflugers Arch. 2004;447(5):666–76. [DOI] [PubMed] [Google Scholar]
- 41.Bush KT, Nagle MA, Truong DM, Bhatnagar V, Kaler G, Eraly SA, et al. Organic anion transporters In: You G, Morris ME, editors. Drug transporters: molecular characterization and role in drug Disposition. Hoboken: John Wiley & Sons; 2014. p. 25–41. [Google Scholar]
- 42.Mori S, Takanaga H, Ohtsuki S, Deguchi T, Kang YS, Hosoya K, et al. Rat organic anion transporter 3 (rOAT3) is responsible for brain-to-blood efflux of homovanillic acid at the abluminal membrane of brain capillary endothelial cells. J Cereb Blood Flow Metab. 2003;23(4):432–40. [DOI] [PubMed] [Google Scholar]
- 43.Ohtsuki S, Asaba H, Takanaga H, Deguchi T, Hosoya K, Otagiri M, et al. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J Neurochem. 2002;83(1):57–66. [DOI] [PubMed] [Google Scholar]
- 44.Ose A, Ito M, Kusuhara H, Yamatsugu K, Kanai M, Shibasaki M, et al. Limited brain distribution of [3R,4R,5S]-4-acetamido-5-amino-3-(1-ethylpropoxy)-1-cyclohexene-1-carboxylate phosphate (Ro 64-0802), a pharmacologically active form of oseltamivir, by active efflux across the blood-brain barrier mediated by organic anion transporter 3 (Oat3/Slc22a8) and multidrug resistance-associated protein 4 (Mrp4/Abcc4). Drug Metab Dispos. 2009;37(2):315–21. [DOI] [PubMed] [Google Scholar]
- 45.Ronaldson PT, Davis TP. Targeted drug delivery to treat pain and cerebral hypoxia. Pharmacol Rev. 2013;65(1):291–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roth M, Obaidat A, Hagenbuch B. OATPs, OATs and OCTs: the organic anion and cation transporters of the SLCO and SLC22A gene superfamilies. Br J Pharmacol. 2012;165(5):1260–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagenbuch B, Meier PJ. Organic anion transporting polypeptides of the OATP/SLC21 family: phylogenetic classification as OATP/ SLCO superfamily, new nomenclature and molecular/functional properties. Pflugers Arch. 2004;447(5):653–65. [DOI] [PubMed] [Google Scholar]
- 48.Hagenbuch B, Meier PJ. The superfamily of organic anion transporting polypeptides. Biochim Biophys Acta. 2003;1609(1):1–18. [DOI] [PubMed] [Google Scholar]
- 49.Gao B, Hagenbuch B, Kullak-Ublick GA, Benke D, Aguzzi A, Meier PJ. Organic anion-transporting polypeptides mediate transport of opioid peptides across blood-brain barrier. J Pharmacol Exp Ther. 2000;294(1):73–9. [PubMed] [Google Scholar]
- 50.Roberts LM, Woodford K, Zhou M, Black DS, Haggerty JE, Tate EH, et al. Expression of the thyroid hormone transporters monocarboxylate transporter-8 (SLC16A2) and organic ion transporter-14 (SLCO1C1) at the blood-brain barrier. Endocrinology. 2008;149(12):6251–61. [DOI] [PubMed] [Google Scholar]
- 51.Gao B, Vavricka SR, Meier PJ, Stieger B. Differential cellular expression of organic anion transporting peptides OATP1A2 and OATP2B1 in the human retina and brain: implications for carrier-mediated transport of neuropeptides and neurosteriods in the CNS. Pflugers Arch. 2015;467(7):1481–93. [DOI] [PubMed] [Google Scholar]
- 52.Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS One. 2010;5(10):e13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao B, Stieger B, Noe B, Fritschy JM, Meier PJ. Localization of the organic anion transporting polypeptide 2 (Oatp2) in capillary endothelium and choroid plexus epithelium of rat brain. J Histochem Cytochem. 1999;47(10):1255–64. [DOI] [PubMed] [Google Scholar]
- 54.Ohtsuki S, Takizawa T, Takanaga H, Hori S, Hosoya K, Terasaki T. Localization of organic anion transporting polypeptide 3 (Oatp3) in mouse brain parenchymal and capillary endothelial cells. J Neurochem. 2004;90(3):743–9. [DOI] [PubMed] [Google Scholar]
- 55.Ose A, Kusuhara H, Endo C, Tohyama K, Miyajima M, Kitamura S, et al. Functional characterization of mouse organic anion transporting peptide 1a4 in the uptake and efflux of drugs across the blood-brain barrier. Drug Metab Dispos. 2010;38(1):168–76. [DOI] [PubMed] [Google Scholar]
