Watch a video presentation of this article
Abbreviations
- BMI
body mass index
- LFT
liver function test
- LPS
lipopolysaccharide
- NAFL
nonalcoholic fatty liver
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- RF
Random Forest
- US
ultrasound
Nonalcoholic fatty liver disease (NAFLD) is the most common cause of liver disease worldwide, affecting approximately 25% of the population, and is quickly becoming the leading indication for liver transplantation.1 NAFLD encompasses a spectrum of liver pathology from steatosis or nonalcoholic fatty liver (NAFL), to the inflammatory state of nonalcoholic steatohepatitis (NASH), and eventually to cirrhosis in a subset of patients.1
Pathophysiology
Current evidence suggests the etiology of NAFLD is multifactorial and includes genetic factors, diet, a disturbance or imbalance in the gut microbiome known as dysbiosis, altered production of gut metabolites, gut luminal barrier dysfunction, and endothelial translocation of proinflammatory substances that results in hepatic inflammation.2 In some patients, an inflammatory cascade leads to histological changes within the liver resulting in NASH, with recurrent insults and parenchymal remodeling resulting in hepatic fibrosis and cirrhosis (Fig. 1).
Figure 1.
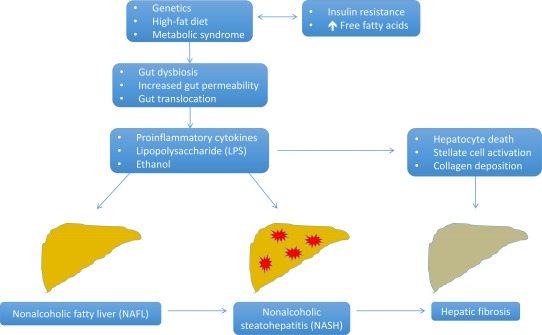
Key factors and pathways involved in the establishment and progression of NAFLD to hepatic fibrosis. Genetics and a high‐fat diet contribute to insulin resistance and an increase in plasma free fatty acids, which can lead to the development of the metabolic syndrome. These factors contribute to dysbiosis of the gut microbiome and increased gut epithelial permeability. The compromised intestinal barrier allows gut translocation of proinflammatory cytokines, ethanol produced from gut bacteria, and LPS derived from the cell wall of gram‐negative bacteria. Delivery of gut‐derived substances directly to the liver via the portal circulation results in an inflammatory cascade with resultant hepatic inflammation and development of NASH in some patients with NAFL. This results in hepatocyte death and activation of hepatic stellate cells, which leads to collagen deposition and subsequent liver fibrosis in a subset of patients with NASH.
Gut Microbiome Dysbiosis in NAFLD
The human gut microbiome is comprised mainly of bacteria, but also includes viral, fungal, and archaeal microorganisms.2 By using 16S rRNA sequencing, it has been shown that two of the most abundant bacterial phyla of the gut microbiome are Bacteroidetes and Firmicutes.3 Human studies examining the alteration of the gut microbiome in patients with NAFLD and NASH compared with healthy control subjects have yielded significant heterogeneity. Children with biopsy‐proven NASH or who were obese demonstrated a significant increase in the phylum of Bacteroidetes and a decrease in Firmicutes compared with healthy control subjects.4 However, similar results have not been found in adults. A study of obese adults with clinically suspected NAFLD showed there was no significant difference in the proportion of Firmicutes to Bacteroidetes between NAFLD and control groups.5 These results were replicated in patients with biopsy‐proven NAFLD versus healthy control subjects with normal liver biopsies, although the relative abundance of Bacteroidetes was significantly lower in the NASH group.6 Among the small number of human studies that sequenced the microbiome of individuals with NAFLD there was significant variation of study designs, inclusion criteria, and conflicting results of bacterial abundance (Table 1). Nonetheless, the gut microbiome appears to be closely associated with the pathophysiology of NALFD development.
Table 1.
