Abstract
Context:
Although screening recommendations for prostate cancer using prostate-specific antigen testing often include shared decision making, the effect of patient decision aids on patients’ intention and uptake is unclear. This study aimed to review the effect of decision aids on men’s screening intention, screening utilization, and the congruence between intentions and uptake.
Evidence acquisition:
Data sources were searched until April 6, 2018, and included MEDLINE, Scopus, CENTRAL, CT.gov, Cochrane report, PsycARTICLES, PsycINFO, and reference lists. This study included RCTs and observational studies of decision aids that measured prostate screening intention or behavior. The analysis was completed in April 2018.
Evidence synthesis:
Eighteen studies (13 RCTs, four before–after studies, one non-RCT) reported data on screening intention for ≅8,400 men and screening uptake for 2,385 men. Compared with usual care, the use of decision aids in any format results in fewer men (aged ≥40 years) planning to undergo prostate-specific antigen testing (risk ratio=0.88, 95% CI=0.81, 0.95, p=0.006, I2=66%, p<0.001, n=8). Many men did not follow their screening intentions during the first year after using a decision aid; however, most men who were planning to undergo screening did so (probability that men who wanted to be screened would receive screening was 95%).
Conclusions:
Integration of decision aids in clinical practice may result in a decrease in the number of men who elect prostate-specific antigen testing, which may in turn reduce screening uptake. To ensure high congruence between intention and screening utilization, providers should not delay the shared decision-making discussion after patients use a decision aid.
CONTEXT
Prostate cancer is the most commonly diagnosed cancer in men globally.1 Its incidence and mortality rates are higher in more developed countries1,2 and increase with age. Estimates compiled by the Surveillance, Epidemiology, and End Results Program of the U.S. National Cancer Institute predicted3,4 that in 2017, prostate cancer would be the main cause of death from cancer in American men, with 161,360 men (9.6% of all cancer cases in the U.S.) diagnosed with prostate cancer and 26,730 men (4.4% of all cancer deaths in the U.S.) dying from it. Five-year survival rates depend on the stage at diagnosis: 100% for men with local disease, and only 29.8% (95% CI=28.8%, 30.9%) for men (all races, all ages) with distant disease.3 Approximately 12 of 100 American men will eventually be diagnosed with an invasive form of prostate cancer during their lifetime.3
The prostate-specific antigen (PSA) test can be used as a screening test in asymptomatic men to detect prostate cancer. However, research suggests that the potential harms of screening with the PSA test can outweigh its benefits. It is estimated that for every 1,000 men aged 55–69 years who are offered PSA-based screening over the next 13 years that 240 will have a positive test and require a biopsy, of whom 100 will be diagnosed with prostate cancer. Potential benefits include approximately one fewer patient dying of prostate cancer and three fewer patients experiencing metastasis, with no change in all-cause mortality.5,6 Potential harms include worry, a high false positive rate, overdiagnosis (treatment of many men whom the prostate cancer never would have harmed), and a high rate of surgical complications including erectile dysfunction and urinary incontinence.7,8
The decision about PSA-based screening is confounded by current prostate cancer screening recommendations, which differ between countries and professional organizations. Although the majority of professional organizations (36 of 42) in developed countries do not recommend or recommend against national mass screening programs, they disagree about opportunistic and selective screening (Appendix Figure 1). Some professional North American organizations recommend against routine PSA-based screening (American College of Preventive Medicine 2016,9 American Academy of Family Physicians 2012,10 Canadian Task Force Preventive Health Care 201411), whereas others suggest shared decision making (SDM) to help patients decide (U.S. Preventive Services Task Force 2018,5,6 American Urological Association 2013 (confirmed in 2015),12 American College of Physicians 2015,13 National Comprehensive Cancer Network 2018,14 American Cancer Society 201615). The final recommendation statement5 from the U.S. Preventive Services Task Force includes new support for SDM among asymptomatic men in the general U.S. population who are aged 55 to 69 years. This change reflects a general shift, with recent and upcoming recommendations converging on the need for SDM (Appendix Figure 1).
Patient decision aids (DAs) are often used to ensure that patients make informed decisions that are congruent to their values and support SDM. None of the certification efforts around DAs focus on intention or uptake as an outcome; however, these outcomes are important because they demonstrate the effect that DAs and SDM have on patient behavior. The effects of DAs on men’s intentions to undergo PSA-based screening, screening utilization, and their congruence remain unclear. Furthermore, the extent to which certain DA formats (audiovisual materials, only visual, only audio, or interactive computer-based) influence these outcomes is unknown. Finally, previous studies have failed to determine whether men indeed follow through with their stated intention.
The purpose of this systematic review and meta-analysis is to determine how the use of DAs in clinical practice affects men’s intention toward PSA screening, screening utilization rates, and the congruence between intention and screening uptake. This review addresses the following key questions (KQs; Appendix Figure 2): KQ1: In men from various racial and age groups, how do DAs, compared with the usual care, affect their intention to undergo PSA screening? KQ2: In men from various racial and age groups, does the use of DAs, compared with the usual care, decrease the number of men who are undecided about their screening plans? KQ3: In men from various racial and age groups, are computer-based interactive DAs, compared with other types of DAs, more influential in changing screening intention? KQ4: For men, are computer-based interactive prostate cancer screening patient DAs compared with other types of DAs more influential in changing utilization of PSA-based screening? KQ5: After using a DA, to what extent is men’s screening intention associated with a change in the PSA-based screening utilization rate?
EVIDENCE ACQUISITION
The protocol for this review was registered in the PROSPERO database, #CRD42017060606.16 This manuscript followed two guidelines: Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)17 for RCTs, and the Meta-analysis Of Observational Studies in Epidemiology (MOOSE)18 for observational studies.
