Watch a video presentation of this article
Watch the interview with the author
Abbreviations
- MELD
Model for End‐Stage Liver Disease
- UNOS
United Network for Organ Sharing
In liver transplantation, ABO‐incompatible liver grafts historically have been used when no suitable identical or compatible grafts were available. In this article, we review the basic immunology of the A1 and A2 phenotypes within blood type A that make this subtype suitable for transplantation in blood type O patients, as well as the clinical outcomes of A2→O liver transplantation. We then explore the current state of A2→O liver transplantation and potential modifications to current allocation policies to promote the use of A2 livers for ABO‐incompatible transplantation.
Immunology
Of the major phenotypes within blood type A, the A1 and A2 phenotypes are distinguished by their reactivity to type B sera, which contains two major antibodies: anti‐A and anti‐A1. In contrast with A1, the A2 phenotype has a lower cell surface expression of the A antigen because of the lower activity of its transferase, which converts the H precursor polysaccharide to A antigen.1 This lower cell surface expression of the A antigen was first exploited in skin grafting, in which cases demonstrated similar survival rates between A2→O and O→O recipients in the 1960s.2 In the 1980s and 1990s, pioneering work conducted by researchers in Midwest Transplant Network demonstrated that A2→O and A2→B kidney grafts had excellent graft and patient survival rates in recipients with low anti‐A titers.3 Given the successful results in kidneys, it was thought that A2 donor livers would also elicit weaker immunogenic responses in ABO‐incompatible recipients (Fig. 1).
Figure 1.
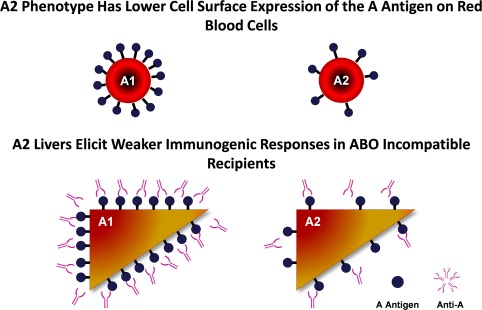
A2 livers elicit weaker immunogenic responses in ABO‐incompatible recipients.
Clinical Outcomes
Initial data from a 1999 single‐center case series of six patients who underwent A2→O liver transplantation with no augmented immunomodulation showed excellent intermediate‐term graft survival at a mean follow‐up of 665 days, but high rates of rejection (nine episodes among the six patients).4 Subsequent case series from Sweden and Canada reported 13 more cases of A2→O liver transplantation with induction immunosuppression; favorable graft and patient survivals rates were reported.5, 6 These encouraging reports prompted a United Network for Organ Sharing (UNOS) registry‐based study of 358 A2 graft recipients from 1990 to 2010.7 Compared with O→O recipients, A2→O recipients were found to have higher Model for End‐Stage Liver Disease (MELD) scores and likelihood of being hospitalized at transplantation.7 Despite this, A2→O recipients had no significant differences in rejection episodes during the index transplant admission or at 12 months. Notably, unadjusted 1‐, 3‐, 5‐, and 10‐year overall survival rates were higher in the A2 group (87%, 80%, 77%, and 63%, respectively) compared with the O group (87%, 79%, 74%, and 61%, respectively), although these results were not statistically significant.7
We recently conducted an analysis of ABO nonidentical liver transplantation using a more contemporary cohort of liver transplant recipients in the United States using data from the UNOS registry from 2013 to 2015. In this cohort, the vast majority (137/154 [87%]) of non‐status 1 ABO‐incompatible transplants were A2→O.8 Additional analyses of posttransplant survival rates demonstrated that the recipients of A2→O grafts had similar 1‐year survival rates (hazard ratio 1.11, 95% confidence interval 0.64‐1.92, P = 0.72) compared with recipients of O→O grafts in adjusted multivariable Cox models (Fig. 2).
Figure 2.
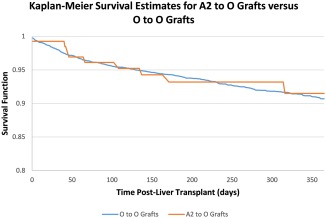
Recipients of A2→O grafts had similar 1‐year posttransplant survival rates compared with recipients of O→O grafts.
Policy Implementations
Even though A2→O liver transplantation has been demonstrated to be safe in retrospective studies, it has been underused across the United States. Blood type O candidates currently suffer with the longest median wait time (380 days) and highest median MELD at transplantation (29). This problem is partly exacerbated by ABO‐compatible transplantation, which disproportionately shunts O livers from O candidates at an estimated 6% net loss in O organs.9 Under current policies, O recipients who voluntarily accept an A2 liver are allocated only five points for blood type matching, the same as an ABO‐compatible match.10 A2→O liver transplantation could decrease wait‐list times and mortality for O candidates, but we found that it is being adopted in a piecemeal fashion. Most numbers of A2→O matching are taking place in regions 5 (23%), 2 (14%), 11 (13%), and 3 (10%); notably, these regions were neither the ones with the highest median MELD scores at transplantation, nor were they similar in the relative utilization of A2→O as a percentage of total O transplants (Fig. 3).8 This demonstrated that MELD score or relative donor scarcity is not the sole driver of A2→O transplantation, and that these differences may reflect center‐level variations or protocols, which warrants further investigation.