- 56.Lin CJ, Tai Y, Huang MT, Tsai YF, Hsu HJ, Tzen KY, et al. Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem. 2010;114(3):717–27. [DOI] [PubMed] [Google Scholar]
- 57.Dahlin A, Royall J, Hohmann JG, Wang J. Expression profiling of the solute carrier gene family in the mouse brain. J Pharmacol Exp Ther. 2009;329(2):558–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cordon-Cardo C, O'Brien JP, Boccia J, Casals D, Bertino JR, Melamed MR. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem. 1990;38(9):1277–87. [DOI] [PubMed] [Google Scholar]
- 59.Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE. The controversial role of ABC transporters in clinical oncology. Essays Biochem. 2011;50(1):209–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Warren MS, Zerangue N, Woodford K, Roberts LM, Tate EH, Feng B, et al. Comparative gene expression profiles of ABC transporters in brain microvessel endothelial cells and brain in five species including human. Pharmacol Res. 2009;59(6):404–13. [DOI] [PubMed] [Google Scholar]
- 61.Beaulieu E, Demeule M, Ghitescu L, Beliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem J. 1997;326(Pt 2):539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Virgintino D, Robertson D, Errede M, Benagiano V, Girolamo F, Maiorano E, et al. Expression of P-glycoprotein in human cerebral cortex microvessels. J Histochem Cytochem. 2002;50(12):1671–6. [DOI] [PubMed] [Google Scholar]
- 63.Bendayan R, Ronaldson PT, Gingras D, Bendayan M. In situ localization of P-glycoprotein (ABCB1) in human and rat brain. J Histochem Cytochem. 2006;54(10):1159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pardridge WM, Golden PL, Kang YS, Bickel U. Brain microvascular and astrocyte localization of P-glycoprotein. J Neurochem. 1997;68(3):1278–85. [DOI] [PubMed] [Google Scholar]
- 65.Stewart PA, Beliveau R, Rogers KA. Cellular localization of P-glycoprotein in brain versus gonadal capillaries. J Histochem Cytochem. 1996;44(7):679–85. [DOI] [PubMed] [Google Scholar]
- 66.Soontornmalai A, Vlaming ML, Fritschy JM. Differential, strain-specific cellular and subcellular distribution of multidrug transporters in murine choroid plexus and blood-brain barrier. Neuroscience. 2006;138(1):159–69. [DOI] [PubMed] [Google Scholar]
- 67.Lam J, Koren G. P-glycoprotein in the developing human brain: a review of the effects of ontogeny on the safety of opioids in neonates. Ther Drug Monit. 2014;36(6):699–705. [DOI] [PubMed] [Google Scholar]
- 68.Choo EF, Leake B, Wandel C, Imamura H, Wood AJ, Wilkinson GR, et al. Pharmacological inhibition of P-glycoprotein transport enhances the distribution of HIV-1 protease inhibitors into brain and testes. Drug Metab Dispos. 2000;28(6):655–60. [PubMed] [Google Scholar]
- 69.Zastre JA, Chan GN, Ronaldson PT, Ramaswamy M, Couraud PO, Romero IA, et al. Up-regulation of P-glycoprotein by HIV protease inhibitors in a human brain microvessel endothelial cell line. J Neurosci Res. 2009;87(4):1023–36. [DOI] [PubMed] [Google Scholar]
- 70.Alms D, Fedrowitz M, Romermann K, Noack A, Loscher W. Marked differences in the effect of antiepileptic and cytostatic drugs on the functionality of P-glycoprotein in human and rat brain capillary endothelial cell lines. Pharm Res. 2014;31(6):1588–604. [DOI] [PubMed] [Google Scholar]
- 71.Chishty M, Reichel A, Siva J, Abbott NJ, Begley DJ. Affinity for the P-glycoprotein efflux pump at the blood-brain barrier may explain the lack of CNS side-effects of modern antihistamines. J Drug Target. 2001;9(3):223–8. [DOI] [PubMed] [Google Scholar]