Data Sources and Searches
The following databases were searched: MEDLINE Epub Ahead of Print, In-Process and Other Non-Indexed Citations, MEDLINE Daily, MEDLINE and Versions, SCOPUS, Cochrane Central Register of Controlled Trials (CENTRAL), PsycINFO, PsycARTICLES, and ClinicalTrial.gov through April 6, 2018, without language restrictions (Appendix Tables 1–3). This search was supplemented by a hand search of reference lists from articles eligible for this review and the Cochrane reports.19–21
Study Selection
Studies were eligible for this systematic review if they met the following predefined criteria: (1) used an intervention involving a DA in any format, (2) reported immediate or deferred intention or utilization data from men aged ≥40 years who were not diagnosed with prostate cancer prior to using a DA, and (3) were RCTs or observational studies (Appendix Table 4). Two reviewers independently screened each title and abstract, and then reviewed full-text articles by using Covidence©, Cochrane’s online systematic review platform. All disagreements were solved through consensus adjudication and the authors confirmed exclusion or inclusion in Covidence©. The authors contacted corresponding authors of potentially eligible papers for further clarifications.
Data Extraction and Quality Assessment
Data extraction was performed with the use of the Covidence© platform. The data were extracted from each eligible study by two reviewers independently. The following data were extracted: study design, study period, patients’ characteristics in each group, numbers of participants enrolled and number with outcome data, ages of participants, details of intervention and comparator, study settings, and other details. Two reviewers independently assessed each study’s risk of bias by using the Cochrane Collaboration tool for assessing the risk of bias for RCTs,22,23 the Risk Of Bias In Non-randomized Studies of Interventions tool24 for a non-RCT and controlled before–after study, and the NIH Quality Assessment Tool25 for uncontrolled before–after studies. Discrepancies were resolved through consensus.
Data Synthesis and Analysis
The overall effect from studies with low or moderate risk of bias was pooled for all RCTs using data from control and intervention groups. Additionally, overall effect across RCTs (baseline data from all groups, after-intervention data in the DA group, and a control group) and before–after studies was pooled. The DerSimonian–Laird random effects model26 with the recommended27 Hartung–Knapp correction were used to calculate risk ratios (RRs) within the “meta” package28 in R, version 3.4.4, accessed with RStudio, Version 1.1.422. The mean difference was used to compare change in patient knowledge. The presence of statistical heterogeneity was assessed with Cochran Q statistics via a chi-square test, and the magnitude of heterogeneity was evaluated with the I2 statistic.29 All studies were stratified based on study design and formats of DAs. Sensitivity analyses included examining the influence of (1) single studies, (2) the used effect model, (3) using before–after intention data in RCTs, and (4) the methodological quality of the studies and their design diversity. The number needed to treat was calculating by using the RR from the statistically significant results (p<0.05) of a meta-analysis.30 Egger’s test31 was used to explore funnel plot asymmetry in meta-analyses with more than ten studies, as recommended.32 Cohen’s κ and its 95% CI were used to evaluate the concordance between men’s screening plans and actual screening behavior during the first year after using a DA. Evidence quality was assessed with the Grading of Recommendations Assessment, Development, and Evaluation (GRADE)33 for RCTs. All conclusions are based on results of the RCTs.
EVIDENCE SYNTHESIS
Study Selection
Of the 1,042 identified records, 20 articles34–53 presenting 18 unique studies met inclusion criteria, 316 records were duplicates, and 706 did not meet the inclusion criteria and were therefore excluded (Figure 1 and Appendix Table 5). Of the 706 excluded articles, 53 were excluded after the full texts were assessed: 22 reported outcomes that were irrelevant to this review, 12 did not indicate a DA as an intervention, seven had an unsuitable study design, seven appeared in the wrong type of publication, and five included populations that did not fit the study protocol. Only one RCT50 and a before–after study40 focused on the effect of DAs on African American men who, compared with white men, are almost twice as likely to be diagnosed with prostate cancer and more than twice as likely to die because of prostate cancer.5,6 For this reason DAs for African American men tend to adopt a tone intended to encourage screening, thus shifting the intention and screening uptake in this population. This RCT50 was not used to pool the overall effect across RCTs that included all races, but was considered separately to evaluate the potential effect of DAs on outcomes in African American men. Both studies40,50 were included in the qualitative synthesis.
Figure 1.
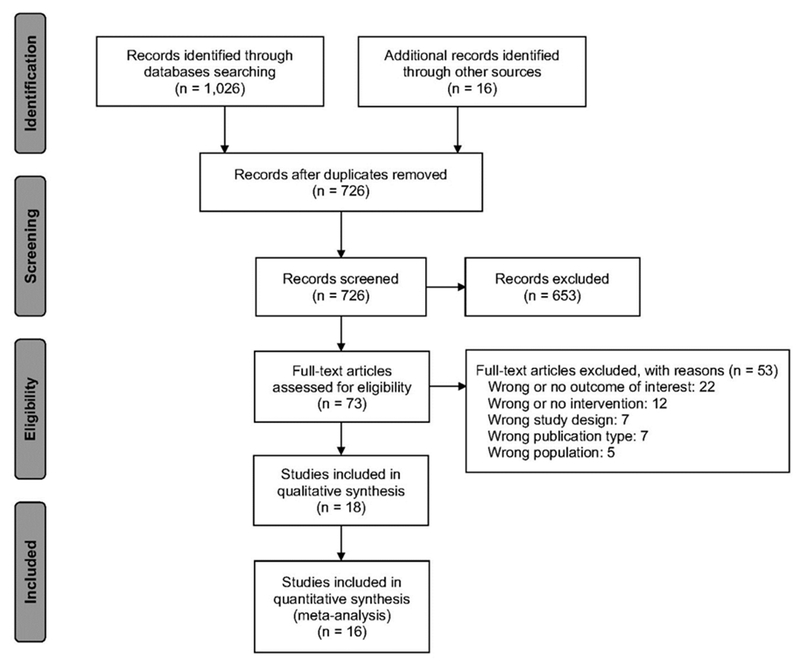
PRISMA flow diagram of study selection.