Summary of Studies Examining the Gut Microbiome and Its Relationship to NAFLD and NASH
| Study | Study Groups | Results |
|---|---|---|
| Boursier et al. (2016)11 |
Biopsy‐proven: 22 Patients with NAFL 35 Patients with NASH |
↑ Bacteroides in NASH versus NAFL ↓ Prevotella in NASH versus NAFL Bacteroides abundance independently associated with NASH Ruminococcus abundance independently associated with fibrosis F ≥ 2 |
| Loomba et al. (2017)7 |
86 Biopsy‐proven NAFLD patients: (G1) 72 Patients with stage 0‐2 fibrosis (G2) 14 Patients with stage 3‐4 fibrosis |
↑ Bacteroidetes in G1
↑ Proteobacteria in G2 Bacteroides vulgatus and Eubacterium rectale were most abundant species in G1 Bacteroides vulgatus and Escherichia coli were most abundant species in G2 |
| Raman et al. (2013)5 |
30 Patients with NAFLD = elevated LFTs > 1.5 × normal, BMI > 30 kg/m2, echogenic liver on US 30 Control patients = BMI < 25 kg/m2, normal LFTs |
↑ Lactobacillus in NAFLD versus control ↓ Oscillibacter, Robinsoniella, Roseburia, and Dorea in NAFLD versus control No difference in Bacteroides |
| Spencer et al. (2011)12 | 15 Healthy female patients, BMI 18‐34 kg/m2; given choline‐deficient diet to induce fatty liver with stool samples before and after |
No change in the abundance of any taxon at any point in the study Each subject's microbiome remained distinct throughout the study course |
| Wong et al. (2013)13 | 16 Patients with biopsy‐proven NASH 22 Control patients = normal LFTs, no chronic liver disease, BMI < 25 kg/m2 |
↑ Phylum Firmicutes in NASH versus control ↑ Parabacteroides and Allisonella in NASH versus control ↓ Faecalibacterium and Anaerosporobacter in NASH versus control |
| Zhu et al. (2013)4 |
22 Children with biopsy‐proven NASH 25 Obese children, BMI > 95th percentile 16 Healthy control subjects, BMI < 85th percentile |
↑ Phylum Bacteroidetes in obese and NASH versus control ↓ Phylum Firmicutes in obese and NASH versus control ↑ Enterobacteriaceae and Escherichia in NASH versus obese |
Abbreviations: LFT, liver function test; US, ultrasound.
Gut Microbiome Signature for Assessment of Liver Fibrosis in NAFLD
The most feared complication of NAFLD is the progression to NASH and subsequently cirrhosis. The presence of advanced fibrosis is the most important predictor for liver‐related events and complications in NAFLD.7 A novel method for noninvasive assessment of liver fibrosis in NAFLD using a gut microbiome‐based metagenomics signature was recently published. The study sequenced the gut microbiome of 86 patients with biopsy‐proven NAFLD. A total of 72 patients with stage 0 to 2 fibrosis were considered mild‐to‐moderate NAFLD, and 14 patients with stage 3 to 4 fibrosis were considered advanced NAFLD. The phylum of gram‐positive Firmicutes was significantly higher in the mild‐to‐moderate NAFLD group, whereas gram‐negative Proteobacteria was higher in the advanced fibrosis group. A trend toward an increase in gram‐negative bacteria in the advanced NAFLD group was also seen at the species level with an increase in Escherichia coli, albeit not statistically significant. A Random Forest (RF) model was generated using 37 bacterial species with Shannon diversity, age, and body mass index (BMI). The diagnostic accuracy for advanced NAFLD using this RF model had an impressive area under the curve of 0.936.7
Increased Intestinal Permeability
The gastrointestinal epithelium forms a mechanical barrier separating the luminal contents, including the commensal organisms of the gut and their proinflammatory products, from the portal circulation, which provides the majority of the blood flow to the liver. One key factor in the development and progression of NAFLD is the integrity of the intestinal barrier, which is in part mediated by the microbiome. A meta‐analysis including 128 patients with NAFLD showed that 39.1% of these patients had evidence for increased intestinal permeability based on urinary excretion of a measured compound compared with only 6.8% of healthy control subjects. In patients with NASH, the percentage of individuals with increased intestinal permeability was even higher at 49.2%.8 It is unclear whether liver injury or compromise of the intestinal epithelium comes first, but nevertheless, current data support a correlation between the two findings.
