Abstract
Background:
There has been a rise in opioid abuse and related injection drug use in the United States and treatment for opioid use disorders may be underutilized. The study aim was to describe utilization of opioid agonist therapy (OAT), and assess factors associated with utilization of OAT, among persons who inject drugs (PWID) in the Seattle metropolitan area.
Methods:
We used data from the 2015 National HIV Behavioral Surveillance (NHBS) system among PWID in the Seattle area. Persons aged ≥18 years who injected drugs in the past year were recruited using respondent-driven sampling. Local supplemental questions assessed whether participants had received methadone or buprenorphine treatment in the past year. The analysis was restricted to participants who reported use of any opioids in the past year. We compared the demographic, health insurance status, duration of injection drug use, prior history of overdose, prior receipt of HCV/HIV testing (self-report), and screening positive for HCV/HIV via study testing between methadone or buprenorphine treated and untreated PWID. Multivariate logistic models were performed to assess adjusted associations with receipt of any OAT.
Results:
The sample included 487 PWID who used opioids in the past year, of whom 27.1% (95% CI: 23.1–31.1) reported past-year treatment with methadone and 4.7% (95% CI: 2.8–6.6) reported treatment with buprenorphine. There were no significant differences in demographics among participants who did and did not report past-year OAT; however, participants who were treated with methadone were more likely to be insured and have hepatitis C. After adjustment for other covariates, having health insurance was strongly associated with receipt of OAT (aOR= 18.6; 95% CI: 2.5–138.7)
Conclusions:
OAT, in particular buprenorphine, has been under-utilized by opioid-using PWID in the Seattle area. Health insurance is a critical factor for enabling PWID to utilize OAT treatment for opioid use disorders.
Keywords: opioid agonist therapy, persons who inject drugs, opioid use disorder
1. Introduction
The past decade and a half has seen a rise in heroin and prescription opioid use disorders in the United States, resulting in major morbidity and mortality.1 In 2014, an estimated 1.9 million American adults had a pharmaceutical opioid use disorder, and 586,000 had a heroin-involved opioid use disorder2; within the same year, 28,647 died from opioid-related overdoses3. Opioid use disorders are closely intertwined with injection drug use, which is a major driver of chronic viral infections such as hepatitis C virus (HCV) and HIV4, 5. In the U.S., there has been an increase of new HIV and HCV cases related to the injection of heroin and prescription opioids in rural areas6 and among young adults7. Effective medications to treat opioid use disorders exist, but may be underutilized even in countries where they are legal and available, such as the U.S.
Methadone and buprenorphine are treatments with demonstrated efficacy for treatment of opioid use disorders. Methadone has been available for decades through federally approved opioid treatment programs (OTPs) that will provide medication dispensation under direct observation. Buprenorphine, which was approved for use by the U.S. Food and Drug Administration in 2002, can be prescribed by any waivered physician in an office-based setting, and as such allows patients to take their medications unsupervised. Both forms of opioid agonist therapy (OAT) have been shown to be effective in reducing craving, illicit opiate use, and injection drug use.8–10 Through reductions in illicit opiate and injection use, OAT can limit the spread of HIV11–13 and HCV.14–16 As such, an argument can be made to make OAT widely available to PWID who have an opioid use disorder both from the patient perspective to treat their addiction and improve quality of life17, 18 and as a public health strategy to prevent HIV and HCV transmission. Yet considerable barriers to treatment with OAT may occur on the level of the patient, the provider, and healthcare system.19 Understanding the current utilization of OAT among persons with opioid use disorder who inject drugs is an important first step in addressing gaps in treatment capacity.
The aim of this study was to describe utilization of methadone and buprenorphine for treatment of opioid use disorders among persons who inject drugs (PWID) in the Seattle metropolitan area. In addition, we sought to examine differences in demographics and other factors among persons who reported past-year treatment with buprenorphine and methadone compared to no treatment, in order to better characterize OAT-treated versus untreated populations.