Study Characteristics
Of the 18 included English-language studies, 1334–38,43–45,47–53 were RCTs, one46 was a non-RCT, and four39–42 were before–after studies. Of all the studies, 1234,36,37,39–43,45–47,50–52 were conducted in the U.S, three44,48,49 in Australia, two35,38 in the United Kingdom, and one53 in France. Studies reported intention to undertake PSA-based screening among men aged 40 to 85 years who: (1) planned to undergo screening,34–42,44,46,48–53 (2) did not wish to be screened,34,38–42,44,48,49,53 (3) were unsure about their screening plans,34,38–42,48,53 or (4) underwent PSA-based screening after using a DA in 1 day,45 243,51 or 347 weeks, 3 months,40 6 months,35 1 year,37,45,50,51 or 2 years.50 The results from individual studies are presented in Table 1 and Appendix Table 6.
Table 1.
Main Characteristics of the Included Studies
| Study, design, country | Study period | N, Population, Age years (mean) or range | Settings | Intervention (I) and comparator (C) | Reported outcomes |
|---|---|---|---|---|---|
| Allen 2010,34 RCT, U.S. |
September 2006–July 2007 | 625, with no prostate cancer, aged 45–70 years | Work (12 work sites with Standard Industrial Classification codes 20 to 39 that represent manufacturing industries were randomly assigned to an intervention or nonintervention comparison condition) | I: interactive DA C: No DA |
Intention, knowledge, decisional conflict |
| Evans 2010,35 RCT, United Kingdom |
NR | 514, men were invited to participate by their general practitioners in South Wales, United Kingdom, mean age not reported, aged 50–75 years | Any Internet based and mailed interventions accessible from home or any setting | I: interactive DA, visual DA C: No DA |
Intention, uptake, knowledge, anxiety, attitude, decisional conflict |
| Frosch 2001,46 Non-RCT, U.S. |
NR | 176, male patients at the Health Appraisal Clinic, in the department of preventive medicine, Kaiser Permanente, San Diego, California, aged >50 years | Outpatient clinic | I: audio-visual DA C: General information about PSA testing (presented orally) |
Uptake, knowledge |
| Frosch 2008,47 RCT, U.S. |
March 2005–May 2006 | 611, Participants were recruited from the Health Appraisal Clinic of Kaiser Permanente, San Diego, California. The clinic conducts preventive screening examinations, aged >50 years | Outpatient clinic | I: interactive DA, visual DA C: Not a DA (men were given links to public websites on prostate cancer screening) |
Uptake, knowledge |
| Gattellari 2003,48 RCT, Australia |
NR | 248, men were recruited from the practices of 13 local general practitioners in Sydney, Australia, ~54, aged 40–70 years | Outpatient clinic (GP’s office) | I: visual DA C: Not a DA (Pamphlet published by Australian Government) |
Intention, knowledge, attitude, decisional conflict |
| Gattellari 2005,49 RCT, Australia |
NR | 421, men recruited by making telephone calls using randomly selected numbers from 29 contiguous postcodes in Sydney, Australia, from the white-page telephone directory, ~58, aged 50–70 years | Community | I: visual DA, audio-visual DA C: Not a DA (Leaflet “Testing for prostate cancer”) |
Intention, knowledge, attitude, decisional conflict |
| Lepore 2012,50 RCT, U.S. |
June 2005–August 2006 | 490, African American men, a high immigrant percentage, 55.04, aged 45–70 years | Community | I: visual DA C: Not a DA (telephone conversation about fruit and vegetable consumption) |
Intention, uptake, knowledge, anxiety, decisional conflict |
| Partin 2004,51,52 RCT, U.S. |
NR | 893, male Veterans with primary care appointments at participating facilities aged ≥50 years | Outpatient clinic (mailed intervention) | I: visual DA, audio-visual DA C: Usual care |
Intention, uptake, knowledge |
| Tran 2015,53 RCT, France |
October 2012–February 2013 | 1,170, men from 86 general practice clinics in France, in both urban and rural environments 61, aged 50–75 years | Outpatient clinic | I: visual DA C: Usual care (physicians answered patients’ questions) |
Intention |
| Volk 1999,36,37 2003, RCT, U.S. |
NR | 158, Male patients seen at primary care at the Family Medicine Center at The University of Texas Medical Branch in Galveston, Texas ~59 years, aged 45–70 years | Outpatient clinic | I: audio-visual DA C: No DA |
Intention, uptake, knowledge |
| Watson 2006,38 RCT, United Kingdom |
NR | 977, men from 11 general practices in England (Oxfordshire and Hertfordshire) and Wales (Cwmbran and Aberystwyth) mean age not reported, aged 40–75 years | Any, (mailed DA) | I: visual DA C: Not a DA (questionnaire only) |
Intention, knowledge, attitude |
| Barry 2015,39 Before after, U.S. |
2009–2013 (data collection) | 1,041, men recruited at primary care practices affiliated with two health systems -Dartmouth-Hitchcock Medical Center and Massachusetts General Hospital, mean age not reported, aged 50– ≥70 years | Any, (mailed DVD) | I: interactive DA C: NA |
Intention, knowledge |
| Frencher 2016,40 Before after, U.S. |
NR | 120, African American men were recruited from 50 barbershops located in South Los Angeles, California, aged ≥40 years | Community (Black Barbershop Health Outreach Program [BBHOP]) | I: audio-visual DA (culturally specific) C: audio-visual DA (not culturally specific) |
Intention, knowledge |
| Ruthman 2004,41 Controlled before-after, U.S. |
NR | 104, Subjects were men who attended a Veterans Administration clinic 66.32, aged 51–77 years | Outpatient clinic | I: audio-visual DA C: No DA |
Intention, Knowledge |
| Sheridan 2004,42 Before-after, U.S. |
NR | 188, men who were presenting to a university internal medicine clinic for routine care, 60, aged 45–85 years | Outpatient clinic | I: visual DA C: NA |
Intention |
| Krist 2007,43 RCT, U.S. |
June 2002–June 2004 | 497, men with no history of prostate cancer, aged 50–70 years | Any, (mailed DA) | I: interactive DA, visual DA C: Usual care |
Uptake, knowledge, decisional conflict |
| Ilic 2008,44 RCT, Australia |
NR | 156, men who have never been screened for prostate cancer, aged >45 years | Any, (mailed DA) | I: audio-visual DA, interactive DA, visual DA C: DAs |
Intention, knowledge, anxiety, decisional conflict |
| Stamm 2017,45 RCT, U.S. |
NR | 329, English speaking men with no history of prostate cancer, men who might have prostate screening more than 1 year ago, aged 50–75 years | Any, (mailed DA) | I: visual DA C: Usual care |
Uptake, knowledge |
NR, not reported; NA, not applicable; PSA, prostate-specific antigen; DA, PSA-based screening patient decision aid.