Figure 3.
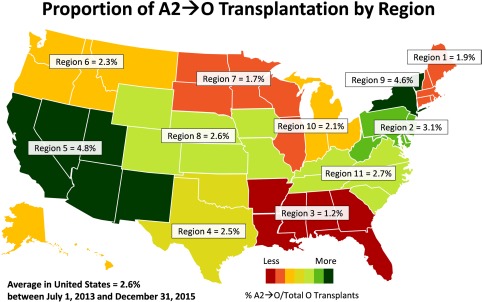
There are still substantial regional variations in the use of A2→O liver transplantation.
A2 Liver Transplantation Across the ABO Barrier
Given that current Organ Procurement and Transplantation Network policies have an underused mechanism for A2→O, the transplantation community should encourage the broader use of A2 livers by O recipients. One possible modification is to treat A2 donor livers as functionally equivalent to O donor livers, awarding 10 points for blood‐type matching rather than 5 points to O recipients who accept A2 livers. Another modification would be to promote A2→B liver transplantation by awarding points to B recipients who voluntarily accept A2 livers. Both of these proposals would allow O candidates to further expand their accessible donor pool and alleviate O→non‐O shunting. However, any policy changes that affect the allocation of A2 donor livers may worsen the shunting of ABO‐compatible donor organs away from type A candidates: type A donor livers are currently made available for ABO nonidentical transplantation at a relatively low median MELD score of 19, compared with the 28 to 34 range for other type livers.9 A2→O and A2→B in these situations may help encourage ABO nonidentical transplantation at higher MELD scores, thereby satisfying the mandates of the US Department of Health and Human Services' Final Rule. Ultimately, we believe that the transplant community should further investigate this topic and develop more comprehensive policies and guidelines regarding A2 liver transplantation across the ABO barrier.
Potential conflict of interest: Nothing to report.
REFERENCES
- 1. Procter J, Crawford J, Bunce M, Welsh KI. A rapid molecular method (polymerase chain reaction with sequence‐specific primers) to genotype for ABO blood group and secretor status and its potential for organ transplants. Tissue Antigens 1997;50:475‐483. [DOI] [PubMed] [Google Scholar]
- 2. Visetti M, Leigheb G, Scudeller G, Ceppellini R. The importance of subgroups A1‐A2 and cross reactions A‐B for the survival of skin allografts. Minerva Dermatol. 1967;42:563‐569. [PubMed] [Google Scholar]
- 3. Alkhunaizi AM, de Mattos AM, Barry JM, Bennett WM, Norman DJ. Renal transplantation across the ABO barrier using A2 kidneys. Transplantation 1999;67:1319‐1324. [DOI] [PubMed] [Google Scholar]
- 4. Fishbein TM, Emre S, Guy SR, Sheiner PA, Kim LH, Schwartz ME, Miller CM. Safe transplantation of blood type A2 livers to blood type O recipients. Transplantation 1999;67:1071‐1073. [DOI] [PubMed] [Google Scholar]
- 5. Skogsberg U, Breimer ME, Friman S, Mjörnstedt L, Mölne J, Olausson M, et al. Successful ABO‐incompatible liver transplantation using A2 donors. Transplant Proc 2006;38:2667‐2670. [DOI] [PubMed] [Google Scholar]
- 6. Toso C, Al‐Qahtani M, Alsaif FA, Bigam DL, Meeberg GA, James Shapiro AM, et al. ABO‐incompatible liver transplantation for critically ill adult patients. Transpl Int 2007;20:675‐681. [DOI] [PubMed] [Google Scholar]
- 7. Kluger MD, Guarrera JV, Olsen SK, Brown RS Jr, Emond JC, Cherqui D. Safety of blood group A2‐to‐O liver transplantation: an analysis of the United Network of Organ Sharing database. Transplantation 2012;94:526‐531. [DOI] [PubMed] [Google Scholar]
- 8. Ge J, Roberts JP, Lai JC. Race/ethnicity is associated with ABO‐nonidentical liver transplantation in the United States. Clin Transplant 2017;31. doi: 10.1111/ctr.13011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lai JC, Roberts JP. ABO‐nonidentical liver transplantation in the United States. Am J Transplant 2016;16:2430‐2436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Organ Procurement and Transplantation Network . OPTN policies. 2016. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Accessed: 6 September 2017.