2. Methods
2.1. Study Sample/Data Source
We used data from the 2015 National HIV Behavioral Surveillance (NHBS) system among PWID in the Seattle area. NHBS is conducted by the U.S. Department of Health and Human Services’ Centers for Disease Control and Prevention (CDC) to help state and local health departments monitor HIV risk behaviors and assess the use of prevention services in three groups: men who have sex with men (MSM), injection drug users (IDU), and persons at high-risk for heterosexually acquired HIV. This study used data collected from the fourth NHBS cycle to focus on PWID (NHBS-IDU4). Persons aged ≥18 years who injected drugs in the past year, who resided in King or Snohomish County, and were able to complete the survey in English were recruited using respondent-driven sampling (RDS). RDS is a form of snowball sampling where participants are paid a small incentive to recruit a limited number of their network members to the study20. For this study, we restricted analyses to participants who reported any opioid use in the past year and answered the treatment questions.
2.2. Data Collection
All data collection activities were conducted at our main field office in the Capitol Hill neighborhood of Seattle and in a small office in South King County. Potential participants were screened for eligibility, which included visual inspection of injection sites and detailed questions about drug preparation and injection. Those who were eligible and provided informed consent were given an interviewer-administered survey. The survey included information about sociodemographic characteristics, sexual and drug-use practices, and health history, including the specific questions on addiction treatment that were part of the local questionnaire and not included in the national survey. All participants, including those who reported previously testing positive, were offered rapid HIV and rapid hepatitis C (HCV) testing (OraSure Technologies). Those with reactive rapid HIV results were offered confirmatory Western Blot tests on whole blood specimens. Participants provided separate consent for the survey and HIV and HCV testing. They received a monetary incentive ($50 for completing survey and HIV testing); condoms; and information about local HIV prevention, health, and social services. No personal identifiers were collected. The study was approved by the Washington State Institutional Review Board.
2.3. Measures
The primary outcome of interest was self-report of recent treatment for opioid use disorders with either methadone or buprenorphine. The NHBS core survey included a question about participation in drug treatment during the past 12 months (i.e., outpatient, inpatient, residential, or drug detoxification programs; methadone treatment; or 12-step programs). At the conclusion of the core survey, interviewers recorded whether a person reported being in drug treatment in the previous 12 months, and if the interviewer selected “yes,” our local questionnaire additionally asked whether the treatment received included methadone, buprenorphine, or naltrexone either as daily oral pills or monthly injections. For participants who reported receiving either methadone or buprenorphine, we asked how long they had been treated during the past 12 months, with possible choices being <1 month, 2–3 months, 4–6 months, and >6 months. The NHBS core survey included a question asking if the participant tried to get into a program to treat drug use in the past 12 months (yes/no). For the local questionnaire, we included questions that asked specifically whether participants had tried but failed to get treatment with either methadone or buprenorphine. Finally, the local questionnaire included questions on whether participants had ever tried buprenorphine without a doctor’s prescription and, if so, whether the intent was to “get high” or to treat withdrawal symptoms (or both).
Other covariates examined included age, sex, race/ethnicity, housing status, health insurance status, duration of injection drug use, prior history of overdose, prior receipt of HCV/HIV testing (self-report), and screening positive for HCV/HIV via study testing.
2.4. Statistical Analysis
We report the number and proportion of participants who reported receiving treatment in the past 12 months with methadone, buprenorphine (buprenorphine alone, or buprenorphine/naloxone), or naltrexone (either oral or injectable), as well as the number and proportion of participants who tried unsuccessfully to get treatment. Prevalence estimates with 95% confidence intervals were calculated for each treatment. For persons who reported being on methadone or buprenorphine, we describe the percentage who reported treatment for <1 month, 2–3 months, 4–6 months, and >6 months. Finally, we performed comparisons of demographics (gender, age, race/ethnicity), housing status, health insurance, years since first injection, overdose in the past year, receipt of prior testing for HCV or HIV test, and screening positive for HCV or HIV between persons who did and did not report treatment with OAT. We used chi-square or Fisher exact tests to assess the association between each covariate and treatment type. Finally, we conducted multivariate logistic regression to examine factors associated with receipt of OAT (combined outcome of either methadone or buprenorphine). Covariates, which were selected a priori based on prior literature and hypothesis that they might influence treatment likelihood, included demographic factors (age, sex and race), homelessness, having health insurance, duration of injection, and past-year overdose.