Risk of bias was assessed (Appendix Tables 7–9) in all studies. Eleven RCTs34–38,44,47–49,51–53 and two non-randomized studies41,46 were considered to have moderate risk of bias. Two RCTs43,45 had a high risk of bias. These two RCTs were omitted from quantitative and qualitative analyses due to high risk of bias; however, the effect of these studies was explored in the sensitivity analysis. Two uncontrolled before–after studies were assigned fair39 and good42 quality rating, and one uncontrolled before–after40 was assigned a poor quality rating.
Prostate cancer screening patient decision aids.
The 18 included studies evaluated 24 DAs (19 unique DAs). Of these 18 studies, 12 studies35,38,42–45,47–53 examined visual DAs (pamphlet or handout), six34,35,39,43,44,47 studies evaluated interactive DAs, and seven36,37,40,41,44,46,49,51,52 assessed audio-visual DAs. Five studies36,40,41,46,51 examined an audio-visual aid called The PSA Decision: What You Need to Know. Some DAs have been updated since the first publication, so the versions of the DA assessed in the studies may not be the same version currently available for patients. For inaccessible DAs evaluations were based solely on available descriptions. Thirteen DAs met all International Patient Decision Aids Standards Qualifying Criteria54 for educational DAs, five lacked sufficient descriptive information to make a determination, and one failed to meet two of the criteria (Appendix Table 10).
Synthesis of the Results
Men’s intention to undergo prostate-specific antigen–based screening.
The overall effect of DAs on men’s willingness to undergo screening was derived from eight RCTs34–38,48,49,51–53 that studied 11 DAs. Compared with control interventions, the use of any format of DA resulted in fewer men planning to undergo PSA-based screening (RR=0.88, 95% CI=0.81, 0.95, p=0.006, I2=66%, p<0.001; Figure 2). This RR was used to calculate that 548 (95% CI=505, 595) of every 1,000 men in the DA group intended to be screened, compared with 623 of 1,000 in the control group. This finding suggests that after using a DA, 75 (95% CI=118, 28) fewer men of 1,000 intended to undergo PSA test, in comparison with usual care (Table 2). With the use of a number needed-to-treat approach, 14 (95% CI=9, 35) men aged 40 to 85 years would need to use any format DA so that one man would reconsider his intention to be screened. Although the evidence was low in quality due to unknown risk of performance bias and considerable inconsistency (Table 2) for this outcome, the analysis of the difference in the number of men at the baseline and after the intervention show a similar significant change in intention for five RCTs34,36,37,48,49,53 (RR=0.82, 95% CI=0.72, 0.93, p<0.01, I2=81%, p<0.01), one non-RCT,46 and one controlled before–after study.41 These findings are consistent.
Figure 2.
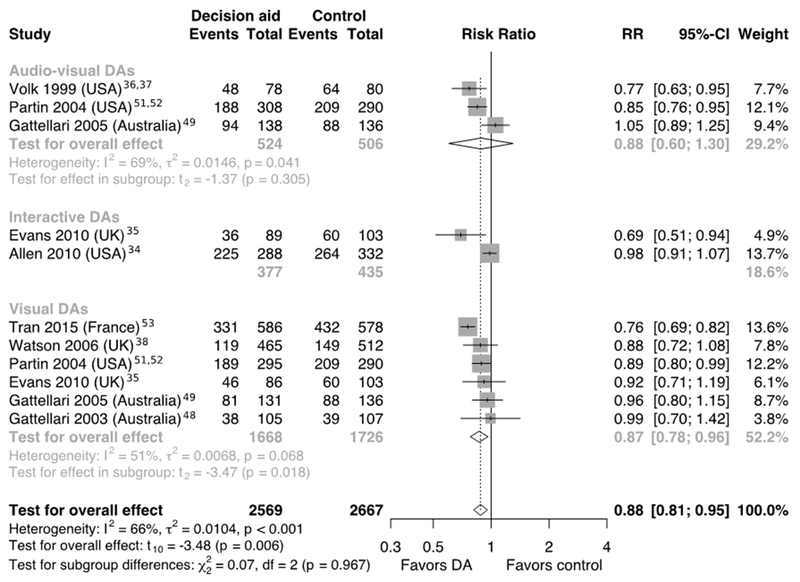
Forest plot of the proportion of men who were planning to undergo PSA-based screening after using various formats of DAs.
aEvents – numbers of men who were intended to undergo screening.