- 72.Pinzon-Daza M, Garzon R, Couraud P, Romero I, Weksler B, Ghigo D, et al. The association of statins plus LDL receptor-targeted liposome-encapsulated doxorubicin increases in vitro drug delivery across blood-brain barrier cells. Br J Pharmacol. 2012;167(7):1431–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maliepaard M, Scheffer GL, Faneyte IF, van Gastelen MA, Pijnenborg AC, Schinkel AH, et al. Subcellular localization and distribution of the breast cancer resistance protein transporter in normal human tissues. Cancer Res. 2001;61(8):3458–64. [PubMed] [Google Scholar]
- 74.Takano M, Yumoto R, Murakami T. Expression and function of efflux drug transporters in the intestine. Pharmacol Ther. 2006;109(1–2):137–61. [DOI] [PubMed] [Google Scholar]
- 75.Eisenblatter T, Huwel S, Galla HJ. Characterisation of the brain multidrug resistance protein (BMDP/ABCG2/BCRP) expressed at the blood-brain barrier. Brain Res. 2003;971(2):221–31. [DOI] [PubMed] [Google Scholar]
- 76.Cooray HC, Blackmore CG, Maskell L, Barrand MA. Localisation of breast cancer resistance protein in microvessel endothelium of human brain. Neuroreport. 2002;13(16):2059–63. [DOI] [PubMed] [Google Scholar]
- 77.Hori S, Ohtsuki S, Tachikawa M, Kimura N, Kondo T, Watanabe M, et al. Functional expression of rat ABCG2 on the luminal side of brain capillaries and its enhancement by astrocyte-derived soluble factor(s). J Neurochem. 2004;90(3):526–36. [DOI] [PubMed] [Google Scholar]
- 78.Tachikawa M, Watanabe M, Hori S, Fukaya M, Ohtsuki S, Asashima T, et al. Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. J Neurochem. 2005;95(1):294–304. [DOI] [PubMed] [Google Scholar]
- 79.Cisternino S, Mercier C, Bourasset F, Roux F, Scherrmann JM. Expression, up-regulation, and transport activity of the multidrug-resistance protein Abcg2 at the mouse blood-brain barrier. Cancer Res. 2004;64(9):3296–301. [DOI] [PubMed] [Google Scholar]
- 80.Lee G, Babakhanian K, Ramaswamy M, Prat A, Wosik K, Bendayan R. Expression of the ATP-binding cassette membrane transporter, ABCG2, in human and rodent brain microvessel endothelial and glial cell culture systems. Pharm Res. 2007;24(7):1262–74. [DOI] [PubMed] [Google Scholar]
- 81.Nies AT, Lang T. Multidrug resistance proteins of the ABCC subfamily In: You G, Morris ME, editors. Drug transporters: molecular characterization and role in drug disposition. Hoboken: John Wiley & Sons; 2014. p. 161–85. [Google Scholar]
- 82.Bosquillon C Drug transporters in the lung—do they play a role in the biopharmaceutics of inhaled drugs? J Pharm Sci. 2010;99(5):2240–55. [DOI] [PubMed] [Google Scholar]
- 83.Nies AT, Jedlitschky G, Konig J, Herold-Mende C, Steiner HH, Schmitt HP, et al. Expression and immunolocalization of the multidrug resistance proteins, MRP1-MRP6 (ABCC1-ABCC6), in human brain. Neuroscience. 2004;129(2):349–60. [DOI] [PubMed] [Google Scholar]
- 84.Carl SM, Lindley DJ, Das D, Couraud PO, Weksler BB, Romero I, et al. ABC and SLC transporter expression and proton oligopeptide transporter (POT) mediated permeation across the human blood–brain barrier cell line, hCMEC/D3 [corrected]. Mol Pharm. 2010;7(4):1057–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dauchy S, Miller F, Couraud PO, Weaver RJ, Weksler B, Romero IA, et al. Expression and transcriptional regulation of ABC transporters and cytochromes P450 in hCMEC/D3 human cerebral microvascular endothelial cells. Biochem Pharmacol. 2009;77(5):897–909. [DOI] [PubMed] [Google Scholar]
- 86.Bauer B, Hartz AM, Lucking JR, Yang X, Pollack GM, Miller DS. Coordinated nuclear receptor regulation of the efflux transporter, Mrp2, and the phase-II metabolizing enzyme, GSTpi, at the blood-brain barrier. J Cereb Blood Flow Metab. 2008;28(6):1222–34. [DOI] [PubMed] [Google Scholar]
- 87.Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G. Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol. 2000;58(6):1357–67. [DOI] [PubMed] [Google Scholar]