3. Results
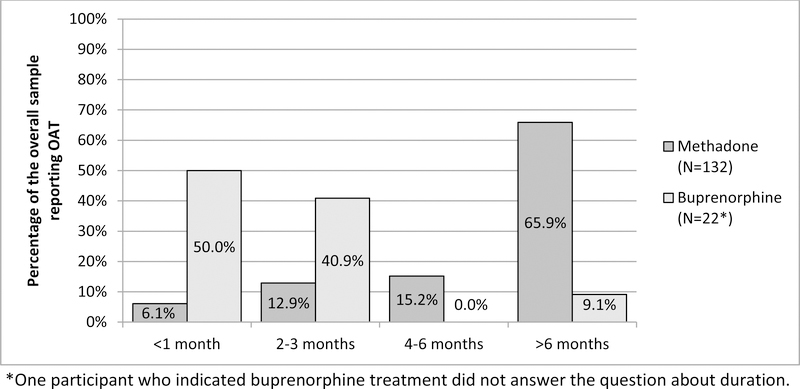
The sample included 487 persons who reported any opioid use in the past year and answered the questions related to OAT. Among this sample of injectors who used opioids, the majority (341/487 or 70.0%) did not report treatment with OAT in the past year. Overall in the sample, 132/487 or 27.1% (95% CI: 23.1–31.1) reported past-year treatment with methadone and 23/487 or 4.7% (95% CI: 2.8–6.6) reported treatment with buprenorphine (9 of the individuals reported both treatments and are included in each group). Of those not on treatment with either methadone or buprenorphine (n=341), 21.1% (16.8–25.4) and 10.0% (6.8–13.2) reported trying to access those respective treatments, but being unsuccessful. Among persons treated with methadone, the majority reported treatment for more than 6 months, whereas the majority of persons treated with buprenorphine reported receiving treatment for only 3 months or less (Figure 1). Illicit use of buprenorphine (i.e., using buprenorphine without a prescription) was reported in 219 persons (45.0%); of those, 81.3% reported using it to treat withdrawal symptoms, 4.1% reported it using to “get high,” 11.4% reported use for both reasons, and 3.2% stated “other” reasons. Only one person reported past-year treatment with naltrexone and was excluded from further analyses.
Figure 1:
Duration of treatment among PWID who use opioids who reported past-year OAT
Table 1 summarizes demographic, medical, and substance use related factors by OAT treatment status compared to PWID who did not report any treatment in the past year. There no significant difference based on gender or race/ethnicity; however, we observed that patients who reported being treated with buprenorphine tended to be younger than those not reporting any treatment (mean age 35.7 v. 40.1, p=0.07). Participants receiving either methadone treatment or buprenorphine were more likely to have health insurance compared to participants not on OAT treatment (99.2% v. 89.6%, p<0.01 and 100% v. 89.6%, p=0.15, respectively). Compared to participants not on OAT, a history of HCV testing was more likely among participants on methadone (93.9% v. 81.1%, p<0.01) and among participants reporting buprenorphine treatment (95.7% v. 81.1%, p=0.09), although the latter association did not reach statistical significance. Furthermore, participants who reported methadone treatment appeared slightly more likely to be HCV infected (75.6% v. 66.5%, p=0.06) compared to those not on OAT. There were no differences in the proportion who tested positive for HIV among participants who were and were not on OAT, and the prevalence of HIV was relatively low in the sample overall (4.3%). In multivariate logistic regression models that examined associations between demographic factors (age, sex, and race), homelessness, having health insurance, duration of injection, and past-year overdose, having health insurance was the only covariate that was significantly associated with past-year receipt of either methadone of buprenorphine (aOR=18.6; 95% CI: 2.5–138.7).