Gut Translocation
A compromised intestinal epithelium leads to translocation of potentially harmful substances derived from the gut microbiota into the portal circulation and directly to the liver. Lipopolysaccharide (LPS), also known as endotoxin, is derived from the cell wall of gram‐negative bacteria. Obese patients with biopsy‐proven NASH were shown to have significantly higher levels of plasma IgG against endotoxin compared with obese patients with a normal liver biopsy. The levels of IgG also correlated with NASH severity on histology.9 Thus, chronic endotoxin exposure in patients with NAFLD appears to induce a proinflammatory cascade within the liver parenchyma that may contribute to the progression of NAFL to NASH and worsen the severity of NASH.
Microbiome‐Directed Therapy in NAFLD
Although it is unclear what specific gut bacterial milieu is most beneficial in preventing or perhaps reversing NAFLD, one therapeutic approach would be to restore gut homeostasis via manipulation of the gut microbiome through the use of probiotics. A meta‐analysis of four randomized trials with a total of 134 patients with NAFLD and NASH demonstrated that administration of probiotics resulted in significantly decreased levels of alanine aminotransferase, aspartate aminotransferase, total cholesterol, high‐density lipoprotein, tumor necrosis factor‐α, and homeostasis model assessment of insulin resistance; however, histological assessments were not performed in any studies.10
Conclusions
Current data suggest gut microbiome dysbiosis is closely related to the onset and progression of NAFLD through multiple mechanisms including disruption of the intestinal epithelial barrier and subsequent gut translocation of proinflammatory substances via the portal circulation to the liver. Studies that have sequenced the gut microbiome in patients with NAFLD demonstrate significant heterogeneity in their design and results, making data interpretation and generalizability difficult.
Although there is no clear predominant bacterium that mediates the development of NAFLD, using the microbiome to identify individuals with NAFLD who have advanced fibrosis may allow for earlier clinical intervention. There appears to be a potential role for probiotics in clinical practice, perhaps as an adjunct to new emerging therapies. Further studies are clearly needed to elucidate the relationship between the gut microbiome and NAFLD.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease: meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73‐84. [DOI] [PubMed] [Google Scholar]
- 2. Leung C, Rivera L, Furness JB, Angus PW. The role of the gut microbiota in NAFLD. Nat Rev Gastroenterol Hepatol 2016;13:412‐425. [DOI] [PubMed] [Google Scholar]
- 3. Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. Non‐alcoholic fatty liver and the gut microbiota. Mol Metab 2016;5:782‐794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu L, Baker SS, Gill C, Liu W, Alkhouri R, Baker RD, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57:601‐609. [DOI] [PubMed] [Google Scholar]
- 5. Raman M, Ahmed I, Gillevet PM, Probert CS, Ratcliffe NM, Smith S, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2013;11:868‐875. [DOI] [PubMed] [Google Scholar]
- 6. Mouzaki M, Comelli EM, Arendt BM, Bonengel J, Fung SK, Fischer SE, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013;58:120‐127. [DOI] [PubMed] [Google Scholar]
- 7. Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome‐based metagenomic signature for non‐invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab 2017;25:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Luther J, Garber JJ, Khalili H, Dave M, Bale SS, Jindal R, et al. Hepatic injury in nonalcoholic steatohepatitis contributes to altered intestinal permeability. Cell Mol Gastroenterol Hepatol 2015;1:222‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verdam FJ, Rensen SS, Driessen A, Greve JW, Buurman WA. Novel evidence for chronic exposure to endotoxin in human nonalcoholic steatohepatitis. J Clin Gastroenterol 2011;45:149‐152. [DOI] [PubMed] [Google Scholar]
- 10. Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta‐analysis. World J Gastroenterol 2013;19:6911‐6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo‐Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 2016;63:764‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology 2011;140:976‐986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wong VW, Tse CH, Lam TT, Wong GL, Chim AM, Chu WC, et al. Molecular characterization of the fecal microbiota in patients with nonalcoholic steatohepatitis ‐ a longitudinal study. PloS One 2013;8:e62885. [DOI] [PMC free article] [PubMed] [Google Scholar]