PSA, prostate-specific antigen; DA, PSA-based prostate cancer screening patient decision aid.
Table 2.
GRADE Summary of Findings and Certainty of the Evidence (Only RCTs)
| Outcomes | Anticipated absolute effectsa (95% CI) |
Relative effect (95% CI) |
Number of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Numbers of men with control | Risk difference with DA | |||||
| Planning to undergo screening after using any format of DAs | 623 per 1,000 |
75 fewer per 1,000 (118 to 28 fewer) |
RR 0.88 (0.81, 0.95) |
5,236 (8 RCTs) |
⨁⨁◯◯ Lowb,c |
This effect is consistent with the before-after results from the RCTs |
| Planning to undergo screening after using visual format of DAs | 566 per 1,000 |
76 fewer per 1,000 (125 to 21 fewer) |
RR 0.87 (0.78, 0.96) |
3,394 (6 RCTs) |
⨁⨁⨁◯ Moderateb |
|
| Do not plan to undergo screening after using any format of DAs | 202 per 1,000 | 53 more per 1,000 (17 fewer to 149 more) |
RR 1.26 (0.92, 1.73) |
3,514 (5 RCTs) |
⨁⨁◯◯ Lowb,cc |
|
| Unsure about undergoing screening after using any format of DAs | 210 per 1,000 | 23 more per 1,000 (27 fewer to 86 more) |
RR 1.11 (0.87, 1.41) |
2,973 (4 RCTs) |
⨁⨁◯◯ Lowb,c |
|
The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
Unknown risk of performance bias: blinding of participants.
Downgraded due considerable inconsistency.
Grades of evidence — High certainty: High confidence that the true effect lies close to that of the estimate of the effect. Moderate certainty: Moderate confidence in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Limited confidence: the true effect may be substantially different from the estimate of the effect. Very low certainty: Very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
DA, decision aid
When comparing the effects of various formats of DAs in RCTs on the change in the number of men aged 40 to 82 years planning to undergo the PSA test, the meta-analysis showed that only visual DAs statistically significantly affected men’s intention (RR=0.87, 95% CI=0.78, 0.96, p=0.018, n=6, I2=51%, p=0.068; Figure 2). Using the above RR, 566 of 1,000 men planned to be screened in control groups, compared with 490 (95% CI=441, 545) of 1,000 after using a visual DA. Therefore, the use of visual DAs can result in 76 (95% CI=125, 21) fewer men of 1,000 intending to undergo PSA testing (Table 2). This effect is similar to the results after using a DA in any format.
The number of men who were reluctant to be screened did not appear to be affected in five RCTs34,38,48,49,53 that tested six DAs (RR=1.26, 95% CI=0.92, 1.73, p=0.12, n=6; Appendix Figure 3). Heterogeneity of this result was considerable (I2=70%, p<0.01) and its level was affected by one French trial53 that showed a large, significant increase in the number of men who were reluctant to be screened after using a visual DA. Furthermore, one large uncontrolled before–after study39 supported this change in intention (Appendix Figure 4). However, the overall effect from three before–after studies39,42,41 and before–after results from five RCTs34,44,48,49,53 that studied eight DAs, suggests a significant increase (p=0.04 and p<0.01, respectively) in the number of men who did not want to be screened after using a DA.
These analyses (Appendix Figure 5) did not identify a significant effect of DAs on the proportion of men who were undecided about their screening strategy. However, the before–after result from RCTs (eight DAs) indicates a lower number of men who were undecided about their screening plans after using a DA (Appendix Figure 6).
Effect on screening utilization.
Five RCTs35–37,45,50–52 evaluated the effect of seven DAs on screening behavior. Four of five RCTs studied men in general populations and provided screening behavior data during 2 weeks,51,52 3 weeks,47 6 months,35 or 1 year after using a DA36,37,51,52 (Appendix Figure 7). Compared with the control, the RCTs indicate that the use of DAs leads to a potential decrease in number of men who would undergo PSA testing during the first 3 weeks (RR=0.94, 95% CI=0.90, 0.97, p=0.02, n=3, I2=0%, p=0.91). However, this effect did not hold through the first 6 months (one RCT) or 1 year (two RCTs with three DAs). Only one36,37 of these four studies reported a statistically significant change in PSA test uptake at the 1-year follow-up and showed that 21% fewer (34% [24/70] vs 55% [37/67], p=0.02) men underwent screening after using an audio-visual DA. One RCT50 included African American men only, and this study showed nonsignificant effect of a visual DA (pamphlet) on changing the odds of men undertaking a PSA test during the first 2 years.
Congruence between screening intentions and screening utilization.
One RCT51,52 with audio-visual and visual DAs demonstrated that 61% (188/308) and 64% (189/295) of men planned to undergo PSA testing after using a DA, respectively. However, 67% (6% and 3% more than were intended initially) of the men in each group underwent screening by the end of the first year after using a DA.51,52
Only one RCT36,37 provided patient-level data about men’s primary intention toward PSA testing and screening utilization during the first year after using an audio-visual DA. The Cohen’s κ coefficient 0.29 (95% CI=0.10, 0.48, p=0.007) demonstrates a slight to moderate strength of agreement between men’s screening intentions and screening utilization by the end of the first year after using this DA. Nevertheless, the probability that men who wanted to be screened would receive screening was high (0.95, 95% CI=0.76, 0.99). This result indicates that it is very likely that men who intend to undergo screening will follow through with screening during the first year after using an audio-visual DA. However, many men will not realize their primary intentions.
Secondary Outcomes
Knowledge.