Table 1:
Demographic, health, and substance use characteristics among persons who inject drugs reporting opioid use in the past year by OAT treatment status
| Past-Year OAT | ||||||
|---|---|---|---|---|---|---|
| All Participantsd | No OAT | Methadone | Buprenorphine | |||
| Characteristic | N=486 n (%) | N=340 n (%) | N=132e n (%) | p-value | N=23e n (%) | p-value f |
| Male | 307 (63.3) | 222 (65.3) | 76 (58.0) | 0.14 | 15 (65.2) | 0.99 |
| Age (years), mean [SD] | 41.1 [12.7] | 40.7 [12.7] | 42.6 [12.1] | 0.14 | 35.7 [13.1] | 0.07 |
| Race/Ethnicity | 0.29 | 0.76 | ||||
| White | 317 (65.5) | 230 (68.1) | 79 (59.9) | 14 (60.9) | ||
| Black | 45 (9.3) | 28 (8.3) | 15 (11.4) | 2 (8.7) | ||
| Hispanic | 45 (9.3) | 32 (9.5) | 12 (9.1) | 3 (13.0) | ||
| Othera | 77 (15.9) | 48 (14.2) | 26 (19.7) | 4 (17.4) | ||
| Currently homeless | 277 (57.2) | 197 (58.3) | 72 (54.6) | 0.46 | 15 (65.2) | 0.51 |
| Has health insurance | 447 (92.6) | 302 (89.6) | 131 (99.2) | <0.01 | 23 (100.0) | 0.15 |
| Years since first injection | 0.10 | 0.66 | ||||
| 0–5 years | 103 (21.2) | 78 (22.9) | 19 (14.4) | 7 (30.4) | ||
| 6–15 years | 85 (17.5) | 60 (17.7) | 23 (17.4) | 4 (17.4) | ||
| >15 years | 298 (61.3) | 202 (59.4) | 90 (68.2) | 12 (52.2) | ||
| Overdosed, past year | 106 (21.8) | 76 (22.4) | 29 (22.0) | 0.93 | 6 (26.1) | 0.68 |
| Prior HCV test, ever | 408 (85.0) | 271 (81.1) | 124 (93.9) | <0.01 | 22 (95.7) | 0.09 |
| HCV antibody positiveb | 325 (68.9) | 218 (66.5) | 99 (75.6) | 0.06 | 17 (77.3) | 0.36 |
| Prior HIV test, past yearc | 216 (48.0) | 143 (45.8) | 65 (52.4) | 0.21 | 14 (63.6) | 0.11 |
| HIV-infectedb | 21 (4.3) | 15 (4.4) | 6 (4.6) | 0.96 | 1 (4.4) | 1.00 |
Includes Asian, Pacific Islander, Native American, and persons who chose multiple races.
Based on tests performed during the study visit.
Among 450 participants who did not report a prior HIV diagnosis.
Excludes 1 person who reported treatment with naltrexone.
9 persons reported both methadone and buprenorphine treatment and are included in both treatment columns.
p-value is for Fisher exact test or Chi-square tests.
Abbreviations: OAT, opioid agonist therapy; SD, standard deviation; HCV, hepatitis C virus; HIV, human immunodeficiency virus
4. Discussion
This study of Seattle-area PWID who use opioids found that the majority did not have any recent (past-year) treatment with either methadone or buprenorphine. These results suggest opportunities for improving access to medication-assisted treatment for opioid use disorders among PWID. In particular, the study demonstrated substantial under-utilization of buprenorphine treatment, less than 5% of the sample. Furthermore, results demonstrated a significant association between having health insurance and receipt of OAT. This highlights the critical need to maintain health care coverage for PWID to ensure access to medication-assisted treatment for opioid use disorders.