- 88.Potschka H, Fedrowitz M, Loscher W. Multidrug resistance protein MRP2 contributes to blood-brain barrier function and restricts antiepileptic drug activity. J Pharmacol Exp Ther. 2003;306(1):124–31. [DOI] [PubMed] [Google Scholar]
- 89.Belinsky MG, Guo P, Lee K, Zhou F, Kotova E, Grinberg A, et al. Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue-induced damage. Cancer Res. 2007;67(1):262–8. [DOI] [PubMed] [Google Scholar]
- 90.Koepsell H. The SLC22 family with transporters of organic cations, anions and zwitterions. Mol Asp Med. 2013;34(2–3):413–35. [DOI] [PubMed] [Google Scholar]
- 91.Kido Y, Tamai I, Ohnari A, Sai Y, Kagami T, Nezu J, et al. Functional relevance of carnitine transporter OCTN2 to brain distribution of L-carnitine and acetyl-L-carnitine across the blood-brain barrier. J Neurochem. 2001;79(5):959–69. [DOI] [PubMed] [Google Scholar]
- 92.Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv. 2016;13(7):963–75. [DOI] [PubMed] [Google Scholar]
- 93.Wollack JB, Makori B, Ahlawat S, Koneru R, Picinich SC, Smith A, et al. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem. 2008;104(6):1494–503. [DOI] [PubMed] [Google Scholar]
- 94.Dinner S, Borkowski J, Stump-Guthier C, Ishikawa H, Tenenbaum T, Schroten H, et al. A choroid plexus epithelial cell-based model of the human blood-cerebrospinal fluid barrier to study bacterial infection from the basolateral side. J Vis Exp. 2016(111). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Haselbach M, Wegener J, Decker S, Engelbertz C, Galla HJ. Porcine choroid plexus epithelial cells in culture: regulation of barrier properties and transport processes. Microsc Res Tech. 2001;52(1):137–52. [DOI] [PubMed] [Google Scholar]
- 96.Takano M, Otani M, Kaji T, Sano K, Hamada-Kanazawa M, Matsuyama S. Proteomic analysis of mouse choroid plexus cell line ECPC-4 treated with lipid a. Inflamm Res. 2016;65(4):295–302. [DOI] [PubMed] [Google Scholar]
- 97.Baehr C, Reichel V, Fricker G. Choroid plexus epithelial monolayers—a cell culture model from porcine brain. Cerebrospinal Fluid Res. 2006;3:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Halwachs S, Lakoma C, Schafer I, Seibel P, Honscha W. The antiepileptic drugs phenobarbital and carbamazepine reduce transport of methotrexate in rat choroid plexus by down-regulation of the reduced folate carrier. Mol Pharmacol. 2011;80(4):621–9. [DOI] [PubMed] [Google Scholar]
- 99.Pardridge WM. Drug transport across the blood-brain barrier. J Cereb Blood Flow Metab. 2012;32(11):1959–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muller J, Heuer H. Expression pattern of thyroid hormone transporters in the postnatal mouse brain. Front Endocrinol (Lausanne). 2014;5:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Uchida Y, Zhang Z, Tachikawa M, Terasaki T. Quantitative targeted absolute proteomics of rat blood-cerebrospinal fluid barrier transporters: comparison with a human specimen. J Neurochem. 2015;134(6):1104–15. [DOI] [PubMed] [Google Scholar]
- 102.Bergersen L, Johannsson E, Veruki ML, N agelhus EA, Halestrap A, Sejersted OM, et al. Cellular and subcellular expression of monocarboxylate transporters in the pigment epithelium and retina of the rat. Neuroscience. 1999;90(1):319–31. [DOI] [PubMed] [Google Scholar]
- 103.Heuer H, Maier MK, Iden S, Mittag J, Friesema EC, Visser TJ, et al. The monocarboxylate transporter 8 linked to human psychomotor retardation is highly expressed in thyroid hormone-sensitive neuron populations. Endocrinology. 2005;146(4):1701–6. [DOI] [PubMed] [Google Scholar]