High-quality evidence was found that the use of DAs increased men’s knowledge.34,36,37,47–49 Six RCTs,34,36–38,47–49 one non-RCT,46 and one before–after study41 showed a significant increase in knowledge when compared with the control group. The overall effect in RCTs across (1) all types of DAs34,36,37,47,48,49 was mean difference of 8.45 (95% CI=3.44, 12.46, p<0.01, I2=78%, p<0.01), and (2) visual DAs36,37,47–49 was mean difference of 11.44 (95% CI=2.40, 20.47, p<0.03, I2=79%, p<0.01; Appendix Figure 8). Only one RCT49 showed an insignificant increase in the number of correctly answered knowledge questions after using an audio-visual DA.
Decisional conflict.
Compared with the control group, men who used DAs in any format reported a reduction in decisional conflict p<0.05 (visual DAs47–49 or interactive DAs34,35). Visual DAs were effective at improving men’s feelings of being informed, feeling clear about values, feeling supported,47 and feeling more certain.48 No difference was found in the levels of decisional conflict in studies that compared various formats (visual DA with audio-visual DA,49 and visual DA with interactive DA35).
Attitude.
The use of either an interactive DAs35 or visual DAs38,49 resulted in less favorable attitudes toward screening compared with the control group (p=0.01). After using a visual DA, men had a less positive attitude49 than after using an audio-visual DA (p=0.01). Men using an interactive DA did not differ in attitude from men who used a visual DA (p=0.39).35 Compared with the control group, men in the audio-visual DA group had a similar attitude toward PSA testing in two RCTs.48,49
Anxiety.
There was no significant difference in men’s anxiety level between the intervention and control groups in one RCT.35
Outcomes for African American men.
The use of DAs by African American men resulted in: (1) reduced decisional conflict (visual DA, p=0.014),50 (2) increased knowledge (p<0.001) for both RCT (visual DA50) and before–after (audio-visual DA40) study designs, and (3) no significant difference in men’s anxiety level between the intervention (visual DA) and control groups.50
Publication Bias and Sensitivity Analyses
There was no identified publication bias as assessed by Egger test (p=0.92 for the proportion of men who were planning to undergo PSA testing after using various types of DAs). Omitting single studies did not affect significance of the RR for number of men who wanted to undergo screening. Pooled effect of visual DAs became nonsignificant after removing either Watson et al.38 or Partin and colleagues51,52 studies. The use of the DerSimonian–Laird random effects model without the Hartung–Knapp correction did not affect the significance of the change in men’s intention.
DISCUSSION
Low-quality evidence was found that the use of DAs in any format resulted in fewer men planning to undergo PSA-based screening. The inconsistent findings across studies limits the strength of these results. However, there is a moderate-quality evidence that the use of visual DAs (pamphlet, leaflet, or brochure) resulted in fewer men planning to undergo PSA testing. The use of DAs appeared not to affect the proportion of men who: (1) were reluctant about screening, (2) were unsure about their screening plans, and (3) underwent screening. The use of DAs in any format resulted in men making more informed screening decisions with less decisional conflict, and these men may develop a less favorable attitude toward screening. There is not enough evidence to conclude that the format of the DA affected secondary outcomes.
Compared with the previous reviews,20,55,56 this work identified additional evidence and provides more detailed analysis of the effect of DAs on the change in men’s screening intentions, actual screening uptake, and the congruence of these two. The data from one study57 used in the Cochrane report was not included here because neither intention nor uptake data were found in the original articles. Another advantage of this research is the use of a more conservative approach for the meta-analysis—Hartung–Knapp small sample correction, in order to minimize the probability of type 1 error27; this correction has not been done in previously published reviews.20,55 Also, additional studies not considered by earlier reviews were included, and outcomes for African American men were analyzed separately, which is unique to this review.
The generalizability of these results may be impacted by the fact that included studies enrolled men with a broader age range than is currently recommended by most of the professional organizations. The screening intentions of men from different age groups could not be explored because of a lack of age-stratified intention data in the identified studies. Only four RCTs reported screening utilization for men from the general population and one RCT for African American men—these data were insufficient to draw a strong conclusion about the effect of DAs on men’s actual screening behavior. More RCTs are needed to determine the generalizable effect of the use of DAs on PSA testing utilization.
CONCLUSIONS
Results of this review indicate that the use of visual DAs may decrease the number of men who choose PSA-based prostate cancer screening compared with men not using DAs, particularly in the short term. The findings suggest that engaging men in SDM shortly after using DAs can result in fewer men choosing to be screened, and more men feeling they have made a choice that aligns with their values. However, more high-quality RCTs are needed to validate the relationship between intentions and screening utilization.
Many modeling studies continue to show that PSA-based screening is not cost effective from a societal point of view. Future research should include decision models to explore the extent to which changes in men’s screening behavior, as affected by their use of DAs, influences the cost effectiveness of PSA-based screening programs.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Andrew Hamilton, MS (Research Librarian, Oregon Health & Science University [OHSU]) for help with the search strategy; Professor Rongwei (Rochelle) F. Fu PhD (Biostatistics, OHSU) and Jack Wiedrick, MS (Biostatistician, OHSU) for a statistical consultation. The authors would also like to thank Professor Heidi D. Nelson, MD, MPH, MACP (OHSU) for the careful review of the first submission. This study was supported by National Library of Medicine and the National Institute of Environmental Health Sciences of NIH under Award #T15LM007088. S.J. was supported by the University of Economics (Prague, Czech Republic) Grants IGS VŠE F1/7/2016 and IP 100040. The grantors had no role in the design, conduct, or reporting of the study. The content is solely the responsibility of the authors and does not necessarily represent the official views of the grantors disclosed above.