This study is consistent with prior research that has shown that, in general, only a small minority of persons with substance use disorders seek and receive treatment for their addictions2 and that the percentage of persons with opioid use disorders seeking any treatment has remained low after accounting for population characteristics21. This study is unique in that it provides specific information on medication-assisted treatment with buprenorphine or methadone in a community sample of PWID. According to a report of the 2014 Treatment Episode Data Set (TEDS)—a dataset of national admissions to substance abuse treatment services—medication-assisted opioid therapy was planned for 28% of heroin admissions22. A 2011 study of 35,240 veterans with opioid use disorders found that 27.3% were treated with OAT, and only 5.1% reported treatment with buprenorphine, which is similar to the proportion reporting buprenorphine treatment in our study23. To our knowledge, this is the first study to estimate OAT treatment specifically among PWID who have use opioids.
It is perhaps surprising that the uptake of buprenorphine was so low in this sample of opioid-using PWID in the Seattle area. Federal law allows buprenorphine to be prescribed by waivered physicians in office-based settings, providing opportunities for expansion of treatment capacity, and since buprenorphine was FDA approved in 2002, prescriptions have steadily increased24. There are numerous advantages to buprenorphine, which is a partial μ-opioid receptor agonist, including less subjective euphoria and lower risk for side effects such as sedation or overdose25. Research suggests that individuals may prefer buprenorphine over methadone for initial treatment of opioid use disorders26–28. Low rates of treatment with buprenorphine may reflect lack of sufficient numbers of providers as well as insurance restrictions to buprenorphine coverage.
It is notable that while a very small minority of this sample reported buprenorphine treatment, nearly half reported illicit buprenorphine use. The majority (81%) of those who used it illicitly reported doing so to treat withdrawal (compared to only 4% who reported its use only to “get high”). These results suggest that many PWID with opioid use disorder are self-treating their addiction with buprenorphine, and diversion of that medication may be filling a treatment gap. We also observed that duration of treatment was much longer among persons who reported treatment with methadone compare to buprenorphine, although results should be interpreted cautiously given the small number of buprenorphine-treated patients. It is unknown whether this difference was due to provider- or patient-level factors. Maintenance treatment with buprenorphine is shown to be more effective than detoxification/taper29, so patients started on buprenorphine should be continued longer term. Prior observational research has suggested that retention rates may be higher among persons treated with methadone compared to buprenorphine30.
Other notable findings in the study include the higher percentage reporting prior HCV testing among the methadone-treated patients. This could reflect increasing efforts to screen for HCV in methadone programs31. Alternatively, the higher prevalence could be related to the slightly higher age among persons reporting methadone treatment, as the prevalence of HCV increases with duration of injection drug use32, 33, and overall national prevalence is highest in the “baby-boomer” cohort born between 1945 and 196534. The high prevalence of HCV among persons who reported past-year methadone treatment, and the fact that most reported engagement in methadone treatment for at least 6 months, attests to a major opportunity to treat and cure HCV in this setting. Finally, our study also found a strong association between insurance status and past-year OAT utilization. This underscores the necessity of maintaining and broadening health insurance coverage to vulnerable populations such as PWID so that they can adequately access effective addiction treatment such as OAT.
There were several limitations to this study. This study was conducted at a single NHBS study site, and results may not be generalizable outside of the Seattle area. Moreover, these analyses did not include adjustments for RDS-based recruitment, thus the precision of our measurements may be overestimated. Our analytic sample of PWID who reported any opioid use in the past year likely includes some people who would not fit the criteria for opioid use disorder, thus our estimates of methadone and buprenorphine utilization may be an underestimate. The study had small numbers of participants on buprenorphine, and therefore, we had limited power to look for differences between that group and the sample overall or with participants on methadone. The questionnaire did not ask about specific barriers to OAT; therefore, we do not know the specific underlying reasons why 15% and 11% of the untreated sample were unable to access methadone and buprenorphine, respectively. Finally, since these questions were not asked in prior years, we are currently unable to provide any information on trends in utilization.