- 104.Trajkovic M, Visser TJ, Mittag J, Horn S, Lukas J, Darras VM, et al. Abnormal thyroid hormone metabolism in mice lacking the monocarboxylate transporter 8. J Clin Invest. 2007;117(3):627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ho HT, Dahlin A, Wang J. Expression profiling of solute carrier gene families at the blood-CSF barrier. Front Pharmacol. 2012;3:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Grijota-Martinez C, Diez D, Morreale de Escobar G, Bernal J, Morte B. Lack of action of exogenously administered T3 on the fetal rat brain despite expression of the monocarboxylate transporter 8. Endocrinology. 2011;152(4):1713–21. [DOI] [PubMed] [Google Scholar]
- 107.Kakinuma H, Itoh M, Takahashi H. A novel mutation in the monocarboxylate transporter 8 gene in a boy with putamen lesions and low free T4 levels in cerebrospinal fluid. J Pediatr. 2005;147(4):552–4. [DOI] [PubMed] [Google Scholar]
- 108.Redzic ZB, Malatiali SA, Grujicic D, Isakovic AJ. Expression and functional activity of nucleoside transporters in human choroid plexus. Cerebrospinal Fluid Res. 2010;7:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kratzer I, Liddelow SA, Saunders NR, Dziegielewska KM, Strazielle N, Ghersi-Egea JF. Developmental changes in the transcriptome of the rat choroid plexus in relation to neuro-protection. Fluids Barriers CNS. 2013;10(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Choudhuri S, Cherrington NJ, Li N, Klaassen CD. Constitutive expression of various xenobiotic and endobiotic transporter mRNAs in the choroid plexus of rats. Drug Metab Dispos. 2003;31(11):1337–45. [DOI] [PubMed] [Google Scholar]
- 111.Alebouyeh M, Takeda M, Onozato ML, Tojo A, Noshiro R, Hasannejad H, et al. Expression of human organic anion transporters in the choroid plexus and their interactions with neurotransmitter metabolites. J Pharmacol Sci. 2003;93(4):430–6. [DOI] [PubMed] [Google Scholar]
- 112.Nagata Y, Kusuhara H, Endou H, Sugiyama Y. Expression and functional characterization of rat organic anion transporter 3 (rOat3) in the choroid plexus. Mol Pharmacol. 2002;61(5):982–8. [DOI] [PubMed] [Google Scholar]
- 113.Nagle MA, Wu W, Eraly SA, Nigam SK. Organic anion transport pathways in antiviral handling in choroid plexus in Oat1 (Slc22a6) and Oat3 (Slc22a8) deficient tissue. Neurosci Lett. 2013;534:133–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sweet DH, Miller DS, Pritchard JB, Fujiwara Y, Beier DR, Nigam SK. Impaired organic anion transport in kidney and choroid plexus of organic anion transporter 3 (Oat3 (Slc22a8)) knockout mice. J Biol Chem. 2002;277(30):26934–43. [DOI] [PubMed] [Google Scholar]
- 115.Xu G, Bhatnagar V, Wen G, Hamilton BA, Eraly SA, Nigam SK. Analyses of coding region polymorphisms in apical and basolateral human organic anion transporter (OAT) genes [OAT1 (NKT), OAT2, OAT3, OAT4, URAT (RST)]. Kidney Int. 2005;68(4):1491–9. [DOI] [PubMed] [Google Scholar]
- 116.Huber RD, Gao B, Sidler Pfandler MA, Zhang-Fu W, Leuthold S, Hagenbuch B, et al. Characterization of two splice variants of human organic anion transporting polypeptide 3A1 isolated from human brain. Am J Physiol Cell Physiol. 2007;292(2):C795–806. [DOI] [PubMed] [Google Scholar]
- 117.Kis B, Isse T, Snipes JA, Chen L, Yamashita H, Ueta Y, et al. Effects of LPS stimulation on the expression of prostaglandin carriers in the cells of the blood-brain and blood-cerebrospinal fluid barriers. J Appl Physiol (1985). 2006;100(4):1392–9. [DOI] [PubMed] [Google Scholar]
- 118.Angeletti RH, Novikoff PM, Juvvadi SR, Fritschy JM, Meier PJ, Wolkoff AW. The choroid plexus epithelium is the site of the organic anion transport protein in the brain. Proc Natl Acad Sci U S A. 1997;94(1):283–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ohtsuki S, Takizawa T, Takanaga H, Terasaki N, Kitazawa T, Sasaki M, et al. In vitro study of the functional expression of organic anion transporting polypeptide 3 at rat choroid plexus epithelial cells and its involvement in the cerebrospinal fluid-to-blood transport of estrone-3-sulfate. Mol Pharmacol. 2003;63(3):532–7. [DOI] [PubMed] [Google Scholar]