Footnotes
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Fitzmaurice C, Allen C, Barber RM, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 32 cancer groups, 1990 to 2015. JAMA Oncol. 2017;3(4):524–548. 10.1001/jamaoncol.2016.5688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen S-L, Wang S-C, Ho C-J, et al. Prostate cancer mortality-to-incidence ratios are associated with cancer care disparities in 35 countries. Sci Rep. 2017;7:40003 10.1038/srep40003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SEER Cancer Stat Facts: Prostate Cancer. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/statfacts/html/prost.html. Published 2017. Accessed December 23, 2017. [Google Scholar]
- 4.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 5.Grossman DC, Curry SJ, Owens DK, et al. Screening for prostate cancer. JAMA. 2018;319(18):1901–1913. 10.1001/jama.2018.3710. [DOI] [PubMed] [Google Scholar]
- 6.Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen–based screening for prostate cancer. JAMA. 2018;319(18):1914–1931. 10.1001/jama.2018.3712. [DOI] [PubMed] [Google Scholar]
- 7.Fenton JJ, Weyrich MS, Durbin S, Liu Y, Bang H, Melnikow J. Prostate-specific antigen–based screening for prostate cancer: a systematic evidence review for the U.S. Preventive Services Task Force. Evidence Synthesis No. 154 AHRQ Publication No. 17–05229-EF-1. Rockville, MD: Agency for Healthcare Research and Quality; 2017. [PubMed] [Google Scholar]
- 8.Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2017: a review of current American Cancer Society guidelines and current issues in cancer screening. Cancer J Clin. 2017;67(2):100–121. 10.3322/caac.21392. [DOI] [PubMed] [Google Scholar]
- 9.Livingston CJ, Freeman RJ, Mohammad A, et al. Choosing Wisely® in preventive medicine. Am J Prev Med. 2016;51(1):141–149. 10.1016/j.amepre.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 10.Mulhem E, Fulbright N, Duncan N. Prostate cancer screening. Am Fam Physician. 2015;92(8):683–688. [PubMed] [Google Scholar]
- 11.Bell N, Gorber SC, Shane A, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. Can Med Assoc J. 2014;186(16):1225–1234. 10.1503/cmaj.140703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter HB. American Urological Association (AUA) Guideline on prostate cancer detection: process and rationale. BJU Int. 2013;112(5):543–547. 10.1111/bju.12318. [DOI] [PubMed] [Google Scholar]
- 13.Wilt TJ, Harris RP, Qaseem A. Screening for cancer: advice for high-value care from the American College of Physicians. Ann Intern Med. 2015;162(10):718–725. 10.7326/M14-2326. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: prostate: prostate cancer early detection (version 2.2018). https://www.nccn.org/professionals/physician_gls/pdf/prostate_detection.pdf. Published 2018. Accessed June 15, 2018. [Google Scholar]
- 15.American Cancer Society. American Cancer Society recommendations for prostate cancer early detection. Prostate cancer prevention and early detection. www.cancer.org/cancer/prostatecancer/moreinformation/prostatecancerearlydetection/prostate-cancer-early-detection-acs-recommendations. Published 2015. Accessed December 7, 2017. [Google Scholar]
- 16.Ivlev I, Eden KB, Mishra M, Jerabkova S. Systematic review and meta-analysis of change in men’s screening behavior after using a prostate cancer screening patient decision aid. Protocol CRD42017060606. www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42017060606. Published 2017. Accessed October 1, 2017. [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman D. The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. JAMA. 2000;283(15):2008–2012. 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 19.Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;(1):CD001431 10.1002/14651858.CD001431.pub4. [DOI] [PubMed] [Google Scholar]
- 20.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431 10.1002/14651858.CD001431.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stacey D, Légaré F, Lewis KB. Patient decision aids to engage adults in treatment or screening decisions. JAMA. 2017;318(7):657–658. 10.1001/jama.2017.10289. [DOI] [PubMed] [Google Scholar]
- 22.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (eds), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0. Cochrane; www.training.cochrane.org/handbook. Published 2017. Accessed October 3, 2017. [Google Scholar]
- 23.Reeves BC, Deeks JJ, Higgins JPT, Wells GA. Chapter 13.5: Assessing risk of bias in non-randomized studies In: Higgins JPT, Green S (eds), Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. The Cochrane Collaboration; www.cochrane-handbook.org. Published 2011. Accessed October 3, 2017. [Google Scholar]
- 24.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Heart Lung and Blood Institute (NHLBI). Quality Assessment Tool for Before-After (Pre-Post) Studies With No Control Group. Study Quality Assessment Tools. www.nhlbi.nih.gov/health-pro/guidelines/in-develop/cardiovascular-risk-reduction/tools/before-after. Published April 2014. Accessed February 3, 2017.