5. Conclusions
In summary, this study of Seattle-area opioid-using PWID found that the majority (70%) reported no medication-assisted treatment with methadone or buprenorphine in the past year. We observed that less than 5% had been treated with buprenorphine in the past year, although twice that number had unsuccessfully sought treatment with buprenorphine. These results underscore a gap in utilization of OAT for PWID and, as such, sub-optimal prevention of HCV and HIV in this high-risk group. We found no differences in demographic factors among PWID who did and did not report past-year OAT use; however, having health insurance was significantly associated with past-year OAT. These results underscore the importance of health insurance to enable PWID to access effective treatment for opioid use disorders, which can ultimately help achieve public health goals of reducing overdose deaths, HIV, and HCV.
Acknowledgments
Role of Funding Source: This work was supported by National HIV Behavioral Surveillance with funding from a cooperative agreement with the Centers for Disease Control and Prevention [5U1BPS003250]. The Centers for Disease Control and Prevention had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Conflict of Interest: There are no conflicts of interest.
References
- 1.Compton WM, Jones CM and Baldwin GT. Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med. 2016;374(2):154–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Center for Behavioral Health Statistics and Quality: Substance Abuse and Mental Health Services Administration. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. 2015.
- 3.Rudd RA, Aleshire N, Zibbell JE and Gladden RM. Increases in drug and opioid overdose deaths--United States, 2000–2014. MMWR Morb Mortal Wkly Rep. 2016;64(50–51):1378–1382. [DOI] [PubMed] [Google Scholar]
- 4.Mathers BM, Degenhardt L, Phillips B, et al. Global epidemiology of injecting drug use and HIV among people who inject drugs: a systematic review. Lancet. 2008;372(9651):1733–1745. [DOI] [PubMed] [Google Scholar]
- 5.Nelson PK, Mathers BM, Cowie B, et al. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: results of systematic reviews. Lancet. 2011;378(9791):571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad C, Bradley HM, Broz D, et al. Community outbreak of HIV infection linked to injection drug use of oxymorphone--Indiana, 2015. MMWR Morb Mortal Wkly Rep. 2015;64(16):443–444. [PMC free article] [PubMed] [Google Scholar]
- 7.Suryaprasad AG, White JZ, Xu F, et al. Emerging epidemic of hepatitis C virus infections among young nonurban persons who inject drugs in the United States, 2006–2012. Clin Infect Dis. 2014;59(10):1411–1419. [DOI] [PubMed] [Google Scholar]
- 8.Fullerton CA, Kim M, Thomas CP, et al. Medication-assisted treatment with methadone: assessing the evidence. Psychiatr Serv. 2014;65(2):146–157. [DOI] [PubMed] [Google Scholar]
- 9.Saxon AJ, Hser YI, Woody G and Ling W. Medication-assisted treatment for opioid addiction: methadone and buprenorphine. J Food Drug Anal. 2013;21(4):S69–s72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mattick RP, Breen C, Kimber J and Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014(2):Cd002207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan LE, Metzger DS, Fudala PJ and Fiellin DA. Decreasing international HIV transmission: the role of expanding access to opioid agonist therapies for injection drug users. Addiction. 2005;100(2):150–158. [DOI] [PubMed] [Google Scholar]
- 12.Gowing L, Farrell M, Bornemann R, Sullivan L and Ali R. Substitution treatment of injecting opioid users for prevention of HIV infection. Cochrane Database Syst Rev. 2008(2):Cd004145. [DOI] [PubMed] [Google Scholar]
- 13.MacArthur GJ, Minozzi S, Martin N, et al. Opiate substitution treatment and HIV transmission in people who inject drugs: systematic review and meta-analysis. BMJ. 2012;345:e5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsui JI, Evans JL, Lum PJ, Hahn JA and Page K. Association of opioid agonist therapy with lower incidence of hepatitis C virus infection in young adult injection drug users. JAMA Intern Med. 2014;174(12):1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nolan S, Dias Lima V, Fairbairn N, et al. The impact of methadone maintenance therapy on hepatitis C incidence among illicit drug users. Addiction. 2014;109(12):2053–2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.White B, Dore GJ, Lloyd AR, Rawlinson WD and Maher L. Opioid substitution therapy protects against hepatitis C virus acquisition in people who inject drugs: the HITS-c study. Med J Aust. 2014;201(6):326–329. [DOI] [PubMed] [Google Scholar]