- 120.Sweet DH, Miller DS, Pritchard JB. Ventricular choline transport: a role for organic cation transporter 2 expressed in choroid plexus. J Biol Chem. 2001;276(45):41611–9. [DOI] [PubMed] [Google Scholar]
- 121.Duan H, Wang J. Impaired monoamine and organic cation uptake in choroid plexus in mice with targeted disruption of the plasma membrane monoamine transporter (Slc29a4) gene. J Biol Chem. 2013;288(5):3535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brandsch M, Knutter I, Bosse-Doenecke E. Pharmaceutical and pharmacological importance of peptide transporters. J Pharm Pharmacol. 2008;60(5):543–85. [DOI] [PubMed] [Google Scholar]
- 123.Smith DE, Clemencon B, Hediger MA. Proton-coupled oligopeptide transporter family SLC15: physiological, pharmacological and pathological implications. Mol Asp Med. 2013;34(2–3):323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shu C, Shen H, Teuscher NS, Lorenzi PJ, Keep RF, Smith DE. Role of PEPT2 in peptide/mimetic trafficking at the blood-cerebrospinal fluid barrier: studies in rat choroid plexus epithelial cells in primary culture. J Pharmacol Exp Ther. 2002;301(3):820–9. [DOI] [PubMed] [Google Scholar]
- 125.Shen H, Smith DE, Keep RF, Brosius FC 3rd. Immunolocalization of the proton-coupled oligopeptide transporter PEPT2 in developing rat brain. Mol Pharm. 2004;1(4):248–56. [DOI] [PubMed] [Google Scholar]
- 126.Kennedy DJ, Leibach FH, Ganapathy V, Thwaites DT. Optimal absorptive transport of the dipeptide glycylsarcosine is dependent on functional Na+/H+ exchange activity. Pflugers Arch. 2002;445(1):139–46. [DOI] [PubMed] [Google Scholar]
- 127.Shen H, Ocheltree SM, Hu Y, Keep RF, Smith DE. Impact of genetic knockout of PEPT2 on cefadroxil pharmacokinetics, renal tubular reabsorption, and brain penetration in mice. Drug Metab Dispos. 2007;35(7):1209–16. [DOI] [PubMed] [Google Scholar]
- 128.Rao VV, Dahlheimer JL, Bardgett ME, Snyder AZ, Finch RA, Sartorelli AC, et al. Choroid plexus epithelial expression of MDR1 P glycoprotein and multidrug resistance-associated protein contribute to the blood-cerebrospinal-fluid drug-permeability barrier. Proc Natl Acad Sci USA. 1999;96(7):3900–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pascale CL, Miller MC, Chiu C, Boylan M, Caralopoulos IN, Gonzalez L, et al. Amyloid-beta transporter expression at the blood-CSF barrier is age-dependent. Fluids Barriers CNS. 2011;8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yasuda K, Cline C, Vogel P, Onciu M, Fatima S, Sorrentino BP, et al. Drug transporters on arachnoid barrier cells contribute to the blood-cerebrospinal fluid barrier. Drug Metab Dispos. 2013;41(4):923–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaur M, Badhan RK. Phytoestrogens modulate breast cancer resistance protein expression and function at the blood-cerebrospinal fluid barrier. J Pharm Pharm Sci. 2015;18(2):132–54. [DOI] [PubMed] [Google Scholar]
- 132.Niehof M, Borlak J. Expression of HNF4alpha in the human and rat choroid plexus: implications for drug transport across the blood-cerebrospinal-fluid (CSF) barrier. BMC Mol Biol. 2009;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Leggas M, Adachi M, Scheffer GL, Sun D, Wielinga P, Du G, et al. Mrp4 confers resistance to topotecan and protects the brain from chemotherapy. Mol Cell Biol. 2004;24(17):7612–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sathyanesan M, Girgenti MJ, Banasr M, Stone K, Bruce C, Guilchicek E, et al. A molecular characterization of the choroid plexus and stress-induced gene regulation. Transl Psychiatry. 2012;2:e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wijnholds J, de Lange EC, Scheffer GL, van den Berg DJ, Mol CA, van der Valk M, et al. Multidrug resistance protein 1 protects the choroid plexus epithelium and contributes to the blood-cerebrospinal fluid barrier. J Clin Invest. 2000;105(3):279–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mercier C, Masseguin C, Roux F, Gabrion J, Scherrmann JM. Expression of P-glycoprotein (ABCB1) and Mrp1 (ABCC1) in adult rat brain: focus on astrocytes. Brain Res. 2004;1021(1):32–40. [DOI] [PubMed] [Google Scholar]