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 27.Cornell JE, Mulrow CD, Localio R, et al. Random-effects meta-analysis of inconsistent effects: a time for change. Ann Intern Med. 2014;160(4):267–270. 10.7326/M13-2886. [DOI] [PubMed] [Google Scholar]
- 28.Schwarzer G meta: An R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 29.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schünemann HJ, Oxman AD, Vist GE, et al. Chapter 12.4.4.2: Computing absolute risk reduction or NNT from a risk ratio (RR) In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (eds), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0. www.training.cochrane.org/handbook. Published 2017. Accessed October 1, 2017. [Google Scholar]
- 31.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 33.Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction - GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 34.Allen JD, Othus MKD, Hart A, et al. A randomized trial of a computer-tailored decision aid to improve prostate cancer screening decisions: results from the Take the Wheel Trial. Cancer Epidemiol Biomarkers Prev. 2010;19(9):2172–2186. 10.1158/1055-9965.EPI-09-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Evans R, Joseph-Williams N, Edwards A, et al. Supporting informed decision making for prostate specific antigen (PSA) testing on the web: An online randomized controlled trial. J Med Internet Res. 2010;12(3):e27 10.2196/jmir.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Volk RJ, Cass AR, Spann SJ. A randomized controlled trial of shared decision making for prostate cancer screening. Arch Fam Med. 1999;8(4):333–340. 10.1001/archfami.8.4.333. [DOI] [PubMed] [Google Scholar]
- 37.Volk RJ, Spann SJ, Cass AR, Hawley ST. Patient education for informed decision making about prostate cancer screening: a randomized controlled trial with 1-year follow-up. Ann Fam Med. 2003;1(1):22–28. 10.1370/afm.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Watson E, Hewitson P, Brett J, et al. Informed decision making and prostate specific antigen (PSA) testing for prostate cancer: A randomised controlled trial exploring the impact of a brief patient decision aid on men’s knowledge, attitudes and intention to be tested. Patient Educ Couns. 2006;63(3):367–379. 10.1016/j.pec.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 39.Barry MJ, Wexler RM, Brackett CD, et al. Responses to a decision aid on prostate cancer screening in primary care practices. Am J Prev Med. 2015;49(4):520–525. 10.1016/j.amepre.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 40.Frencher SK, Sharma AK, Teklehaimanot S, et al. PEP talk: Prostate Education Program, “Cutting through the uncertainty of prostate cancer for black men using decision support instruments in barbershops.” J Cancer Educ. 2016;31(3):506–513. 10.1007/s13187-015-0871-7. [DOI] [PubMed] [Google Scholar]
- 41.Ruthman JL, Ferrans CE. Efficacy of a video for teaching patients about prostate cancer screening and treatment. Am J Health Promot. 2004;18(4):292–295. 10.4278/0890-1171-18.4.292. [DOI] [PubMed] [Google Scholar]
- 42.Sheridan SL, Felix K, Pignone MP, Lewis CL. Information needs of men regarding prostate cancer screening and the effect of a brief decision aid. Patient Educ Couns. 2004;54(3):345–351. 10.1016/j.pec.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 43.Krist AH, Woolf SH, Johnson RE, Kerns JW. Patient education on prostate cancer screening and involvement in decision making. Ann Fam Med. 2007;5(2):112–119. 10.1370/afm.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ilic D, Egberts K, McKenzie JE, Risbridger G, Green S. Informing men about prostate cancer screening: a randomized controlled trial of patient education materials. J Gen Intern Med. 2008;23(4):466–471. 10.1007/s11606-007-0466-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stamm AW, Banerji JS, Wolff EM, et al. A decision aid versus shared decision making for prostate cancer screening: results of a randomized, controlled trial. Can J Urol. 2017;24(4):8910–8917. [PubMed] [Google Scholar]
- 46.Frosch DL, Kaplan RM, Felitti V. Evaluation of two methods to facilitate shared decision making for men considering the prostate-specific antigen test. J Gen Intern Med. 2001;16(6):391–398. 10.1046/j.1525-1497.2001.016006391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frosch DL, Bhatnagar V, Tally S, Hamori CJ, Kaplan RM. Internet patient decision support: A randomized controlled trial comparing alternative approaches for men considering prostate cancer screening. Arch Intern Med. 2008;168(4):363–369. 10.1001/archinternmed.2007.111. [DOI] [PubMed] [Google Scholar]
- 48.Gattellari M, Ward JE. Does evidence-based information about screening for prostate cancer enhance consumer decision-making? A randomised controlled trial. J Med Screen. 2003;10(1):27–39. 10.1258/096914103321610789. [DOI] [PubMed] [Google Scholar]
- 49.Gattellari M, Ward JE. A community-based randomised controlled trial of three different educational resources for men about prostate cancer screening. Patient Educ Couns. 2005;57(2):168–182. 10.1016/j.pec.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 50.Lepore SJ, Wolf RL, Basch CE, et al. Informed decision making about prostate cancer testing in predominantly immigrant black men: a randomized controlled trial. Ann Behav Med. 2012;44(3):320–330. 10.1007/s12160-012-9392-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Partin MR, Nelson D, Radosevich D, et al. Randomized trial examining the effect of two prostate cancer screening educational interventions on patient knowledge, preferences, and behaviors. J Gen Intern Med. 2004;19(8):835–842. 10.1111/j.1525-1497.2004.30047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Partin MR, Nelson D, Flood AB, Friedemann-Sanchez G, Wilt TJ. Who uses decision aids? Subgroup analyses from a randomized controlled effectiveness trial of two prostate cancer screening decision support interventions. Health Expect. 2006;9(3):285–295. 10.1111/j.1369-7625.2006.00400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tran VT, Kisseleva-Romanova E, Rigal L, Falcoff H. Impact of a printed decision aid on patients’ intention to undergo prostate cancer screening: a multicentre, pragmatic randomised controlled trial in primary care. Br J Gen Pract. 2015;65(634):e295–e304. 10.3399/bjgp15X684817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Durand M-A, Witt J, Joseph-Williams N, et al. Minimum standards for the certification of patient decision support interventions: feasibility and application. Patient Educ Couns. 2015;98(4):462–468. 10.1016/j.pec.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 55.Volk RJ, Hawley ST, Kneuper S, et al. Trials of decision aids for prostate cancer screening. Am J Prev Med. 2007;33(5):428–434. 10.1016/j.amepre.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 56.Evans R, Edwards A, Brett J, et al. Reduction in uptake of PSA tests following decision aids: systematic review of current aids and their evaluations. Patient Educ Couns. 2005;58(1):13–26. 10.1016/j.pec.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 57.Wolf AM, Nasser JF, Wolf AM, Schorling JB. The impact of informed consent on patient interest in prostate-specific antigen screening. Arch Intern Med. 1996;156(12):1333–1336. 10.1001/archinte.1996.00440110105014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