- 17.Nosyk B, Bray JW, Wittenberg E, et al. Short term health-related quality of life improvement during opioid agonist treatment. Drug Alcohol Depend. 2015;157:121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitchell SG, Gryczynski J, Schwartz RP, et al. Changes in quality of life following buprenorphine treatment: Relationship with treatment retention and illicit opioid use. J Psychoactive Drugs. 2015;47(2):149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oliva EM, Maisel NC, Gordon AJ and Harris AH. Barriers to use of pharmacotherapy for addiction disorders and how to overcome them. Curr Psychiatry Rep. 2011;13(5):374–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heckathorn DD. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–199. [Google Scholar]
- 21.Saloner B and Karthikeyan S. Changes in substance abuse treatment use among individuals with opioid use disorders in the United States, 2004–2013. JAMA. 2015;314(14):1515–1517. [DOI] [PubMed] [Google Scholar]
- 22.Substance Abuse and Mental Health Services Administration CfBHSaQ. Treatment Episode Data Set (TEDS) 2004 – 2014: National Admissions to Substance Abuse Treatment Services. In: Services DoHaH, ed. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2016. [Google Scholar]
- 23.Oliva EM, Harris AH, Trafton JA and Gordon AJ. Receipt of opioid agonist treatment in the Veterans Health Administration: facility and patient factors. Drug Alcohol Depend. 2012;122(3):241–246. [DOI] [PubMed] [Google Scholar]
- 24.Greene P Outpatient drug utilization trends for buprenorphine years 2002–2009. 2010;Available at: http://buprenorphine.samhsa.gov/bwns/2010_presentations_pdf/09_Greene_508.pdf. Accessed: 2014–03-24 (Archived by WebCite® at http://www.webcitation.org/6OJVKGwyg). Accessed March 24, 2014.
- 25.Center for Substance Abuse Treatment. Clinical guidelines for the use of buprenorphine in the treatment of opioid addiction Treatment Improvement Protocol (TIP) Series 40. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2004. [PubMed] [Google Scholar]
- 26.Korthuis PT, Gregg J, Rogers WE, McCarty D, Nicolaidis C and Boverman J. Patients’ reasons for choosing office-based buprenorphine: preference for patient-centered care. J Addict Med. 2010;4(4):204–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gryczynski J, Jaffe JH, Schwartz RP, et al. Patient perspectives on choosing buprenorphine over methadone in an urban, equal-access system. Am J Addict. 2013;22(3):285–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pinto H, Maskrey V, Swift L, Rumball D, Wagle A and Holland R. The SUMMIT trial: a field comparison of buprenorphine versus methadone maintenance treatment. J Subst Abuse Treat. 2010;39(4):340–352. [DOI] [PubMed] [Google Scholar]
- 29.Fiellin DA, Schottenfeld RS, Cutter CJ, Moore BA, Barry DT and O’Connor PG. Primary care-based buprenorphine taper vs maintenance therapy for prescription opioid dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(12):1947–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frimpong JA, D’Aunno T and Jiang L. Determinants of the availability of hepatitis C testing services in opioid treatment programs: results from a national study. Am J Public Health. 2014;104(6):e75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hagan H, Pouget ER, Williams IT, et al. Attribution of hepatitis C virus seroconversion risk in young injection drug users in 5 US cities. J Infect Dis. 2010;201(3):378–385. [DOI] [PubMed] [Google Scholar]
- 33.Amon JJ, Garfein RS, Ahdieh-Grant L, et al. Prevalence of hepatitis C virus infection among injection drug users in the United States, 1994–2004. Clin Infect Dis. 2008;46(12):1852–1858. [DOI] [PubMed] [Google Scholar]
- 34.Smith BD, Morgan RL, Beckett GA, et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. MMWR Recomm Rep. 2012;61(Rr-4):1–32. [PubMed] [Google Scholar]