- 137.Zhang Y, Schuetz JD, Elmquist WF, Miller DW. Plasma membrane localization of multidrug resistance-associated protein homologs in brain capillary endothelial cells. J Pharmacol Exp Ther. 2004;311(2):449–55. [DOI] [PubMed] [Google Scholar]
- 138.Coyle B, Kessler M, Sabnis DH, Kerr ID. ABCB1 in children’s brain tumours. Biochem Soc Trans. 2015;43(5):1018–22. [DOI] [PubMed] [Google Scholar]
- 139.On NH, Mitchell R, Savant SD, Bachmeier CJ, Hatch GM, Miller DW. Examination of blood-brain barrier (BBB) integrity in a mouse brain tumor model. J Neuro-Oncol. 2013;111(2):133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Shawahna R. Physical and metabolic integrity of the blood-brain barrier in HIV infection: a special focus on intercellular junctions, influx and efflux transporters and metabolizing enzymes. Curr Drug Metab. 2015;16(2):105–23. [DOI] [PubMed] [Google Scholar]
- 141.Hayashi K, Pu H, Andras IE, Eum SY, Yamauchi A, Hennig B, et al. HIV-TAT protein upregulates expression of multidrug resistance protein 1 in the blood-brain barrier. J Cereb Blood Flow Metab. 2006;26(8):1052–65. [DOI] [PubMed] [Google Scholar]
- 142.Hayashi K, Pu H, Tian J, Andras IE, Lee YW, Hennig B, et al. HIV-TAT protein induces P-glycoprotein expression in brain microvascular endothelial cells. J Neurochem. 2005;93(5):1231–41. [DOI] [PubMed] [Google Scholar]
- 143.de Almeida SM, Rotta I, Ribeiro CE, Smith D, Wang R, Judicello J, et al. Blood-CSF barrier and compartmentalization of CNS cellular immune response in HIV infection. J Neuroimmunol. 2016;301:41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Erickson MA, Banks WA. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J Cereb Blood Flow Metab. 2013;33(10):1500–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Shibata M, Yamada S, Kumar SR, Calero M, Bading J, Frangione B, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106(12):1489–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Vogelgesang S, Cascorbi I, Schroeder E, Pahnke J, Kroemer HK, Siegmund W, et al. Deposition of Alzheimer’s beta-amyloid is inversely correlated with P-glycoprotein expression in the brains of elderly non-demented humans. Pharmacogenetics. 2002;12(7):535–41. [DOI] [PubMed] [Google Scholar]
- 147.Vautier S, Fernandez C. ABCB1: the role in Parkinson’s disease and pharmacokinetics of antiparkinsonian drugs. Expert Opin Drug Metab Toxicol. 2009;5(11):1349–58. [DOI] [PubMed] [Google Scholar]
- 148.Bartels AL, Willemsen AT, Kortekaas R, de Jong BM, de Vries R, de Klerk O, et al. Decreased blood-brain barrier P-glycoprotein function in the progression of Parkinson’s disease, PSP and MSA. J Neural Transm (Vienna). 2008;115(7):1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cen J, Liu L, Li MS, He L, Wang LJ, Liu YQ, et al. Alteration in P-glycoprotein at the blood-brain barrier in the early period of MCAO in rats. J Pharm Pharmacol. 2013;65(5):665–72. [DOI] [PubMed] [Google Scholar]
- 150.Ennis SR, Keep RF. The effects of cerebral ischemia on the rat choroid plexus. J Cereb Blood Flow Metab. 2006;26(5):675–83. [DOI] [PubMed] [Google Scholar]
- 151.Renu A, Laredo C, Lopez-Rueda A, Llull L, Tudela R, San-Roman L, et al. Vessel wall enhancement and blood-cerebrospinal fluid barrier disruption after mechanical thrombectomy in acute ischemic stroke. Stroke. 2017;48(3):651–7. [DOI] [PubMed] [Google Scholar]